Emerging Human Fascioliasis in India: Review of Case Reports, Climate Change Impact, and Geo-Historical Correlation Defining Areas and Seasons of High Infection Risk
Abstract
1. Introduction
- Adult stage and egg shedding: The definitive host is infected by ingestion of metacercariae [13]. After excystment, juvenile flukes migrate from the intestine to the liver, where they develop into adult flukes, which attain sexual maturity in 3–4 months inside biliary canals and/or the gallbladder (invasive, migratory, or acute phase), following a life span of between 9 and 13.5 years (biliary, obstructive, or chronic phase), during which adults produce eggs that reach the external milieu by way of the bile, intestines, and stool. This phase is crucial for the maintenance of prevalences and intensities of the disease in an endemic area [14,15], and for its wide intercontinental geographical spread, as occurred with human-guided movements of pack animals for thousands of years from the Neolithic Age [1], and as recently occurred due to livestock management in the countrywide spread of human fascioliasis in Vietnam without any influence of climate change [16].
- Egg and miracidium: The transition between definitive mammal host and intermediate snail host includes the long resistance phase of the egg and the short active phase of the miracidium. The fluke eggs shed with the mammalian feces will continue their development in freshwater of appropriate physicochemical characteristics.
- Intramolluscan development and cercariae shedding: The development at snail level includes miracidium penetration, the sporocyst stage, redial generation, the production of cercariae, and shedding of the latter into water.
- Cercariae and metacercariae: The transition between snail and mammal hosts includes the short swimming phase of cercariae and the long resistance phase of metacercariae. Cercariae are shed by the snail and swim for a short time until contacting a solid support, mostly leaves of water plants above or below the water line, to attach and give rise to the metacercarial cyst, which becomes infective within 24 h.
2. Materials and Methods
2.1. Literature Search for Human Infection Reports
2.2. Review and Quality Assessment
2.3. Climate Analyses
2.4. Checking and Completion of Missing Data
2.5. Climatic Forecast Indices
2.6. Spatial and Statistical Analyses
2.7. Geo-Historical Analyses
3. Results
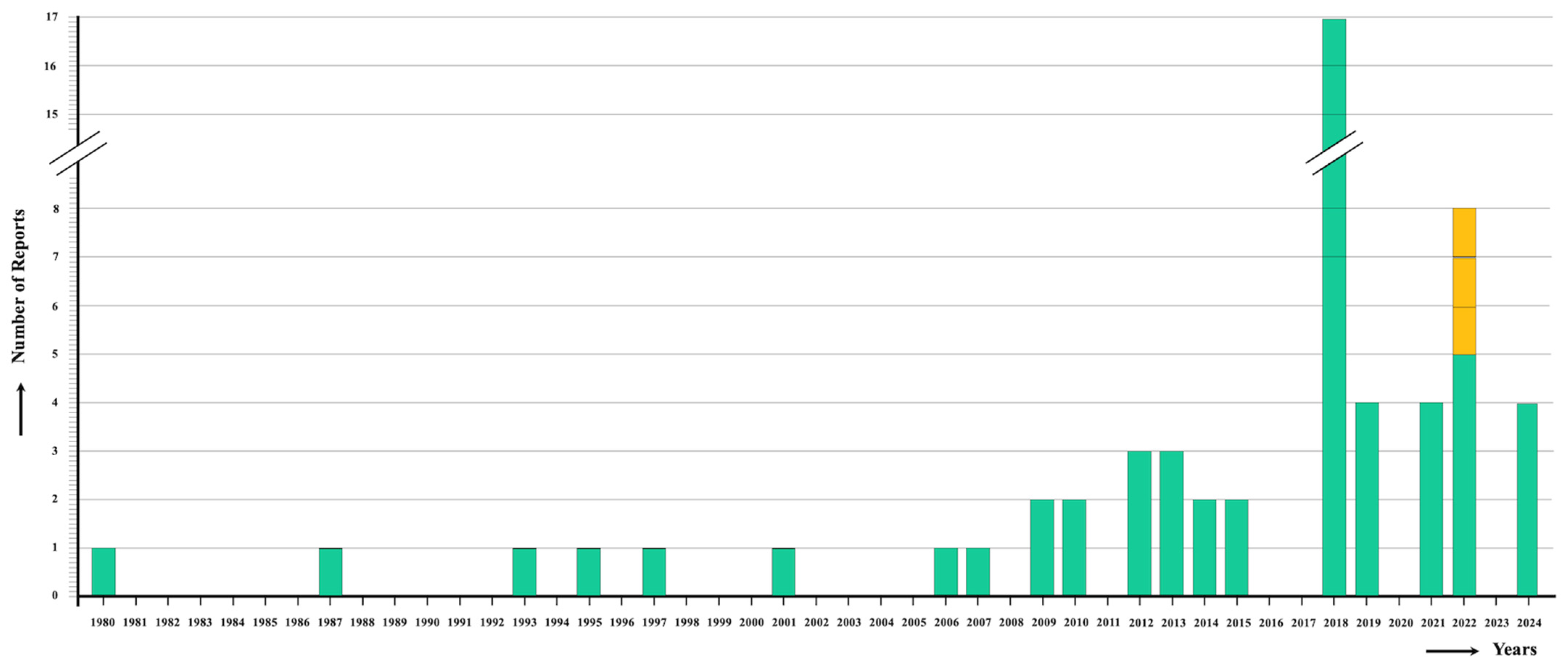
3.1. Review of Case Reports
3.2. Climatic Analyses
- The analytical studies have allowed us to understand the influences of climate factors on the transmission of fascioliasis throughout India and of climate change on the emergence of human fascioliasis in India:
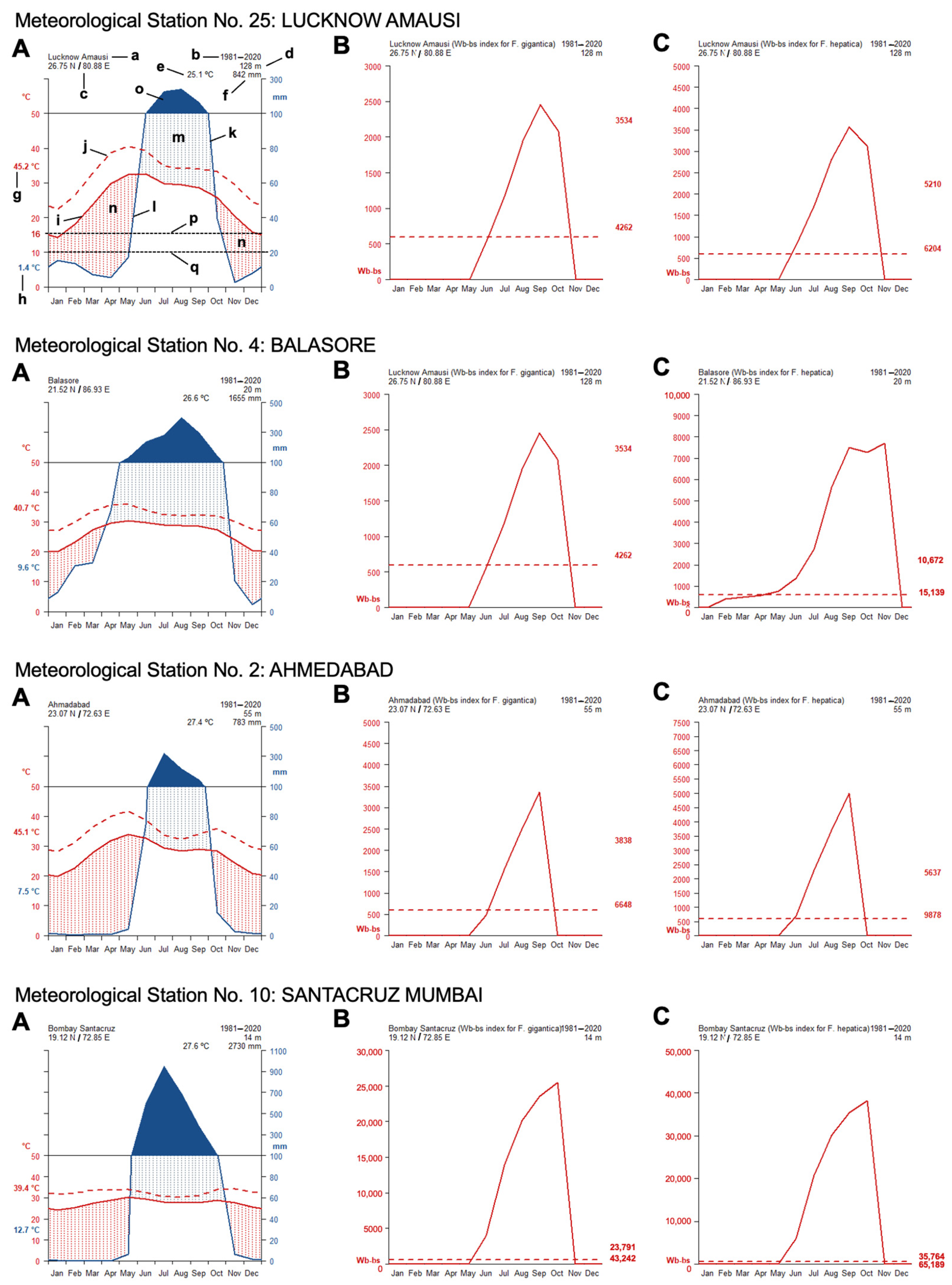
- The climate diagrams covering the 1980–2020 period show that temperatures are higher than the minimum fascioliasis transmission thresholds of both F. gigantica and F. hepatica along a whole-year period (Figure 5A).
- The Wb-bs climatic forecast index analyzed throughout the aforementioned four-decade period indicates that fascioliasis transmission in India follows a marked seasonal pattern, including a potential transmission window from June–July up to October–November, with peaks between September and October. This applies for both F. gigantica (Figure 5B) and F. hepatica (Figure 5C).
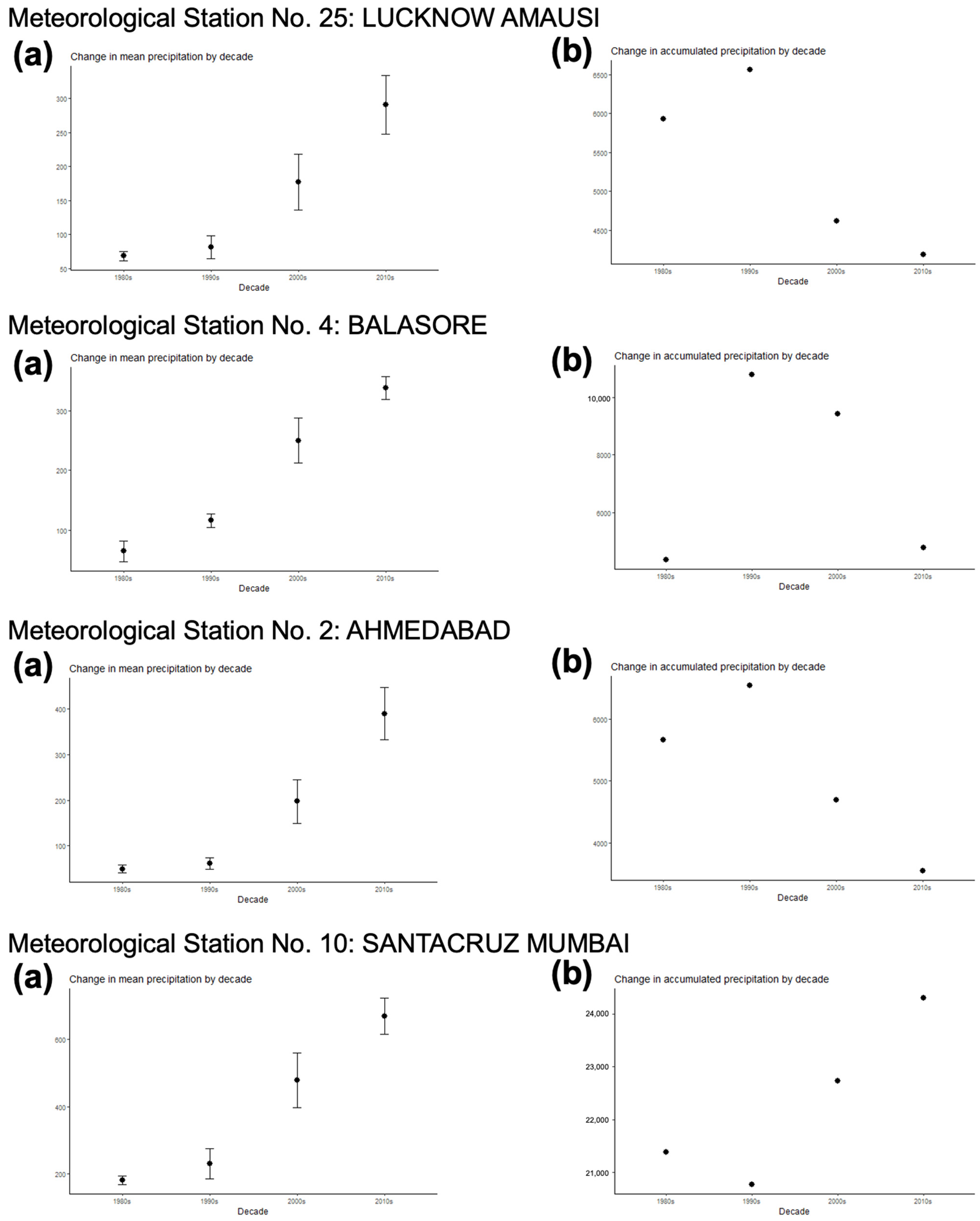
- The analysis of the monthly values of the Wb-bs risk index along the 1980–2020 period shows a gradual, progressively increasing value for both F. gigantica (Figure 6a) and F. hepatica (Figure 6b) in the meteorological stations located in the four areas where most human fascioliasis patients have been diagnosed: meteorological station No. 25 of Lucknow Amausi (Uttar Pradesh), station No. 4 of Balasore (in Odisha, very close to West Bengal), station No. 2 of Ahmedabad (Gujarat), and station No. 10 of Santacruz Mumbai (Maharashtra).
- The aforementioned marked seasonality and progressively increasing Wb-bs value therefore appear to be related to precipitation and surface water availability, allowing for lymnaeid snail vector activity and liver fluke transmission, and, consequently, human fascioliasis infection risk. An evident pattern is observed when considering the mean monthly precipitation by decade (Figure 7). The mean monthly precipitation presents a marked increase in every meteorological station, and is particularly evident after 1980 (Figure 7a), whereas the total accumulated precipitation by decade evinces a negative trend in almost every location assessed (Figure 7b).
3.3. Geo-Historical Assessments
3.3.1. The Grand Trunk Road
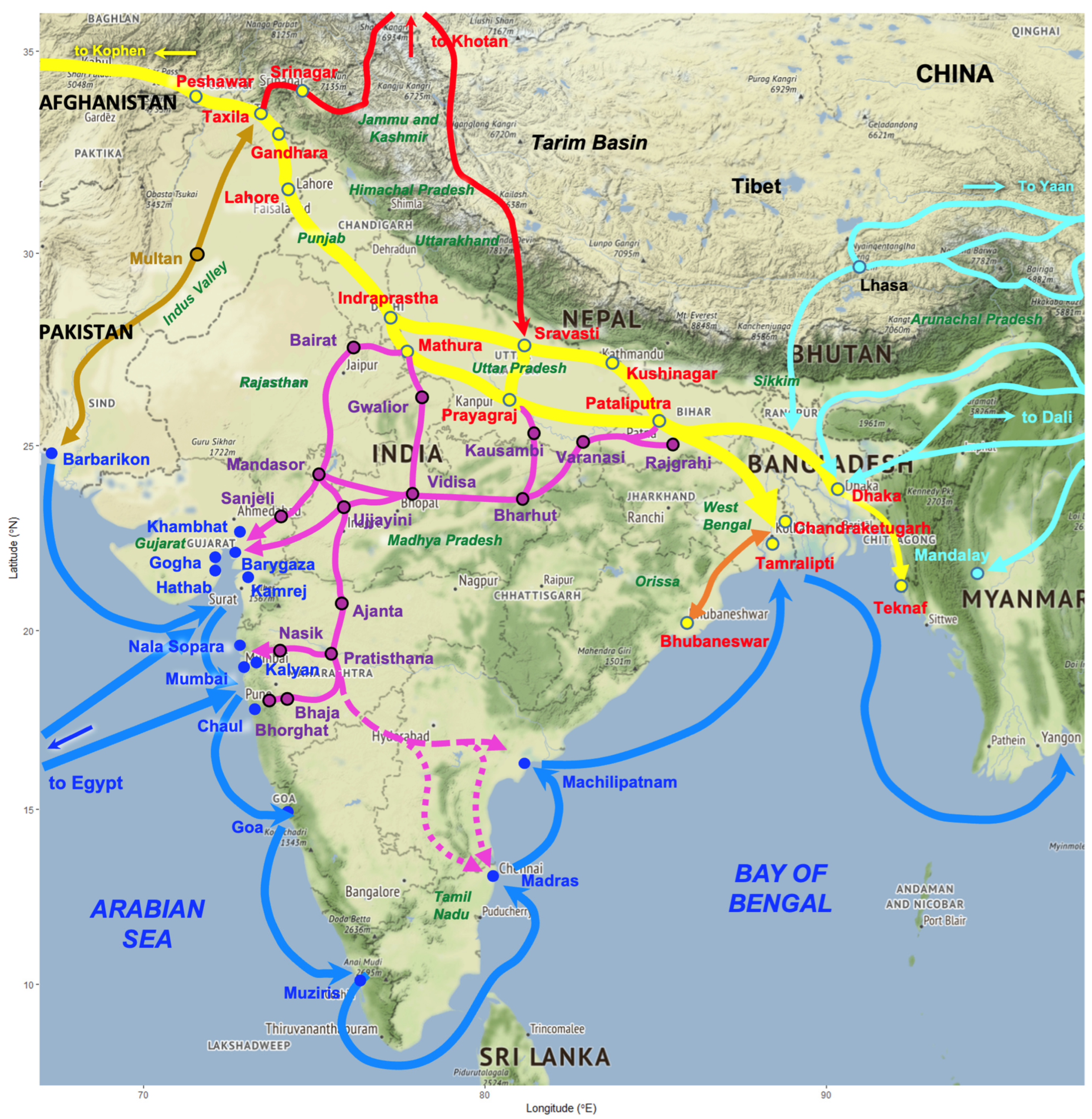
3.3.2. Southward Connections from the Grand Trunk Road
3.3.3. Northward Connections from the Grand Trunk Road
3.3.4. Geographically Extreme Connections from the Grand Trunk Road
4. Discussion
4.1. Human Infection Reports
- (A)
- Specific diagnosis: In patients in whom the causal agent was classified at species level, the high number of cases ascribed to F. hepatica is surprising. Indeed, the original Fasciola species of the whole subcontinent of India is F. gigantica, except in the northern high-altitude areas, where F. hepatica is known to occur, such as in (i) the western Kashmir valley, (ii) the area of Sikkim located between Nepal, Bhutan, and Bangladesh, and (iii) the eastern state of Arunachal Pradesh [1].
- (B)
- Geographical origin of the infection: The second problem found in almost all reports concerns the absence of the locality where the patient was living. In several reports, only the state of origin of the patient is given [35,39,50,53,56], and for 15 patients diagnosed in the hospital of Chennai between 2010 and 2016, it is only noted that they were from “northeastern India,” without any detail about even the state [45].
- (C)
- Potential infection sources: Another piece of information usually lacking concerns data about the infection source, obtained during patient anamnesis. This makes it difficult to assess epidemiological interpretations regarding the infection sources of highest risk in India. There has been no way, except in a few cases, to assess whether certain traditional foods, attitudes, habits, behavior, housing, social aspects, or professional activities, such as those of livestock management practices or farmers working in field cultures of vegetables needing intense irrigation, may be linked to disease transmission and infection sources in India, as is known in other countries [10].
- (D)
- Family/community infections: Except in a case of a Gujarati male patient whose wife was also diagnosed as being infected by Fasciola [54], in no other report were efforts made to verify whether other members of the family or community were also infected by liver flukes. In fascioliasis, subjects living close to others share the infection probability because of the use of the same traditional foods, attitudes, habits, behavior, housing, social aspects, and livestock management practices, mainly in rural areas but also in urban areas where metacercaria-carrying vegetables are sold in uncontrolled city markets or people share the same piped water supply at home [4,10,105].
4.2. Clinical Pictures, Symptomatology, and Pathology
- (A)
- Absence of massive infections: In no fascioliasis patient infected in India were numerous liver flukes reported. This resembles the situation in Vietnam [16,107], but differs from the high intensities found in children of countries such as Bolivia [10,108,109,110], Peru [111,112], and Egypt [105]. The usual low intensity in Indian patients may indicate the absence of vegetables carrying high concentrations of attached metacercariae and the absence of reinfections leading to fluke accumulation because of the lack of premunition in human fascioliasis [113], with both absences suggesting low local transmission rates. This would indeed fit a situation of the first step of an emerging trend, as concluded when compared to the long period before 1992 in which only two cases were reported (Figure 1).
- (B)
- Early diagnosis of most patients: In 28 patients, the time elapsed between symptom onset and diagnosis is noted. In only six cases was it of one year or longer (12 months: 1 case; 18 months: 1; 2 years: 1; 3 years: 2; 5 years: 1). In the remaining cases, this interval varied between 10 days and 7 months. This early diagnosis suggests that many patients were probably (i) in the invasive, migratory or acute phase, which explains the absence of eggs in stool, and duodenal and biliary aspirates, and justifies the cases diagnosed based on the clinical pictures, image methods, and/or serological techniques, or (ii) at the beginning of the biliary, obstructive, or chronic phase. This would underlie the still relatively small size of the flukes recovered from the patients, which could justify potential confusion regarding still-growing specimens of F. gigantica with F. hepatica. The usual early diagnosis of patients in India resembles the situation in Vietnam, where early diagnosis was facilitated by radio broadcasting [16] and explains the extreme rarity of disorders that typically appear in the long-term advanced chronicity of fascioliasis [107]. The opposite situation of long-term delayed diagnosis was frequent in Argentina and explained extreme clinical pictures and surgical interventions due to lithiasis suspicion [114]. In non-endemic countries or areas, an early correct diagnosis avoids frequent misdiagnoses and unnecessary surgery [115].
- (C)
- Higher infection rate in females: The analysis shows an infection rate 1.57 times higher in females (n = 33) than in males (n = 21). A higher prevalence rate in females is well known in human fascioliasis-endemic areas, such as in Vietnam [16], southern China [116], Egypt [117], and Peru [112]. Similarly, infection intensities estimated by fecal egg counts (eggs per gram of stool = epg) also proved to be higher in females in Bolivia [110] and Egypt [105]. Unfortunately, egg quantification was conducted in no Indian patients. Indian health centers will need to use a quantitative analysis tool such as the Kato–Katz test (or any other quantitative diagnostic technique) to quantify epg in the patients, mainly to assess the adequate treatment drug dose to avoid the potential risk of bile duct obstruction due to the accumulation of dragged flukes and consequent colic. A quantity of 300 epg was established by the WHO as the colic risk limit, so a divided drug dose should be administered when surpassed [4]. This limit was increased to 400 epg for the human hyperendemic area of the Northern Bolivian Altiplano, where massive infections were frequently detected in children [118,119]. Interestingly, however, biliary colic was reported in two Indian patients, a 40-year-old female from Saharanpur, Uttar Pradesh [24], and a 40-year-old male from Alwar, Rajasthan [41], in both of whom only one adult fluke was found after surgery and ERCP, respectively.
- (D)
- Patient age peaking in adults: The age of infected patients in India showed a mean of 33.1 years. This agrees with the situation in Vietnam, where infection rates peak in the age groups of 31–40-year-old and 41–50-year-old subjects [16], and southern China, where the age mean is 38.54 ± 15.68 years [116]. Such age-related infection rates pronouncedly differ from human hyperendemic areas in Andean countries [110,112] and the Nile Delta in Egypt [117], where the highest infection rates concentrate in 5–15-year-old schoolchildren.
4.3. Problems, Exceptions, and Clarifications
4.4. Assessments by Combining Climatic and Geo-Historical Analyses
4.4.1. Species of Fasciola Infecting Humans
- The northeastern altitudes of Arunachal Pradesh, where the past connections through the Tea-Horse Road also allowed for the arrival of F. hepatica and lymnaeid-transmitting species today known in Nepal, southern China, and southeastern Asia [1].
- Northeastern Indian lowlands and low altitudes surrounding Bangladesh, mainly West Bengal, Sikkim, Assam, and Arunachal Pradesh, where livestock importation has been seen to underlie the introduction of F. hepatica from foreign countries of other continents that are endemic only for F. hepatica [17]. This man-made activity leads to fluke hybridization, giving rise to nuclear rDNA admixed Fasciola hybrids infecting both domestic ruminants [17] and humans [18] in the aforementioned states of India. In agreement with this scenario, intermediate forms have also been found in high-altitude areas of Sikkim [134]. The consequent increase in fascioliasis transmission rates is most probably related to the high number of liver fluke-infected patients reported from this northeastern part of India [45].
- Radix luteola is a mid-sized lymnaeid (adults of around 1.5–2 cm in length) recently proposed to be included in the genus Racesina [135]. The life cycle of this radicine species fits environmental conditions of pH 7 and a temperature between 20 and 35 °C, and is unable to complete its life cycle at 10 °C and 15 °C [136]. The preference for warmer temperatures agrees with the hot conditions reached by the lowlands throughout India up to the lowest latitudes [137,138]. This species shows an amphibious trend, which underlies its preference for irrigated lands and channels, such as, for instance, in rice fields, although it may also be found in ponds [138]. Radix luteola is often found in temporary water bodies that dry up in summer, surviving unfavorable conditions by burying inside the mud. When compared to lymnaeid species that are well known to be involved in human infection, such as the amphibious species G. truncatula in Europe, Lymnaea neotropica in South America [139], and Radix viridis in Vietnam [16], the similar behavioral characteristics of R. luteola suggests that it is the most important vector regarding human infection in India.
- The superspecies Radix auricularia includes species or varieties differing in shell forms, among which the Indian R. acuminata has recently been proposed to be included in the South Asian species Radix rufescens [135]. Radix acuminata is a more aquatic and large-sized lymnaeid species (with adults reaching a length greater than 2 cm), typically occurring in permanent water bodies with abundant vegetation, such as in small to large ponds, where it reaches very high prevalences of infection by F. gigantica in ponds frequented by buffaloes [140]. The life cycle of this radicine species shows a longer survival and higher multiplication rates at the lower temperatures of 15 °C and 20 °C, whereas its lifespan and egg production are markedly reduced at the higher temperatures of 25 °C and 30 °C [141]. This species, and also R. luteola, have been reported in the southernmost areas of India, such as Tamil Nadu [142]. The ecology of R. acuminata favors human infection by freshwater drinking and is suggested to be less involved in human infection.
4.4.2. Transmission Seasonality and Evolution of the Yearly Infection Risk
- Higher temperatures favoring the warm condition-preferring species R. luteola, which is the lymnaeid species showing ecological and behavioral characteristics most appropriate for fascioliasis transmission.
- A marked increase in the mean monthly precipitation, which has been evident since 1980, which is due to fewer rainy days but more days of extreme rainfall events, leading to increasing amounts of rain per event and, consequently, more frequent floodings and increased surface water availability. This favors population dynamics of amphibious lymnaeids such as R. luteola and fascioliasis transmission in temporary transmission foci. Despite the generally decreasing precipitation, scarcer temporal sources of water may persist for longer, concentrating the populations of both the lymnaeid snail vectors and of the livestock reservoir hosts and thereby favoring fascioliasis transmission. Public health problems posed by human fascioliasis have already been reported from semiarid–arid areas, where water availability depends on located water sources. In such drought situations, disease transmission factors are concentrated in small areas where humans and animals go for water supply, vegetable cultures, and livestock farming [144], epidemiologically resembling desert oases [145].
4.4.3. Geographical Distribution of Human Infection Hotspots
5. Conclusions
- (A)
- Physicians in charge of fascioliasis patients should henceforth consider the following key points:
- Adequate efforts should be made to reach a specific diagnosis by describing the morphology and length/width of adult worms if obtained by ERCP or surgery, and/or by describing and measuring the length/width of eggs if found in stools or bile/duodenum aspirates; moreover, authors of case reports are requested to add a scale in photographs illustrating worms and/or eggs.
- When eggs are found in the patient’s stool, epgs should be quantified by using Kato–Katz or any other quantitative technique, and thereby assessing the appropriate drug dose to avoid post-treatment colic.
- Physicians should consider a specific serological test, several of which are commercially available [4], for patients not shedding eggs; overlooked or misdiagnosed human infection cases in India cannot be ruled out.
- The locality where the patient is living should be provided to obtain accurate information about the geographical risk of human infection.
- In anamnesis, patients should be questioned about potential infection sources, i.e., which suspicious vegetables form part of their diet and the origin of these vegetables (sylvatic, agricultural fields, uncontrolled city markets), and water-drinking habits (from natural collections shared with livestock, wells, irrigation canals, streams or rivers, traditional beverages).
- The patient should also be asked about other members of the family or community sharing food and drinking traditions [4], and carry out their diagnosis if the information suggests their possible infection.
- The recently described complete clinical picture caused by F. gigantica [107] may be henceforth useful for clinicians in India.
- (B)
- Epidemiologists should consider the conclusions of the present multidisciplinary assessments:
- Current climate trends throughout India suggest a future increasing emergence of human fascioliasis.
- The seasonal risk of human infection ranging from June–July up to October–November in India allows for the definition of prevention and control measures.
- The geographical zones of high risk of human fascioliasis defined by archeological–historical analyses, mainly linked to the Indo-Gangetic Plains and corridors used by the old Grand Trunk Road and old Daksinapatha Road, but also in northern mountainous areas, as well as the hinterlands of western and eastern seaport cities involved in the past Maritime Silk Road [1], also allow for the design of specific control measures.
- (C)
- Responsible government officers should also consider several conclusions for the necessary improvement in certain crucial items:
- Meteorological stations should assure the continuity of climate factor measurement and make efforts to provide available data; climate change trends in India indicate a future worse situation, and meteorological data will be increasingly needed.
- Measures should be implemented to reduce uncontrolled livestock exchange with neighboring countries and apply appropriate animal quarantines and treatments for livestock imported from foreign endemic countries to avoid F. hepatica introduction, risky fasciolid hybridization, or the introduction of lymnaeid vectors attached to the hooves of imported ruminants [16,17,18].
- Human health administration officers should seek the official registration of triclabendazole for human use (Egaten® from Novartis Pharma, Basel, Switzerland), and recommend drugs different from triclabendazole for animal treatment to avoid the appearance of resistance to this drug.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a.s.l. | Above sea level |
| BC | Before Christ |
| DF | Number of days with frost |
| DP | Number of days with precipitation |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Extreme maximum temperature |
| EmT | Extreme minimum temperature |
| epg | Eggs per gram of stool |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| ETD | Extreme temperature difference |
| GDD | Growing degree-days |
| MET | Mean environmental temperature |
| MMT | Mean maximum temperature |
| MmT | Mean minimum temperature |
| MTD | Maximum temperature difference |
| mtDNA | Mitochondrial DNA |
| NTD | Neglected tropical diseases |
| PET | Total potential evapotranspiration |
| Prcp | Precipitation |
| rDNA | Ribosomal DNA |
| Wb-bs index | Water budget-based system index |
References
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Human and animal fascioliasis: Origins and worldwide evolving scenario. Clin. Microbiol. Rev. 2022, 35, e0008819. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Halajian, A.; Artigas, P.; Luus-Powell, W.J.; Valero, M.A.; Mas-Coma, S. Paleobiogeographical origins of Fasciola hepatica and F. gigantica in light of new DNA sequence characteristics of F. nyanzae from hippopotamus. Front. Vet. Sci. 2022, 9, 990872. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.D.; Skuce, P.J.; McNeilly, T.N. The influence of liver fluke infection on production in sheep and cattle: A meta-analysis. Int. J. Parasitol. 2021, 51, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Diagnosis of human fascioliasis by stool and blood techniques: Update for the present global scenario. Parasitology 2014, 141, 1918–1946. [Google Scholar] [CrossRef]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases; Department of Control of Neglected Tropical Diseases, World Health Organization, WHO Headquarters: Geneva, Switzerland, 2013; pp. 1–153. Available online: https://iris.who.int/handle/10665/77950 (accessed on 13 March 2025).
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2021; pp. 1–196. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 13 March 2025).
- Valero, M.A.; Bargues, M.D.; Khoubbane, M.; Artigas, P.; Quesada, C.; Berinde, L.; Ubeira, F.M.; Mezo, M.; Hernandez, J.L.; Agramunt, V.H.; et al. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: Experimental long-term follow-up of biochemical markers. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 55–66. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Agramunt, V.H.; Valero, M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014, 84, 27–149. [Google Scholar] [CrossRef]
- Rondelaud, D.; Dreyfuss, G.; Vignoles, P. Clinical and biological abnormalities in patients after fasciolosis treatment. Méd. Mal. Infect. 2006, 36, 466–468. [Google Scholar] [CrossRef]
- Angles, R.; Buchon, P.; Valero, M.A.; Bargues, M.D.; Mas-Coma, S. One Health action against human fascioliasis in the Bolivian Altiplano: Food, water, housing, behavioural traditions, social aspects, and livestock management linked to disease transmission and infection sources. Int. J. Environ. Res. Public Health 2022, 19, 1120. [Google Scholar] [CrossRef]
- Bargues, M.D.; Valero, M.A.; Trueba, G.A.; Fornasini, M.; Villavicencio, A.F.; Guaman, R.; De Elias-Escribano, A.; Perez-Crespo, I.; Artigas, P.; Mas-Coma, S. DNA multi-marker genotyping and CIAS morphometric phenotyping of Fasciola gigantica-sized flukes from Ecuador, with an analysis of the Radix absence in the New World and the evolutionary lymnaeid snail vector filter. Animals 2021, 11, 2495. [Google Scholar] [CrossRef]
- Mas-Coma, S. Human fascioliasis: Epidemiological patterns in human endemic areas of South America, Africa and Asia. Southeast Asian J. Trop. Med. Public Health 2004, 35 (Suppl. S1), 1–11. [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Artigas, P.; Valero, M.A.; Bargues, M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: Experimental transmission capacity, field epidemiology, and control within a One Health initiative in Bolivia. Front. Vet. Sci. 2020, 7, 583204. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Funatsu, I.R.; Angles, R.; Buchon, P.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: Experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. One Health 2021, 13, 100249. [Google Scholar] [CrossRef] [PubMed]
- De, N.V.; Minh, P.N.; Le, T.H.; Dung, D.T.; Duon, T.T.; Tuan, B.V.; Dong, L.T.; Chau, N.V.V.; Cuervo, P.F.; Bargues, M.D.; et al. A multidisciplinary analysis of over 53,000 fascioliasis patients along the 1995–2019 countrywide spread in Vietnam defines a new epidemiological baseline for One Health approaches. One Health 2024, 19, 100869. [Google Scholar] [CrossRef]
- Ahasan, S.A.; De Elías-Escribano, A.; Artigas, P.; Alam, M.Z.; Mondal, M.M.H.; Blair, D.; Chowdhury, E.H.; Bargues, M.D.; Mas-Coma, S. Wide variation of heterozygotic genotypes of recent fasciolid hybrids from livestock in Bangladesh assessed by rDNA internal transcribed spacer region sequencing and cloning. One Health 2023, 17, 100614. [Google Scholar] [CrossRef]
- Bargues, M.D.; Artigas, P.; Varghese, G.M.; John, T.J.; Ajjampur, S.S.R.; Ahasan, S.A.; Chowdhury, E.H.; Gabrielli, A.F.; Mas-Coma, S. Human fascioliasis emergence in southern Asia: Complete nuclear rDNA spacer and mtDNA gene sequences prove Indian patient infection related to fluke hybridization in northeastern India and Bangladesh. One Health 2024, 18, 100675. [Google Scholar] [CrossRef]
- Fuentes, M.V.; Malone, J.B. Development of a forecast system for fasciolosis in central Chile using remote sensing and climatic data in a geographic information system. Res. Rev. Parasitol. 1999, 59, 129–134. [Google Scholar]
- Afshan, K.; Fortes-Lima, C.A.; Artigas, P.; Valero, M.A.; Qayyum, M.; Mas-Coma, S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health 2014, 8, 317–334. [Google Scholar] [CrossRef]
- Cuervo, P.F.; Bargues, M.D.; Artigas, P.; Buchon, P.; Angles, R.; Mas-Coma, S. Global warming induced spread of the highest human fascioliasis hyperendemic area. Parasit. Vectors 2024, 17, 434. [Google Scholar] [CrossRef]
- World Health Organization. Control of Neglected Tropical Diseases. Application for Donated Triclabendazole. Fascioliasis. World Health Organization Website. 2024. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/foodborne-trematode-infections/application-for-donated-triclabendazole (accessed on 13 March 2025).
- Bhambal, S.S.; Bhandari, N.R.; Bajpai, R. Liver-fluke infestation (Fasciola hepatica). Ind. Pediatr. 1980, 17, 469–471. [Google Scholar]
- Gupta, N.M. Fasciola gigantica in gallbladder. Ind. J. Gastroenterol. 1987, 6, 113. [Google Scholar]
- Stark, M.E.; Herrington, D.A.; Hillyer, G.V.; McGill, D.B. An international traveller with fever, abdominal pain, eosinophilia, and a liver lesion. Gastroenterology 1993, 105, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, A.; Chaturvedi, S. Obstructive jaundice due to Fasciola hepatica. Ind. J. Gastroenterol. 1995, 14, 79–80. [Google Scholar]
- Narain, K.; Biswas, D.; Rajguru, S.K.; Mahanta, J. Human distomatosis due to Fasciola hepatica infection in Assam, India. J. Commun. Dis. 1997, 29, 161–165. [Google Scholar]
- Elhence, V.; Mehta, B.; Gupta, R.K. Fascioliasis: A case from central Uttar Pradesh. Ind. J. Gastroenterol. 2001, 20, 164–165. [Google Scholar]
- Vatsal, D.K.; Kapoor, S.; Venkatesh, V.; Vatsal, P.; Husain, N. Ectopic fascioliasis in the dorsal spine: Case report. Neurosurgery 2006, 59, E706–E707. [Google Scholar] [CrossRef]
- Das, K.; Sakuja, P.; Aggarwal, A.; Puri, A.S.; Tatke, M. Non-resolving liver abscess with Echinococcus cross-reactivity in a non-endemic region. Ind. J. Gastroenterol. 2007, 26, 92–93. [Google Scholar]
- Patil, K.; Kulkarni, S.; Gorad, K.; Panchal, A.; Arora, S.; Gautam, R. Acute fascioliasis–Rare cause of obstructive jaundice–A case report. Bombay Hosp. J. 2009, 51, 398–400. [Google Scholar]
- Misra, V.; Debnath, S.; Misra, S.P.; Singh, P.A. Significance of Charcot Leyden crystals in hepatic aspirates. J. Cytol. 2009, 26, 77–79. [Google Scholar] [CrossRef]
- Gandhi, V.; Jain, P.; Rathod, V.; Nagral, S. Endoscopic ultrasound in biliary fasciolosis. Ind. J. Gastroenterol. 2010, 29, 128. [Google Scholar] [CrossRef][Green Version]
- Goenka, M.K.; Majumder, S.; Sethy, P.K.; Kumar, S.; Goenka, U. Hepatobiliary fascioliasis treated at endoscopic retrograde cholangiopancreatography. Endoscopy 2010, 42 (Suppl. S2), E103. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, J.; Ajjampur, S.; Chandramohan, A.; Varghese, G.M. Cases of human fascioliasis in India: Tip of the iceberg. J. Postgrad. Med. 2012, 58, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Thatte, U.M.; Gogtay, N.J.; Sridharan, K.; Oak, S.N. Access to medicines for orphan diseases: Experience in the management of a case of Fasciola hepatica in Mumbai, Maharashtra. Nat. Med. J. India 2012, 25, 234–235. [Google Scholar]
- Parelkar, S.V.; Oak, S.N.; Maydeo, A.; Sanghvi, B.V.; Joshi, P.B.; Chaubal, N.; Patil, R.T.; Sahoo, S.K.; Lal, P.J.; Sampath, N.; et al. Biliary fascioliasis: Management in a child using endoscopic retrograde cholangiopancreatography. J. Indian Assoc. Pediatr. Surg. 2013, 18, 23–24. [Google Scholar] [CrossRef]
- Jha, A.K.; Goenka, M.K.; Goenka, U.; Chakrabarti, A. Hepatobiliary fascioliasis in non-endemic zones: A surprise diagnosis. Arab. J. Gastroenterol. 2013, 14, 29–30. [Google Scholar] [CrossRef]
- Kumari, V.; Banerjee, T.; Negi, N.; Gupta, M.I.; Tiwari, K.; Gupta, M. Human fascioliasis with biliary complications. J. Commun. Dis. 2013, 45, 91–93. [Google Scholar]
- Ghildiyal, J.P.; Singh, D.P.; Goyal, R.K. Cutaneous images of interest of fasciolosis from India. Ind. J. Pathol. Microbiol. 2014, 57, 499–500. [Google Scholar] [CrossRef]
- Ashdhir, P.; Sharma, S.S.; Sharma, G. Biliary colic with dilated common bile duct: Simple “sheepish” problem? J. Ind. Med. Assoc. 2014, 112, 122–123. [Google Scholar]
- Madhumitha, R.; Gohel, S.; Latha Vishwanathan, L.; Gopalakrishnan, R. Liver lesions, fever and eosinophilia caused by Fasciola hepatica in a 15-year-old girl. Ind. J. Pediatr. 2015, 82, 967–968. [Google Scholar] [CrossRef]
- Menon, P.; Sinha, A.K.; Rao, K.L.; Khurana, S.; Lal, S.; Thapa, B.R. Biliary Fasciola gigantica infestation in a nonendemic area -- An intraoperative surprise. J. Pediatr. Surg. 2015, 50, 1983–1986. [Google Scholar] [CrossRef]
- Verma, M.; Sarangi, Y.; Sudhir Vashist, M.G.; Data, S.; Kumar, S. Incidental human fascioliasis in a patient of blunt trauma abdomen: First case report. Int. J. Sci. Res. 2018, 8, 20–21. [Google Scholar]
- Ramanan, R.V.; Dhus, U.; Ramamurthy, A.; Parameswaran, S.A.; Piramanayagam, P.; Gopalakrishnan, R. Human fascioliasis: Diagnosis by typical computed tomography features and response to nitazoxanide in 16 patients from India. Trop. Gastroenterol. 2018, 39, 149–176. Available online: https://scispace.com/pdf/human-fascioliasis-diagnosis-by-typical-computed-tomography-2ngiddf5z3.pdf (accessed on 13 March 2025). [CrossRef]
- Patel, K.P.; Shah, A.; Shetty, A.; Rodrigues, C. Hepatobiliary fascioliasis: A neglected tropical disease. Ind. J. Case Rep. 2019, 5, 597–599. [Google Scholar] [CrossRef]
- Kelgeri, C.; Valamparampil, J.; Shanmugam, N.; Srinivas Reddy, M.; Swaminathan, S.; Rela, M. An unusual cause of graft loss in pediatric liver transplant recipient–Fasciola hepatica. Pediatr. Transplant. 2019, 23, e13521. [Google Scholar] [CrossRef]
- Abdullah, I.; Tak, H.; Ganie, S.A. Parasitic zoonosis prevalent in Jammu and Kashmir: A review. In Horizons in Zoological Studies; Ahmad, F., Bakhtiyar, Y., Eds.; Department of Zoology, University of Kashmir: Srinagar, India, 2019; Chapter 22; pp. 170–178. [Google Scholar]
- Malingham, H.V.; Ramachandran, R.; Rajp, V.B.R.; Radhakrishnan, P.R.; Sai, V. A rare case of Fasciola hepatica and its treatment. J. Clin. Diagn. Res. 2021, 15, TD01–TD03. [Google Scholar]
- Yattoo, G.N.; Dar, G.A.; Jan, K.; Sodhi, J.S.; Rasool, Z.; Kaushik, S.; Gorka, S. Human fascioliasis: Report of two cases from Kashmir Valley. J. Clin. Exp. Hepatol. 2021, 11, 747–750. [Google Scholar] [CrossRef]
- Swain, B.; Otta, S.; Sahu, M.K.; Uthasingh, K. Fasciola hepatica association with gallbladder malignancy: A rare case report. Trop. Parasitol. 2021, 11, 42–45. [Google Scholar] [CrossRef]
- Patel, A.K.; Patel, K.K.; Patel, K. A young female with fever, abdominal pain and eosinophilia. Ind. J. Med. Microbiol. 2022, 40, 449–450. [Google Scholar] [CrossRef]
- Chavda, H.J.; Usha Ramakrishna, K.; Patel, K.R.; Patel, K.K.; Patel, A.K. Liver fluke infection: Case series from a single centre in western India. Trop. Gastroenterol. 2022, 43, 228–230. [Google Scholar]
- Ray, D.; Ganesh, C.P.; Kumar, Y.; Prasad Dhibar, D.; Mewara, A.; De, A. Eosinophilia in an Indian patient with helminthic infection unresponsive to albendazole and diethylcarbamazine: An enigmatic case of human fascioliasis. J. Gastrointest. Infect. 2024, 14, 20–23. [Google Scholar] [CrossRef]
- Meher, M.K.; Gupta, C.; Misra, S.P.; Baranwal, V.; Patidar, S.; Bharti, P.; Khan, F. Hepatic space occupying lesion caused by Fasciola hepatica: A case report from North India. In 32nd Annual Meeting of Indian National Association for the Study of the Liver (INASL 2024), S159, Abstract ID 487. J. Clin. Exp. Hepatol. 2024, 14 (Suppl. S1), 101995. [Google Scholar] [CrossRef]
- Pal, D. Liver fluke causing obstructive jaundice in a student from Jharkhand. J. Clin. Infect. Dis. Soc. 2024, 1, 316–317. [Google Scholar] [CrossRef]
- Adler, R.F.; Huffman, G.J.; Chang, A.; Ferraro, R.; Xie, P.; Janowiak, J.; Rudolf, B.; Schneider, U.; Curtis, S.; Bolvin, D.; et al. The Version 2 Global Precipitation Climatology Project (GPCP) Monthly Precipitation Analysis (1979-Present). J. Hydrometeor. 2003, 4, 1147–1167. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, P.; Kessler, M. Climatologies at high resolution for the Earth land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Harris, I.C.; Jones, P.D.; Osborn, T. CRU TS4.08: Climatic Research Unit (CRU) Time-Series (TS) Version 4.08 of high-Resolution Gridded Data of Month-By-Month Variation in Climate (January 1901–December 2023); NERC EDS Centre for Environmental Data Analysis, University of East Anglia Climatic Research Unit: Norwich, UK, 2024; Available online: https://catalogue.ceda.ac.uk/uuid/715abce1604a42f396f81db83aeb2a4b/ (accessed on 28 January 2025).
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef]
- Ollerenshaw, C.B.; Rowlands, W.T. A method of forecasting the incidence of fascioliasis in Anglesey. Vet. Rec. 1959, 71, 591–598. [Google Scholar]
- Ollerenshaw, C.B. Quelques aspects des relations entre le climat et l’incidence de la fasciolose en Europe. Cah. Med. Vet. 1971, 40, 303–319. [Google Scholar]
- Ollerenshaw, C.B. A comment on the epidemiology of Fasciola hepatica in Italy. Ann. Fac. Med. Vet. Univ. Torino 1973, 20, 83–121. [Google Scholar]
- Malone, J.B.; Williams, T.E.; Muller, R.A.; Geaghan, J.P.; Loyacano, A.F. Fascioliasis in cattle in Louisiana: Development of a system to predict disease risk by climate, using the Thornthwaite water budget. Am. J. Anim. Vet. Sci. 1987, 48, 1167–1170. [Google Scholar] [CrossRef]
- Malone, J.B.; Gommes, R.; Hansen, J.; Yilma, J.M.; Slingenberg, J.; Snijders, F.; Ataman, E. A geographic information system on the potential distribution and abundance of Fasciola hepatica and F. gigantica in east Africa based on Food and Agriculture Organization databases. Vet. Parasitol. 1998, 78, 87–101. [Google Scholar] [CrossRef]
- Kendall, S.B.; McCullough, F.S. The emergence of the cercariae of Fasciola hepatica from the snail Limnaea truncatula. J. Helminthol. 1951, 25, 77–92. [Google Scholar] [CrossRef]
- Dinnik, J.A.; Dinnik, N.N. Effects of seasonal variations of temperature on development of Fasciola gigantica in the snail host in the Kenyan highlands. Bull. Epiz. Dis. Afr. 1963, 11, 197–207. [Google Scholar]
- Fuentes, M.V.; Valero, M.A.; Bargues, M.D.; Esteban, J.G.; Angles, R.; Mas-Coma, S. Analysis of climatic data and forecast indices for human fascioliasis at very high altitude. Ann. Trop. Med. Parasitol. 1999, 93, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Yilma, J.; Malone, J.B. A geographic information system forecast model for strategic control of fasciolosis in Ethiopia. Vet. Parasitol. 1998, 78, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, G.H.; Samani, Z.A. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Samani, Z. Estimating solar radiation and evapotranspiration using minimum climatological data. J. Irrig. Drain. Eng. 2000, 126, 265–267. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Allen, R.G. History and evaluation of Hargreaves evapotranspiration equation. J. Irrig. Drain. Eng. 2003, 129, 53–63. [Google Scholar] [CrossRef]
- Vanderlinden, K.; Giraldez, J.V.; Van Meirvenne, M. Assessing reference evapotranspiration by the Hargreaves method in southern Spain. J. Irrig. Drain. Eng. 2004, 130, 184–191. [Google Scholar] [CrossRef]
- Cuervo, P.F.; Bargues, M.D.; Artigas, P.; Buchon, P.; Angles, R.; Mas-Coma, S. Heterogeneous zonal impacts of climate change on a wide hyperendemic area of human and animal fascioliasis assessed within a One Health action for prevention and control. PLoS Negl. Trop. Dis. 2025, 19, e0012820. [Google Scholar] [CrossRef]
- Neelis, J. Trade Networks in ancient South Asia. In Early Buddhist Transmission and Trade Networks; Series Dynamics in the History of Religion, Vol. 2, Brill E-Book Collection; Koninklijke Brill NV: Leiden, The Netherlands, 2011; Chapter 3; pp. 183–228. [Google Scholar] [CrossRef]
- Neelis, J. Long-distance transmission to Central Asian Silk Routes and China. In Early Buddhist Transmission and Trade Networks; Series Dynamics in the History of Religion, Vol. 2, Brill E-Book Collection; Koninklijke Brill NV: Leiden, The Netherlands, 2011; Chapter 6; pp. 289–309. [Google Scholar] [CrossRef]
- Neelis, J. Old roads in the northwestern borderlands. In Early Buddhist Transmission and Trade Networks; Series Dynamics in the History of Religion, Vol. 2, Brill E-Book Collection; Koninklijke Brill NV: Leiden, The Netherlands, 2011; Chapter 4; pp. 229–256. [Google Scholar] [CrossRef]
- History of Bhubaneswar. Bhubaneswar Guide. A Part of India; Pan India Internet Pvt Ltd.: Bhubaneswar, India, 2023; Available online: https://www.bhubaneswaronline.in/guide/history-of-bhubaneswar (accessed on 13 March 2025).
- Nyima, T. The western Himalaya. In Transhumant Grazing Systems in Temperate Asia; Suttie, J.M., Reynolds, S.G., Eds.; Plant Production and Protection Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Chapter 9; Available online: https://www.fao.org/4/Y4856E/y4856e0e.htm#bm14 (accessed on 13 March 2025).
- Pastoralist and Aadhaar Survey; Draft Report; Centre for Pastoralism, Chaudhari Prem Singh House: New Delhi, India, 2019; pp. 1–36. Available online: https://centreforpastoralism.org/wp-content/uploads/2021/09/Pastoralist-and-Aadhaar-Survey-May-2019.pdf (accessed on 13 March 2025).
- Malhotra, A. Supporting Pastoralism in India. Blog 211-Moo’ing Forward. Blogs Livestock Extension, Agricultural Extension in South Asia; Centre for Research on Innovation and Science Policy (CRISP): Hyderabad-Telangana, India, 2024; Available online: https://aesanetwork.org/blog-211-mooing-forward-supporting-pastoralism-in-india/ (accessed on 13 March 2025).
- Gooch, P. Transhumant pastoralism in northern India: The Gujar case. Nomadic Peoples 1992, 30, 84–96. Available online: https://www.jstor.org/stable/43123359 (accessed on 13 March 2025).
- Jithendran, K.P. Helminth parasites—A constraint in animal health management in Himachal Pradesh. ENVIS Bull. Himalayan Ecol. Dev. 2000, 8, 1–19. [Google Scholar]
- Bimal, M. Migratory goat and sheep rearing in Himachal Pradesh, India. In Transhumant Grazing Systems in Temperate Asia; Suttie, J.M., Reynolds, S.G., Eds.; Plant Production and Protection Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Chapter 10; Available online: https://www.fao.org/4/y4856e/y4856e0f.htm#bm15 (accessed on 13 March 2025).
- Shardha, M. Pastoralism in India: A Case Study of Shepherd’s Community of Kullu District in Himachal Pradesh. Int. Res. J. Commer. Arts Sci. 2023, 14, 42–50. [Google Scholar] [CrossRef]
- Mishra, H.; Pandey, B.W.; Mukwada, G.; De Los Rios, P.; Nigam, N.; Sahu, N. Trapped within nature: Climatic variability and its impact on traditional livelihood of Gaddi transhumance of Indian Himalayas. Local Environ. 2023, 28, 547–563. [Google Scholar] [CrossRef]
- Choudhary, S. Mapping Transhumance in J&K. The Indian Tribal; Madtri Ventures Pvt Ltd.: Ghaziabad, India, 2024; Available online: https://theindiantribal.com/2022/01/23/mapping-transhumance-in-j-and-k/ (accessed on 13 March 2025).
- Sanaullah, K.; Mukhtar, A. The eastern Himalaya. In Transhumant Grazing Systems in Temperate Asia; Suttie, J.M., Reynolds, S.G., Eds.; Plant Production and Protection Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Chapter 13; Available online: https://www.fao.org/4/Y4856E/y4856e0i.htm#bm18 (accessed on 13 March 2025).
- Gyaltsen, T.; Bhattarai, B.N. Transhumant cattle raising in western Bhutan. In Transhumant Grazing Systems in Temperate Asia; Suttie, J.M., Reynolds, S.G., Eds.; Plant Production and Protection Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; Chapter 14; Available online: https://www.fao.org/4/Y4856E/y4856e0j.htm#bm19 (accessed on 13 March 2025).
- Chakrabarti, A. Transhumance, Livelihood and Sustainable Development and Conflict between Formal Institution and Communal Governance: An Evaluative Note on East Himalyan State of Sikkim. In 2011 International Conference on Social Science and Humanity; IACSIT Press: Singapore, 2011; Volume VI, pp. 1–7. [Google Scholar]
- Feroze, S.M.; Ray, L.I.P.; Singh, K.J.; Singh, R. Pastoral yak rearing system is changing with change in climate: An exploration of North Sikkim in Eastern Himalaya. Clim. Change 2019, 157, 483–498. [Google Scholar] [CrossRef]
- Namgay, K.; Millar, J.; Black, R.; Samdup, T. Transhumant agro-pastoralism in Bhutan: Exploring contemporary practices and socio-cultural traditions. Pastor. Res. Policy Pract. 2013, 3, 13. [Google Scholar] [CrossRef]
- Bhattacharya, B.K. Climate Change Threatens Traditional Way of Life of Brokpa Herders in Arunachal Pradesh. Arunachal Pradesh, Conservation News. 15 January 2020. Available online: https://india.mongabay.com/2020/01/climate-change-threatens-traditional-way-of-life-of-brokpa-herders-in-arunachal-pradesh/#:~:text=“The%20Brokpa%20yak%20pastoralists%20in,the%20School%20of%20Environment%20and (accessed on 13 March 2025).
- Choephel, T.; Garg, A.; Mall, M. Yak milk production in North-East India: Prospect of yak herding in Arunachal Pradesh. Ind. J. Anim. Prod. Manag. 2024, 40, 237–242. [Google Scholar]
- Dorjee, T. Yak pastoralist (Brokpa) of Arunachal Pradesh: Mobility and institutional arrangement regarding regulation of seasonal use. Int. J. Innovat. Res. Dev. 2015, 4, 27–33. Available online: https://www.internationaljournalcorner.com/index.php/ijird_ojs/article/view/135611/94735 (accessed on 13 March 2025).
- Norbu, L.; Riba, T.; Nimasow, G. Pasture and grazing fee management system of the transhumant Brokpa communities of Arunachal Pradesh (India). Rajiv Gandhi Univ. Res J. 2018, 17, 27–38. [Google Scholar]
- Singh, R.K.; Sureja, A.K.; Maiti, S.; Tsering, D. Grazing and rangeland management: Trans-human adaptations by Brokpa community in fragile. Ecosystems of Arunachal Pradesh. Ind. J Tradit. Knowl. 2018, 17, 550–558. [Google Scholar]
- Mitra, S. Transhumance Sheepmasters: Gadariya Pastoralists of the Rarh. Ways of Living, Sahapedia, New Delhi, 2021. Available online: https://map.sahapedia.org/gallery/Transhumance-Sheepmasters:-Gadariya-Pastoralists-of-the-Rarh/10775 (accessed on 13 March 2025).
- Challenges for Pastoral Communities in Eastern India; India Water Portal (IWP), Arghyam: Bengaluru, India, 2022; Available online: https://www.indiawaterportal.org/agriculture/livelihoods/challenges-pastoral-communities-eastern-india#:~:text=These%20nomadic%20pastoralists%20traverse%20with,entering%20forest%20and%20grazing%20lands (accessed on 13 March 2025).
- ActionAid. Zonal Level Convention Addresses Challenges Faced by Pastoral Communities in Eastern India. Ranchi ActionAid Association India: New Delhi, India, 2023. Available online: https://www.csrmandate.org/zonal-level-convention-addresses-challenges-faced-by-pastoral-communities-in-eastern-india/ (accessed on 13 March 2025).
- Adhikari, S. Pastoral Farming: Rejuvenating the Rural Economy and Creating Alternative Livelihoods for Rural Communities in Jharkhand; LinkedIn: Sunnyvale, CA, USA, 2024; pp. 1–5. Available online: https://www.linkedin.com/pulse/pastoral-farming-rejuvenating-rural-economy-creating-santu-adhikari-le7af (accessed on 13 March 2025).
- Valero, M.A.; Perez-Crespo, I.; Periago, M.V.; Khoubbane, M.; Mas-Coma, S. Fluke egg characteristics for the diagnosis of human and animal fascioliasis by Fasciola hepatica and F. gigantica. Acta Trop. 2009, 111, 150–159. [Google Scholar] [CrossRef]
- Subba Rao, N.V. Handbook of Freshwater Mollusks of India, Zoological Survey of India; Government of India: Calcutta, India, 1989; pp. 1–289.
- Ramakrishna Dey, A. Handbook on Indian Freshwater Molluscs. Zoological Survey of India; Government of India: Kolkata, India, 2007; pp. 1–399.
- Periago, M.V.; Valero, M.A.; Artigas, P.; Agramunt, V.H.; Bargues, M.D.; Curtale, F.; Mas-Coma, S. Very high fascioliasis intensities in schoolchildren of Nile Delta governorates: The Old World highest burdens found in lowlands. Pathogens 2021, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.G.; Mott, K.E. Progress in assessment of morbidity due to Fasciola hepatica infection: A review of recent literature. Trop. Dis. Bull. 1990, 87, R1–R38. [Google Scholar]
- De, N.V.; Minh, P.N.; Le, T.H.; Dung, D.T.; Duon, T.T.; Tuan, B.V.; Dong, L.T.; Chau, N.V.V.; Cuervo, P.F.; Bargues, M.D.; et al. The clinical picture caused by Fasciola gigantica: Analysis of 3,250 patients along the 1995–2019 countrywide spread in Vietnam. Open Forum Infect. Dis. 2025, 12, ofaf116. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Angles, R.; Strauss, W.; Aguirre, C.; Mas-Coma, S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop. Med. Int. Health 1997, 2, 695–699. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Aguirre, C.; Strauss, W.; Angles, R.; Mas-Coma, S. 1997b. Presence of very high prevalence and intensity of infection with Fasciola hepatica among Aymara children from the Northern Bolivian Altiplano. Acta Trop. 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Angles, R.; Mas-Coma, S. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 151–156. [Google Scholar] [CrossRef]
- Esteban, J.G.; Gonzalez, C.; Bargues, M.D.; Angles, R.; Sanchez, C.; Naquira, C.; Mas-Coma, S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health 2002, 7, 339–348. [Google Scholar] [CrossRef]
- Gonzalez, L.C.; Esteban, J.G.; Bargues, M.D.; Valero, M.A.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Hyperendemic human fascioliasis in Andean valleys: An altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 2011, 120, 119–129. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. One Health for fascioliasis control in human endemic areas. Trends Parasitol. 2023, 39, 650–667. [Google Scholar] [CrossRef]
- Mera y Sierra, R.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasit. Vectors 2011, 4, 104. [Google Scholar] [CrossRef]
- Mowlavi, G.; Bargues, M.D.; Najafi, F.; Naddaf, S.R.; Salehabadi, A.; Vejdan, A.K.; Salimi, M.; Fadavi, A.; Arab-Mazar, Z.; Mas-Coma, S. Fasciola infection unexpectedly found during cholecystectomy: Review on how to avoid increasing surgery interventions in non-human endemic areas. Acta Parasitol. 2023, 68, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, F.; Su, H.; Luo, J.; Gu, W. Emerging human fascioliasis: A retrospective study of epidemiological findings in Dali, Yunnan province, China (2012–2021). Med. Sci. Monit. 2023, 29, e940581. [Google Scholar] [CrossRef]
- Esteban, J.G.; Gonzalez, C.; Curtale, F.; Muñoz-Antoli, C.; Valero, M.A.; Bargues, M.D.; El Sayed, M.; El Wakeel, A.; Abdel-Wahab, Y.; Montresor, A.; et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am. J. Trop. Med. Hyg. 2003, 69, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Villegas, F.; Angles, R.; Barrientos, R.; Barrios, G.; Valero, M.A.; Hamed, K.; Gruemingr, H.; Ault, S.K.; Montresor, A.; Engels, D.; et al. Administration of triclabendazole is safe and effective in controlling fascioliasis in an endemic community of the Bolivian Altiplano. PLoS Negl. Trop. Dis. 2012, 6, e1720. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Periago, M.V.; Perez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef]
- De, N.V.; Le, T.H.; Agramunt, V.H.; Mas-Coma, S. Early postnatal and preschool age infection by Fasciola spp.: Report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020, 103, 1578–1589. [Google Scholar] [CrossRef]
- Le, T.H.; De, N.V.; Agatsuma, T.; Blair, D.; Vercruysse, J.; Dorny, P.; Nguyen, T.G.T.; McManus, D.P. Molecular confirmation that Fasciola gigantica can undertake aberrant migrations in human hosts. J. Clin. Microbiol. 2007, 45, 648–650. [Google Scholar] [CrossRef]
- Eliachar, E.; Tassy, R. Distomatose compliquée de péritonite chez un enfant de 13 ans. Ann. Pédiatr. Paris 1960, 42-43, 433–435. [Google Scholar]
- Tanir, G.; Karaman, A.; Tüfekci, S.B.; Erdogan, D.; Tuygun, N.; Özkan, A.T. A case of ectopic intraabdominal fascioliasis presented with acute abdomen. Turk. J. Gastroenterol. 2011, 22, 347–350. [Google Scholar] [CrossRef]
- Gonzalez-Miguel, J.; Valero, M.A.; Reguera-Gomez, M.; Mas-Bargues, C.; Bargues, M.D.; Simon-Martin, F.; Mas-Coma, S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology 2019, 146, 284–298. [Google Scholar] [CrossRef]
- Serrat, J.; Becerro-Recio, D.; Torres-Valle, M.; Simón, F.; Valero, M.A.; Bargues, M.D.; Mas-Coma, S.; Siles-Lucas, M.; González-Miguel, J. Fasciola hepatica juveniles interact with the host fibrinolytic system as a potential early-stage invasion mechanism. PLoS Negl. Trop. Dis. 2023, 17, e0010936. [Google Scholar] [CrossRef] [PubMed]
- Chougar, L.; Mas-Coma, S.; Artigas, P.; Harhoura, K.; Aissi, M.; Agramunt, V.H.; Bargues, M.D. Genetically “pure” Fasciola gigantica discovered in Algeria: DNA multimarker characterization, trans-Saharan introduction from a Sahel origin and spreading risk into northwestern Maghreb countries. Transb. Emerg. Dis. 2020, 67, 2190–2205. [Google Scholar] [CrossRef]
- Fonti, N.; Parisi, F.; Mancianti, F.; Freer, G.; Poli, A. Cancerogenic parasites in veterinary medicine: A narrative literature review. Infect. Agents Cancer 2023, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Khadka, S.; Lakhey, P.J.; Pradhan, S.; Shah, N.P.; Singh, Y.P.; Mas-Coma, S. Human case of Fasciola gigantica-like infection, review of human fascioliasis reports in Nepal, and epidemiological analysis within the South Central Asia. Acta Parasitol. 2018, 63, 435–443. [Google Scholar] [CrossRef]
- Howell, A.; Mugisha, L.; Davies, J.; LaCourse, E.J.; Claridge, J.; Williams, D.J.L.; Kelly-Hope, L.; Betson, M.; Kabatereine, N.B.; Stothard, R.J. Bovine fasciolosis at increasing altitudes: Parasitological and malacological sampling on the slopes of Mount Elgon, Uganda. Parasit. Vectors 2012, 5, 196. [Google Scholar] [CrossRef]
- Joan, N.; Stephen, M.; Bashir, M.; Kiguli, J.; Orikiriza, P.; Bazira, J.; Itabangi, H.; Stanley, I. Prevalence and economic impact of bovine fasciolosis at Kampala city abattoir, Central Uganda. Br. Microbiol. Res. J. 2015, 7, 109–117. [Google Scholar] [CrossRef]
- Mohan, A.; Mirdha, B.R.; Vishaka, M. Fascioliasis in a pregnant woman. Ind. J. Gastroenterol. 1996, 15, 155. [Google Scholar]
- Kendall, S.B. Fascioliasis in Pakistan. Ann. Trop. Med. Parasitol. 1954, 43, 307–313. [Google Scholar] [CrossRef]
- Qureshi, A.W.; Zeb, A.; Mansoor, A.; Hayat, A.; Mas-Coma, S. Fasciola hepatica infection in children actively detected in a survey in rural areas of Mardan district, Khyber Pakhtunkhawa province, northern Pakistan. Parasitol. Int. 2019, 69, 39–46. [Google Scholar] [CrossRef]
- Lyngdoh, D.; Sharma, S.; Roy, B.; Tandon, V. Animal fascioliasis: Perspectives from high altitudinal regions. Vet. Parasitol. 2016, 232, 21–31. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Aksenova, O.V.; Bolotov, I.N. Taxonomic assessment of genetically-delineated species of radicine snails (Mollusca, Gastropoda, Lymnaeidae). Zoosyst. Evol. 2020, 96, 577–608. [Google Scholar] [CrossRef]
- Aziz, M.A.; Raut, S.K. Thermal effect on the life-cycle parameters of the medically important freshwater snail species Lymnaea (Radix) luteola (Lamarck). Mem. Inst. Oswaldo Cruz 1996, 91, 119–128. [Google Scholar] [CrossRef]
- Raman, M.; Jeyathilakan, R.S.N.; Soundararajan, C.; Alex, A.; Ravikumar, G. Maintenance of Radix snails and artificial infection with Fasciola gigantica miracidium in laboratory conditions. Tamilnadu J. Vet. Anim. Sci. 2012, 8, 360–367. [Google Scholar]
- Latchumikanthan, A.; Vimalraj, P.G.; Kumar, P.P.; Vadhana, A.P.; Jithin, M.V.; Soundararajan, C. A preliminary study on occurrence of fresh water snails in different snail habitats in some parts of Puducherry. J. Entomol. Zool. Stud. 2019, 7, 975–980. Available online: https://www.entomoljournal.com/archives/2019/vol7issue2/PartP/7-2--9-876.pdf (accessed on 13 March 2025).
- Bargues, M.D.; Gayo, V.; Sanchis, J.; Artigas, P.; Khoubbane, M.; Birriel, S.; Mas-Coma, S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005352. [Google Scholar] [CrossRef]
- Sunita, K.; Mas-Coma, S.; Bargues, M.D.; Sadaf Khan, M.A.; Habib, M.; Mustafa, S.; Husain, S.A. Buffalo infection by Fasciola gigantica transmitted by Radix acuminata in Uttar Pradesh, India: A molecular tool to improve snail vector epidemiology assessments and control surveillance. Acta Parasitol. 2021, 66, 1396–1405. [Google Scholar] [CrossRef]
- Misra, T.K.; Raut, S.K. Thermal effects on a medically important snail species Lymnaea (Radix) acuminata Lamarck (Gastropoda: Lymnaeidae). Appl. Phys. Expr. (APEX) 1997, 12, 101–108. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.vliz.be/imisdocs/publications/288547.pdf&ved=2ahUKEwiC2pqA0IeMAxUbRPEDHcQpN6YQFnoECBsQAQ&usg=AOvVaw1xPmFVy7C2KQR87MT9ICPU (accessed on 13 March 2025).
- Soundararajan, C.; Venkatesan, R.; Nagarajan, K. Prevalence of snails in north eastern and hilly zones of Tamil Nadu, India. Int. J. Zool. Stud. 2018, 3, 29–32. Available online: https://www.zoologyjournals.com/assets/archives/2018/vol3issue3/3-3-19-283.pdf (accessed on 13 March 2025).
- Lalrinkima, H.; Lalchhandama, C.; Jacob, S.S.; Raina, O.K.; Lallianchhunga, M.C. Fasciolosis in India: An overview. Exp. Parasitol. 2021, 222, 108066. [Google Scholar] [CrossRef]
- Bargues, M.D.; Malandrini, J.B.; Artigas, P.; Soria, C.C.; Velasquez, J.N.; Carnevale, S.; Mateo, L.; Khoubbane, M.; Mas-Coma, S. Human fascioliasis endemic areas in Argentina: Multigene characterisation of the lymnaeid vectors and climatic-environmental assessment of the transmission pattern. Parasit. Vectors 2016, 9, 306. [Google Scholar] [CrossRef][Green Version]
- Arafa, W.M.; Hassan, A.I.; Snousi, S.A.M.; El-Dakhly, K.M.; Holman, P.J.; Craig, T.M.; Aboelhadid, S.M. Fasciola hepatica infections in cattle and the freshwater snail Galba truncatula from Dakhla Oasis, Egypt. J. Helminthol. 2018, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global Climate Risk Index 2021; Germanwatch e.V.: Bonn, Germany, 2021; pp. 1–49. Available online: https://www.germanwatch.org/en/19777 (accessed on 13 March 2025).
- Krishnan, R.; Sanjay, J.; Gnanaseelan, C.; Mujumdar, M.; Kulkarni, A.; Chakraborty, S. (Eds.) Assessment of Climate Change over the Indian Region; A Report of the Ministry of Earth Sciences (MoES), Government of India; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 1–226. [Google Scholar] [CrossRef]
- Tugjamba, N.; Walkerden, G.; Miller, F. Adapting nomadic pastoralism to climate change. Clim. Change 2023, 176, 28. [Google Scholar] [CrossRef]
- Revadekar, J.V.; Varikoden, H.; Murumkar, P.K.; Ahmed, S.A. On the relationship between sea surface temperatures, circulation parameters and temperatures over west coast of India. Sci. Total Environ. 2016, 551–552, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, A.K.; Singh, V.; Bhambak, S.R. Relationship between sea surface temperature and surface air temperature over Arabian Sea, Bay of Bengal and Indian Ocean. J. Ind. Geophys. Union 2012, 16, 41–53. Available online: https://iguonline.in/journal/Archives/16-2/paper2_jaswal.pdf (accessed on 13 March 2025).
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar] [CrossRef]
- Mera y Sierra, R.; Neira, G.; Bargues, M.D.; Cuervo, P.F.; Artigas, P.; Logarzo, L.; Cortiñas, G.; Ibaceta, D.E.J.; Lopez Garrido, A.; Bisutti, E.; et al. Equines as reservoirs of human fascioliasis: Transmission capacity, epidemiology and pathogenicity in Fasciola hepatica infected mules. J. Helminthol. 2020, 94, e189. [Google Scholar] [CrossRef]
- Askari, Z.; Mas-Coma, S.; Bouwman, A.S.; Boenke, N.; Stöllner, T.; Aali, A.; Rezaiian, M.; Mowlavi, G. Fasciola hepatica eggs in paleofaeces of the Persian onager Equus hemionus onager, a donkey from Chehrabad archaeological site, dating back to the Sassanid Empire (224-651 AD), in ancient Iran. Infect. Genet. Evol. 2018, 62, 233–243. [Google Scholar] [CrossRef]
- Williams, T. The Silk Roads: An ICOMOS Thematic Study. International Council on Monuments and Sites–ICOMOS. United Nations Educational, Scientific and Cultural Organization–UNESCO; World Heritage Centre: Charenton-le-Pont, France, 2014; pp. 1–152. Available online: https://publ.icomos.org/publicomos/jlbSai?html=Bur&base=technica&ref=42555&file=1225.pdf&path=ICOMOS_WHThematicStudy_SilkRoads_final_lv_201406.pdf (accessed on 13 March 2025).
- Pandya, S.S.; Hasnani, J.J.; Patel, P.V. Morphological and histological identification of Fasciola gigantica recovered from liver of infected buffaloes. Int. J. Res.–Granthaaalayah 2015, 3, 25–31. [Google Scholar] [CrossRef]
- Pandya, S.S.; Hasnani, J.J.; Patel, P.V.; Chauhan, V.D.; Hirani, N.D.; Shukla, R.; Dhamsaniya, H.B. Study on prevalence of fasciolosis in buffaloes at Anand and Ahmedabad districts, Gujarat, India. Vet. World 2015, 8, 870–874. [Google Scholar] [CrossRef]
- Soundararajan, C.; Ani! Kumar, R.; Raman, M.; Iyue, M. Prevalence of fasciolosis in sheep in Nilgiris. Ind. J. Anim. Res. 2000, 34, 73–74. [Google Scholar]
- Latchumikanthan, A.; Soundararajan, C.; Abdul Basith, S.; Raj, G. Serodiagnosis of bovine fasciolosis by Dot-enzyme immuno assay in Chennai, Tamil Nadu. J. Parasit. Dis. 2012, 38, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, K.R.; Tewari, A.K.; Sreekumar, C.; Gupta, S.C.; Rao, J.R. Elucidation of genetic variability among different isolates of Fasciola gigantica (giant liver fluke) using random-amplified polymorphic DNA polymerase chain reaction. Parasitol. Res. 2008, 103, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.K.; Tandon, V.; Biswal, D.K.; Goswami, L.M.; Chatterjee, A. Use of sequence motifs as barcodes and secondary structures of Internal Transcribed spacer 2 (ITS2, rDNA) for identification of the Indian liver fluke, Fasciola (Trematoda: Fasciolidae). Bioinformation 2009, 3, 314–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, P.K.; Goswami, L.M.; Tandon, V.; Chatterjee, A. PCR-based molecular characterization and insilico analysis of food-borne trematode parasites Paragonimus westermani, Fasciolopsis buski and Fasciola gigantica from Northeast India using ITS2 rDNA. Bioinformation 2011, 6, 64–68. [Google Scholar] [CrossRef]
- Raina, O.K.; Jacob, S.S.; Sankar, M.; Bhattacharya, D.; Bandyopadyay, S.; Varghese, A.; Chamuah, I.K. Genetic characterization of Fasciola gigantica from different geographical regions of India by ribosomal DNA markers. J. Parasit. Dis. 2015, 39, 27–32. [Google Scholar] [CrossRef]
- Chamuah, J.K.; Raina, O.K.; Lalrinkima, H.; Jacob, S.S.; Sankar, M.; Sakhrie, A.; Lama, S.; Banerjee, P.S. Molecular characterization of veterinary important trematode and cestode species in the mithun Bos frontalis from north-east India. J. Helminthol. 2015, 90, 577–582. [Google Scholar] [CrossRef]
- Hayashi, K.; Ichikawa-Seki, M.; Mohanta, U.K.; Singh, T.S.; Shoriki, T.; Sugiyama, H.; Itagaki, T. Molecular phylogenetic analysis of Fasciola flukes from eastern India. Parasitol. Int. 2015, 64, 334–338. [Google Scholar] [CrossRef]
- Hayashi, K.; Mohanta, U.K.; Neeraja, T.; Itagaki, T. Molecular characterization of Fasciola gigantica in Delhi, India and its phylogenetic relation to the species from South Asian countries. J. Vet. Med. Sci. 2016, 78, 1529–1532. [Google Scholar] [CrossRef][Green Version]
- Lyngdoh, D.; Shabong, G.C.; Warjri, C.D.; Zas, P. Documentation of Radix acuminata as the intermediate host for Fasciola gigantica in Meghalaya, Northeast India. Ann. Parasitol. 2024, 70, 137–145. [Google Scholar] [CrossRef]
- Sharma, R.L.; Dhar, D.N.; Raina, O.K. Studies on the prevalence and laboratory transmission of fascioliasis in animals in the Kashmir Valley. Br. Vet. J. 1989, 145, 57–61. [Google Scholar] [CrossRef]
- Pandit, B.A.; Mir, A.S.; Nasreen, S.; Khand, A.A. Epidemiology of ovine fascioliasis in J and K State. J. Vet. Parasitol. 1989, 3, 13–15. [Google Scholar]
- Khajuria, J.K.; Kapoor, P.R. Prevalence of parasites in sheep and goats at Kathua-Jammu. J. Vet. Parasitol. 2003, 17, 121–126. [Google Scholar]
- Yadav, A.; Khajuria, J.K.; Raina, A.K. Gastrointestinal parasitic infestation profile of bovines at R.S. Pura, Jammu. J. Vet. Parasitol. 2004, 18, 167–169. [Google Scholar]
- Blench, R. Pastoralism in the New Millennium; Food and Agriculture Organisation of the United Nations. Overseas Development Institute: London, UK, 2001; pp. 1–104. Available online: https://www.fao.org/4/y2647e/y2647e00.htm (accessed on 13 March 2025).
- Sharma, V.P.; Köhler-Rollefson, I.; Morton, J. Pastoralism in India: A Scoping Study; Report; Centre for Management in Agriculture Indian Institute of Management: Ahmedabad, India; Natural Resources Institute, University of Greenwich: London, UK, 2016; pp. 1–63. Available online: https://assets.publishing.service.gov.uk/media/57a08ce2e5274a31e00014fa/ZC0181b.pdf (accessed on 13 March 2025).


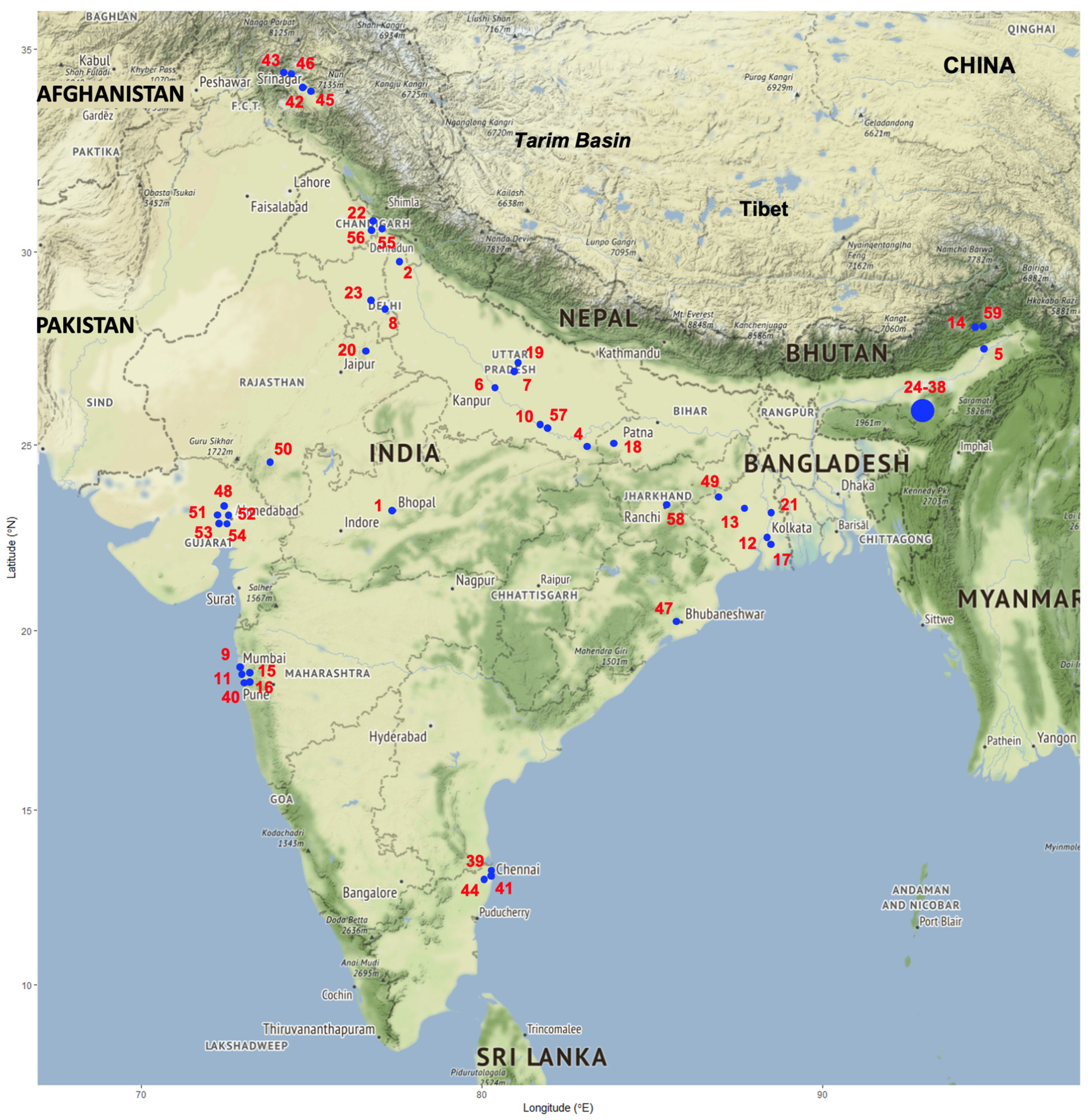



| Location on Map | Station | International Station Code | Geographical Coordinates | Altitude (m) | Available Data (%) |
|---|---|---|---|---|---|
| 1 | Agartala | IN021010100 | 23.88° N–91.25° E | 16 | 79.88 |
| 2 | Ahmedabad | IN005010600 | 23.07° N–72.63° E | 55 | 80.57 |
| 3 | Amritsar | INM00042071 | 31.71° N–74.80° E | 230.4 | 70.28 |
| 4 | Balasore | IN017010300 | 21.52° N–86.93° E | 20 | 78.17 |
| 5 | Bangalore | IN009010100 | 12.97° N–77.58° E | 921 | 85.2 |
| 6 | Begumpet Observatory | IN001080500 | 17.45° N–78.47° E | 527 | 83.74 |
| 7 | Bhopal Bairagarh | IN011351500 | 23.28° N–77.35° E | 523 | 82.11 |
| 8 | Bhuj Rudramata | IN005120501 | 23.25° N–69.67° E | 80 | 76.63 |
| 9 | Bikaner | IN019070100 | 28.00° N–73.30° E | 224 | 70.35 |
| 10 | Santacruz Mumbai | IN012070800 | 19.12° N–72.85° E | 14 | 87.24 |
| 11 | Chitradurga | IN009070100 | 14.23° N–76.43° E | 733 | 80.45 |
| 12 | Cuddalore | IN020020300 | 11.77° N–79.77° E | 12 | 80.65 |
| 13 | Dehradun | IN023160900 | 30.32° N–78.03° E | 682 | 58.28 |
| 14 | Gadag | IN009090300 | 15.42° N–75.63° E | 650 | 79.23 |
| 15 | Goa Panjim | IN022030600 | 15.48° N–73.82° E | 60 | 84.39 |
| 16 | Gorakhpur | IN023261300 | 26.75° N–83.37° E | 77 | 56.14 |
| 17 | Hissar | IN006031000 | 29.17° N–75.73° E | 221 | 75.73 |
| 18 | Indore | IN011170400 | 22.72° N–75.80° E | 567 | 81.87 |
| 19 | Jabalpur | IN011180800 | 23.20° N–79.95° E | 393 | 73.46 |
| 20 | Jaipur Sanganer | IN019131301 | 26.82° N–75.80° E | 390 | 80.81 |
| 21 | Jodhpur | IN019180500 | 26.30° N–73.02° E | 224 | 72.48 |
| 22 | Kakinada | IN001050200 | 16.95° N–82.23° E | 8 | 81.46 |
| 23 | Kozhikode | IN010050700 | 11.25° N–75.78° E | 5 | 71.87 |
| 24 | Kurnool | IN001120100 | 15.80° N–78.07° E | 281 | 69.43 |
| 25 | Lucknow Amausi | IN023351400 | 26.75° N–80.88° E | 128 | 80.65 |
| 26 | Machilipatnam | IN001111200 | 16.20° N–81.15° E | 3 | 83.74 |
| 27 | Madras Minambakkam | IN020040900 | 13.00° N–80.18° E | 16 | 83.98 |
| 28 | Mangalore Bajpe | IN009130300 | 12.92° N–74.88° E | 102 | 87.97 |
| 29 | Nellore | IN001160200 | 14.45° N–79.98° E | 20 | 80.24 |
| 30 | New Delhi Safdarjun | IN022021900 | 28.58° N–77.20° E | 216 | 81.79 |
| 31 | Patiala | IN018103100 | 30.33° N–76.47° E | 251 | 76.46 |
| 32 | Pbo Anantapur | IN001020700 | 14.58° N–77.63° E | 364 | 80.98 |
| 33 | Pendra Road | IN011060800 | 22.77° N–81.90° E | 625 | 70.69 |
| 34 | Poona | IN012190100 | 18.53° N–73.85° E | 559 | 85.28 |
| 35 | Raipur | IN011291000 | 21.22° N–81.67° E | 294 | 38.58 |
| 36 | Rajkot | IN005150100 | 22.30° N–70.78° E | 138 | 80.41 |
| 37 | Ratnagiri | IN012201100 | 16.98° N–73.33° E | 67 | 86.91 |
| 38 | Satna | IN011340100 | 24.57° N–80.83° E | 317 | 55.2 |
| 39 | Solapur | IN012230300 | 17.67° N–75.90° E | 479 | 79.84 |
| 40 | Srinagar | IN008010200 | 34.08° N–74.83° E | 1587 | 64.27 |
| 41 | Surat | IN005171200 | 21.20° N–72.83° E | 12 | 80.81 |
| 42 | Tezpur | IN003020100 | 26.62° N–92.78° E | 79 | 56.54 |
| 43 | Thiruvananthapuram | IN010100400 | 8.48° N–76.95° E | 64 | 87.28 |
| 44 | Tiruchchirapalli | IN020130700 | 10.77° N–78.72° E | 88 | 80.89 |
| Origin of Reports and Cases: | No. | States with Diagnosed Cases: | No. |
|---|---|---|---|
| - Reports of cases (in 35 articles + 3 requests to the WHO) | 59 | - Northeastern India—state(s) n.s. | 15 |
| - Individual fascioliasis patients (–4 repeatedly reported) | 55 | - Uttar Pradesh | 7 |
| - Cases in which the locality of the patient was given | 11 | - Gujarat | 5 |
| - Cases including only the locality of the hospital | 44 | - West Bengal | 5 |
| - Cases imported from other countries | 3 | - Maharashtra | 5 |
| - Punjab | 3 | ||
| Specific diagnosis of causal agent: | - Tamil Nadu | 3 | |
| - Infected by Fasciola hepatica | 31 | - Jammu and Kashmir | 2 |
| - Infected by Fasciola gigantica | 6 | - Rajasthan | 2 |
| - Infected by admixed Fasciola hybrid | 1 | - Haryana | 1 |
| - Without specific diagnosis | 17 | - Delhi | 1 |
| - Bihar | 1 | ||
| Altitude of patient’s locality or hospital’s locality: | - Jharkhand | 1 | |
| - Between 1 and 500 m a.s.l. | 35 | - Assam | 1 |
| - Between 500 and 600 m a.s.l. | 2 | - Arunachal Pradesh | 1 |
| - Higher than 1000 m a.s.l. | 2 | - Madhya Pradesh | 1 |
| - Unknown because of lack of patient’s or hospital’s locality | 16 | - Odisha | 1 |
| - State n.s. in traveler | 1 | ||
| Sex and age of patient: | |||
| - Male/female | 21/33 | Drugs used for patient’s treatment: | |
| - Sex n.s. | 1 | - Triclabendazole | 30 |
| - Age—extreme values | 30 m–71 y | - Albendazole | 10 |
| - Age—mean (in years) | 33.1 | - Nitazoxanide | 21 |
| - Age—mean for males/for females (in years) | 33.4/32.7 | - Diethylcarbamazine | 1 |
| - Praziquantel | 4 | ||
| Time elapsed between symptom onset and diagnosis: | - Antibiotics plus steroids | 2 | |
| - Extreme values | 10 d–5 y | - Bitionol | 1 |
| - Mean | 9.2 m | - Ivermectine | 6 |
| Diagnostic method: | Coinfecting parasites: | ||
| - By egg finding (in stool, or duodenal or bile aspirate) | 12 | - Giardia intestinalis | 1 |
| - By adult finding (in ERCP, surgery, or liver biopsy) | 28 | - Hymenolepis nana | 1 |
| - By serology | 3 | - Ascaris lumbricoides | 1 |
| - By clinics and image techniques (US, CT, MRCP) | 12 | - Trichuris trichiura | 1 |
| Routes | Category | Main Nodes (see Map in Figure 8) | Starting Period | Purpose/Use | References |
|---|---|---|---|---|---|
| Grand Trunk Road (= Uttarapatha; = northern route) | Main route | Kophen, Peshawar, Taxila, Gandhara, Lahore, Indraprastha, Mathura, Sravasti, Prayagraj, Kushinagar, Pataliputra, Chandraketugarh, Tamralipti, Teknaf | 500 y BC | Long-distance goods transport | [1,75] |
| Daksinapatha Road (= southern route) | Main route | – Main route: Rajgrahi, Varanasi, Bharhut, Vidisa, Ujjayini, Ajanta, Pratisthana – Connections with the Grand Trunk Road: Kausambi, Gwalior, Mandasor, Bairat. – Connections with seaports of the Maritime Silk Road: Sanjeli, Nasik, Bhorgat, Bhaja | 500 y BC | Long-distance goods transport | [75] |
| Silk Road | Main route | Taxila–Srinagar–northern Silk Road; Sravasti–Khotan in northern Silk Road | 2500–2000 y BC | Long-distance goods transport | [1,76] |
| Tea-Horse Road | Main route | Sikkim state–Lhasa–Yaan; Dhaka–Dali; Mandalay–Dali | 700 y BC | Long-distance goods transport | [1] |
| Maritime Silk Road | Main route | – West Indian coast: Barbarikon, Hayhab, Gogha, Khambhat, Barygaza, Kamrej, Nala Sopera, Mumbai, Kalyan, Chaul, Goa, Muziris – East Indian coast: Madras, Machilipatnam | 200 y BC | Long-distance goods transport, seaport to hinterland | [1,75] |
| Sea–Grand Trunk Road connection | Secondary route | Barbaricon–Multan/Indus Valley–Taxila | 300 y BC– 100 y AD | Mid-distance goods transport | [1,75,77] |
| Orissa–Bangladesh connection | Secondary route | Bhubaneswar–eastern end of Grand Trunk Road and Tea-Horse Road | 200 y BC | Long-distance goods transport | [78] |
| Arabian Sea– hinterland connections | Tertiary route | – Barygaza (and neighboring seaports) – Sanjeli, Mandasor, Ujjayini – Nala Sopara/Mumbai, Kalyan, Nasik, Pratisthana – Chaul, Bhorgat, Bhaja, Pratisthana | 500 y BC | Short-distance goods transport | [1,75] |
| Bay of Bengal–Daksinapatha | Tertiary route | Madras–Pratisthana; Machilipatnam–Pratisthana | 1500 y AD | Mid-distance goods transport | [75] |
| Gujarat intra-/inter-state movements | Quaternary route | Gujarat state lowlands | Horizontal lowland seasonal transhumance | [79,80,81] | |
| Rajasthan intra-/interstate movements | Quaternary route | Rajasthan state lowlands | Horizontal lowland seasonal transhumance | [79,80] | |
| Madhya Pradesh intra-/interstate movements | Quaternary route | Madhya Pradesh state lowlands | Horizontal lowland seasonal transhumance | [79,80] | |
| Uttar Pradesh Intra-/interstate movements | Quaternary route | Uttar Pradesh state lowlands | Horizontal lowland seasonal transhumance | [79,82] | |
| Tamil Nadu intra-/interstate movements | Quaternary route | Tamil Nadu state lowlands | Horizontal lowland seasonal transhumance | [79] | |
| Punjab intra-/inter- state movements | Quaternary route | Punjab state lowlands | Horizontal lowland seasonal transhumance | [79] | |
| Western Himalaya vertical movements | Quaternary route | Jammu and Kashmir–Himachal Pradesh–Uttarakhand | Vertical altitudinal seasonal transhumance | [79,80,82,83,84,85,86,87] | |
| Central Himalaya vertical movements | Quaternary route | Eastern Nepal/Sikkim–Lhasa/Bhutan | Vertical altitudinal seasonal transhumance | [88,89,90,91,92] | |
| Eastern Himalaya vertical movements | Quaternary route | Arunachal Pradesh–Tea-Horse Road | Vertical altitudinal seasonal transhumance | [93,94,95,96,97] | |
| Eastern states | Quaternary routes | Bihar, Jharkhand, Orissa, Chhattisgarh, and West Bengal | Nomadic pastoralism | [98,99,100,101] |
| States of India | State Subzones | Transhumant, Nomadic, or Pastoralist Groups | Moving Livestock Reservoir Species | Ruminant Prevalences Reported |
|---|---|---|---|---|
| Andhra Pradesh | Golla | Cattle | Cattle & buffaloes: 3.8% | |
| Kuruma | Sheep | |||
| Arunachal Pradesh | Tawang and Kemeng districts | Monpa | Cattle, yak | Mithun: 4.08–28.9% |
| Assam | Cattle: 34.0% | |||
| Bihar | Gaddi Muslim/Ghosi | Cattle | Sheep: 8.98%; goats: 12.87% | |
| Chhattisgarh | Cattle: 0.3%; buffaloes: 0.6% | |||
| Delhi | Sheep, goats, cattle, buffaloes: 0.8–2.1% | |||
| Goa | Gavli | Cattle | n.d. | |
| Gujarat | Bharwad | Sheep, goats, cattle | Cattle & buffaloes: 12.0–42.0% Buffaloes: 25.0–33.0% | |
| Gir forest region | Charan | Cattle | ||
| Gavli | Cattle | |||
| Kutch region | Jath | Cattle, camelids | ||
| Saurashtra region | Mer | Cattle, camelids | ||
| Rebari/Reika | Goats, cattle, camelids | |||
| Haryana | Gaderia | Sheep, goats | Buffaloes: 1.3% | |
| Himachal Pradesh | Gaddis | Sheep, goats | Sheep & goats: 8.8–9.6% Cattle & buffaloes: 36.0–48.7% | |
| Gujjar | Buffalo, cattle | |||
| Kinnaur district | Kinnaura | Sheep, goats | ||
| Jharkhand | Pashu Sakhis | Mostly goats | n.d. | |
| Jammu and Kashmir | Bakarwal | goats | Sheep: 15.0% Sheep & goats: 2.0–4.2% Cattle: 4.5–85.0% Cattle & buffaloes: 2.6–6.9% | |
| Zanskar | Changpa | Yak | ||
| Gaddis | Sheep, goats | |||
| Gujjar | Buffalo, cattle | |||
| Karnataka | Dhangar/Kuruba | Sheep | Cattle: 2.4–26.6% Buffaloes: 1.5–22.5% | |
| Gavli/Toda | Cattle | |||
| Kerala | Toda | Cattle | n.d. | |
| Madhya Pradesh | Dhangar | Sheep | Goats: 3.5–25.3%; cattle: 6.02%; buffaloes: 3.51% | |
| Gaderia | Sheep, goats | |||
| Maharashtra | Dhangar | Sheep | Sheep: 31.8%; goats: 19.4%; cattle: 17.5%; buffaloes: 23.8% | |
| Gavli/Golla | Cattle | |||
| Manipur | Farmers | Goats, cattle | n.d. | |
| Meghalaya | Farmers, Scheduled Tribe | Sheep, goats, cattle, buffaloes | Cattle: 52.2% | |
| Mizoram | Farmers | Cattle | n.d. | |
| Nagaland | Farmers | Sheep, cattle, buffaloes | n.d. | |
| Orissa (= Odisha) | Cattle: 4.3% | |||
| Punjab | Banjaras | Cattle | Sheep: 72.9%; goats: 56.8% Cattle: 1.9–18.0%; buffaloes: 21.0% | |
| Rajasthan | Gaddi Muslim/Ghosi | Cattle | Cattle: 2.4% | |
| Mewar in the south | Gayri | Sheep, cattle | ||
| Gujjar | Buffalo, cattle | |||
| Ganganagar and Bikaner districts | Rath | Cattle | ||
| Rebari/Reika | Goats, cattle, camelids | |||
| Marwar region | Sindhi | Sheep, cattle, camelids | ||
| Jaisalmer | Sindhi | Sheep, cattle, camelids | ||
| Sikkim | North district | Bhutia | Sheep, goats, cattle | Goat & cattle: 19.3–83.0% |
| Tamil Nadu | Nilgiri region | Toda | Cattle | Sheep: 50.0% |
| Telangana | Mahabubnagar dist. | Farmers | Sheep, goats, cattle, buffaloes | n.d. |
| Tripura | Farmers | Sheep, goats, cattle, buffaloes | n.d. | |
| Uttar Pradesh | Gaddi Muslim | Cattle | Sheep: 1.7% Goats: 2.0–3.6% Buffaloes: 32.8–94.0% | |
| Gaderia | Sheep, goats | |||
| Ghosi | Cattle | |||
| Van Gujar | Buffalo | |||
| Uttarakhand | Garhwal, Kumaon | Bhotia | Sheep, goats, cattle | Sheep: 2.5–4.5%; goats: 1.2–3.5%; buffaloes: 57.4–78.0% |
| Van Gujar | Buffalo | |||
| West Bengal | Cattle: 0.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Coma, S.; Cuervo, P.F.; Chetri, P.B.; Tripathi, T.; Gabrielli, A.F.; Bargues, M.D. Emerging Human Fascioliasis in India: Review of Case Reports, Climate Change Impact, and Geo-Historical Correlation Defining Areas and Seasons of High Infection Risk. Trop. Med. Infect. Dis. 2025, 10, 123. https://doi.org/10.3390/tropicalmed10050123
Mas-Coma S, Cuervo PF, Chetri PB, Tripathi T, Gabrielli AF, Bargues MD. Emerging Human Fascioliasis in India: Review of Case Reports, Climate Change Impact, and Geo-Historical Correlation Defining Areas and Seasons of High Infection Risk. Tropical Medicine and Infectious Disease. 2025; 10(5):123. https://doi.org/10.3390/tropicalmed10050123
Chicago/Turabian StyleMas-Coma, Santiago, Pablo F. Cuervo, Purna Bahadur Chetri, Timir Tripathi, Albis Francesco Gabrielli, and M. Dolores Bargues. 2025. "Emerging Human Fascioliasis in India: Review of Case Reports, Climate Change Impact, and Geo-Historical Correlation Defining Areas and Seasons of High Infection Risk" Tropical Medicine and Infectious Disease 10, no. 5: 123. https://doi.org/10.3390/tropicalmed10050123
APA StyleMas-Coma, S., Cuervo, P. F., Chetri, P. B., Tripathi, T., Gabrielli, A. F., & Bargues, M. D. (2025). Emerging Human Fascioliasis in India: Review of Case Reports, Climate Change Impact, and Geo-Historical Correlation Defining Areas and Seasons of High Infection Risk. Tropical Medicine and Infectious Disease, 10(5), 123. https://doi.org/10.3390/tropicalmed10050123










