A Case Report and Literature Review of Prostatic Tuberculosis Masquerading as Prostate Cancer: A Diagnostic Challenge in a Tuberculosis-Endemic Region
Abstract
1. Introduction
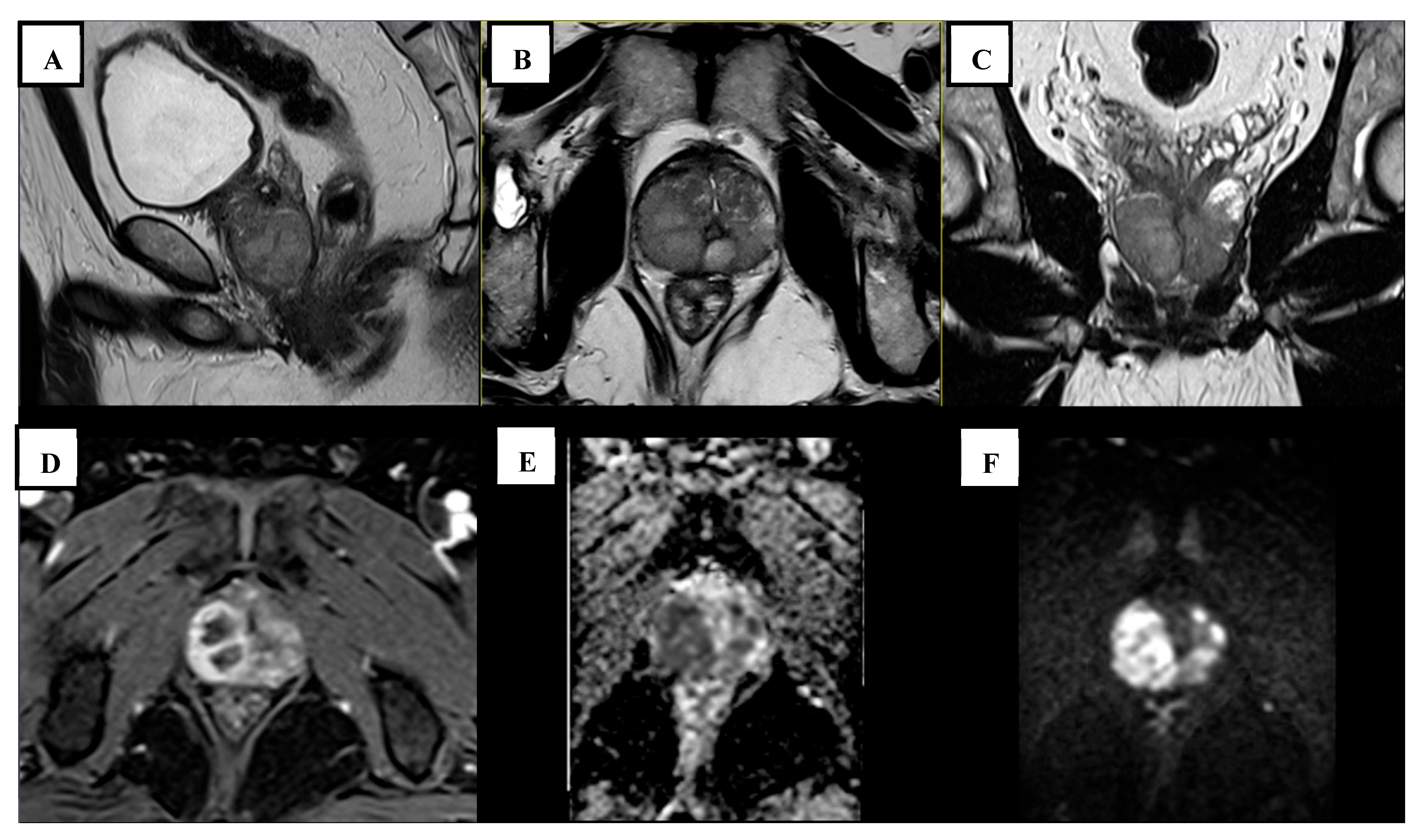
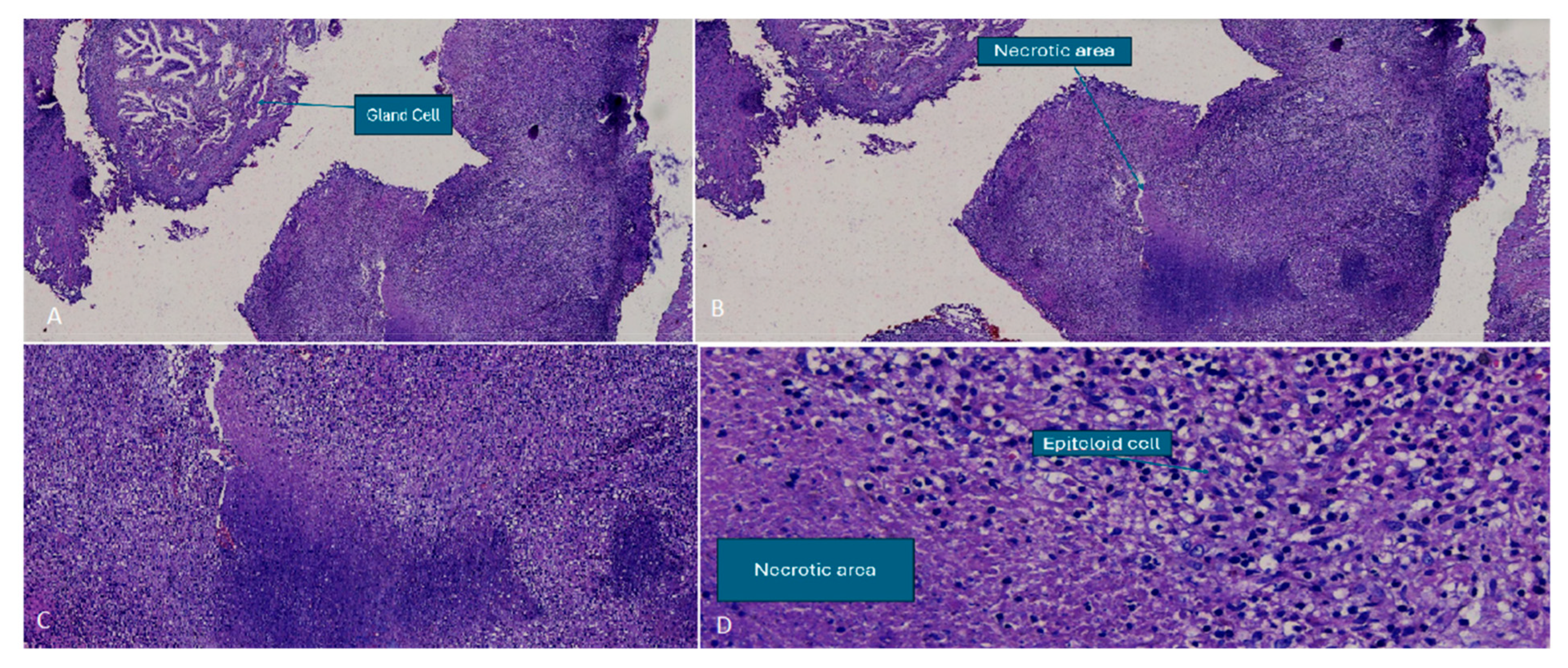
2. Case Report
3. Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent Diffusion Coefficient |
| BCG | Bacillus Calmette–Guérin |
| BPH | Benign Prostatic Hyperplasia |
| DCE | Dynamic Contrast-Enhanced |
| DWI | Diffusion-Weighted Imaging |
| IGRA | Interferon-Gamma Release Assay |
| LAMP | Loop-Mediated Isothermal Amplification |
| LPA | Line Probe Assay |
| LUTS | Lower Urinary Tract Symptoms |
| MP-MRI | Multiparametric Magnetic Resonance Imaging |
| MRI | Magnetic Resonance Imaging |
| N/A | Not Available |
| PI-RADS | Prostate Imaging-Reporting and Data System |
| PCR | Polymerase Chain Reaction |
| PSA | Prostate-Specific Antigen |
| PTB | Prostatic Tuberculosis |
| TB | Tuberculosis |
| TST | Tuberculin skin testing |
| T1WI | T1-Weighted Imaging |
| T2WI | T2-Weighted Imaging |
| TURP | Transurethral Resection of the Prostate |
| TWIST-VIBE | Time-Resolved Interleaved Stochastic Trajectory 3D T1-Weighted Spoiled Gradient Echo |
References
- Ministry of Health Republic of Indonesia. Tuberculosis Control in Indonesia 2022. Available online: https://tbindonesia.or.id/wp-content/uploads/2023/02/Factsheet-Country-Profile-Indonesia-2022.pdf (accessed on 29 September 2024).
- Yang, G.; Ruan, L. Imaging findings of prostate tuberculosis by transrectal contrast-enhanced ultrasound and comparison with 2D ultrasound and pathology. Br. J. Radiol. 2022, 95, 20210713. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dan, S.; Yang, F.; He, X.; He, L.; Yue, W. Prostate tuberculosis mimicking prostate cancer: Case report and literature review. Medicine 2023, 102, e36172. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.A.; Lopes, H.E.; Barreto, A.d.A.; Fanni, V.S.S.; Netto, J.M.B. Prostate Tuberculosis: Six forms of clinical presentation. Int. Braz. J. Urol. 2024, 50, 80–86. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, J.J.; Wang, H.Y.; Sun, L.J.; Zhang, G.M. Granulomatous prostatitis after bacille Calmette-Guérin instillation resembles prostate carcinoma: A case report and review of the literature. World J. Clin. Cases 2023, 11, 2051–2059. [Google Scholar] [CrossRef]
- Li, Y.; Mongan, J.; Behr, S.C.; Sud, S.; Coakley, F.V.; Simko, J.; Westphalen, A.C. Beyond Prostate Adenocarcinoma: Expanding the Differential Diagnosis in Prostate Pathologic Conditions. Radiographics 2016, 36, 1055–1075. [Google Scholar] [CrossRef]
- Gupta, N.; Mandal, A.K.; Singh, S.K. Tuberculosis of the prostate and urethra: A review. Indian J. Urol. 2008, 24, 388–391. [Google Scholar] [PubMed]
- Bellouki, O.; Boughaleb, A.; Soufiani, I.; Boualaoui, I.; El Sayegh, H.; Nouini, Y. Isolated prostate tuberculosis: A rare condition. Urol. Case Rep. 2024, 53, 102669. [Google Scholar] [CrossRef]
- Koesbandono; Lukito, A.A.; Muljadi, R.; Yuniarti, M.; Sindunata, N.A.; Sarikie, A.; Pratama, T.A.; Thio, R.S.; Christanti, J.; Octavius, G.S. High Prevalence of Myocardial Bridging Detected in an Indonesian Population Using Multi-Detector Computed Tomography. Medicina 2024, 60, 794. [Google Scholar] [CrossRef]
- Lestari, B.W.; Nijman, G.; Larasmanah, A.; Soeroto, A.Y.; Santoso, P.; Alisjahbana, B.; Chaidir, L.; Andriyoko, B.; Crevel, R.v.; Hill, P.C. Management of drug-resistant tuberculosis in Indonesia: A four-year cascade of care analysis. Lancet Reg. Health-Southeast Asia 2024, 22, 100294. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, L.; Zhang, X.; Ji, Q.; Shen, W. Multiparametric Magnetic Resonance Imaging Characteristics of Prostate Tuberculosis. Korean J. Radiol. 2015, 16, 846–852. [Google Scholar] [CrossRef]
- Srivastava, A.; Chandra, M.; Saha, A. Use of multiparametric magnetic resonance imaging in prostate cancer: A review. Meas. Sens. 2024, 33, 101128. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Guo, Y. Proton Magnetic Resonance Spectroscopy in Prostate Tuberculosis. Urology 2010, 75, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Kostakopoulos, A.; Economou, G.; Picramenos, D.; Macrichoritis, C.; Tekerlekis, P.; Kalliakmanis, N. Tuberculosis of the prostate. Int. Urol. Nephrol. 1998, 30, 153–157. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liang, C.; Zhu, F.P.; Zhou, T.R.; Li, J.; Wang, Z.J.; Liu, B.J. Improving the understanding of PI-RADS in practice: Characters of PI-RADS 4 and 5 lesions with negative biopsy. Asian J. Androl. 2023, 25, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Van Hau, H.; Van Hieu, N.; Dinh Lien, N.; Truong Thanh, D. A Rare Case of Urinary Retention Due to Prostate Tuberculosis in Vietnam. Cureus 2022, 14, e28574. [Google Scholar] [CrossRef]
- Fonseca, E.; Kaufmann, O.G.; Leão, L.R.S.; Tridente, C.F.; Yamauchi, F.I.; Baroni, R.H. Incidentally detected tuberculous prostatitis with microabscess. Int. Braz. J. Urol. 2018, 44, 397–399. [Google Scholar] [CrossRef]
- Legesse, T.K.; Issa, S.A.; Yaynishet, Y.A.; Dessie, T.A.; Gebremariam, T.Y.; Reta, B.K. Isolated Prostate Tuberculosis Mimicking Prostate Cancer. Ethiop. J. Health Sci. 2024, 34, 67–72. [Google Scholar]
- Aslan, S.; Eryuruk, U.; Ozdemir, B. Tuberculous prostatitis mimicking metastatic prostate cancer. Rev. Soc. Bras. Med. Trop. 2022, 55, e07262021. [Google Scholar] [CrossRef]
- Smani, S.; Jalfon, M.; Sundaresan, V.; Lokeshwar, S.D.; Nguyen, J.; Halstuch, D.; Khajir, G.; Cavallo, J.A.; Sprenkle, P.C.; Leapman, M.S.; et al. Inter-reader reliability and diagnostic accuracy of PI-RADS scoring between academic and community care networks: How wide is the gap? Urol. Oncol. Semin. Orig. Investig. 2024, 43, 396.e1–396.e7. [Google Scholar] [CrossRef]
- Sauvat, L.; Lhermite, Q.; Desplechain, C.; Long, B.; Vidal, M. An ambivalent prostate nodule after Bacillus Calmette-Guérin therapy. IDCases 2021, 26, e01338. [Google Scholar] [CrossRef]
- Fujikawa, K.; Matsui, Y.; Fukuzawa, S.; Soeda, A.; Takeuchi, H. A case of tuberculosis of the prostate. Scand. J. Urol. Nephrol. 1999, 33, 268–269. [Google Scholar] [PubMed]
- Rachapalli, V.; Natale, S.; Awasthi, R. Tuberculosis of the prostate. Br. J. Hosp. Med. 2006, 67, 211. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Singh, A.; Kishore, K.; Kant, S. A rare presentation of disseminated tuberculosis: Prostatic abscess. Indian J. Tuberc. 2017, 64, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [Google Scholar] [CrossRef]
| Author (Year) | Age (Years) | Constitutional Symptoms * | PSA Level (ng/mL) | Previous TURP | PIRADS Score | MRI Findings |
|---|---|---|---|---|---|---|
| Legesse (2024) [18] | 69 | None | 1768 | Yes | 5 | The transitional zone appeared enlarged with mixed signal intensity on T2WI. A focal hypointense lesion was detected in the right basal and midzone of the prostate, extending into the peripheral zone. This lesion was accompanied by capsular obliteration and extraprostatic extension. It exhibited restricted diffusion on DWI and the ADC. |
| Aslan (2022) [19] | 80 | None | N/A | No | N/A | A poorly defined, low-signal intensity lesion was identified in the left anterolateral peripheral zone on T2WI. The lesion exhibited restricted diffusion and early ring enhancement. |
| Li (2023) [3] | 52 | None | 12.4 | No | N/A | A mass-like abnormal signal shadow was detected in the peripheral zone, appearing isointense on T1WI, slightly hyperintense on fat-suppressed T2WI, and hyperintense on DWI. Additionally, the lesion had invaded the left seminal vesicle gland, while the left peripheral zone exhibited significant heterogeneous enhancement, and pelvic lymph nodes were observed. |
| Fonseca (2018) [17] | 73 | None | 6.54 | No | 2 | A small nodule with abnormal diffusion restriction was detected in the left posterior mid-third of the transition zone. It exhibited intense peripheral post-contrast enhancement with a liquefied center, indicative of a microabscess. |
| Hau (2022) [16] | 62 | None | 4.5 | No | 3 | Multiple lesions were present in the transitional zone and bilateral peripheral zone of the prostate. On T2-weighted imaging, the entire gland’s parenchyma appeared slightly elevated with irregular signals. It showed no early enhancement after contrast administration and exhibited heterogeneous enhancement in the late phase. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
William, Y.; Sugiono, M.; Diana Prasetiyo, P.; Erico, A.; Octavius, G.S. A Case Report and Literature Review of Prostatic Tuberculosis Masquerading as Prostate Cancer: A Diagnostic Challenge in a Tuberculosis-Endemic Region. Trop. Med. Infect. Dis. 2025, 10, 145. https://doi.org/10.3390/tropicalmed10050145
William Y, Sugiono M, Diana Prasetiyo P, Erico A, Octavius GS. A Case Report and Literature Review of Prostatic Tuberculosis Masquerading as Prostate Cancer: A Diagnostic Challenge in a Tuberculosis-Endemic Region. Tropical Medicine and Infectious Disease. 2025; 10(5):145. https://doi.org/10.3390/tropicalmed10050145
Chicago/Turabian StyleWilliam, Yonathan, Marto Sugiono, Patricia Diana Prasetiyo, Adelbertus Erico, and Gilbert Sterling Octavius. 2025. "A Case Report and Literature Review of Prostatic Tuberculosis Masquerading as Prostate Cancer: A Diagnostic Challenge in a Tuberculosis-Endemic Region" Tropical Medicine and Infectious Disease 10, no. 5: 145. https://doi.org/10.3390/tropicalmed10050145
APA StyleWilliam, Y., Sugiono, M., Diana Prasetiyo, P., Erico, A., & Octavius, G. S. (2025). A Case Report and Literature Review of Prostatic Tuberculosis Masquerading as Prostate Cancer: A Diagnostic Challenge in a Tuberculosis-Endemic Region. Tropical Medicine and Infectious Disease, 10(5), 145. https://doi.org/10.3390/tropicalmed10050145








