Changes in Lipid Profile Secondary to Asymptomatic Malaria in Migrants from Sub-Saharan Africa: A Retrospective Analysis of a 2010–2022 Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.1.1. Study Population
- -
- Patients with asymptomatic malaria infection (AMI): without any symptoms suggestive of malaria (fever, cephalea, myalgias, arthralgias, or other symptoms that patients associate with previous malaria infections) or any previous treatment for malaria within the past three months and with a positive polymerase chain reaction (PCR) result for Plasmodium spp.
- -
- Patients without asymptomatic malaria infection (non-AMI): without any symptoms suggestive of malaria and with a negative PCR for Plasmodium spp.
2.1.2. Variables Collected
2.1.3. Statistical Analysis
2.2. Ethical Aspects
3. Results
3.1. Results of Screening for Malaria
3.2. Results of Screening for Other Infectious Diseases
3.3. Hemogram and Screening for Hemoglobinopathies
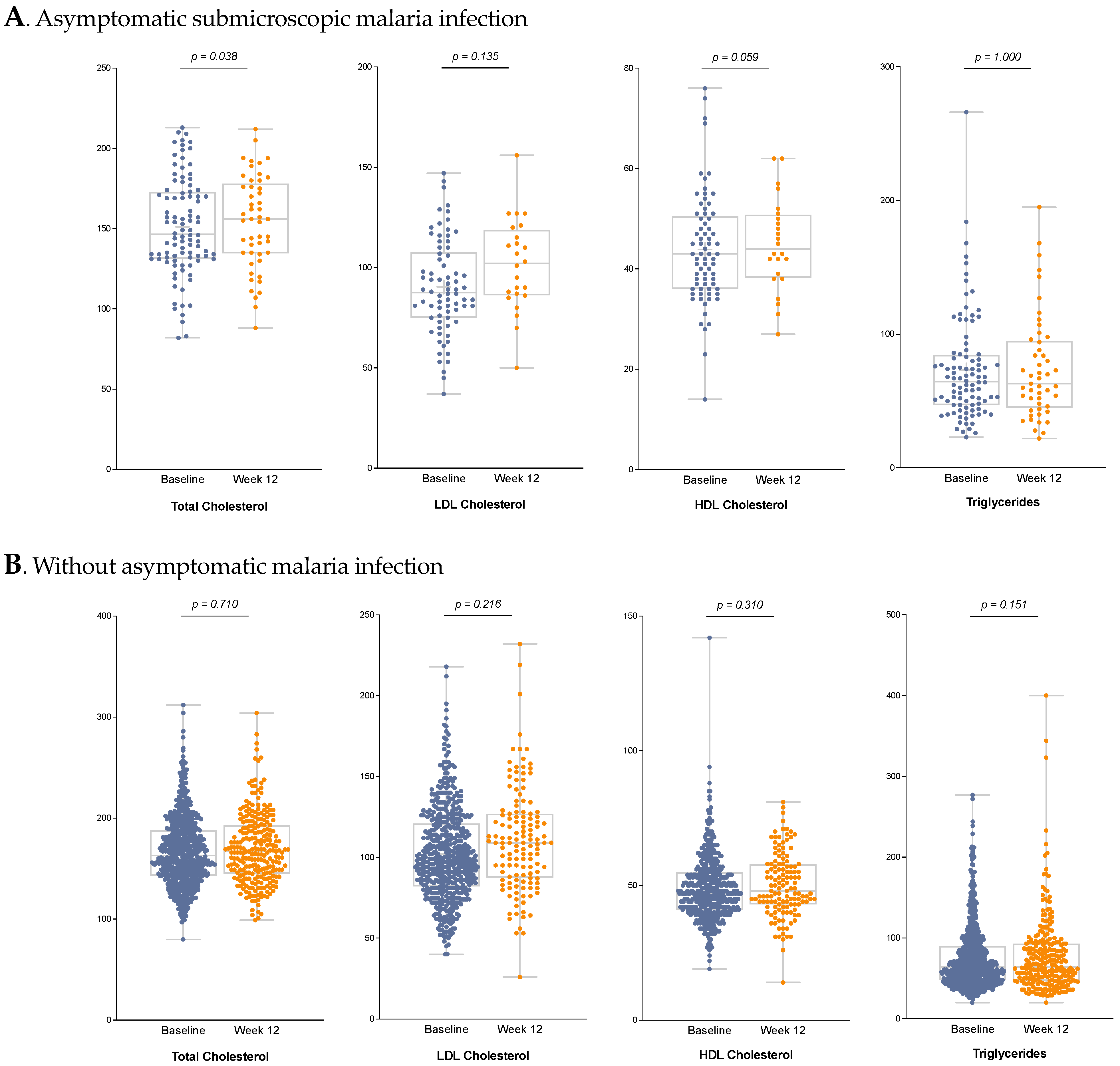
3.4. Lipid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 18 March 2025).
- European Centre for Disease Prevention and Control. Malaria Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/malaria-annual-epidemiological-report-2022 (accessed on 18 March 2025).
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef] [PubMed]
- Hofmaenner, D.A.; Kleyman, A.; Press, A.; Bauer, M.; Singer, M. The Many Roles of Cholesterol in Sepsis: A Review. Am. J. Respir. Crit. Care Med. 2022, 205, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Fraunberger, P.; Schaefer, S.; Werdan, K.; Walli, A.K.; Seidel, D. Reduction of Circulating Cholesterol and Apolipoprotein Levels during Sepsis. Clin. Chem. Lab. Med. 1999, 37, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Escolar Castellón, J.L.; Pinzón Martín, J.L. Infección y lipoproteínas. Enferm. Infecc. Microbiol. Clin. 2000, 18, 83–86. (In Spanish) [Google Scholar] [PubMed]
- Kumar, R.; Uddin Md, J.; Mehdi, M.D.; Agrawal, P.K.; Abhishek, K. Hospital Based Study of Lipid Profile in Newly Diagnosed Cases of Plasmodium Falciparum Infected Malaria Patients in Kosi Region of Bihar. J. Evol. Med. Dent. Sci. 2015, 4, 1996–2000. [Google Scholar] [CrossRef]
- Dias, R.M.; Vieira, J.L.F.; Cabral, B.D.C.; Da Silva, I.R.P.; Brasil, L.M.B.F.; Araújo, E.D.C.; De Andrade, M.A. Lipid profile of children with malaria by Plasmodium vivax. J. Trop. Med. 2016, 2016, 9052612. [Google Scholar] [CrossRef]
- Vliegenthart-Jongbloed, K.J.; Koelewijn, R.; Tielens, A.G.M.; Sauerwein, R.W.; van Hellemond, J.J.; van Genderen, P.J.J. The decrease in plasma cholesterol during Plasmodium falciparum infections is not caused by cholesterol utilization by the parasites but by an infection-induced acute-phase response. J. Infect. 2023, 86, 617–619. [Google Scholar] [CrossRef]
- Visser, B.J.; De Vries, S.G.; Vingerling, R.; Gritter, M.; Kroon, D.; Aguilar, L.C.; Kraan, R.B.J.; Wieten, R.W.; Danion, F.; Sjouke, B.; et al. Serum lipids and lipoproteins during uncomplicated malaria: A cohort study in Lambaréné, Gabon. Am. J. Trop. Med. Hyg. 2017, 96, 1205–1214. [Google Scholar] [CrossRef]
- Visser, B.J.; Wieten, R.W.; Nagel, I.M.; Grobusch, M.P. Serum lipids and lipoproteins in malaria—A systematic review and meta-analysis. Malar. J. 2013, 12, 442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neves, F.A.R.; Ventura, A.M.R.S.; Filho, M.G.S.; Libonati, R.M.F. Lipid parameters in a hyperendemic area for malaria. Lipids Health Dis. 2013, 12, 162. [Google Scholar] [CrossRef]
- Maier, A.G.; van Ooij, C. The role of cholesterol in invasion and growth of malaria parasites. Front. Cell. Infect. Microbiol. 2022, 12, 984049. [Google Scholar] [CrossRef]
- Koch, M.; Cegla, J.; Jones, B.; Lu, Y.; Mallat, Z.; Blagborough, A.M.; Angrisano, F.; Baum, J. The effects of dyslipidaemia and cholesterol modulation on erythrocyte susceptibility to malaria parasite infection. Malar. J. 2019, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- Tokumasu, F.; Hayakawa, E.H.; Fukumoto, J.; Tokuoka, S.M.; Miyazaki, S. Creative interior design by Plasmodium falciparum: Lipid metabolism and the parasite’s secret chamber. Parasitol. Int. 2021, 83, 102369. [Google Scholar] [CrossRef]
- Hoffmeister, B.; Aguilar Valdez, A.D. Hypertension is associated with an increased risk for severe imported falciparum malaria: A tertiary care hospital based observational study from Berlin, Germany. Malar. J. 2019, 18, 410. [Google Scholar] [CrossRef]
- Orimadegun, A.E.; Orimadegun, B.E. Serum apolipoprotein-A1 and cholesterol levels in Nigerian children with plasmodium falciparum infection. Med. Princ. Pract. 2015, 24, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Corbacho-Loarte, M.D.; Crespillo-Andújar, C.; Chamorro-Tojeiro, S.; Norman, F.; Pérez-Molina, J.A.; Martín, O.; Rubio, J.M.; Gullón-Peña, B.; López-Vélez, R.; Monge-Maillo, B. Screening of imported malaria infection in asymptomatic migrants from Sub-Saharan Africa: A retrospective analysis of a 2010–2019 cohort. Travel. Med. Infect. Dis. 2022, 49, 102411. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.P.; Chitnis, C.E. Lipid peroxidation and its repair in malaria parasites. Trends Parasitol. 2023, 39, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, E.; Arese, P.; Skorokhod, O.A. Role of the lipoperoxidation product 4-hydroxynonenal in the pathogenesis of severe malaria anemia and malaria immunodepression. Oxid. Med. Cell Longev. 2015, 2015, 638416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monge-Maillo, B.; López-Vélez, R. Migration and malaria in Europe. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012014. [Google Scholar] [CrossRef]
- Ta, T.H.; Hisam, S.; Lanza, M.; Jiram, A.I.; Ismail, N.; Rubio, J.M. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar. J. 2014, 13, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez, A.M.; Tang, T.H.T.; Suárez, M.L.; Fernández, A.Á.; García, C.M.; Hisam, S.; Rubio, J.M. Assessment of Commercial Real-Time PCR Assays for Detection of Malaria Infection in a Non-Endemic Setting. Am. J. Trop. Med. Hyg. 2021, 105, 1732–1737. [Google Scholar] [CrossRef]
- Shalmiev, G.; Ginsburg, H. The Susceptibility of The Malarial Parasite Plasmodzum falczparum to Quinoline-Containing Drugs is Correlated to the Lipid Composition of the Infected Erythrocyte Membranes. Biochem. Pharmacol. 1993, 46, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, R.; Low, H.; Mukhamedova, N.; Fu, Y.; Lai, S.-J.; Sasaoka, M.; Hara, A.; Yamazaki, A.; Kameda, T.; Horiuchi, Y.; et al. Cholesterol transport between red blood cells and lipoproteins contributes to cholesterol metabolism in blood. J. Lipid Res. 2020, 61, 1577–1588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murray, M.J.; Murray, A.B.; Murray, N.J.; Murray, M.B. Diet and cerebral malaria: The effect of famine and refeeding. Am. J. Clin Nutr. 1978, 31, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Koopman, J.P.R.; Lule, S.A.; Zziwa, C.; Akurut, H.; Lubyayi, L.; Nampijja, M.; Akello, F.; Balungi, P.; Tumusiime, J.; Oduru, G.; et al. The determinants of lipid profiles in early adolescence in a Ugandan birth cohort. Sci. Rep. 2021, 11, 16503. [Google Scholar] [CrossRef]
- Getaneh, F.; Zeleke, A.J.; Lemma, W.; Tegegne, Y. Malaria Parasitemia in Febrile Patients Mono- and Coinfected with Soil-Transmitted Helminthiasis Attending Sanja Hospital, Northwest Ethiopia. J. Parasitol. Res. 2020, 2020, 9891870. [Google Scholar] [CrossRef]
- Roussilhon, C.; Brasseur, P.; Agnamey, P.; Pérignon, J.L.; Druilhe, P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS ONE 2010, 5, e9309. [Google Scholar] [CrossRef]
- Degarege, A.; Legesse, M.; Medhin, G.; Animut, A.; Erko, B. Malaria and related outcomes in patients with intestinal helminths: A cross-sectional study. BMC Infect. Dis. 2012, 12, 291. Available online: http://www.biomedcentral.com/1471-2334/12/291 (accessed on 18 March 2025). [CrossRef]
- Jackson, J.A.; Friberg, I.M.; Little, S.; Bradley, J.E. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology 2009, 126, 18–27, Erratum in: Immunology 2009, 126, 446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, X.; Mohandas, N. Red cell membrane and malaria. Transfus. Clin. Biol. 2010, 17, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N.; An, X. Malaria and human red blood cells. Med. Microbiol. Immunol. 2012, 201, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Kuesap, J.; Chaijaroenkul, W.; Rungsihirunrat, K.; Pongjantharasatien, K.; Na-Bangchang, K. Coexistence of malaria and thalassemia in malaria endemic areas of Thailand. Korean J. Parasitol. 2015, 53, 265–270. [Google Scholar] [CrossRef]
- Taylor, S.M.; Parobek, C.M.; Fairhurst, R.M. Haemoglobinopathies and the clinical epidemiology of malaria: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Esoh, K.; Wonkam, A. Evolutionary history of sickle-cell mutation: Implications for global genetic medicine. Hum. Mol. Genet. 2021, 30, R119–R128. [Google Scholar] [CrossRef]
- Jeenduang, N.; Horpet, D.; Plyduang, T.; Nuinoon, M. Association of thalassemia, hemoglobinopathies, and vitamin D levels with lipid profile in adults: Community-based research in southern Thai population. Heliyon 2024, 10, e31374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shalev, H.; Kapelushnik, J.; Moser, A.; Knobler, H.; Tamary, H. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am. J. Hematol. 2007, 82, 199–202. [Google Scholar] [CrossRef]
- Brotons, C.; Ribera, A.; Perich, R.M.; Abrodos, D.; Magaña, P.; Pablo, S.; Terradas, D.; Fernández, F.; Permanyer, G. Worldwide distribution of blood lipids and lipoproteins in childhood and adolescence: A review study. Atherosclerosis 1998, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fedoryak, O.; Arama, C.; Diarra, I.; Kouriba, B.; Chrétien, M.; Mbikay, M. Association of the rs562556 PCSK9 Gene Polymorphism with Reduced Mortality in Severe Malaria among Malian Children. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 9340480. [Google Scholar] [CrossRef]
- Rougeron, V.; Woods, C.M.; Tiedje, K.E.; Bodeau-Livinec, F.; Migot-Nabias, F.; Deloron, P.; Luty, A.J.F.; Fowkes, F.J.I.; Day, K.P. Epistatic Interactions between Apolipoprotein E and Hemoglobin S Genes in Regulation of Malaria Parasitemia. PLoS ONE 2013, 8, e76924. [Google Scholar] [CrossRef]
- Sein, K.K.; Aikawa, M. The prime role of plasma membrane cholesterol in the pathogenesis of immune evasion and clinical manifestations of falciparum malaria. Med. Hypotheses 1998, 51, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Frimpong, A.; Amponsah, J.; Adjokatseh, A.S.; Agyemang, D.; Bentum-Ennin, L.; Ofori, E.A.; Kyei-Baafour, E.; Akyea-Mensah, K.; Adu, B.; Mensah, G.I.; et al. Asymptomatic Malaria Infection Is Maintained by a Balanced Pro- and Anti-inflammatory Response. Front. Microbiol. 2020, 11, 559255, Erratum in Front. Microbiol. 2021, 12, 686435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, D.; Perucha, E. Cholesterol metabolism: A new molecular switch to control inflammation. Clin. Sci. 2021, 135, 1389–1408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshida, Y.; Saito, Y.; Hayakawa, M.; Habuchi, Y.; Imai, Y.; Sawai, Y.; Niki, E. Levels of lipid peroxidation in human plasma and erythrocytes: Comparison between fatty acids and cholesterol. Lipids 2007, 42, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Noguchi, N. 7-Hydroxycholestrol as a possible biomarker of cellular lipid peroxidation: Difference between cellular and plasma lipid peroxidation. Biochem. Biophys. Res. Commun. 2014, 446, 741–744. [Google Scholar] [CrossRef] [PubMed]

| Variable | Non-Malaria Infection N = 696 | Malaria Infection N = 104 | p-Value |
|---|---|---|---|
| Gender, male % | 596 (85.6) | 94 (90.4) | 0.333 |
| Age, mean * | 27.1 (10.4) | 24.5 (8.8) | 0.017 |
| Pre-consultation period (months) Median ** | 4.5 [1.9; 9.5] | 3.0 [1.1; 6.9] | 0.003 |
| Transient time (months) Median ** | 4 [1; 11] | 3 [1; 8] | 0.04 |
| Time from consultation to laboratory testing (weeks) Median ** | 1.3 [0.9; 2.0] | 1.1 [0.6; 2.1] | 0.272 |
African endemic area (%)
| 458 (65.8) 215 (30.9) 16 (2.3) 7 (1.0) | 65 (62.5) 39 (37.5) 0 (0.0) 0 (0.0) |
| Plasmodium spp. | Pre-Consultation Time | Total | ||
|---|---|---|---|---|
| <12 Months | 12–24 Months | >24 Months | ||
| P. falciparum | 63 | 5 | 2 | 70 (67.3%) |
| P. malariae | 15 | 1 | 3 * | 19 (18.3%) |
| P. ovale | 8 | 2 | 0 | 10 (9.6%) |
| P. vivax | 0 | 0 | 0 | - |
| P. falciparum + P. ovale | 4 | 0 | 0 | 4 (3.8%) |
| P. malariae + P. ovale | 1 | 0 | 0 | 1 (1%) |
| Total | 91 (87.5%) | 8 (7.7%) | 5 (4.8%) | 104 (100%) |
| Variable | Non-Malaria Infection N = 696 | Malaria Infection N = 104 | p-Value |
|---|---|---|---|
| Hepatitis B Chronic infection Past infection Vaccinated Isolated anti-HBc Negative Not performed | 109 (15.7) 260 (37.4) 24 (3.4) 92 (13.2) 182 (26.1) 29 (4.2) | 14 (13.5) 52 (50.0) 3 (2.9) 10 (9.6) 23 (22.1) 2 (1.9) | 0.252 p < 0.001 * |
| Strongyloides spp. Positive Negative Not performed | 141 (20.3) 482 (69.3) 73 (10.5) | 43 (41.3) 47 (45.2) 14 (13.5) | <0.001 |
| Filariae Positive Loa Loa Onchocerca spp. Mansonella spp. Negative Not performed | 13 (1.9) 5 (0.7) 1 (0.1) 7 (1.0) 168 (24.1) 515 (74.0) | 8 (7.7) 2 (1.9) 0 (0.0) 6 (5.8) 27 (26.0) 69 (66.3) | 0.004 |
| Baseline Measurement | Control Measurement | |||||
|---|---|---|---|---|---|---|
| Variable Median | Non-Malaria Infection N = 676 | Malaria Infection N = 96 | p-Value | Non-Malaria Infection N = 260 | Malaria Infection N = 49 | p-Value |
| Total cholesterol * (mg/dL), | 163 [143; 188] | 146 [131; 173] | <0.001 | 166 [143.5; 192] | 156 [135; 176] | 0.003 |
| HDL cholesterol (mg/dL), ** | 47 [41; 55] | 43 [36; 50] | <0.001 | 47.5 [42; 57] | 44 [38.5; 50.5] | 0.048 |
| LDL cholesterol (mg/dL), ** | 98 [83; 121] | 87.5 [75; 107] | <0.001 | 108.5 [87; 127] | 102 [86.5; 117.5] | 0.208 |
| Triglycerides (mg/dL), * | 64 [47.5; 89] | 65 [47; 85] | 0.744 | 63 [47; 93] | 63 [46; 94] | 0.811 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayoso-Cantero, D.; Corbacho-Loarte, M.D.; Crespillo-Andújar, C.; Chamorro-Tojeiro, S.; Norman, F.; Perez-Molina, J.A.; González-Sanz, M.; Martín, O.; Rubio, J.M.; Gullón-Peña, B.; et al. Changes in Lipid Profile Secondary to Asymptomatic Malaria in Migrants from Sub-Saharan Africa: A Retrospective Analysis of a 2010–2022 Cohort. Trop. Med. Infect. Dis. 2025, 10, 134. https://doi.org/10.3390/tropicalmed10050134
Gayoso-Cantero D, Corbacho-Loarte MD, Crespillo-Andújar C, Chamorro-Tojeiro S, Norman F, Perez-Molina JA, González-Sanz M, Martín O, Rubio JM, Gullón-Peña B, et al. Changes in Lipid Profile Secondary to Asymptomatic Malaria in Migrants from Sub-Saharan Africa: A Retrospective Analysis of a 2010–2022 Cohort. Tropical Medicine and Infectious Disease. 2025; 10(5):134. https://doi.org/10.3390/tropicalmed10050134
Chicago/Turabian StyleGayoso-Cantero, Diego, María Dolores Corbacho-Loarte, Clara Crespillo-Andújar, Sandra Chamorro-Tojeiro, Francesca Norman, Jose A. Perez-Molina, Marta González-Sanz, Oihane Martín, José Miguel Rubio, Beatriz Gullón-Peña, and et al. 2025. "Changes in Lipid Profile Secondary to Asymptomatic Malaria in Migrants from Sub-Saharan Africa: A Retrospective Analysis of a 2010–2022 Cohort" Tropical Medicine and Infectious Disease 10, no. 5: 134. https://doi.org/10.3390/tropicalmed10050134
APA StyleGayoso-Cantero, D., Corbacho-Loarte, M. D., Crespillo-Andújar, C., Chamorro-Tojeiro, S., Norman, F., Perez-Molina, J. A., González-Sanz, M., Martín, O., Rubio, J. M., Gullón-Peña, B., del Campo Albendea, L., López-Vélez, R., & Monge-Maillo, B. (2025). Changes in Lipid Profile Secondary to Asymptomatic Malaria in Migrants from Sub-Saharan Africa: A Retrospective Analysis of a 2010–2022 Cohort. Tropical Medicine and Infectious Disease, 10(5), 134. https://doi.org/10.3390/tropicalmed10050134






