Baseline Seroprevalence of Arboviruses in Liberia Using a Multiplex IgG Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Sample Collection
2.2. Antigen
2.3. Arbovirus Microsphere Panel
2.4. Microsphere Immunoassay
2.5. Statistical Analysis
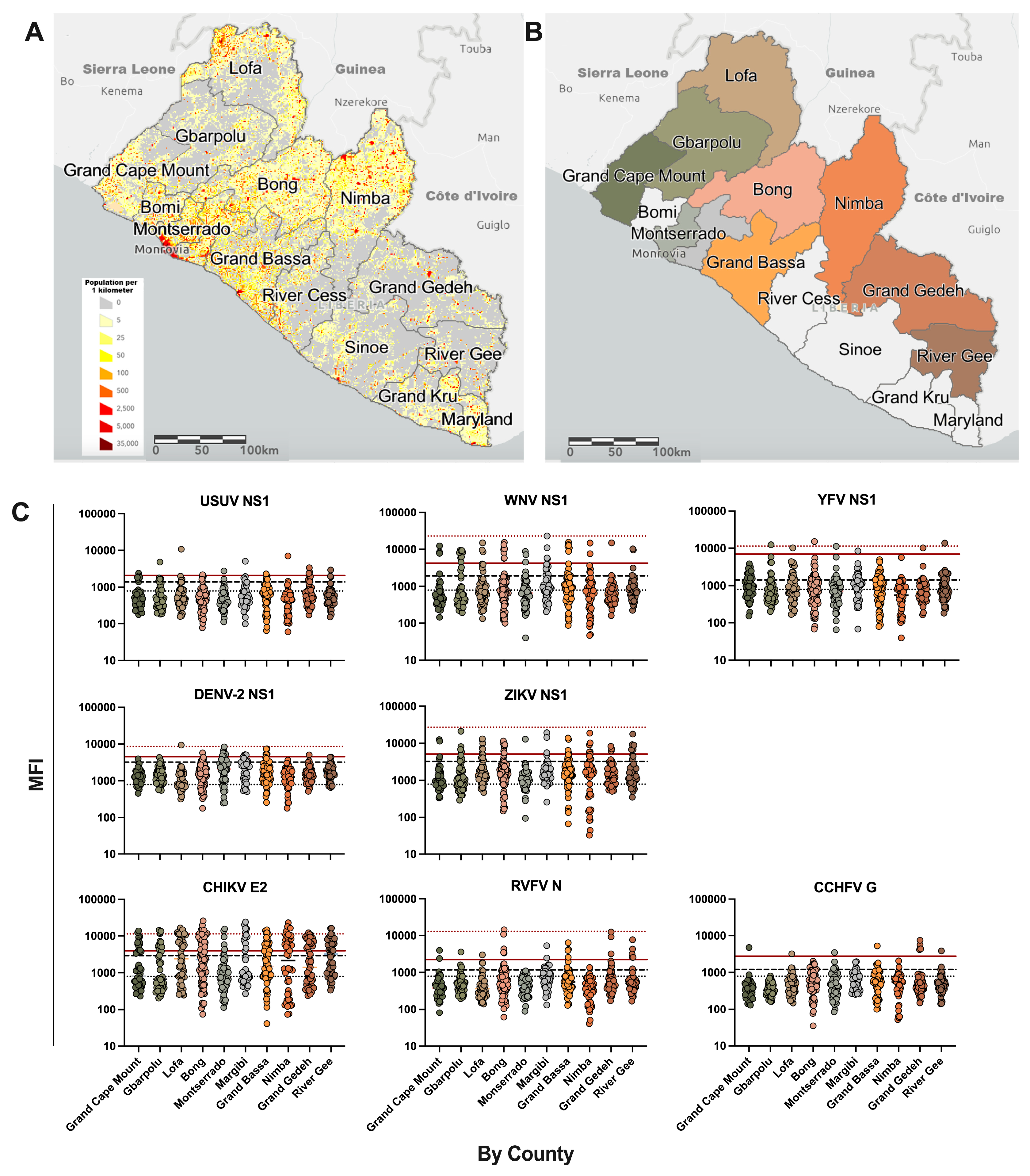
3. Results
| County | n | USUV NS1 | WNV NS1 | YFV NS1 | DENV-2 NS1 | ZIKV NS1 | RVFV N | CHIKV E2 | CCHFV Gc |
|---|---|---|---|---|---|---|---|---|---|
| Grand Cape Mount | 49 | 1 (2.0%) | 3 (6.1%) | - | - | 2 (4.1%) | 2 (4.1%) | 13 (26.5%) | 1 (2.0%) |
| Gbarpolu | 50 | 1 (2.0%) | 9 (18.0%) | 1 (2.0%) | - | 4 (8.0%) | 1 (2.0%) | 12 (24.0%) | - |
| Lofa | 50 | 1 (2.0%) | 3 (6.0%) | 1 (2.0%) | 1 (2.0%) | 5 (10.0%) | 1 (2.0%) | 22 (44.0%) | 1 (2.0%) |
| Bong | 79 | 1 (1.3%) | 8 (10.1%) | 1 (1.3%) | 1 (2.0%) | 5 (6.3%) | 5 (6.3%) | 29 (36.7%) | - |
| Montserrado | 51 | 1 (2.0%) | 3 (5.9%) | 1 (2.0%) | 7 (13.7%) | 1 (2.0%) | - | 5 (9.0%) | 1 (2.0%) |
| Margibi | 40 | 1 (2.5%) | 4 (10.0%) | 1 (2.5%) | 2 (5.0%) | 3 (7.5%) | 2 (5.0%) | 11 (27.5%) | - |
| Grand Bassa | 66 | 1 (1.5%) | 6 (9.1%) | - | 4 (6.1%) | 3 (4.5%) | 4 (6.1%) | 20 (30.3%) | 1 (1.5%) |
| Nimba | 48 | 1 (2.1%) | 2 (4.2%) | - | - | 6 (12.5%) | - | 21 (43.8%) | - |
| Grand Gedeh | 49 | 3 (6.1%) | 1 (2.0%) | 1 (2.0%) | 1 (2.0%) | 4 (8.2%) | 3 (6.1%) | 17 (34.7.0%) | 4 (8.2%) |
| River Gee | 50 | 1 (2.0%) | 2 (4.0%) | 1 (2.0%) | 1 (2.0%) | 5 (10.0%) | 5 (10.0%) | 23 (46.0%) | 1 (2.0%) |
| Countrywide Total | 532 | 12 | 41 | 7 | 17 | 38 | 23 | 173 | 9 |
| Percentage | - | 2.3% | 7.7% | 1.3% | 3.2% | 7.1% | 4.3% | 32.5% | 1.7% |
| County | n | Single Flavivirus Infection | Multiple (≥2) Flavivirus Infections | Total Flavivirus Infection | Single Flavivirus w/CHIKV Infection | Multiple Flavivirus w/CHIKV Infection | CHIKV with RVFV Infection | CHIKV with CCHFV Infection |
|---|---|---|---|---|---|---|---|---|
| Grand Cape Mount | 49 | 6 (12.2%) | - | 6 (12.2%) | 2 (4.1%) | - | - | - |
| Gbarpolu | 50 | 13 (26.0%) | 2 (4.0%) | 15 (30.0%) | 6 (12.0%) | - | 1 (2.0%) | - |
| Lofa | 50 | 9 (18.0%) | 2 (4.0%) | 11 (22.0%) | 4 (8.0%) | - | 1s (2.0%) | - |
| Bong | 79 | 11 (13.9%) | 5 (6.3%) | 16 (20.3%) | 8 (10.1%) | 4 (5.1%) | 3 (1s) (3.8%) | - |
| Montserrado | 51 | 12 (23.5%) | 1 (2.0%) | 13 (25.5%) | 1 (2.0%) | 1 (2.0%) | - | 1m (2.0%) |
| Margibi | 40 | 7 (17.5%) | 4 (10.0%) | 11 (27.5%) | - | 2 (5.0%) | 1 (2.5%) | - |
| Grand Bassa | 66 | 11 (16.7%) | 3 (4.5%) | 14 (21.2%) | 4 (6.1%) | 2 (3.0%) | - | 1m (1.5%) |
| Nimba | 48 | 9 (18.8%) | - | 9 (18.8%) | 4 (8.3%) | - | - | - |
| Grand Gedeh | 49 | 7 (14.3%) | 3 (6.1%) | 10 (20.4%) | 2 (4.1%) | 2 (4.1%) | - | 3 (6.1%) |
| River Gee | 50 | 10 (20.0%) | - | 10 (20.0%) | 3 (6.0%) | - | 2 (1s) (4.0%) | 1s (2.0%) |
| Countrywide Total | 532 | 95 | 20 | 115 | 34 | 11 | 8 | 6 |
| Percentage | - | 17.9% | 3.8% | 21.6% | 6.4% | 2.1% | 1.5% | 1.1% |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liberia Institute of Statistics and Geo-Information Services. Liberia Demographic and Health Survey 2019–2020; Liberia Institute of Statistics and Geo-Information Services (LISGIS): Monrovia, Liberia, 2020.
- Ndede, P.O.; Senkungu, J.K.; Shakpeh, J.K.; Jones, T.E.; Sky, R.; McDonnell, S. Health Services and Infrastructure Recovery of a Major Public Hospital in Liberia During the 2014–2016 Ebola Epidemic. Disaster Med. Public Health Prep. 2019, 13, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sow, A.; Loucoubar, C.; Diallo, D.; Faye, O.; Ndiaye, Y.; Senghor, C.S.; Dia, A.T.; Faye, O.; Weaver, S.C.; Diallo, M.; et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar. J. 2016, 15, 47. [Google Scholar] [CrossRef]
- Baba, M.; Logue, C.H.; Oderinde, B.; Abdulmaleek, H.; Williams, J.; Lewis, J.; Laws, T.R.; Hewson, R.; Marcello, A.; D’Agaro, P. Evidence of arbovirus co-infection in suspected febrile malaria and typhoid patients in Nigeria. J. Infect. Dev. Ctries. 2013, 7, 51–59. [Google Scholar] [CrossRef]
- Van der Waals, F.W.; Asher, D.M.; Goudsmit, J.; Pomeroy, K.L.; Karabatsos, N.; Gajdusek, D.C. Post-encephalitic epilepsy and arbovirus infections in an isolated rainforest area of central Liberia. Trop. Geogr. Med. 1986, 38, 203–208. [Google Scholar] [PubMed]
- Fox, R.M. Man-biting mosquitoes in coastal Liberia. Am. J. Trop. Med. Hyg. 1958, 7, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Liberia-Country Summary, The World Factbook. Available online: https://www.cia.gov/the-world-factbook/countries/liberia/ (accessed on 2 January 2024).
- Christensen, D.; Hartman, A.C.; Samii, C. Citizen monitoring promotes informed and inclusive forest governance in Liberia. Proc. Natl. Acad. Sci. USA 2021, 118, e2015169118. [Google Scholar] [CrossRef]
- Tyson, J.; Tsai, W.Y.; Tsai, J.J.; Massgard, L.; Stramer, S.L.; Lehrer, A.T.; Nerurkar, V.R.; Wang, W.K. A high-throughput and multiplex microsphere immunoassay based on non-structural protein 1 can discriminate three flavivirus infections. PLoS Negl. Trop. Dis. 2019, 13, e0007649. [Google Scholar] [CrossRef]
- Surtees, R.; Stern, D.; Ahrens, K.; Kromarek, N.; Lander, A.; Kreher, P.; Weiss, S.; Hewson, R.; Punch, E.K.; Barr, J.N.; et al. Development of a multiplex microsphere immunoassay for the detection of antibodies against highly pathogenic viruses in human and animal serum samples. PLoS Negl. Trop. Dis. 2020, 14, e0008699. [Google Scholar] [CrossRef]
- Belij-Rammerstorfer, S.; Limon, G.; Maze, E.A.; Hannant, K.; Hughes, E.; Tchakarova, S.R.; Alexandrov, T.; Mmbaga, B.T.; Willett, B.; Booth, G.; et al. Development of anti-Crimean-Congo hemorrhagic fever virus Gc and NP-specific ELISA for detection of antibodies in domestic animal sera. Front. Vet. Sci. 2022, 9, 913046. [Google Scholar] [CrossRef]
- To, A.; Medina, L.O.; Mfuh, K.O.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Lai, C.Y.; Kumar, M.; Nerurkar, V.R.; et al. Recombinant Zika Virus Subunits Are Immunogenic and Efficacious in Mice. mSphere 2018, 3, e00576-17. [Google Scholar] [CrossRef]
- Haun, B.K.; To, A.; Williams, C.A.; Ball, A.; Fong, K.; Wong, T.A.S.; Shobayo, B.; Teahton, J.; Ching, L.; Kamara, V.; et al. A Serological Multiplexed Immunoassay (MIA) Detects Antibody Reactivity to SARS-CoV-2 and Other Viral Pathogens in Liberia and Is Configurable as a Multiplexed Inhibition Test (MINT). Immuno 2024, 4, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.J. Analysis of serological surveys using mixture models: Application to a survey of parvovirus B19. Stat. Med. 1996, 15, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. mixR: Finite Mixture Modeling for Raw and Binned Data, R Package Version 0.2.0. 2022. Available online: https://www.theoj.org/joss-papers/joss.04031/10.21105.joss.04031.pdf (accessed on 3 July 2023).
- Nikolay, B.; Diallo, M.; Boye, C.S.; Sall, A.A. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011, 11, 1417–1423. [Google Scholar] [CrossRef]
- Pisano, M.R.; Nicoli, J.; Tolou, H. Homogeneity of yellow fever virus strains isolated during an epidemic and a post-epidemic period in West Africa. Virus Genes 1997, 14, 225–234. [Google Scholar] [CrossRef]
- Adjogoua, E.V.; Coulibaly-Guindo, N.; Diaha-Kouame, C.A.; Diane, M.K.; Kouassi, R.; Coulibaly, J.T.; Dosso, M. Geographical Distribution of Ticks Ixodidae in Cote d’Ivoire: Potential Reservoir of the Crimean-Congo Hemorrhagic Fever Virus. Vector Borne Zoonotic Dis. 2021, 21, 628–634. [Google Scholar] [CrossRef]
- Mediannikov, O.; Diatta, G.; Zolia, Y.; Balde, M.C.; Kohar, H.; Trape, J.F.; Raoult, D. Tick-borne rickettsiae in Guinea and Liberia. Ticks Tick. Borne Dis. 2012, 3, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dieng, I.; Ndiaye, M.; Ndione, M.H.D.; Sankhe, S.; Diagne, M.M.; Sagne, S.N.; Gaye, A.; Balde, D.; Barry, A.; Fall, G.; et al. Molecular characterization of circulating Dengue virus 2 during an outbreak in Northern Senegal’s Saint-Louis region in 2018. J. Med. Virol. 2023, 95, e28347. [Google Scholar] [CrossRef]
- Yonga, M.G.; Yandai, F.H.; Sadeuh-Mba, S.; Abdallah, A.H.; Ouapi, D.; Gamougam, K.; Abanda, N.N.; Endengue-Zanga, M.C.; Demanou, M.; Njouom, R. Molecular characterization of chikungunya virus from the first cluster of patients during the 2020 outbreak in Chad. Arch. Virol. 2022, 167, 1301–1305. [Google Scholar] [CrossRef]
- Faye, O.; de Lourdes Monteiro, M.; Vrancken, B.; Prot, M.; Lequime, S.; Diarra, M.; Ndiaye, O.; Valdez, T.; Tavarez, S.; Ramos, J.; et al. Genomic Epidemiology of 2015–2016 Zika Virus Outbreak in Cape Verde. Emerg. Infect. Dis. 2020, 26, 1084–1090. [Google Scholar] [CrossRef]
- Leaders de l’Actualité Africaine. Burkina Faso: 688 Décès Liés à la Dengue Depuis le Début de l’Année. Available online: https://www.faapa.info/blog/burkina-faso-688-deces-lies-a-la-dengue-depuis-le-debut-de-lannee/ (accessed on 2 January 2023).
- Nigeria Centre for Disease Control and Prevention. NCDC on Alert Following the Confirmation of the Dengue Fever Outbreak in Sokoto State. Available online: https://ncdc.gov.ng/news/506/ncdc-on-alert-following-the-confirmation-of-the-dengue-fever-outbreak-in-sokoto-state (accessed on 2 January 2024).
- Padane, A.; Tegally, H.; Ramphal, Y.; Seyni, N.; Sarr, M.; Diop, M.M.; Diedhiou, C.K.; Mboup, A.; Diouf, N.D.; Souare, A.; et al. An emerging clade of Chikungunya West African genotype discovered in real-time during 2023 outbreak in Senegal. medRxiv 2023. [Google Scholar] [CrossRef]
- Hoze, N.; Diarra, I.; Sangare, A.K.; Pastorino, B.; Pezzi, L.; Kouriba, B.; Sagara, I.; Dabo, A.; Djimde, A.; Thera, M.A.; et al. Model-based assessment of Chikungunya and O’nyong-nyong virus circulation in Mali in a serological cross-reactivity context. Nat. Commun. 2021, 12, 6735. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Ridde, V.; Agnandji, S.T.; Lell, B.; Yaro, S.; Yang, J.S.; Hoinard, D.; Weaver, S.C.; Vanhomwegen, J.; Salje, H.; et al. Seroepidemiological Reconstruction of Long-term Chikungunya Virus Circulation in Burkina Faso and Gabon. J. Infect. Dis. 2023, 227, 261–267. [Google Scholar] [CrossRef]
- Ansumana, R.; Jacobsen, K.H.; Leski, T.A.; Covington, A.L.; Bangura, U.; Hodges, M.H.; Lin, B.; Bockarie, A.S.; Lamin, J.M.; Bockarie, M.J.; et al. Reemergence of chikungunya virus in Bo, Sierra Leone. Emerg. Infect. Dis. 2013, 19, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Agboli, E.; Zahouli, J.B.Z.; Badolo, A.; Jost, H. Mosquito-Associated Viruses and Their Related Mosquitoes in West Africa. Viruses 2021, 13, 891. [Google Scholar] [CrossRef]
- Schoepp, R.J.; Rossi, C.A.; Khan, S.H.; Goba, A.; Fair, J.N. Undiagnosed acute viral febrile illnesses, Sierra Leone. Emerg. Infect. Dis. 2014, 20, 1176–1182. [Google Scholar] [CrossRef]
- Dariano, D.F.; Taitt, C.R.; Jacobsen, K.H.; Bangura, U.; Bockarie, A.S.; Bockarie, M.J.; Lahai, J.; Lamin, J.M.; Leski, T.A.; Yasuda, C.; et al. Surveillance of Vector-Borne Infections (Chikungunya, Dengue, and Malaria) in Bo, Sierra Leone, 2012–2013. Am. J. Trop. Med. Hyg. 2017, 97, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, A.W.; Bowen, E.T.; Platt, G.S. Viral infections in travellers from tropical Africa. Br. Med. J. 1978, 1, 956–958. [Google Scholar] [CrossRef]
- Jentes, E.S.; Robinson, J.; Johnson, B.W.; Conde, I.; Sakouvougui, Y.; Iverson, J.; Beecher, S.; Bah, M.A.; Diakite, F.; Coulibaly, M.; et al. Acute arboviral infections in Guinea, West Africa, 2006. Am. J. Trop. Med. Hyg. 2010, 83, 388–394. [Google Scholar] [CrossRef]
- Bane, S.; Rosenke, K.; Feldmann, F.; Meade-White, K.; Diawara, S.; Keita, M.; Maiga, O.; Diakite, M.; Safronetz, D.; Doumbia, S.; et al. Seroprevalence of Arboviruses in a Malaria Hyperendemic Area in Southern Mali. Am. J. Trop. Med. Hyg. 2024, 111, 107–112. [Google Scholar] [CrossRef]
- Sow, A.; Nikolay, B.; Faye, O.; Cauchemez, S.; Cano, J.; Diallo, M.; Faye, O.; Sadio, B.; Ndiaye, O.; Weaver, S.C.; et al. Changes in the Transmission Dynamic of Chikungunya Virus in Southeastern Senegal. Viruses 2020, 12, 196. [Google Scholar] [CrossRef]
- Tonto, P.B.; Sy, M.; Ndiaye, I.M.; Toure, M.; Gaye, A.; Aidara, M.; Mbaye, A.M.; Dia, A.K.; Diallo, M.A.; Gomis, J.F.; et al. Seroprevalence of chikungunya and o’nyong-nyong viruses in Senegal, West Africa. medRxiv 2024. [Google Scholar] [CrossRef]
- Nanfack Minkeu, F.; Vernick, K.D. A Systematic Review of the Natural Virome of Anopheles Mosquitoes. Viruses 2018, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Posey, D.L.; O’Rourke, T.; Roehrig, J.T.; Lanciotti, R.S.; Weinberg, M.; Maloney, S. O’Nyong-nyong fever in West Africa. Am. J. Trop. Med. Hyg. 2005, 73, 32. [Google Scholar]
- Safronetz, D.; Sacko, M.; Sogoba, N.; Rosenke, K.; Martellaro, C.; Traore, S.; Cisse, I.; Maiga, O.; Boisen, M.; Nelson, D.; et al. Vectorborne Infections, Mali. Emerg. Infect. Dis. 2016, 22, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Boisen, M.L.; Schieffelin, J.S.; Goba, A.; Oottamasathien, D.; Jones, A.B.; Shaffer, J.G.; Hastie, K.M.; Hartnett, J.N.; Momoh, M.; Fullah, M.; et al. Multiple circulating infections can mimic the early stages of viral hemorrhagic fevers and possible human exposure to filoviruses in Sierra Leone prior to the 2014 outbreak. Viral Immunol. 2015, 28, 19–31. [Google Scholar] [CrossRef]
- Diarra, I.; Nurtop, E.; Sangare, A.K.; Sagara, I.; Pastorino, B.; Sacko, S.; Zeguime, A.; Coulibaly, D.; Fofana, B.; Gallian, P.; et al. Zika Virus Circulation in Mali. Emerg. Infect. Dis. 2020, 26, 945–952. [Google Scholar] [CrossRef]
- Bayandin, R.B.; Makenov, M.T.; Boumbaly, S.; Stukolova, O.A.; Gladysheva, A.V.; Shipovalov, A.V.; Skarnovich, M.O.; Camara, O.; Toure, A.H.; Svyatchenko, V.A.; et al. The First Case of Zika Virus Disease in Guinea: Description, Virus Isolation, Sequencing, and Seroprevalence in Local Population. Viruses 2023, 15, 1620. [Google Scholar] [CrossRef]
- Sene, O.; Sagne, S.N.; Bob, N.S.; Mhamadi, M.; Dieng, I.; Gaye, A.; Ba, H.; Dia, M.; Faye, E.T.; Diop, S.M.; et al. Re-Emergence of Rift Valley Fever Virus Lineage H in Senegal in 2022: In Vitro Characterization and Impact on Its Global Emergence in West Africa. Viruses 2024, 16, 1018. [Google Scholar] [CrossRef]
- Ba, Y.; Sall, A.A.; Diallo, D.; Mondo, M.; Girault, L.; Dia, I.; Diallo, M. Re-emergence of Rift Valley fever virus in Barkedji (Senegal, West Africa) in 2002–2003: Identification of new vectors and epidemiological implications. J. Am. Mosq. Control Assoc. 2012, 28, 170–178. [Google Scholar] [CrossRef]
- Troupin, C.; Ellis, I.; Doukoure, B.; Camara, A.; Keita, M.; Kagbadouno, M.; Bart, J.M.; Diallo, R.; Lacote, S.; Marianneau, P.; et al. Seroprevalence of brucellosis, Q fever and Rift Valley fever in domestic ruminants in Guinea in 2017–2019. BMC Vet. Res. 2022, 18, 64. [Google Scholar] [CrossRef]
- Kanoute, Y.B.; Gragnon, B.G.; Schindler, C.; Bonfoh, B.; Schelling, E. Epidemiology of brucellosis, Q Fever and Rift Valley Fever at the human and livestock interface in northern Cote d’Ivoire. Acta Trop. 2017, 165, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Favier, C.; Chalvet-Monfray, K.; Sabatier, P.; Lancelot, R.; Fontenille, D.; Dubois, M.A. Rift Valley fever in West Africa: The role of space in endemicity. Trop. Med. Int. Health 2006, 11, 1878–1888. [Google Scholar] [CrossRef]
- Clark, M.H.A.; Warimwe, G.M.; Di Nardo, A.; Lyons, N.A.; Gubbins, S. Systematic literature review of Rift Valley fever virus seroprevalence in livestock, wildlife and humans in Africa from 1968 to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006627. [Google Scholar] [CrossRef]
- Ebogo-Belobo, J.T.; Kenmoe, S.; Abanda, N.N.; Bowo-Ngandji, A.; Mbaga, D.S.; Magoudjou-Pekam, J.N.; Kame-Ngasse, G.I.; Tchatchouang, S.; Menkem, E.Z.; Okobalemba, E.A.; et al. Contemporary epidemiological data of Rift Valley fever virus in humans, mosquitoes and other animal species in Africa: A systematic review and meta-analysis. Vet. Med. Sci. 2023, 9, 2309–2328. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.P.; Vallee, L.; de Pina, J.J.; Tolou, H. Isolation of a dengue type 1 virus from a soldier in West Africa (Cote d’Ivoire). Emerg. Infect. Dis. 2000, 6, 83–84. [Google Scholar] [CrossRef]
- Saluzzo, J.F.; Cornet, M.; Castagnet, P.; Rey, C.; Digoutte, J.P. Isolation of dengue 2 and dengue 4 viruses from patients in Senegal. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 5. [Google Scholar] [CrossRef]
- Bonney, J.H.K.; Pratt, D.; Ofori, M.; Hayashi, T.; Abankwa, A.; Awuku-Larbi, Y.; Kumordjie, S.; Agbodzi, B.; Salisu, M.; Mante, A.A.O.; et al. Molecular detection of dengue virus from febrile patients in Ghana. BMC Infect. Dis. 2024, 24, 1382. [Google Scholar] [CrossRef]
- Bonney, J.H.K.; Hayashi, T.; Dadzie, S.; Agbosu, E.; Pratt, D.; Nyarko, S.; Asiedu-Bekoe, F.; Ido, E.; Sarkodie, B.; Ohta, N.; et al. Molecular detection of dengue virus in patients suspected of Ebola virus disease in Ghana. PLoS ONE 2018, 13, e0208907. [Google Scholar] [CrossRef]
- Dieng, I.; Diagne, M.M.; Ndione, M.H.D.; Hedible, B.G.; Diop, M.; Adjoguoua, E.V.; Sylla, Y.; Kadjo, H.; Loucoubar, C.; Sall, A.A.; et al. Dengue virus serotype 2 imported case from Cote d’Ivoire to Senegal, 2017. Transbound. Emerg. Dis. 2022, 69, 3035–3040. [Google Scholar] [CrossRef]
- L’Azou, M.; Succo, T.; Kamagate, M.; Ouattara, A.; Gilbernair, E.; Adjogoua, E.; Luxemburger, C. Dengue: Etiology of acute febrile illness in Abidjan, Cote d’Ivoire, in 2011–2012. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 717–722. [Google Scholar] [CrossRef]
- Ouattara, C.A.; Traore, S.; Sangare, I.; Traore, T.I.; Meda, Z.C.; Savadogo, L.G.B. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health 2022, 22, 462. [Google Scholar] [CrossRef]
- de Araujo Lobo, J.M.; Mores, C.N.; Bausch, D.G.; Christofferson, R.C. Short Report: Serological Evidence of Under-Reported Dengue Circulation in Sierra Leone. PLoS Negl. Trop. Dis. 2016, 10, e0004613. [Google Scholar] [CrossRef]
- Manu, S.K.; Bonney, J.H.K.; Pratt, D.; Abdulai, F.N.; Agbosu, E.E.; Frimpong, P.O.; Adiku, T.K. Arbovirus circulation among febrile patients at the greater Accra Regional Hospital, Ghana. BMC Res. Notes 2019, 12, 332. [Google Scholar] [CrossRef]
- de Brito Arduino, M.; Mucci, L.F.; Serpa, L.L.; Rodrigues Mde, M. Effect of salinity on the behavior of Aedes aegypti populations from the coast and plateau of southeastern Brazil. J. Vector Borne Dis. 2015, 52, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, J.; Kien, D.T.; Nguyen, N.M.; Nguyen, H.L.; Kyrylos, P.P.; Carrington, L.B.; Tran, C.N.; Quyen, N.T.; Thi, L.V.; Le Thi, D.; et al. Comparative Susceptibility of Aedes albopictus and Aedes aegypti to Dengue Virus Infection After Feeding on Blood of Viremic Humans: Implications for Public Health. J. Infect. Dis. 2015, 212, 1182–1190. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Vloet, R.P.M.; Kant, J.; van Keulen, L.; Gonzales, J.L.; Visser, T.M.; Koenraadt, C.J.M.; Vogels, C.B.F.; Kortekaas, J. Reproducing the Rift Valley fever virus mosquito-lamb-mosquito transmission cycle. Sci. Rep. 2021, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J.; Linthicum, K.J.; Patrican, L.A.; Davies, F.G.; Kairo, A.; Bailey, C.L. Vector competence of selected African mosquito (Diptera: Culicidae) species for Rift Valley fever virus. J. Med. Entomol. 2008, 45, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Vasquez, C.; Carvajal, A.; Moreno, M.; Fuller, D.O.; Cardenas, G.; Petrie, W.D.; Beier, J.C. Urbanization favors the proliferation of Aedes aegypti and Culex quinquefasciatus in urban areas of Miami-Dade County, Florida. Sci. Rep. 2021, 11, 22989. [Google Scholar] [CrossRef]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef]
- Kamgang, B.; Wilson-Bahun, T.A.; Irving, H.; Kusimo, M.O.; Lenga, A.; Wondji, C.S. Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res. 2018, 3, 79. [Google Scholar] [CrossRef]
- Guindo-Coulibaly, N.; Adja, A.M.; Coulibaly, J.T.; Kpan, M.D.S.; Adou, K.A.; Zoh, D.D. Expansion of Aedes africanus (Diptera: Culicidae), a sylvatic vector of arboviruses, into an urban environment of Abidjan, Cote d’Ivoire. J. Vector Ecol. 2019, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T. West Nile virus and its vectors. Curr. Opin. Insect Sci. 2017, 22, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Haun, B.K.; Kamara, V.; Dweh, A.S.; Garalde-Machida, K.; Forkay, S.S.E.; Takaaze, M.; Namekar, M.; Wong, T.A.S.; Bell-Gam Woto, A.E.R.; Humphreys, P.; et al. Serological evidence of Ebola virus exposure in dogs from affected communities in Liberia: A preliminary report. PLoS Negl. Trop. Dis. 2019, 13, e0007614. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, A.; Kamara, V.M.; Tekah, D.M.; Jalloh, M.A.; Kamara, S.B.; Wong, T.A.S.; Ball, A.H.; Mayerlen, L.I.; Ishikawa, K.M.; Ahn, H.J.; et al. Baseline Seroprevalence of Arboviruses in Liberia Using a Multiplex IgG Immunoassay. Trop. Med. Infect. Dis. 2025, 10, 92. https://doi.org/10.3390/tropicalmed10040092
To A, Kamara VM, Tekah DM, Jalloh MA, Kamara SB, Wong TAS, Ball AH, Mayerlen LI, Ishikawa KM, Ahn HJ, et al. Baseline Seroprevalence of Arboviruses in Liberia Using a Multiplex IgG Immunoassay. Tropical Medicine and Infectious Disease. 2025; 10(4):92. https://doi.org/10.3390/tropicalmed10040092
Chicago/Turabian StyleTo, Albert, Varney M. Kamara, Davidetta M. Tekah, Mohammed A. Jalloh, Salematu B. Kamara, Teri Ann S. Wong, Aquena H. Ball, Ludwig I. Mayerlen, Kyle M. Ishikawa, Hyeong Jun Ahn, and et al. 2025. "Baseline Seroprevalence of Arboviruses in Liberia Using a Multiplex IgG Immunoassay" Tropical Medicine and Infectious Disease 10, no. 4: 92. https://doi.org/10.3390/tropicalmed10040092
APA StyleTo, A., Kamara, V. M., Tekah, D. M., Jalloh, M. A., Kamara, S. B., Wong, T. A. S., Ball, A. H., Mayerlen, L. I., Ishikawa, K. M., Ahn, H. J., Shobayo, B., Teahton, J., Haun, B. K., Wang, W.-K., Berestecky, J. M., Nerurkar, V. R., Humphrey, P. S., & Lehrer, A. T. (2025). Baseline Seroprevalence of Arboviruses in Liberia Using a Multiplex IgG Immunoassay. Tropical Medicine and Infectious Disease, 10(4), 92. https://doi.org/10.3390/tropicalmed10040092









