Impact of Human Granulocytic Anaplasmosis in Spain from 1997 to 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Case Selection and Data Collection
2.2. Data Analysis
3. Results
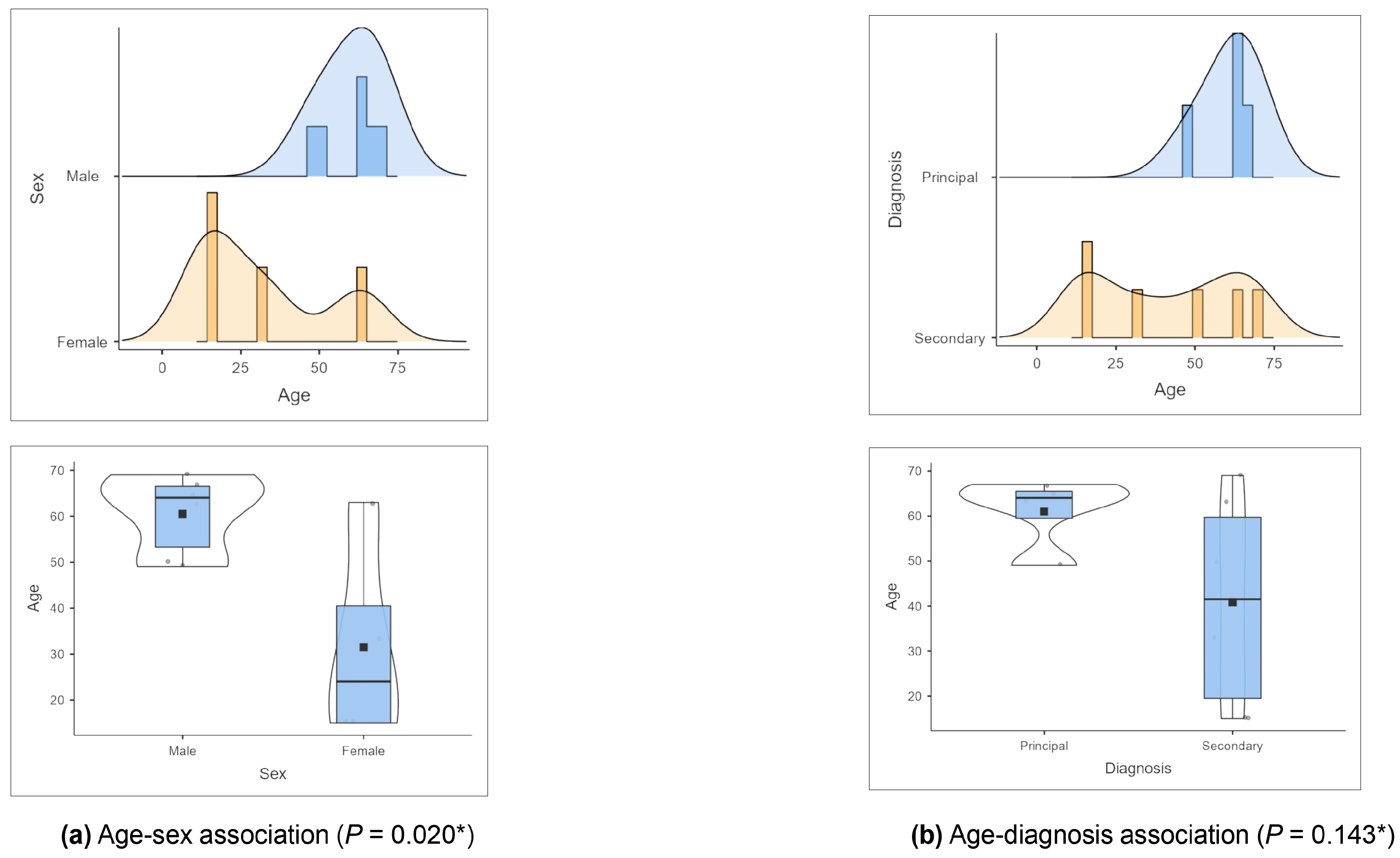
3.1. Epidemiological Features of the Case Series
3.2. Clinical Features of the Infection Episodes
3.3. Incidence Data
4. Discussion
5. Conclusions
Limitations and Strengths
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dumler, J.S.; Choi, K.-S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human Granulocytic Anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-B.; Tang, T.; Chen, J.-J.; Zhang, Y.-Y.; Lv, C.-L.; Xu, Q.; Wang, G.-L.; Zhu, Y.; Wei, Y.-H.; Hay, S.I.; et al. The global distribution and risk prediction of Anaplasmataceae species: A systematic review and geospatial modelling analysis. EBioMedicine 2025, 115, 105722. [Google Scholar] [CrossRef] [PubMed]
- Prusinski, M.; O’Connor, C.; Russell, A.; Sommer, J.; White, J.; Rose, L.; Falco, R.; Kokas, J.; Vinci, V.; Gall, W.; et al. Associations of Anaplasma phagocytophilum Bacteria Variants in Ixodes scapularis Ticks and Humans, New York, USA. Emerg. Infect. Dis. 2023, 29, 540–550. [Google Scholar] [CrossRef]
- Huhn, C.; Winter, C.; Wolfsperger, T.; Wüppenhorst, N.; Strašek Smrdel, K.; Skuballa, J.; Pfäffle, M.; Petney, T.; Silaghi, C.; Dyachenko, V.; et al. Analysis of the population structure of Anaplasma phagocytophilum using multilocus sequence typing. PLoS ONE 2014, 9, e93725. [Google Scholar] [CrossRef]
- Langenwalder, D.B.; Schmidt, S.; Silaghi, C.; Skuballa, J.; Pantchev, N.; Matei, I.A.; Mihalca, A.D.; Gilli, U.; Zajkowska, J.; Ganter, M.; et al. The absence of the drhm gene is not a marker for human-pathogenicity in European Anaplasma phagocytophilum strains. Parasites Vectors 2020, 13, 238. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Vieira Lista, M.C.; Belhassen-García, M.; Vicente Santiago, M.B.; Sánchez-Montejo, J.; Pedroza Pérez, C.; Monsalve Arteaga, L.C.; Herrador, Z.; Del Álamo-Sanz, R.; Benito, A.; Soto López, J.D.; et al. Identification and Distribution of Human-Biting Ticks in Northwestern Spain. Insects 2022, 13, 469. [Google Scholar] [CrossRef]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, 34. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.-P.; Gray, J. Human Babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef]
- Naranjo, V.; Ruiz-Fons, F.; Höfle, U.; Fernández de Mera, I.G.; Villanúa, D.; Almazán, C.; Torina, A.; Caracappa, S.; Kocan, K.M.; Gortázar, C.; et al. Molecular epidemiology of human and bovine anaplasmosis in southern Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 95–99. [Google Scholar] [CrossRef] [PubMed]
- García, J.C.; Núñez, M.J.; Castro, B.; Fraile, F.J.; López, A.; Mella, M.C.; Blanco, A.; Sieira, C.; Loureiro, E.; Portillo, A.; et al. Human anaplasmosis: The first Spanish case confirmed by PCR. Ann. N. Y. Acad. Sci. 2006, 1078, 545–547. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Biggs, H.M.; Behravesh, C.B.; Bradley, K.K.; Dahlgren, F.S.; Drexler, N.A.; Dumler, J.S.; Folk, S.M.; Kato, C.Y.; Lash, R.R.; Levin, M.L.; et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States. MMWR Recomm. Rep. 2016, 65, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Wang, H.-H.; Mogg, M.; Derouen, Z.; Borski, J.; Grant, W.E. Increasing Incidence of Anaplasmosis in the United States, 2012 Through 2016. Vector Borne Zoonotic Dis. 2020, 20, 855–859. [Google Scholar] [CrossRef]
- Dahlgren, F.S.; Heitman, K.N.; Drexler, N.A.; Massung, R.F.; Behravesh, C.B. Human granulocytic anaplasmosis in the United States from 2008 to 2012: A summary of national surveillance data. Am. J. Trop. Med. Hyg. 2015, 93, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Tickborne Diseases of the United States: A Reference Manual for Healthcare Providers, 6th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2022. Available online: https://www.cdc.gov/ticks/media/pdfs/2025/03/tickborne-diseases-manual-508.pdf (accessed on 10 January 2024).
- Belongia, E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002, 2, 265–273. [Google Scholar] [CrossRef]
- Nordberg, M. Tick-Borne Infections in Humans: Aspects of Immunopathogenesis, Diagnosis and Co-Infections with Borrelia burgdorferi and Anaplasma phagocytophilum; Linköping University Electronic Press: Linköping, Sweden, 2012. [Google Scholar]
- von Wissmann, B.; Hautmann, W.; Sing, A.; Hizo-Teufel, C.; Fingerle, V. Assessing the risk of human granulocytic anaplasmosis and lyme borreliosis after a tick bite in Bavaria, Germany. Int. J. Med. Microbiol. 2015, 305, 736–741. [Google Scholar] [CrossRef]
- Kowalski, J.; Hopfenmüller, W.; Fingerle, V.; Malberg, H.; Eisenblätter, M.; Wagner, J.; Miksits, K.; Hahn, H.; Ignatius, R. Seroprevalence of human granulocytic anaplasmosis in Berlin/Brandenburg, Germany: An 8-year survey. Clin. Microbiol. Infect. 2006, 12, 924–927. [Google Scholar] [CrossRef]
- Wang, F.; Yan, M.; Liu, A.; Chen, T.; Luo, L.; Li, L.; Teng, Z.; Li, B.; Ji, Z.; Jian, M.; et al. The seroprevalence of Anaplasma phagocytophilum in global human populations: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2020, 67, 2050–2064. [Google Scholar] [CrossRef]
- Lillini, E.; Macrì, G.; Proietti, G.; Scarpulla, M. New findings on anaplasmosis caused by infection with Anaplasma phagocytophilum. Ann. N. Y. Acad. Sci. 2006, 1081, 360–370. [Google Scholar] [CrossRef]
- García, J.C.; Núñez, M.J.; Portillo, A.; Oteo, J.A. Anaplasmosis humana: Comunicación de 2 casos. Enfermedades Infecc. Y Microbiol. Clínica 2015, 33, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis—A Systematic Review of Published Cases. Microorganisms 2022, 10, 1433. [Google Scholar] [CrossRef] [PubMed]
- Vidal Alzate, J.; Acevedo Castro, T.; Osorno Palacio, D.; Urán Velázquez, J.; Cardona-Arias, J.A. Prevalência de Anaplasma spp. em humanos: Revisão sistemática da literatura entre 2000 e 2017. Investig. Andin. 2019, 21, 239–252. [Google Scholar] [CrossRef]
- Ministerio de Sanidad Registro de Actividad de Atención Especializada (RAE-CMBD). Tablas Nacionales 2022; Gobierno de España, Ministerio de Sanidad: Madrid, Spain, 2024; Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/docs/TablasSIAE2022/Tablas_Nacionales_2022.pdf (accessed on 14 June 2025).
- Población por Comunidades y Ciudades Autónomas y Sexo (2853). Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=2853 (accessed on 14 June 2025).
- Millán, J.; Proboste, T.; Fernández de Mera, I.G.; Chirife, A.D.; de la Fuente, J.; Altet, L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human–wildlife interface. Ticks Tick-Borne Dis. 2016, 7, 284–290. [Google Scholar] [CrossRef]
- Heo, D.-H.; Hwang, J.-H.; Choi, S.H.; Jeon, M.; Lee, J.-H.; Lee, J.-H.; Hwang, S.-D.; Lee, K.-A.; Lee, S.-H.; Lee, C.-S. Recent Increase of Human Granulocytic Anaplasmosis and Co-Infection with Scrub Typhus or Korean Hemorrhagic Fever with Renal Syndrome in Korea. J. Korean Med. Sci. 2019, 34, e87. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wei, F.; Liu, Q.; Qian, J. Epidemiology and Control of Human Granulocytic Anaplasmosis: A Systematic Review. Vector-Borne Zoonotic Dis. 2012, 12, 269–274. [Google Scholar] [CrossRef]
- de la Fuente, J.; Naranjo, V.; Ruiz-Fons, F.; Höfle, U.; Fernández De Mera, I.G.; Villanúa, D.; Almazán, C.; Torina, A.; Caracappa, S.; Kocan, K.M.; et al. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonotic Dis. 2005, 5, 390–401. [Google Scholar] [CrossRef]
- Jahfari, S.; Coipan, E.C.; Fonville, M.; van Leeuwen, A.D.; Hengeveld, P.; Heylen, D.; Heyman, P.; van Maanen, C.; Butler, C.M.; Földvári, G.; et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasites Vectors 2014, 7, 365. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Cascio, A.; Bosilkovski, M.; Rodriguez-Morales, A.J.; Pappas, G. The socio-ecology of zoonotic infections. Clin. Microbiol. Infect. 2011, 17, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Juanes, H.M.L.; Carbonell, C.; Sendra, B.F.; López-Bernus, A.; Bahamonde, A.; Orfao, A.; Lista, C.V.; Ledesma, M.S.; Negredo, A.I.; Rodríguez-Alonso, B.; et al. Crimean-Congo Hemorrhagic Fever, Spain, 2013–2021. Emerg. Infect. Dis. 2023, 29, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Portillo, A.; Palomar, A.M.; Santibáñez, P.; Oteo, J.A. Epidemiological Aspects of Crimean-Congo Hemorrhagic Fever in Western Europe: What about the Future? Microorganisms 2021, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Jánová, E. Emerging and threatening vector-borne zoonoses in the world and in Europe: A brief update. Pathog. Glob. Health 2019, 113, 49–57. [Google Scholar] [CrossRef]
- Sánchez-Seco, M.P.; Sierra, M.J.; Estrada-Peña, A.; Valcárcel, F.; Molina, R.; de Arellano, E.R.; Olmeda, A.S.; San Miguel, L.G.; Jiménez, M.; Romero, L.J.; et al. Widespread Detection of Multiple Strains of Crimean-Congo Hemorrhagic Fever Virus in Ticks, Spain. Emerg. Infect. Dis. 2021, 28, 394–402. [Google Scholar] [CrossRef]

| 1. Epidemiological features of the case series (N = 9) | ||
|---|---|---|
| Qualitative variables | n (%) | |
| Gender | Male | 6 (66.7) |
| Female | 3 (33.3) | |
| Regions, Autonomous Community | Andalucía | 1 (11.1) |
| Aragón | 1 (11.1) | |
| Castilla y León | 2 (22.2) | |
| Cataluña | 1 (11.1) | |
| Galicia | 1 (11.1) | |
| Madrid | 2 (22.2) | |
| Murcia | 1 (11.1) | |
| Quantitative variables | Mean (± SD), median (IQR), (range, min. value-max. value) | |
| Age | 53 (± 18.2), 63 (66–41), (range, 15–69) | |
| 2. Clinical features of the infection episodes (N = 10) | ||
| Qualitative variables | n (%) | |
| Type of admission | Urgent | 8 (80.0) |
| Programmed | 2 (20.0) | |
| Service | Internal medicine | 8 (80.0) |
| Nephrology | 1 (10.0) | |
| Urology | 1 (10.0) | |
| Hospital discharge | Home | 10 (100.0) |
| Diagnosis | Principal diagnosis | 4 (40.0) |
| Secondary diagnosis | 5 (50.0) | |
| Recurrence/reactivation | 1 (10.0) | |
| Quantitative variables | Mean (± SD), median (IQR), (range, min. value-max. value) | |
| Hospital stays, days | 7 (±3.5), 6 (9–4), (range, 3–13) | |
| Cost, EUR | 4554.06 (±1032.16), 4331.83 (5319.61–3902.50), (range, 3020.82–6459.88) | |
| Number of Cases | Year | Month | Gender | Age | Origin | Admission Type | Hospital Service | Diagnosis | Comorbidity | Hospital Discharge | Hospital Stays, Days | Cost (€) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 2008 | July | Male | 67 | Madrid | Urgent | Internal medicine | Principal | Heart failure, muscular dystrophy, prostate hyperplasia | Home | 3538.14 | |
| Case 2 | 2009 | January | Male | 69 | Murcia | Programmed | Urology | Secondary | Bladder neoplasia, hypertension, chronic kidney disease | Home | 4126.58 | |
| Case 3 | 2013 | May | Female | 33 | Castilla y Leon | Programmed | Nephrology | Secondary | Connective tissue disease, HTA | Home | 3020.82 | |
| Case 4 | 2015 | September | Male | 50 | Madrid | Urgent | Internal medicine | Secondary | Cirrhosis, Ischemic heart disease | Home | 4768.43 | |
| Case 5 | 2016 | May | Male | 65 | Galicia | Urgent | Internal medicine | Principal | No comorbidity | Home | 4 | 4023.96 |
| Case 6 | 2017 | August | Male | 63 | Andalucía | Urgent | Internal medicine | Secondary | Atrial fibrillation, hypertension, pacemaker | Home | 13 | 5778.79 |
| Case 7 | 2018 | August | Female | 15 | Castilla y Leon | Urgent | Internal medicine | Secondary | Autoimmune lymphoproliferative syndrome | Home | 3 | 6459.88 |
| Case 8 | 2018 | October | Female | 15 | Castilla y Leon | Urgent | Internal medicine | Secondary | Hepatosplenomegaly | Home | 6 | 4120.34 |
| Case 9 | 2021 | June | Male | 49 | Aragón | Urgent | Internal medicine | Principal | Purpura | Home | 8 | 5166.56 |
| Case 10 | 2022 | November | Female | 63 | Cataluña | Urgent | Internal medicine | Principal | Syncope | Home | 6 | 4537.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, H.; Alonso-Sardón, M.; Rodríguez-Alonso, B.; López-Bernus, A.; Romero-Alegría, Á.; Velasco-Tirado, V.; Muro, A.; Belhassen-García, M. Impact of Human Granulocytic Anaplasmosis in Spain from 1997 to 2022. Trop. Med. Infect. Dis. 2025, 10, 183. https://doi.org/10.3390/tropicalmed10070183
Almeida H, Alonso-Sardón M, Rodríguez-Alonso B, López-Bernus A, Romero-Alegría Á, Velasco-Tirado V, Muro A, Belhassen-García M. Impact of Human Granulocytic Anaplasmosis in Spain from 1997 to 2022. Tropical Medicine and Infectious Disease. 2025; 10(7):183. https://doi.org/10.3390/tropicalmed10070183
Chicago/Turabian StyleAlmeida, Hugo, Montserrat Alonso-Sardón, Beatriz Rodríguez-Alonso, Amparo López-Bernus, Ángela Romero-Alegría, Virginia Velasco-Tirado, Antonio Muro, and Moncef Belhassen-García. 2025. "Impact of Human Granulocytic Anaplasmosis in Spain from 1997 to 2022" Tropical Medicine and Infectious Disease 10, no. 7: 183. https://doi.org/10.3390/tropicalmed10070183
APA StyleAlmeida, H., Alonso-Sardón, M., Rodríguez-Alonso, B., López-Bernus, A., Romero-Alegría, Á., Velasco-Tirado, V., Muro, A., & Belhassen-García, M. (2025). Impact of Human Granulocytic Anaplasmosis in Spain from 1997 to 2022. Tropical Medicine and Infectious Disease, 10(7), 183. https://doi.org/10.3390/tropicalmed10070183






