Abstract
The antimicrobial resistance (AMR) surveillance network has been monitoring bloodstream bacterial pathogens and their resistance since 1999 in Tunisia. We report the long-term trends in the distribution of bloodstream bacterial pathogens and their resistance patterns from this surveillance database. We analyzed antibiotic resistance rates in 11 tertiary teaching hospital laboratories under the AMR surveillance network during 2011–2023, focusing on six priority bacterial pathogens, using the Cochrane–Armitage test for trend analysis. Of 22,795 isolates, K. pneumoniae (38.5%) was the most common, followed by S. aureus (20.4%), E. coli (13.6%), and A. baumannii (10.3%). Carbapenem resistance was highest in A. baumannii (77%), followed by Pseudomonas aeruginosa (29.3%), K. pneumoniae (19.4%), and Enterobacter cloacae (6.8%). Carbapenem-resistant Enterobacterales and third-generation cephalosporin-resistant Enterobacterales (3GCREB) increased from 10.6% to 26.3% (p-value < 0.001), and from 39% to 50.2%, respectively, during 2011–2023 (p-value < 0.001). Vancomycin resistance (38.3%) and the emergence of linezolid resistance in 2019 (2.4%) were reported in E. faecium isolates. Resistance to carbapenems and 3GC is a major challenge to controlling BSI in Tunisia. The national AMR surveillance network helps monitor annual patterns and guides empirical therapy. An integrated database combining clinical profiles and resistance data via real-time data-sharing platforms could improve clinical decision-making.
1. Introduction
Bloodstream infections (BSIs) are serious life-threatening infections that significantly contribute to morbidity, mortality, and healthcare costs worldwide [1]. Bacterial pathogens account for over 90% of all bloodstream infections (BSIs), with a considerable proportion arising from the six priority pathogens identified by the World Health Organization (WHO) [2,3]. Unfortunately, the global spread of antimicrobial resistance (AMR) and the emergence and spread of multidrug-resistant (MDR) bacterial strains, both in hospital and community settings, further complicate the management of these infections [4]. In 2021, bacterial AMR alone killed 1.14 million people (95% uncertainty interval 1.00–1.28), with nearly three-quarters of these deaths being caused by six common bacterial pathogens, also known as the ESKAPE pathogens [5,6].
In the Eastern Mediterranean Region, antimicrobial-resistant isolates are being increasingly reported, with carbapenem-resistant Acinetobacter species being the highest (70.3%), followed by K. pneumoniae resistant to third-generation cephalosporins (66.3%) in 2019 [7]. BSIs remain a major health burden in the region, with high rates of morbidity, mortality, and prolonged hospital stay, especially among vulnerable populations [8]. BSIs claimed nearly 159,000 lives and loss of 6.7 million Disability-Adjusted Life Years (DALYs) in the Middle East and North Africa (MENA) Region in 2019 [9].
Tunisia, located in the MENA region, has one of the highest rates of antibiotic consumption, with 38 defined doses per 1000 individuals per day. This rate is second only to Greece within the region and is approximately four times higher than the global average [10]. This, coupled with factors such as limited infection prevention and control measures, sanitation, and hygiene, could further accelerate the high AMR rates in the country. According to the latest report of the Global Antimicrobial Resistance and Use Surveillance System (GLASS) in 2020 in Tunisia, a substantial proportion of K. pneumoniae isolates from blood cultures were resistant to cefotaxime (68.4%) and carbapenems (27.7%). A. baumannii strains isolated from blood were resistant to carbapenems in 82.5% of cases, whereas S. aureus strains resistant to methicillin ranged from 10 to 30% [11]. The proportion of resistant isolates exhibiting these specific AMR phenotypes is concerning and has critical implications for clinical management and patient survival, necessitating immediate action.
In view of this imminent threat, surveillance of bacterial pathogens causing BSI and their AMR patterns started in Tunisia in 1999 by a voluntary initiative from a group of university microbiologists who formed a surveillance network called “L’Antibiorésistance en Tunisie” (LART). It began with four laboratories from Tunisian university hospitals, and since 2011, 11 laboratories have consistently tested and reported blood cultures from patients. These tests cover a wide range of bacterial pathogens and assess their sensitivity to various antibiotics following standard recommendations [12]. The test results were maintained using a laboratory information system (LIS). However, there is no published literature analyzing the laboratory surveillance data to document the long-term trends of the resistance patterns in Tunisia.
The previous literature in Tunisia on bacterial pathogens causing BSIs and their AMR patterns is limited to single-hospital studies over shorter periods and with limited sample sizes [13,14]. Information about long-term trends in AMR patterns of the most frequent bacteria causing BSI in Tunisia is useful for clinicians and policymakers in implementing empirical antibiotic protocols and infection control programs and informing them about emerging bacterial resistance threats.
Thus, we analyzed the laboratory-based AMR sentinel surveillance database in Tunisia to describe the long-term longitudinal trends in the (i) number of positive bacterial isolates, disaggregated by Gram-positive and Gram-negative pathogens; (ii) distribution of pathogens causing BSI; and (iii) their antimicrobial resistance patterns (among ESKAPE pathogens only) from to 2011 to 2023, with a focus on the specific AMR phenotypes of concern [third-generation cephalosporin-resistant (3GCREB) and carbapenem-resistant Enterobacterales CRE), carbapenem-resistant A. baumannii (CRAB), carbapenem-resistant P. aeruginosa, methicillin-resistant S. aureus (MRSA), and vancomycin-resistant Enterococci (VRE)], all of which are in line with the WHO global research priorities for AMR [15].
2. Materials and Methods
2.1. Study Design
This is a cross-sectional study involving the analysis of secondary aggregate data from the laboratory-based AMR surveillance network database.
2.2. Study Population
All blood culture bacterial strains were isolated and tested in the laboratories of Tunisian university hospitals that were part of the AMR surveillance network during the period 2011–2023.
2.3. Study Setting
- General setting
Tunisia is a North African country on the southern coast of the Mediterranean, inhabited by approximately 11 million people [14]. Tunisia has a great north–south environmental difference, defined by sharply decreasing southward rainfall from any point. Tunisia’s climate is Mediterranean in the north, whereas the south of the country is desert. The country is divided into four regions: the Greater Tunis (24% of the population), the northern region, the central region, and the southern region. Health services are provided by the public and private sectors, with public facilities representing 56% of the total bed capacity in Tunisia, mainly located in “Grand Tunis” (40%) and the Central East (33%) [16].
- Specific setting: AMR surveillance network
AMR surveillance was initiated in 1999 by the “LART” network, “L’Antibio-Résistance en Tunisie,” a group of volunteer microbiologists from university hospitals. During 1999–2010, AMR surveillance was carried out in 4 public sector university hospitals, which expanded to 8 in 2011 and was further extended to 10 in 2012. Since 2019, a total of 11 AMR surveillance sites have been functional: 8 in the Grand Tunis region and 3 in the Central East region.
In 1999, nine bacterial species were being tested for; these were E. coli, K. pneumoniae, P. aeruginosa, Salmonella spp., E. faecalis, S. pyogenes, S. aureus, S. pneumoniae, and H. influenzae. This spectrum has been expanded to include A. baumannii and E. faecalis in 2008 and E. cloacae in 2011. In addition, the capacity of the network for testing and reporting antibiotics has expanded over the years according to international recommendations [12].
2.4. Bacterial Species Identification and Antimicrobial Susceptibility Testing
Blood samples are collected from hospitals enrolled in the AMR surveillance system for culture under aseptic conditions from hospitalized patients and outpatients with suspected fever or sepsis. All blood culture vials received at the laboratories are incubated in an automated system (Bact/Alert bioMérieux®, Marcy-l’Étoile, France or BD Bactec Blood Culture® Becton, Dickinson and Company, France, depending on the laboratories) for 5 to 7 days.
When a vial is detected as positive by automation, a Gram stain is immediately performed to provide a preliminary idea of the microorganism(s) present. Following this, blood is subcultured on appropriate agar media, including blood agar and chocolate agar (incubated in 5–10% CO2), at 35–37 °C for 18–24 h. Bacterial identification is based on morphological characteristics (colony morphology and Gram staining), biochemical tests (manual and automated), and antigenic characteristics: Enterobacterales, oxidase-positive Gram-negative bacilli, and Enterococci are identified by API (BioMérieux, Marcy-l’Étoile, France) or Vitek®2 Compact (BioMérieux, Marcy-l’Étoile, France), staphylococci by tube coagulase; β-haemolytic streptococci by Lancefield antigen testing; and Salmonella by serotyping according to the White–Kauffmann–Le Minor scheme by the polyvalent O and H, O4, O9, Hd, Hg, Hi, Hm, and Vi antisera. This process of identifying bacterial species takes approximately 24–48 h.
Depending on the surveillance site, antibiotic susceptibility is determined either through disc diffusion on Mueller Hinton agar or by automated techniques (Vitek®2 Compact (BioMérieux, Marcy-l’Étoile, France), according to the guidelines of the “Comité de l’antibiogramme de la Société Française de Microbiologie” (CA-SFM) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) that were applicable at the time of sample collection [12,17].
Interpretation of susceptibility is performed according to the CA-SFM/EUCAST guidelines, using the version at the time of isolate testing over the 13-year study period.
All the laboratories participating in the surveillance program followed a comparable methodology regarding bacterial species identification, antibiotic susceptibility testing, and interpretation.
2.5. Recording and Reporting System
The results of blood culture testing were entered daily into the LIS by a microbiologist in the respective laboratory. Aggregate data for specific indicators were extracted annually from the LIS in Excel format and entered into the surveillance network database at each laboratory. This surveillance database, which receives aggregate data from 11 sentinel surveillance sites in Tunisia, was the source of data for this study.
2.6. Data Collection and Variables
Between 2011 and 2023, aggregate data on blood culture isolates of bacterial pathogens and their resistance profiles were downloaded from a surveillance database. To avoid duplication, we only considered the first isolated strain isolated from the patient for the bacteraemia episode.
The following annual aggregate data were extracted: total number of isolates positive for bacterial pathogens, number of isolates positive by the type of bacterial pathogen, number of bacterial isolates tested for resistance to selected antibiotics (ESKAPE pathogen-wise), number of bacterial isolates resistant to selected antibiotics (ESKAPE pathogen-wise), and number of bacterial isolates positive for specific AMR phenotypes.
The list of antibiotic–pathogen combinations is given below:
- -
- E. cloacae, K. pneumoniae: Amoxicillin-clavulanic acid (AMC), cefotaxime (CTX), gentamicin (GEN), amikacin (AMK), imipenem (IMP), ertapenem (ERT), and ciprofloxacin (CIP).
- -
- S. aureus: Cefoxitin (FOX), GEN, AMK, ofloxacin (OFL), vancomycin (VAN), and linezolid (LIN).
- -
- E. faecium: Ampicillin (AMP), gentamicin high-charged (GHC), VAN, LIN, and tigecycline (TIG).
- -
- P. aeruginosa and A. baumannii: Piperacillin–tazobactam (PTZ), ceftazidime (CAZ), GEN, IMP, CIP, and AMK.
2.7. Data Analysis
Aggregate data from each hospital in Excel format were collated and imported into STATA v.13 for analysis. Trends in the number of positive bacterial isolates and the proportion of bacterial isolates resistant to selected antibiotics (pathogen-wise) are depicted as line diagrams. The proportion of bacterial isolates resistant to various antibiotics (pathogen-wise) is shown using vertical bar diagrams. The distribution of bacterial pathogens responsible for BSI among all the positive isolates is displayed using a stacked vertical bar diagram. The Cochran–Armitage test with continuity correction was used to test linear trends in the proportion of bacterial isolates (ESKAPE pathogens) resistant to selected antibiotics and specific AMR phenotypes, which yields a p-value. A p-value < 0.05 represents a statistically significant linear trend.
3. Results
3.1. Trends in Bacterial Isolates
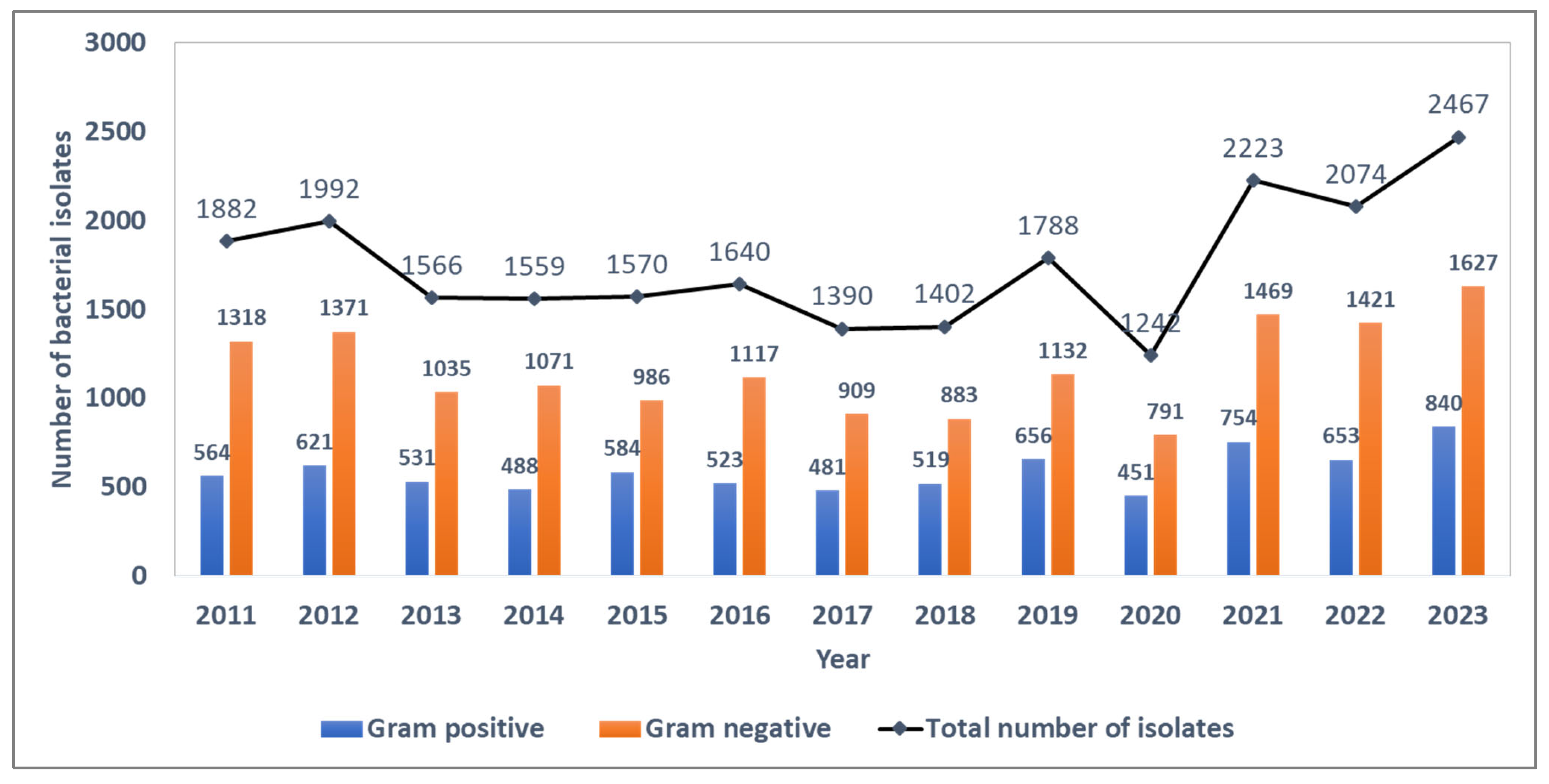
Between 2011 and 2023, 22,795 blood culture bacterial isolates were identified, of which 7665 (33.6%) were Gram-positive and 15,130 (66.4%) were Gram-negative bacterial strains. The ratio of Gram-negative to Gram-positive strains during the surveillance period was ~2.0, with annual estimates ranging from 1.9 to 2.3 during the surveillance period. The absolute number of bacterial isolates per year was fairly stable until the COVID-19 pandemic in 2020, when the number of isolates showed a sudden dip followed by a sharp rise in subsequent years (Figure 1).
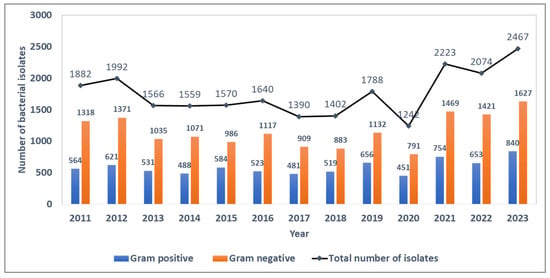
Figure 1.
Trends in the number of blood-culture-positive bacterial isolates and the distribution of Gram-positive and Gram-negative isolates from the antimicrobial resistance sentinel surveillance laboratories in Tunisia, 2011–2023.
3.2. Trends in Distribution of Bacterial Pathogens
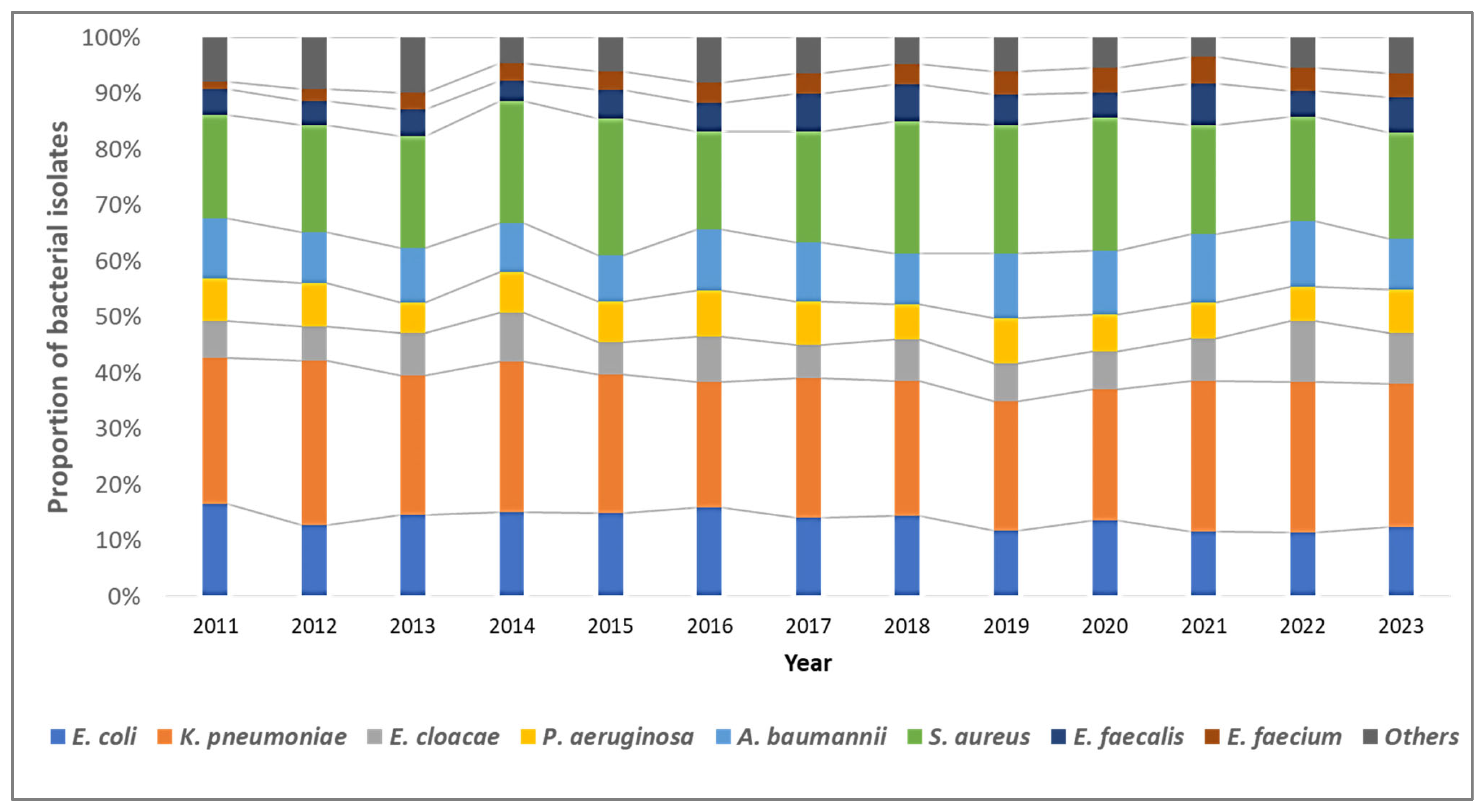
Overall, K. pneumoniae isolates (n = 5830, 38.5%) were the most common, followed by S. aureus (n = 4650, 20.4%), E. coli (n = 3098, 13.6%), and A. baumannii (n = 2352, 10.3%). The distribution of bacterial pathogens isolated from blood cultures remained stable over the study period (Figure 2).
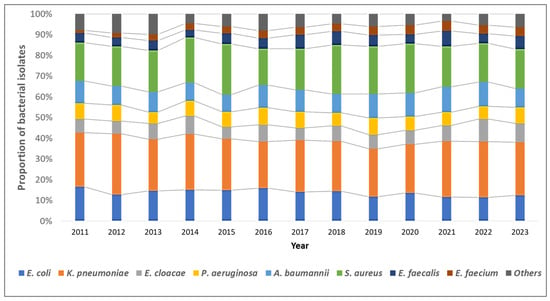
Figure 2.
Trends in the distribution of the type of bacterial pathogens among blood-culture-positive bacterial isolates from the antimicrobial resistance sentinel surveillance laboratories in Tunisia, 2011–2023.
3.3. Patterns of Antibiotic Resistance and Their Trends
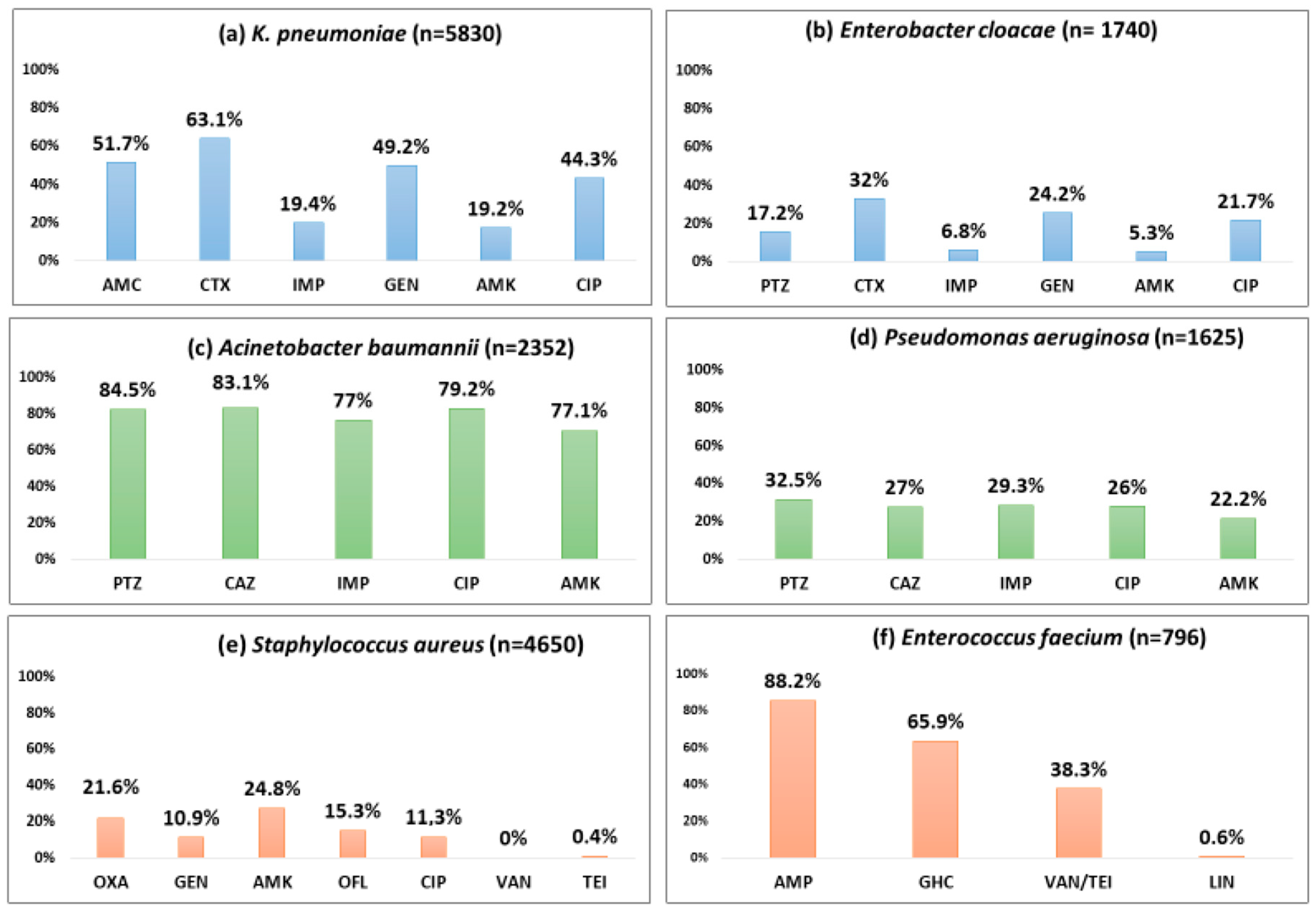
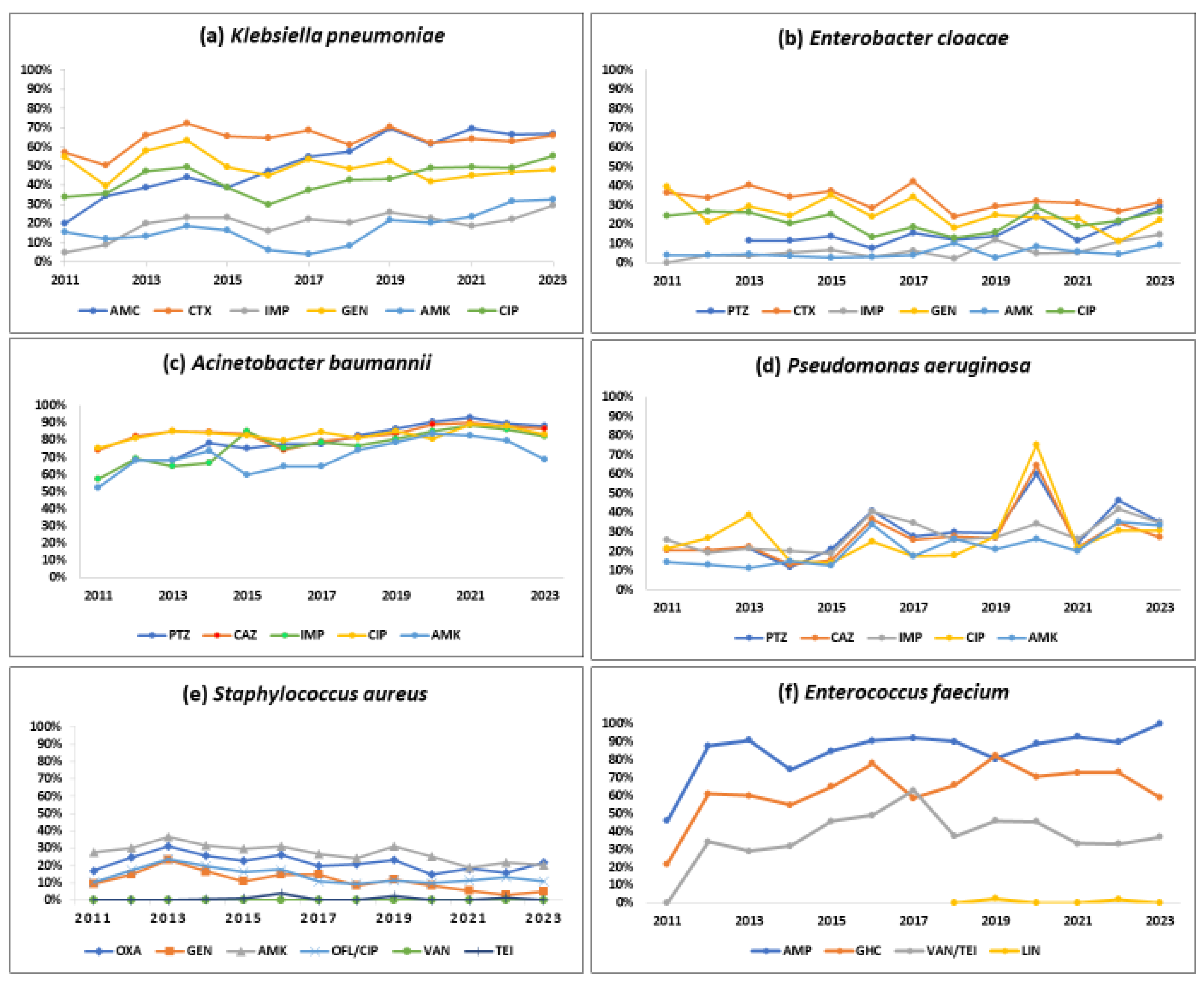
Table 1 show the number of isolates tested and the number and proportion positive for resistance to various antibiotics by Gram-positive and Gram-negative pathogens. Figure 3 and Figure 4 describe the overall proportion of AMR and the time trends of AMR to selected antimicrobials by the type of ESKAPE pathogens during 2011–2023, respectively.

Table 1.
Number of ESKAPE blood culture Gram-negative isolates tested positive for resistance to selected antibiotics by the type of pathogen from the antimicrobial resistance surveillance laboratories in Tunisia during 2011–2023.
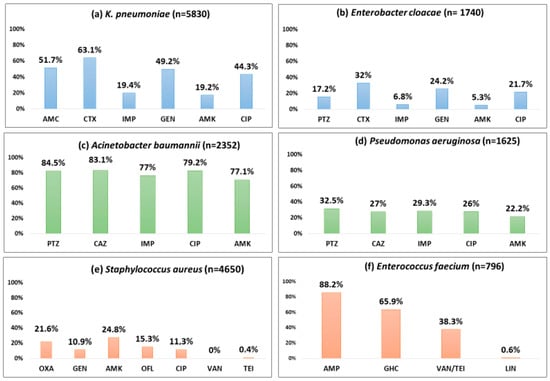
Figure 3.
Proportion of blood-culture-positive bacterial isolates resistant to selected antibiotics by the type of pathogen (ESKAPE) from the antimicrobial resistance sentinel surveillance laboratories in Tunisia, 2011–2023. Abbreviation: ampicillin (AMP); amoxicillin-clavulanic acid (AMC); piperacillin–tazobactam (PTZ); cefotaxime (CTX); ceftazidime (CAZ); imipenem (IMP); gentamicin (GEN); gentamicin high-charged 30 µg (GHC); amikacin (AMK); ciprofloxacin (CIP); ofloxacin (OFL); oxacillin (OXA); vancomycin (VAN); teicoplanin (TEI), linezolid (LIN).
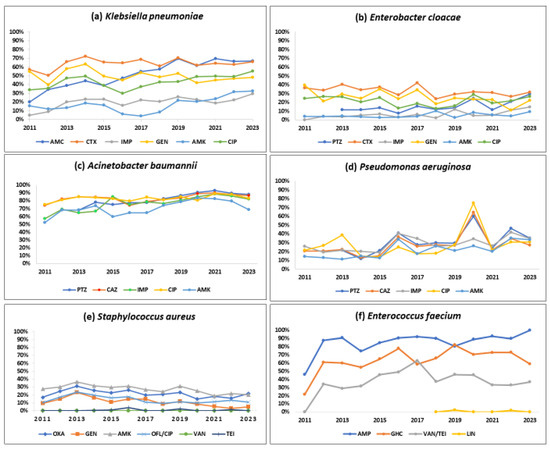
Figure 4.
Trends in the proportion of positive bacterial isolates resistant to selected antibiotics by the type of pathogen (ESKAPE) from the antimicrobial resistance sentinel surveillance laboratories in Tunisia, 2011–2023. Abbreviation: amoxicillin-clavulanic acid (AMC); cefotaxime (CTX); imipenem (IMP); gentamicin (GEN); amikacin (AMK); ciprofloxacin (CIP); ofloxacin (OFL); oxacillin (OXA); vancomycin (VAN); teicoplanin (TEI); ampicillin (AMP); gentamicin high-charged 30 µg (GHC); linezolid (LIN).
3.3.1. Klebsiella pneumoniae
About half of the K. pneumoniae strains reported resistance against amoxicillin-clavulanate (51.7%), gentamicin (49.2%), and ciprofloxacin (44.3%). Cefotaxime and imipenem resistance were seen in 63.1%, and 19.4%, respectively. A statistically significant (p < 0.01) rising trend in the resistance rates was observed during the study period for amoxicillin-clavulanate (18.9% to 66.8%), cefotaxime (57.1% to 65.8%), imipenem (4.7% to 29.5%), amikacin (15.7% to 32.3%), and ciprofloxacin (34% to 55.3%).
3.3.2. Enterobacter cloacae
One-third of the isolates (32%) were resistant to cefotaxime, a 3GC; 24.2% of these were resistant to gentamicin, and 6.8% were resistant to imipenem. Imipenem resistance was first detected in 2012 in 4% of the E. cloacae isolates, and this proportion increased to 14.5% in 2023 (p-value for trend < 0.001).
3.3.3. Acinetobacter baumannii
More than 80% of the isolates were resistant to piperacillin-tazobactam (84.5%), ceftazidime (83.4%), and ciprofloxacin (79.2%). Resistance to imipenem (also known as CRAB) and amikacin was seen in 77% and 71.1%, respectively with a significant increasing trend over time (p-value < 0.01). CRAB rose from 57.2% to 82.1% during 2011–2023 (p-value < 0.001).
3.3.4. Pseudomonas aeruginosa
Three out of ten isolates were found to be resistant to piperacillin-tazobactam (32.5%), ceftazidime (27%), and imipenem (29.3%), respectively, and one-fifth were resistant to amikacin (22.2%). These rates showed a statistically significant rising trend over the last 13 years (p < 0.001). Resistance to piperacillin-tazobactam and amikacin increased from 21.5% to 35.1% and from 14.4% to 33.5%, respectively.
3.3.5. Stapylococcus aureus
One out of five S. aureus isolates was MRSA (21.6%), and this proportion remained stable over time with no significant trend. S. aureus remained susceptible to vancomycin and linezolid with no reported resistance. However, the emergence of resistance to teicoplanin (0.4%) has been reported.
3.3.6. Enterococcus faecium
Resistance to ampicillin, vancomycin (also known as VRE), and a high level of resistance to gentamicin were seen in 88.2%, 38.3%, and 65.9% of the isolates, respectively. Resistance to linezolid was reported for the first time in 2019 in 2.4% of the isolates. A statistically significant (p < 0.001) rising trend in the resistance rates was observed during the study period for ampicillin (45.8% to 100%), gentamicin (21.7% to 59%), and vancomycin (0% to 36.8%).
3.3.7. Other Specific AMR Phenotypes
CRE and 3GCREB increased from 10.6% to 26.3% (p-value < 0.001) and from 39% to 50.2%, respectively, during 2011–2023 (p-value < 0.001).
4. Discussion
This is the first study from Tunisia comprehensively describing the blood culture bacterial pathogen profile and AMR patterns trends over a 13-year period. There were some interesting findings. First, resistance to carbapenems and 3GC among A. baumannii and Enterobacterales was high and increased over the last decade, which has important clinical implications, especially for patients in critical settings, such as intensive care units. Second, high vancomycin resistance and the emergence of linezolid resistance in E. faecium isolates limit therapeutic options. Third, the stable MRSA resistance rates and vancomycin susceptibility in S. aureus isolates are encouraging but necessitate the judicious use of vancomycin for MRSA isolates. Fourth, although the current surveillance system provides useful information on antibiotic resistance trends and patterns, it lacks integration with clinical data to make rapid and meaningful clinical decisions.
CRE increased nearly six times over the last decade in Tunisia. Nearly one out of five Klebsiella isolates were imipenem-resistant. This is a significant finding as carbapenems are considered one of the “last-resort” agents in the face of limited treatment options for CRE pathogens. An analysis of surveillance data in the UAE in 2021 reported that carbapenem resistance among K. pneumoniae isolates was as high as 67.6%, 76.2%, and 91.6% for imipenem, meropenem, and ertapenem, respectively. CRE has also been associated with higher mortality (relative risk, 6.3) and ICU admission (relative risk, 3.9) [18]. Countries such as Belarus, Georgia, Greece, Moldova, Romania, Russia, Serbia, and Ukraine have also reported CRE in >50% of the isolates [19]. The Global Burden of Disease estimates showed that among Gram-negative bacteria, resistance to carbapenems increased more than any other antibiotic class during 1990–2021 [6].
Along with rising carbapenem resistance, approximately two-thirds of K. pneumoniae and one-third of E. cloacae isolates are also resistant to cefotaxime (3GC). It is deeply concerning as 3GC drugs are widely used, often with limited access to alternative agents. A systematic review of 28 studies in sub-Saharan African countries found that nearly 54% of Klebsiella isolates were resistant to 3GCs [20]. A total of 19 countries in the WHO European Region, particularly in the Southern and Eastern parts of the region, also reported resistance to 3GC in >50% of Klebsiella isolates [19]. The high resistance to both carbapenems and 3GCs among Enterobacterales is a challenge to patient management and survival, especially among patients with severe infections and those critically ill in ICUs, because they are the cornerstone of antibiotic therapy in such settings.
According to our findings, nearly 80 percent of Acinetobacter baumannii strains were found to be resistant to carbapenems (these are known as CRAB), which constitutes a great challenge for clinical practice. Similar rates of resistance to carbapenems (up to 90%) have been reported in other Mediterranean countries, including Tunisia [21,22]. In a recent study in Lebanon, all A. baumannii isolates were resistant to carbapenem, with a high 30-day mortality rate of 72% [23]. The present study also highlighted the significant increase in CRAB trends over time, as described worldwide [19,24]. A. baumannii has emerged as a significant multidrug-resistant (MDR) pathogen in hospitals, leading to increased length of intensive care unit (ICU) stays and high mortality, which is why the WHO has put the pathogen in the critical group of bacterial priority pathogens [15], for which new antibiotics and vaccines are urgently needed. The long-term persistence of the organism in the hospital environment makes cross-contamination through air and inanimate objects very likely. This emphasizes the importance of environmental cleaning and disinfection, isolation, and enhanced contact precautions to prevent cross-transmission of CRAB.
Regarding S. aureus, the study findings are in alignment with the situation in the majority of European Union countries where MRSA seems to be stabilizing or even decreasing [25]. This could be due to the cyclical success of some methicillin-sensitive S. aureus clones that tend to replace dominant MRSA clones [26]. The susceptibility of S. aureus to vancomycin was encouraging. However, judicious use of this drug for culture-proven MRSA infections that are not susceptible to routine or alternative agents is necessary to prevent the development of vancomycin-resistant S. aureus.
More than one-third of the E. faecium isolates were vancomycin-resistant (VRE), which is much higher than reported in other countries of the region, such as Egypt and the UAE, with 23.1% and 8.1%, respectively [27,28]. This could be due to the emergence of novel clones of the bacteria in Tunisia, which is a precursor to the rapid evolution of these resistant bacteria [18]. However, a number of other European countries have reported high rates of vancomycin resistance among E. faecium isolates, ranging from 27 to 66% [29]. Our study also reported the emergence of linezolid-resistant E. faecium isolates as high as 2.4%. With linezolid among the few remaining therapeutic options, this is a major challenge to managing nosocomial infections due to VRE [30,31].
Strengths and Limitations
The major strength of this study is the analysis of a large number of bacterial isolates (n = 22,795) from the nationwide AMR surveillance network. The network covered the majority of the high-burden public sector tertiary teaching hospitals in the country that followed standardized laboratory protocols for testing blood specimens for bacterial identification and antimicrobial testing over a long 13-year period, making the findings representative of what is happening in the country’s public health sector.
However, the study had a few limitations. First, the surveillance network does not capture the scenario in the private sector, which caters to a substantial proportion of the population. Second, we could not calculate and compare incidence rates of BSIs due to a lack of a defined catchment area/population. Third, the lack of individual-level clinico-demographic data, such as age, gender, length of stay, clinical profile, resistance profile, diagnosis, etc., did not allow us to explore the factors associated with AMR and multidrug resistance. The lack of patient outcome data also limits our understanding of mortality associated with AMR. Fourth, using aggregate data for analysis rules out the possibility of data validation.
There are four important implications for policy and practice from this study. First, this nationwide AMR surveillance data over a long period will inform protocols for empirical antibiotic use in common bloodstream bacterial pathogens in Tunisia. Second, treatment practices should be individualized based on susceptibility testing to ensure the most effective antibiotics are used. This requires rapid point-of-care diagnostics for bacterial identification and susceptibility testing to aid quick decision-making by clinicians. Third, infection prevention and control (IPC) measures should be strengthened in healthcare settings to reduce the spread of resistant bacteria. Fourth, the lack of clinical data from patients with drug-resistant infections is a major knowledge gap. The AMR surveillance system in Tunisia must integrate antibiotic resistance data with clinical data through real-time data-sharing platforms that allow for the immediate exchange of information across healthcare providers and settings. This will generate big data and will allow us to use machine learning tools to analyze patterns and trends and make useful predictive models for quick and real-time clinical decision-making, and this will eventually lead to improved patient outcomes. User-friendly dashboards that visualize integrated data for clinicians, microbiologists, and public health officials can be helpful.
5. Conclusions
The increasing resistance to critically important antibiotics, particularly carbapenems and third-generation cephalosporins (3GCs) among Gram-negative bacteria responsible for bloodstream infections (BSIs), presents a significant and urgent challenge to the management of these infections in Tunisia, as it severely restricts effective therapeutic options. Furthermore, the emerging resistance to linezolid among Gram-positive cocci constitutes an additional substantial threat, necessitating urgent interventions to limit the irrational use of these life-saving drugs. Despite certain limitations, including the scope of the surveillance network and the lack of patient-level clinical information, Tunisia’s national AMR surveillance network has supplied essential foundational data for tracking annual AMR trends and informing empirical antibiotic prescriptions, rational antibiotic use, and stewardship policy in the country.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/tropicalmed10060164/s1. Figure S1 reports the AMR pattern to selected antimicrobials among E. coli and E. faecalis isolates. Figure S2 shows the trends in the proportion of positive E. coli and E. faecalis isolates resistant to selected antibiotics 2011–2023 (p-value < 0.001).
Author Contributions
Conceptualization, L.K., B.D.F., J.P.T. and I.B.-B.B.; methodology, L.K., B.D.F. and J.P.T.; software, L.K., B.D.F. and J.P.T.; validation, L.K., B.D.F., J.P.T. and I.B.-B.B.; formal analysis, L.K., B.D.F. and J.P.T.; investigation, L.K., K.M., M.Z., S.M., A.F., Y.C., M.H., H.R., Y.K., O.B., S.F. (Siwar Frigui), S.F. (Sana Ferjani), E.M., A.G., N.B.A., F.M., Y.B.L., S.K., B.M., H.N., M.M. (Manel Marzouk), S.D., H.K., S.B. (Safa Bouazra), H.B., R.O., L.T., J.B., F.B., S.B. (Sophia Besbes), L.S., W.A., M.M. (Maha Mastouri), A.H., H.S. and I.B.-B.B.; data curation, L.K.; writing—original draft preparation, L.K., B.D.F. and J.P.T.; writing—review and editing, I.B.-B.B.; visualization, L.K., B.D.F. and J.P.T.; supervision, I.B.-B.B.; project administration, I.B.-B.B. and R.O.; funding acquisition I.B.-B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This SORT IT program was funded by TDR, ADPHC and UAEU. TDR is able to conduct its work thanks to the commitment and support from a variety of funders. A full list of TDR donors is available at: https://tdr.who.int/about-us/our-donors, accessed on 30 November 2024. L’Antibiorésistance en Tunisie” (LART) was supported by the Ministry of Health, Tunisia.
Institutional Review Board Statement
Permission to use the laboratory data was obtained from the Director of the National Reference Laboratory (NRL), and ethics approval was sought from the local ethics committee of Charles Nicolle Hospital and the Union Ethics Advisory Group at the International Union against Tuberculosis and Lung Disease, Paris, France.
Informed Consent Statement
Only aggregated national-level estimates are presented without disclosing the names of the hospitals.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by TDR, the Special Programme for Research and Training in Tropical Diseases hosted at the World Health Organization (WHO). The specific SORT IT program that led to this publication included a SORT IT partnership with the Abu Dhabi Public Health Center (ADPHC), United Arab Emirates, The United Arab Emirates University (UAEU), Al Ain, United Arab Emirates, The International Union Against Tuberculosis and Lung Disease, Paris and India offices, The Damien Foundation, Brussels, Belgium, All India Institute of Medical Sciences, Nagpur, India, All India Institute of Medical Sciences, Deoghar, India, The Tuberculosis Research and Prevention Center Non-Governmental Organization, Armenia, The NarotamSekhsaria Foundation, Mumbai, India, The Ministry of Health of Iran and, The WHO Regional Office for the Eastern Mediterranean, and the WHO country office of Egypt. Data collection and validation during the study period were provided by the different participants from the laboratories involved in the LART surveillance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMR | Antimicrobial resistance |
| AMC | Amoxicillin-clavulanic acid |
| AMK | Amikacin |
| AMP | Ampicillin |
| BSI | Bloodstream infections |
| CIP | Ciprofloxacin |
| CRE | Carbapenem-resistant Enterobacterales |
| CRAB | Carbapenem-resistant A. baumannii |
| CTX | Cefotaxime |
| CAZ | Ceftazidime |
| ERT | Ertapenem |
| DALYs | Disability-Adjusted Life Years |
| ESKAPE | E. cloacae, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, E. faecium |
| FOX | Cefoxitin |
| GEN | Gentamicin |
| GHC | Gentamicin high-charged |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| ICU | Intensive care unit |
| IMP | Imipenem |
| LART | L’Antibiorésistance en Tunisie |
| LIN | Linezolid |
| LIS | Laboratory information system |
| MDR | Multidrug resistant |
| MENA | Middle East and North Africa |
| MRSA | Methicillin-resistant S. aureus |
| OFL | Ofloxacin |
| PTZ | Piperacillin–tazobactam |
| TIG | Tigecycline |
| 3GC | Third-generation cephalosporin |
| 3GCREB | Third-generation cephalosporin-resistant Enterobacterales |
| VAN | Vancomycin |
| VRE | Vancomycin-resistant Enterococci |
| WHO | World Health Organization |
References
- Stewardson, A.J.; Allignol, A.; Beyersmann, J.; Graves, N.; Schumacher, M.; Meyer, R.; Tacconelli, E.; De Angelis, G.; Farina, C.; Pezzoli, F.; et al. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: A multicentre retrospective cohort study. Eurosurveillance 2016, 21, 30319. [Google Scholar] [CrossRef] [PubMed]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 26 November 2023).
- Marchello, C.S.; Dale, A.P.; Pisharody, S.; Rubach, M.P.; Crump, J.A. A Systematic Review and Meta-analysis of the Prevalence of Community-Onset Bloodstream Infections among Hospitalized Patients in Africa and Asia. Antimicrob. Agents Chemother. 2019, 64, e01974-19. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Hsueh, P.-R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; E Wool, E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Talaat, M.; Zayed, B.; Tolba, S.; Abdou, E.; Gomaa, M.; Itani, D.; Hutin, Y.; Hajjeh, R. Increasing Antimicrobial Resistance in World Health Organization Eastern Mediterranean Region, 2017–2019. Emerg. Infect. Dis. 2022, 28, 717–724. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; Alrand, H.; Everett, D.B.; Senok, A.; Menezes, G.A.; Ayoub Moubareck, C. Epidemiology and antimicrobial resistance trends of Acinetobacter species in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2024, 11, 1245131. [Google Scholar] [CrossRef]
- Zha, L.; Li, S.; Guo, J.; Hu, Y.; Pan, L.; Wang, H.; Zhou, Y.; Xu, Q.; Lu, Z.; Kong, X.; et al. Global and regional burden of bloodstream infections caused by carbapenem-resistant Gram-negative bacteria in 2019: A systematic analysis from the MICROBE database. Int. J. Infect. Dis. 2025, 153, 107769. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; A Kumaran, E.P.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Available online: https://www.who.int/publications/book-orders (accessed on 26 November 2023).
- Comité de l’Antibiograme de la Société Française de Microbiologie. Available online: https://www.sfm-microbiologie.org/categorie-produit/livres/ (accessed on 26 November 2023).
- Saïdani, M.; Boutiba, I.; Ghozzi, R.; Kammoun, A.; Redjeb, S.B. Communication brève Profil bactériologique des bactériémies à germes multirésistants à l’hôpital Charles-Nicolle de Tunis Bacteriologic profile of bacteremia due to multi-drug resistant bacteria at Charles-Nicolle hospital of Tunis. Médecine Mal. Infect. 2006, 36, 163–166. [Google Scholar] [CrossRef]
- Mellouli, A.; Chebbi, Y.; El Fatmi, R.; Raddaoui, A.; Lakhal, A.; Torjmane, L.; Abdeljelil, N.B.; Belloumi, D.; Ladeb, S.; Othmen, T.B. Multidrug resistant bacteremia in hematopoietic stem cell transplant recipients Bactériémies à bactéries multirésistantes chez les greffés de cellules souches-hématopoïétiques. Tunis Med. 2021, 99, 269–276. [Google Scholar] [PubMed]
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe 2024, 5, 100902. [Google Scholar] [CrossRef] [PubMed]
- Brayek, A.; Bouguerra, H. Carte Sanitaire Tunisie 2021. Available online: https://santetunisie.rns.tn/fr/carte-sanitaire/carte-sanitaire-2011 (accessed on 26 November 2023).
- EUCAST: European Committee of Antimicrobial Susceptbility Testing. Available online: https://www.eucast.org (accessed on 26 November 2023).
- Dziri, R.; El Kara, F.; Barguellil, F.; Ouzari, H.-I.; El Asli, M.S.; Klibi, N. Vancomycin-Resistant Enterococcus faecium in Tunisia: Emergence of Novel Clones. Microb. Drug Resist. 2019, 25, 469–474. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2021 (accessed on 29 November 2023).
- Lester, R.; Musicha, P.; van Ginneken, N.; Dramowski, A.; Hamer, D.H.; Garner, P.; A Feasey, N. Prevalence and outcome of bloodstream infections due to third-generation cephalosporin-resistant Enterobacteriaceae in sub-Saharan Africa: A systematic review. J. Antimicrob. Chemother. 2019, 75, 492–507. [Google Scholar] [CrossRef]
- Ma, C.; McClean, S. Mapping global prevalence of Acinetobacter baumannii and recent vaccine development to tackle it. Vaccines 2021, 9, 570. [Google Scholar] [CrossRef]
- Abukhalil, A.; Barakat, S.; Mansour, A.; Al-Shami, N.; Naseef, H. ESKAPE Pathogens: Antimicrobial Resistance Patterns, Risk Factors, and Outcomes a Retrospective Cross-Sectional Study of Hospitalized Patients in Palestine. Infect. Drug Resist. 2024, 17, 3813–3823. [Google Scholar] [CrossRef]
- Itani, R.; Khojah, H.M.J.; Karout, S.; Rahme, D.; Hammoud, L.; Awad, R.; Abu-Farha, R.; Mukattash, T.L.; Raychouni, H.; El-Lakany, A. Acinetobacter baumannii: Assessing susceptibility patterns, management practices, and mortality predictors in a tertiary teaching hospital in Lebanon. Antimicrob. Resist. Infect. Control 2023, 12, 136. [Google Scholar] [CrossRef]
- Bandy, A.; Almaeen, A.H. Pathogenic spectrum of blood stream infections and resistance pattern in Gram negative bacteria from Aljouf region of Saudi Arabia. PLoS ONE 2020, 15, e0233704. [Google Scholar] [CrossRef]
- Surveillance of Antimicrobial Resistance in Europe, 2023 Data: Executive Summary. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-ECDC-WHO-executive-summary-2023-data.pdf (accessed on 29 November 2024).
- Rolain, J.-M.; Abat, C.; Brouqui, P.; Raoult, D. Worldwide decrease in methicillin-resistant Staphylococcus aureus: Do we understand something? Clin. Microbiol. Infect. 2015, 21, 515–517. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzak, N.M.; AlRand, H.; Menezes, G.A.; Moubareck, C.A.; Everett, D.B.; Senok, A.; Podbielski, A. Epidemiology of vancomycin-resistant enterococci in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2023, 11, 1275778. [Google Scholar] [CrossRef]
- Moemen, D.; Tawfeek, D.; Badawy, W. Healthcare-associated vancomycin resistant Enterococcus faecium infections in the Mansoura University Hospitals intensive care units. Egypt. Braz. J. Microbiol. 2015, 46, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Surveillance Atlas of Infectious Diseases. Available online: https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases (accessed on 29 November 2023).
- O’Toole, R.F.; Leong, K.W.; Cumming, V.; Van Hal, S.J. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).