Differential Emodepside Efficacy in Drug-Resistant and Drug-Susceptible Ancylostoma caninum Highlights Variability in Potassium Channel Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasite Isolates Source and Maintenance
2.2. Drug Selection and Preparation
2.2.1. Selection of Concentrations
2.2.2. Preparation of Stock Solutions
2.3. Experimental Design of Survival Assays
2.3.1. Setup and Assessment of Survival Assays: A. caninum L3
2.3.2. Evaluation of SLO-1 Modulation Using Penitrem A
2.4. Data Collection and Analysis
3. Results
3.1. A. Caninum L3 Survival Assays
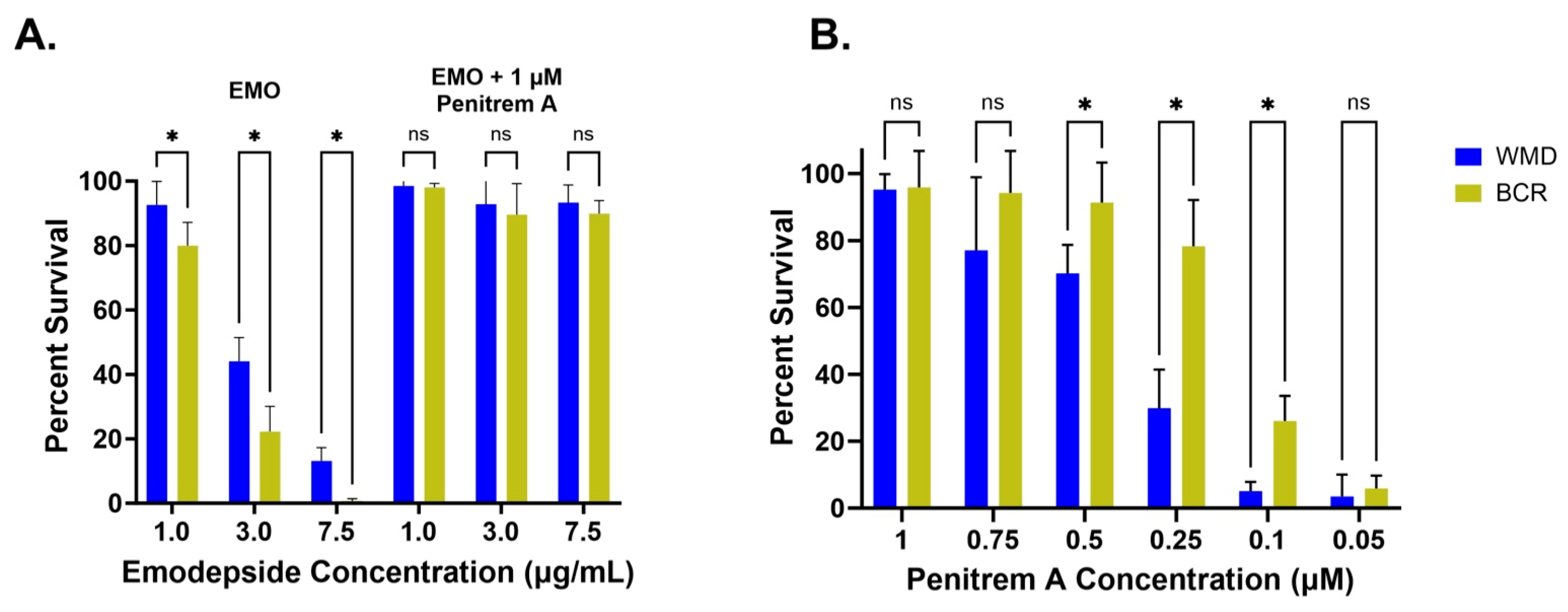
3.2. Emodepside + Penitrem A Survival Assays
3.2.1. Emodepside + 1 µM Penitrem A
3.2.2. Penitrem A Titration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MADR | Multi-anthelmintic drug resistant |
| WMD | Drug-susceptible Ancylostoma caninum isolate |
| BCR | Triple-anthelmintic resistant Ancylostoma caninum isolate |
| Cmax | Maximum blood plasma concentration |
| L3 | Third-stage larvae |
| BK channel | Calcium-gated potassium channel |
| FECR | Fecal egg count reduction |
References
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Castro, P.D.; Howell, S.B.; Schaefer, J.J.; Avramenko, R.W.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasites Vectors 2019, 12, 576. [Google Scholar] [CrossRef]
- Jimenez Castro, P.D.; Mansour, A.; Charles, S.; Hostetler, J.; Settje, T.; Kulke, D.; Kaplan, R.M. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Castro, P.D.; Venkatesan, A.; Redman, E.; Chen, R.; Malatesta, A.; Huff, H.; Zuluaga Salazar, D.A.; Avramenko, R.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in hookworms infecting greyhound dogs in the USA. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.; Jimenez Castro, P.D.; Morosetti, A.; Horvath, H.; Chen, R.; Redman, E.; Dunn, K.; Collins, J.B.; Fraser, J.S.; Andersen, E.C.; et al. Molecular evidence of widespread benzimidazole drug resistance in Ancylostoma caninum from domestic dogs throughout the USA and discovery of a novel β-tubulin benzimidazole resistance mutation. PLoS Pathog. 2023, 19, e1011146. [Google Scholar] [CrossRef]
- Kitchen, S.; Ratnappan, R.; Han, S.; Leasure, C.; Grill, E.; Iqbal, Z.; Granger, O.; O’Halloran, D.M.; Hawdon, J.M. Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum. Int. J. Parasitol. 2019, 49, 397–406. [Google Scholar] [CrossRef]
- McKean, E.L.; Grill, E.; Choi, Y.-J.; Mitreva, M.; O’Halloran, D.M.; Hawdon, J.M. Altered larval activation response associated with multidrug resistance in the canine hookworm Ancylostoma caninum. Parasitology 2023, 151, 271–281. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Barankin, B.; Hon, K.L.E. Cutaneous Larva Migrans. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 11, 2–11. [Google Scholar] [CrossRef]
- Hawdon, J.M.; Wise, K.A. Ancylostoma caninum and other canine hookworms. In Dog Parasites Endangering Human Health; Strube, C., Mehlhorn, H., Eds.; Springer: Cham, Switzerland, 2021; pp. 147–193. [Google Scholar]
- Marsh, A.E.; Lakritz, J. Reflecting on the past and fast forwarding to present day anthelmintic resistant Ancylostoma caninum—A critical issue we neglected to forecast. Int. J. Parasitol. Drugs Drugs Resist. 2023, 22, 36–43. [Google Scholar] [CrossRef]
- Sasaki, T.; Takagi, M.; Yaguchi, T.; Miyadoh, S.; Okada, T.; Koyama, M. A new anthelmintic cyclodepsipeptide, PF1022A. J. Antibiot. 1992, 45, 692–697. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Cyclic Depsipeptides from Fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef] [PubMed]
- Guest, M.; Bull, K.; Walker, R.J.; Amliwala, K.; O’Connor, V.; Harder, A.; Holden-Dye, L.; Hopper, N.A. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int. J. Parasitol. 2007, 37, 1577–1588. [Google Scholar] [CrossRef]
- Welz, C.; Krüger, N.; Schniederjans, M.; Miltsch, S.M.; Krücken, J.; Guest, M.; Holden-Dye, L.; Harder, A.; von Samson-Himmelstjerna, G. SLO-1-Channels of Parasitic Nematodes Reconstitute Locomotor Behaviour and Emodepside Sensitivity in Caenorhabditis elegans slo-1 Loss of Function Mutants. PLoS Pathog. 2011, 7, e1001330. [Google Scholar] [CrossRef]
- Karpstein, T.; Pasche, V.; Häberli, C.; Scandale, I.; Neodo, A.; Keiser, J. Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections. Parasites Vectors 2019, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.D.J.; Durrence, K.; Durrence, S.; Giannechini, L.S.; Collins, J.; Dunn, K.; Kaplan, R.M. Multiple anthelmintic drug resistance in hookworms (Ancylostoma caninum) in a Labrador breeding and training kennel in Georgia, USA. J. Am. Veter.-Med. Assoc. 2023, 261, 342–347. [Google Scholar] [CrossRef]
- Taylor, L.; Ahmada, A.A.; Ali, M.S.; Ali, S.M.; Hattendorf, J.; Mohammed, I.S.; Keiser, J. Efficacy and safety of emodepside compared with albendazole in adolescents and adults with hookworm infection in Pemba Island, Tanzania: A double-blind, superiority, phase 2b, randomised controlled trial. Lancet 2024, 404, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Schad, G.A.; Page, M.R. Ancylostoma caninum: Adult worm removal, corticosteroid treatment, and resumed development of arrested larvae in dogs. Exp. Parasitol. 1982, 54, 303–309. [Google Scholar] [CrossRef]
- Krepp, J.; Gelmedin, V.; Hawdon, J.M. Characterisation of hookworm heat shock factor binding protein (HSB-1) during heat shock and larval activation. Int. J. Parasitol. 2010, 41, 533–543. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Moore, T.A. 42—Drugs for Helminths. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Saunders, W.B., Eds.; 2015; pp. 519–527. [Google Scholar] [CrossRef]
- Arion, A.; Fernández-Varón, E.; Cárceles, C.M.; Gagyi, L.; Ognean, L. Pharmacokinetics of praziquantel and pyrantel pamoate combination following oral administration in cats. J. Feline Med. Surg. 2017, 20, 900–904. [Google Scholar] [CrossRef]
- Peregrine, A.S. Hookworms in Small Animals—Digestive System. MSD Veterinary Manual. July 2023. Available online: https://www.msdvetmanual.com/digestive-system/gastrointestinal-parasites-of-small-animals/hookworms-in-small-animals (accessed on 21 May 2025).
- Quintana, T.A.; Karwath, G.N.; Mayhue, E.J.; Jugan, M.C.; Jesudoss Chelladurai, J.R.J.; Martinez, S.E. Topical and oral emodepside formulations for last-line treatment of multianthelmintic drug-resistant hookworms when given orally to dogs are not bioequivalent. Am. J. Vet. Res. 2025, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mrimi, E.C.; Welsche, S.; Ali, S.M.; Hattendorf, J.; Keiser, J. Emodepside for Trichuris trichiura and Hookworm Infection. N. Engl. J. Med. 2023, 388, 1863–1875. [Google Scholar] [CrossRef]
- Gillon, J.; Dennison, J.; Berg, F.v.D.; Delhomme, S.; Cheeseman, K.D.; Rossi, C.P.; Wourgaft, N.S.; Specht, S.; Pedrique, B.; Monnot, F.; et al. Safety, tolerability and pharmacokinetics of emodepside, a potential novel treatment for onchocerciasis (river blindness), in healthy male subjects. Br. J. Clin. Pharmacol. 2021, 87, 3949–3960. [Google Scholar] [CrossRef] [PubMed]
- Hawdon, J.M.; Schad, G.A. Albumin and a Dialyzable Serum Factor Stimulate Feeding In vitro by Third-Stage Larvae of the Canine Hookworm Ancylostoma caninum. J. Parasitol. 1991, 77, 587–591. [Google Scholar] [CrossRef]
- Kotze, A.C.; McCarthy, J.S.; O’grady, J.; Clifford, S.; Behnke, J.M. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am. J. Trop. Med. Hyg. 2004, 71, 608–616. [Google Scholar] [CrossRef]
- Satou, T.; Koga, M.; Koike, K.; Tada, I.; Nikaido, T. Nematocidal activities of thiabendazole and ivermectin against the larvae of Strongyloides ratti and S. venezuelensis. Veter Parasitol. 2001, 99, 311–322. [Google Scholar] [CrossRef]
- Kopp, S.R.; Kotze, A.C.; McCarthy, J.S.; Coleman, G.T. High-level pyrantel resistance in the hookworm Ancylostoma caninum. Veter Parasitol. 2007, 143, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ellis, B.L.; Yiu, Y.Y.; Miller, M.M.; Urban, J.F.; Shi, L.Z.; Aroian, R.V.; Hofmann, A. An Extensive Comparison of the Effect of Anthelmintic Classes on Diverse Nematodes. PLoS ONE 2013, 8, e70702. [Google Scholar] [CrossRef]
- Keiser, J.; Häberli, C. Evaluation of Commercially Available Anthelminthics in Laboratory Models of Human Intestinal Nematode Infections. ACS Infect. Dis. 2021, 7, 1177–1185. [Google Scholar] [CrossRef]
- Bah, G.S.; Schneckener, S.; Hahnel, S.R.; Bayang, N.H.; Fieseler, H.; Schmuck, G.M.; Krebber, R.; Sarr, A.; Terjung, C.; Ngangyung, H.F.; et al. Emodepside targets SLO-1 channels of Onchocerca ochengi and induces broad anthelmintic effects in a bovine model of onchocerciasis. PLOS Pathog. 2021, 17, e1009601. [Google Scholar] [CrossRef]
- Njeshi, C.N.; Robertson, A.P.; Martin, R.J. Emodepside: The anthelmintic’s mode of action and toxicity. Front. Parasitol. 2024, 3, 1508167. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2011.
- (United States Department of Agriculture), U.S.D.A. Animal Welfare Act; U.S. Government Publishing Office: Washington, DC, USA, 2018.
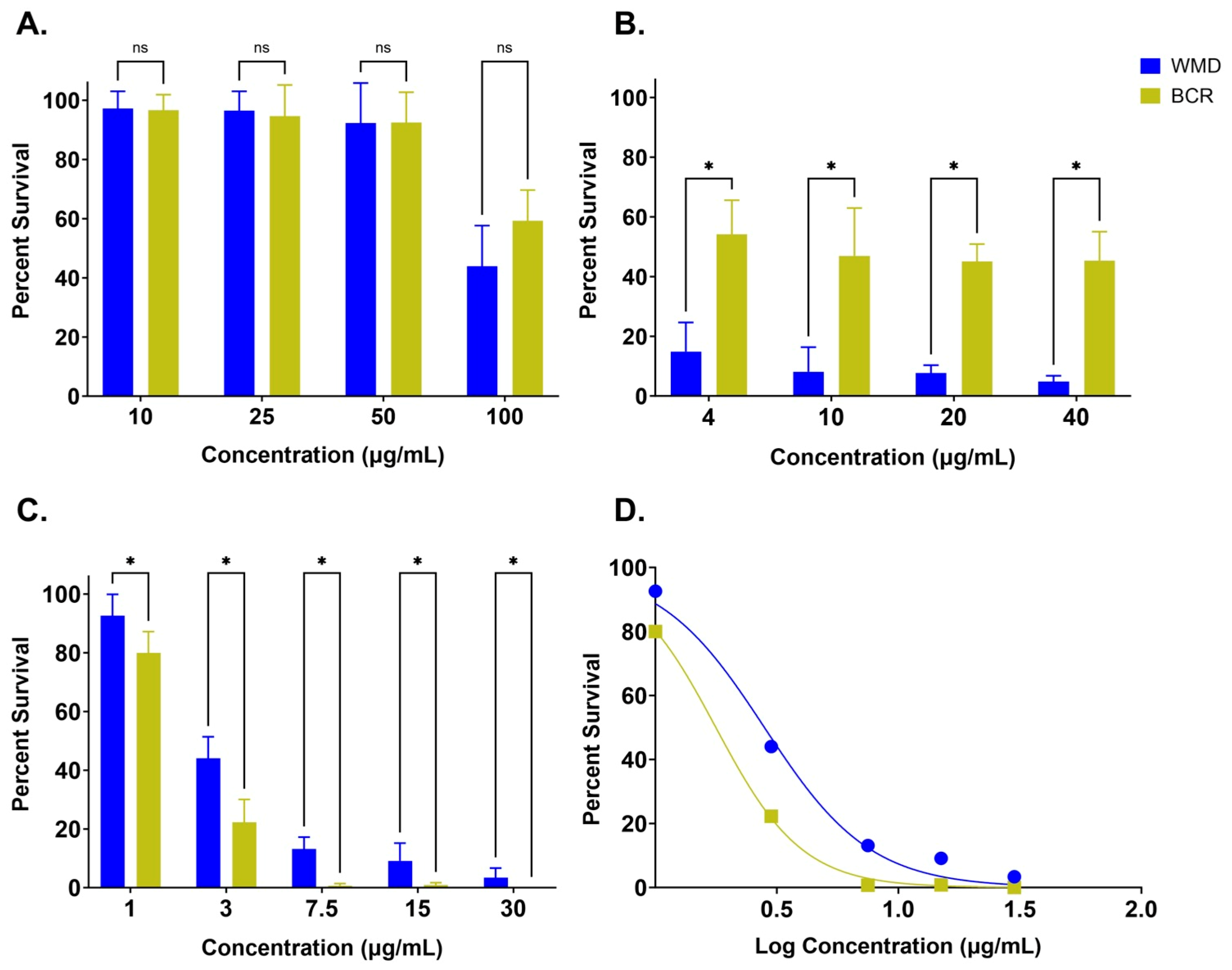
| Drug | Administered Dose | Corresponding Cmax (µg/mL) | X1000 (µg/mL) | Testable Range (µg/mL) |
|---|---|---|---|---|
| Pyrantel | 100 mg/kg | 0.11 | 110 | 10, 25, 50, 100 |
| Ivermectin | 150–200 µg/kg | 0.03–0.04 | 30–40 | 4, 10, 20, 40 |
| Emodepside | 2.5 mg | 0.037 | 37.6 | 1, 3, 7.5, 15, 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, C.A.; McKean, E.L.; Hawdon, J.M. Differential Emodepside Efficacy in Drug-Resistant and Drug-Susceptible Ancylostoma caninum Highlights Variability in Potassium Channel Activity. Trop. Med. Infect. Dis. 2025, 10, 181. https://doi.org/10.3390/tropicalmed10070181
Jackson CA, McKean EL, Hawdon JM. Differential Emodepside Efficacy in Drug-Resistant and Drug-Susceptible Ancylostoma caninum Highlights Variability in Potassium Channel Activity. Tropical Medicine and Infectious Disease. 2025; 10(7):181. https://doi.org/10.3390/tropicalmed10070181
Chicago/Turabian StyleJackson, Catherine A., Elise L. McKean, and John M. Hawdon. 2025. "Differential Emodepside Efficacy in Drug-Resistant and Drug-Susceptible Ancylostoma caninum Highlights Variability in Potassium Channel Activity" Tropical Medicine and Infectious Disease 10, no. 7: 181. https://doi.org/10.3390/tropicalmed10070181
APA StyleJackson, C. A., McKean, E. L., & Hawdon, J. M. (2025). Differential Emodepside Efficacy in Drug-Resistant and Drug-Susceptible Ancylostoma caninum Highlights Variability in Potassium Channel Activity. Tropical Medicine and Infectious Disease, 10(7), 181. https://doi.org/10.3390/tropicalmed10070181






