Modelling Adaptation through Social Allostasis: Modulating the Effects of Social Touch with Oxytocin in Embodied Agents
Abstract
:1. Introduction
1.1. Background
1.2. Related Work
1.2.1. Social Adaptation in Embodied Agents
1.2.2. Social Adaptation with Allostasis
1.2.3. Social Behaviours and Oxytocin
1.2.4. Our Previous Work: Oxytocin and the Social Salience Hypotheses
1.2.5. Affective Touch
2. Materials and Methods
2.1. Aim of Study
2.2. Hypothesis
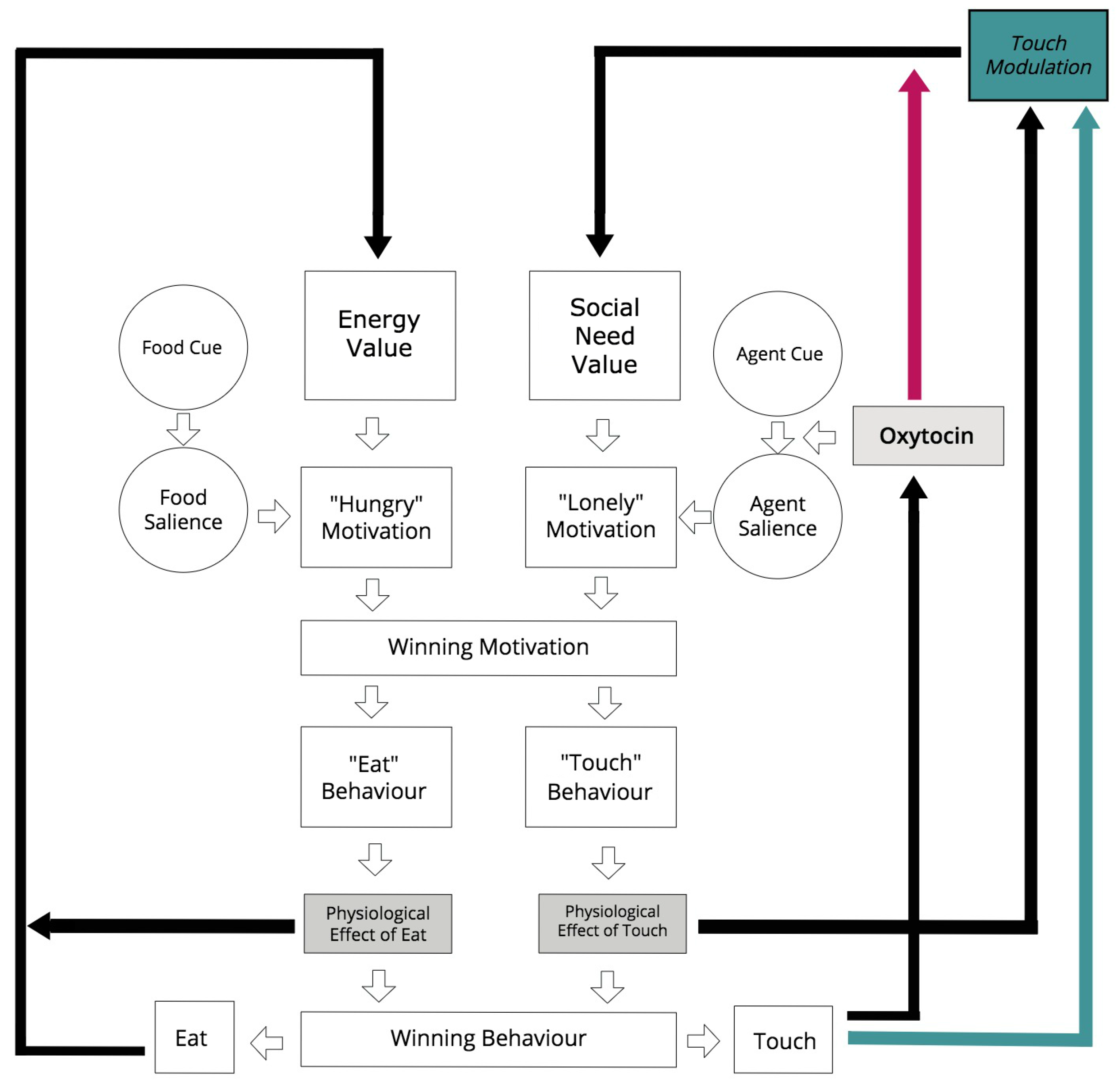
2.3. Agent Model
2.3.1. Action Selection Architecture
- Eat, to satisfy their deficiency,
- Touch, to satisfy their .
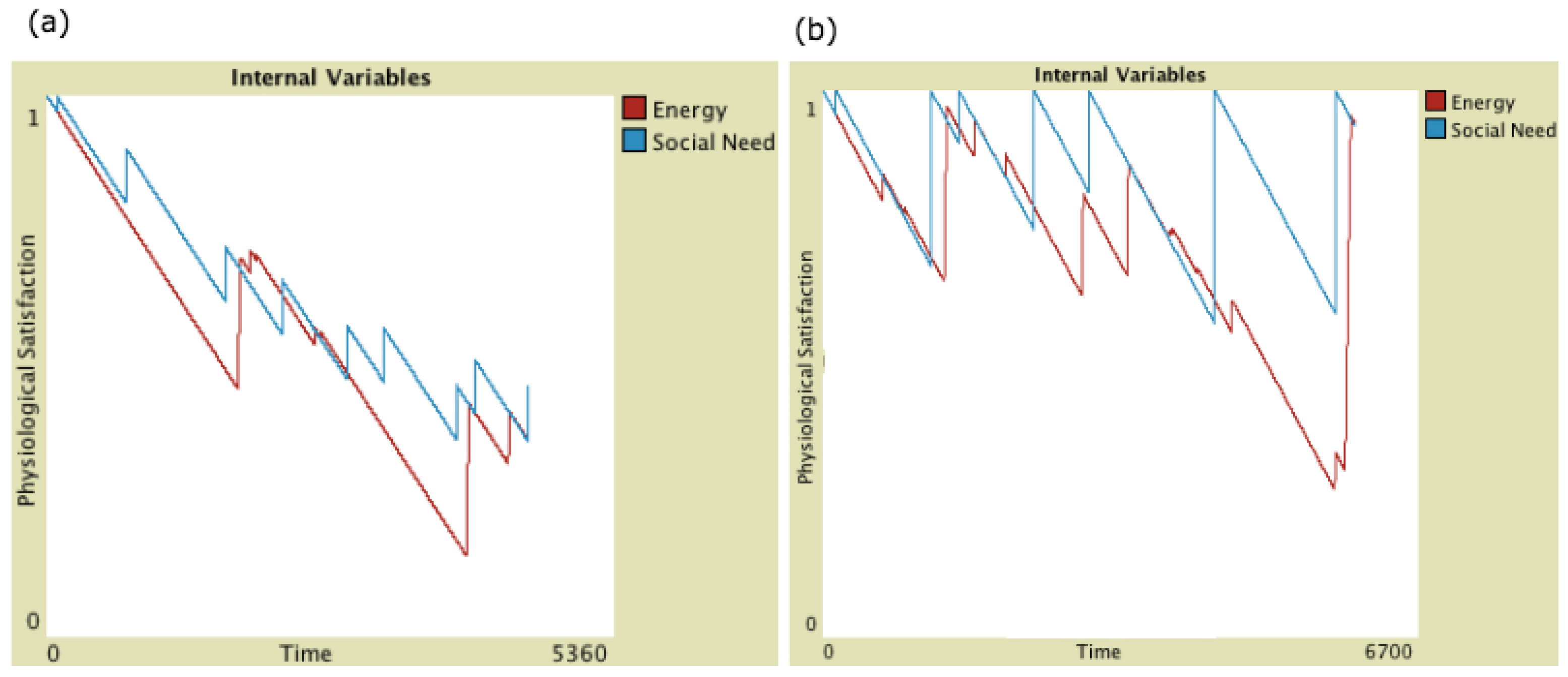
2.3.2. Physiological Effects of Behaviour
2.3.3. Roles of Oxytocin
- On the one hand, it is applied to the function that calculates the social salience in our action selection architecture, and
- On the other hand, it modulates the rate of physiological satisfaction of social behaviours (social touch).
- None, where oxytocin does not affect social salience.
- Increase Social Salience: Salience of social cues is increased, using:
- Decrease Social Salience: Salience of social cues is decreased, using:
- Direct Modulation: where the intensity of physiological effect is directly correlated with the level of OT. This is calculated as:
- Inverse Modulation: where the intensity of physiological effect is inversely correlated with the level of OT This is calculated as:
2.4. Experimental Setup and Conditions
- Easy conditions are defined as a world with an abundance of food resources (18) distributed in small clusters throughout the world.
- Challenging conditions are defined as a world with scarce food resources (6) in two clusters, placed towards the corners of the world.
- Super Challenging conditions are exactly the same as Challenging conditions, but the food resources have half of the nutritional value.
2.5. Metrics Definition
- (i)
- Life Length: The simulation time steps that an agent survived, i.e., remained viable by keeping its , given as a percentage of the maximum simulation length (30,000 time steps).
- (ii)
- Average Comfort: The average level of satisfaction of internal variables throughout an agent’s lifetime.
2.6. Statistical Significance Testing
3. Experiments and Results
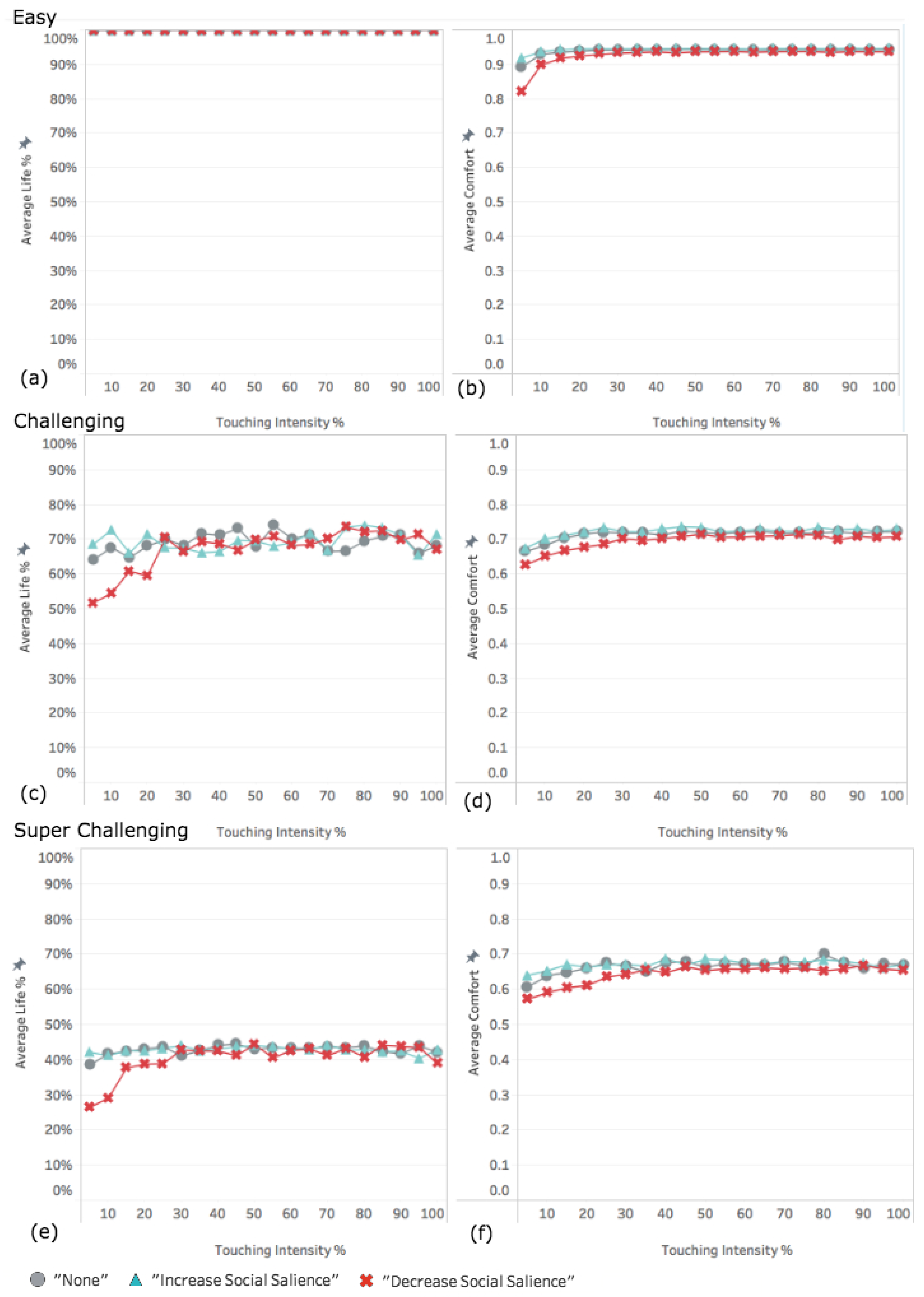
3.1. Experiment 1: Varying the Intensity of the Effect of Touch
3.1.1. Easy World Conditions
3.1.2. Challenging World Conditions
3.1.3. Super Challenging World Conditions
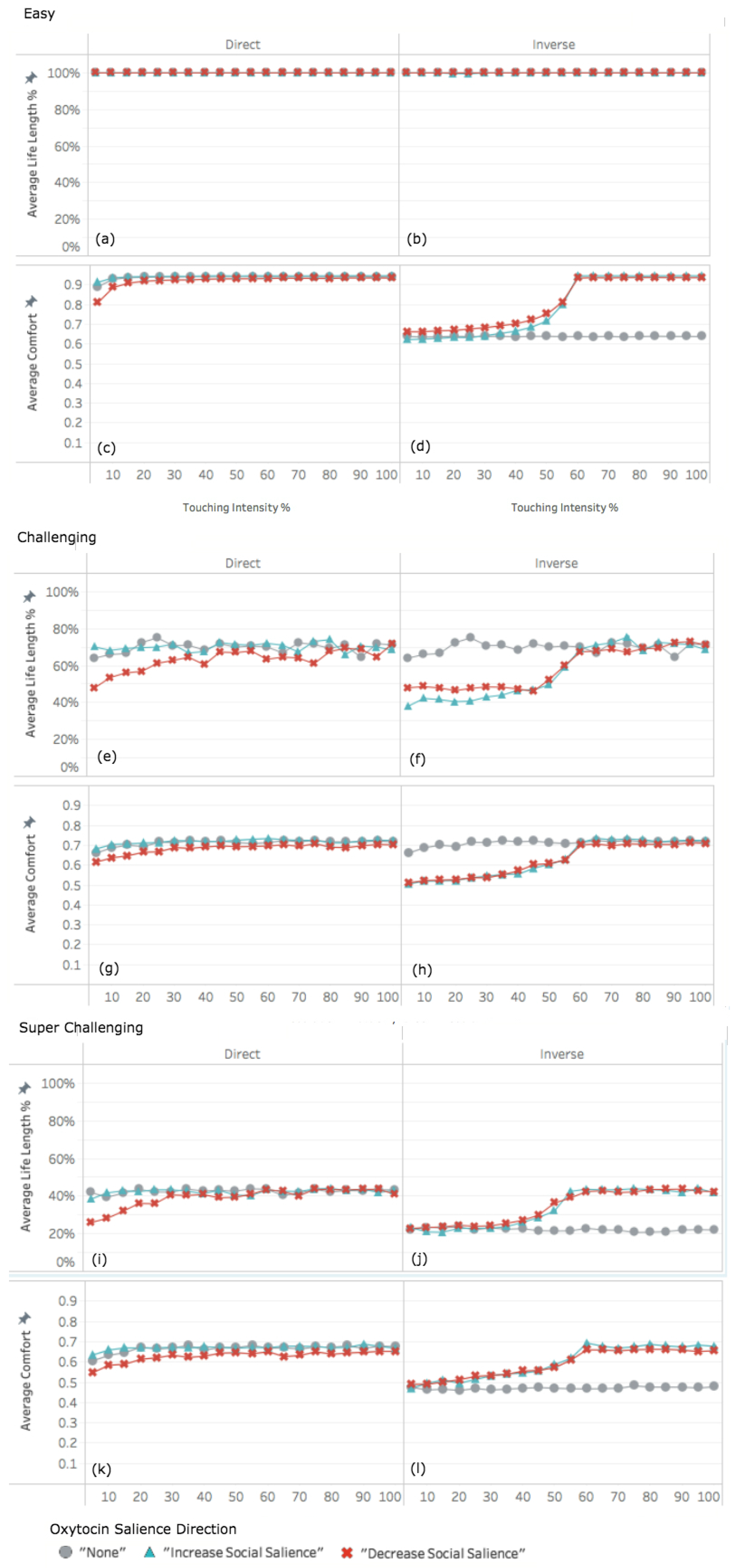
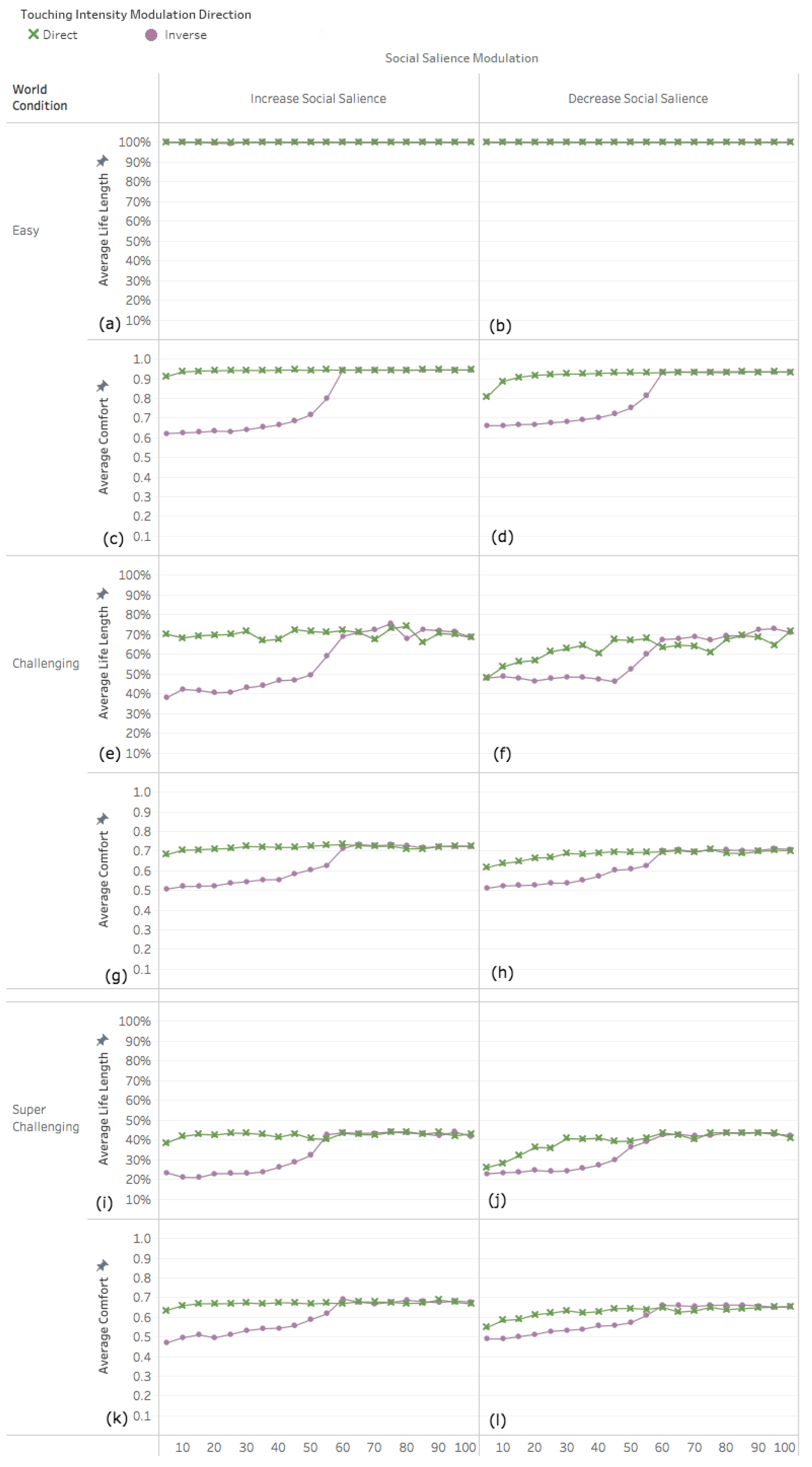
3.2. Experiment 2: Adapting Touch Intensity with Oxytocin
3.2.1. Easy World Conditions
3.2.2. Challenging World Conditions
3.2.3. Super Challenging World Conditions
4. Discussion
4.1. Decreased Social Salience Is Detrimental When Touch Intensity Is Low
4.2. Interplay among Parameters
4.3. Infrequent Social Contact Intensifies the Effects of Tactile Contact
4.4. Modelling Approach
5. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | Action Selection Architecture |
| OT | Oxytocin |
Appendix A. Mathematical Notation of the Action Selection Architecture
- (i)
- Calculate the drive/error for each internal variable. Errors are calculated as the difference between the current values of the variables from their ideal value:where is the error value, is the ideal value for that internal variable, and is the current value of that internal variable.
- (ii)
- Calculate the cues, i.e., the value of each external stimulus. The value of food cues is calculated as:where is the total nutritional amount of food resources seen in an agent’s vision.The value of agent cues is calculated as:where is the total count of agents in an agent’s vision.
- (iii)
- Calculate the salience of each stimulus. The value of food salience is the same as food cues, therefore:The value of social salience is calculated as:where is the effect of oxytocin defined in the next section.
- (iv)
- Calculate the intensity of each motivation, Hungry and Lonely, as follows:where is the error of internal variable v and is the stimuli linked to that motivation. The motivation with the highest intensity is selected as the winning motivation:
- (v)
- Calculate the intensity of both behaviours by multiplying the winning motivation by the physiological effect of each of the behaviours:where is the winning motivation and is the physiological effect that has on behaviour
- (vi)
- The behaviour with the highest intensity is the winning behaviour, and the one that is executed:
Appendix B. Mathematical Definitions of Our Metrics
- (i)
- Life Length: The simulation time steps that an agent survived—remained viable by keeping its —as a percentage of the maximum simulation length (30,000 time steps). Calculated using:where is the total time steps that the agent remained viable (alive) and is the maximum length of the simulation (30,000 time steps).
- (ii)
- Average Comfort: The average level of satisfaction of internal variables throughout an agent’s lifetime. At each time step, comfort levels are calculated bywhere is the sum of errors of the agent’s two internal variables at time t, and as the sum of the maximum errors of all the internal variables. The average comfort level per experimental run is then given by:
References
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Khan, I.; Lewis, M.; Cañamero, L. Adaptation and the Social Salience Hypothesis of Oxytocin: Early Experiments in a Simulated Agent Environment. In Proceedings of the 2nd Symposium on Social Interactions in Complex Intelligent Systems (SICIS), Liverpool, UK, 4–6 April 2018. [Google Scholar]
- Schulkin, J. Social allostasis: Anticipatory regulation of the internal milieu. Front. Evol. Neurosci. 2011, 2, 111. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. Principles of allostasis: Optimal design, predictive regulation, pathophysiology, and rational. In Allostasis, Homeostasis, and the Costs of Physiological Adaptation; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Zagoory-Sharon, O.; Leckman, J.F.; Feldman, R. Oxytocin and the development of parenting in humans. Biol. Psychiatry 2010, 68, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bethlehem, R.A.; Baron-Cohen, S.; van Honk, J.; Auyeung, B.; Bos, P.A. The oxytocin paradox. Front. Behav. Neurosci. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- De Dreu, C.K.; Kret, M.E. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiatry 2016, 79, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psychiatry 2016, 79, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ebitz, R.B.; Platt, M.M. An evolutionary perspective on the behavioral consequences of exogenous oxytocin application. Front. Behav. Neurosci. 2014, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, D.M.; Wessberg, J.; Chelnokova, O.; Olausson, H.; Laeng, B.; Leknes, S. In touch with your emotions: Oxytocin and touch change social impressions while others’ facial expressions can alter touch. Psychoneuroendocrinology 2014, 39, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.G.; Knapp, D.E.; Huemmrich, K.F. Physically based classification and satellite mapping of biophysical characteristics in the southern boreal forest. J. Geophys. Res. 1997, 102, 29567–29580. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.E.; Yarbrough, A.E. A naturalistic study of the meanings of touch. Commun. Monogr. 1985, 52, 19–56. [Google Scholar] [CrossRef]
- Onaka, T.; Ikeda, K.; Yamashita, T.; Honda, K. Facilitative role of endogenous oxytocin in noradrenaline release in the rat supraoptic nucleus. Eur. J. Neurosci. 2003, 18, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.S.; Meintjes, R.A. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 2003, 165, 296–301. [Google Scholar] [CrossRef]
- Declerck, C.H.; Boone, C.; Kiyonari, T. The effect of oxytocin on cooperation in a prisoner’s dilemma depends on the social context and a person’s social value orientation. Soc. Cogn. Affect. Neurosci. 2013, 9, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.R.; Insel, T.R.; Harbaugh, C.R.; Carter, C.S. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J. Neuroendocrinol. 1994, 6, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Barraza, J.A.; Zak, P.J. Empathy toward Strangers Triggers Oxytocin Release and Subsequent Generosity. Ann. N. Y. Acad. Sci. 2009, 1167, 182–189. [Google Scholar] [CrossRef] [PubMed]
- De Dreu, C.K.; Greer, L.L.; Handgraaf, M.J.; Shalvi, S.; Van Kleef, G.A.; Baas, M.; Ten Velden, F.S.; Van Dijk, E.; Feith, S.W. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 2010, 328, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Algoe, S.B.; Kurtz, L.E.; Grewen, K. Oxytocin and social bonds: The role of oxytocin in perceptions of romantic partners’ bonding behavior. Psychol. Sci. 2017, 28, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Fischer, M.; Dvash, J.; Harari, H.; Perach-Bloom, N.; Levkovitz, Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol. Psychiatry 2009, 66, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Fiske, S.T. What we know now about bias and intergroup conflict, the problem of the century. Curr. Dir. Psychol. Sci. 2002, 11, 123–128. [Google Scholar] [CrossRef]
- Cho, M.M.; DeVries, A.C.; Williams, J.R.; Carter, C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 1999, 113, 1071. [Google Scholar] [CrossRef] [PubMed]
- Declerck, C.H.; Boone, C.; Kiyonari, T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm. Behav. 2010, 57, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 1998, 23, 779–818. [Google Scholar] [CrossRef]
- Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 2012, 37, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Dreu, C.K. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm. Behav. 2012, 61, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ashby, W.R. Design for a Brain; Chapman and Hall: London, UK, 1952. [Google Scholar]
- Hertenstein, M.J.; Verkamp, J.M.; Kerestes, A.M.; Holmes, R.M. The communicative functions of touch in humans, nonhuman primates, and rats: A review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006, 132, 5–94. [Google Scholar] [CrossRef] [PubMed]
- Gallace, A.; Spence, C. The science of interpersonal touch: An overview. Neurosci. Biobehav. Rev. 2010, 34, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Yohanan, S.; MacLean, K.E. The role of affective touch in human-robot interaction: Human intent and expectations in touching the haptic creature. Int. J. Soc. Robot. 2012, 4, 163–180. [Google Scholar] [CrossRef]
- Morrison, I. Keep calm and cuddle on: Social touch as a stress buffer. Adapt. Hum. Behav. Physiol. 2016, 2, 344–362. [Google Scholar] [CrossRef]
- Shiota, M.N.; Campos, B.; Oveis, C.; Hertenstein, M.J.; Simon-Thomas, E.; Keltner, D. Beyond happiness: Building a science of discrete positive emotions. Am. Psychol. 2017, 72, 617. [Google Scholar] [CrossRef] [PubMed]
- Stiehl, W.D.; Breazeal, C.; Han, K.H.; Lieberman, J.; Lalla, L.; Maymin, A.; Salinas, J.; Fuentes, D.; Toscano, R.; Tong, C.H.; et al. The huggable: A therapeutic robotic companion for relational, affective touch. In Proceedings of the ACM SIGGRAPH 2006 Emerging Technologies, Boston, MA, USA, 30 July–3 August 2006; p. 15. [Google Scholar]
- Saldien, J.; Goris, K.; Vanderborght, B.; Vanderfaeillie, J.; Lefeber, D. Expressing emotions with the social robot probo. Int. J. Soc. Robot. 2010, 2, 377–389. [Google Scholar] [CrossRef]
- McGlone, F.; Vallbo, A.B.; Olausson, H.; Loken, L.; Wessberg, J. Discriminative touch and emotional touch. Can. J. Exp. Psychol. 2007, 61, 173. [Google Scholar] [CrossRef] [PubMed]
- Terris, E.T.; Beavin, L.E.; Barraza, J.A.; Schloss, J.; Zak, P.J. Endogenous oxytocin release eliminates in-group bias in monetary transfers with perspective-taking. Front. Behav. Neurosci. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.C.J.; Anumandla, S.R.; Thibeault, C.M.; Hoang, R.V.; Goodman, P.H.; Dascalu, S.M.; Bryant, B.D.; Harris, F.C., Jr. Real-time human–robot interaction underlying neurorobotic trust and intent recognition. Neural Netw. 2012, 32, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Cañamero, D. Modeling Motivations and Emotions as a Basis for Intelligent Behavior. In Proceedings of the First International Symposium on Autonomous Agents (Agents’97), Marina del Rey, CA, USA, 5–8 February 1997; Johnson, W.L., Ed.; The ACM Press: Marina del Rey, CA, USA, 1997; pp. 148–155. [Google Scholar]
- Avila-Garcia, O.; Cañamero, L. Using hormonal feedback to modulate action selection in a competitive scenario. Anim. Animats 2004, 8, 243–252. [Google Scholar]
- Lewis, M.; Canamero, L. Hedonic quality or reward? A study of basic pleasure in homeostasis and decision making of a motivated autonomous robot. Adapt. Behav. 2016, 24, 267–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilensky, U. NetLogo 5.3.1; CCL: Miami, FL, USA, 2000. [Google Scholar]
- Avila-Garcıa, O.; Canamero, L. A comparison of behavior selection architectures using viability indicators. In Proceedings of the International Workshop on Biologically-Inspired Robotics: The Legacy of W. Grey Walter, Edinburgh, UK, 14–16 August 2002; pp. 14–16. [Google Scholar]
- Cos, I.; Cañamero, L.; Hayes, G.M.; Gillies, A. Hedonic value: Enhancing adaptation for motivated agents. Adapt. Behav. 2013, 21, 465–483. [Google Scholar] [CrossRef] [Green Version]
| Behaviour | Stimuli | Energy | Social | Oxytocin OT | Time To Execute |
|---|---|---|---|---|---|
| Eat | Food | +0.01 | 0 | 0 | Variable |
| Touch | Agent | 0 | 0.01 | +1 | Immediate |
| Internal Variable | Decay Rate | Motivation | Behaviour | Stimuli | Physiological Effect |
|---|---|---|---|---|---|
| Energy | −0.0003 | Hungry | Eat | Food | + Energy |
| Social | −0.0003 | Lonely | Touch | Agent | + Social, + Oxytocin |
| Oxytocin* | −0.0005 | - | - | - | - |
| World Name | # of Food Resources (Items) | # of Clusters | Nutritional Value/Item | Max. Nutrition In World |
|---|---|---|---|---|
| Easy | 20 | 10 | 2 | 40 |
| Challenging | 6 | 2 | 2 | 12 |
| Super Challenging | 6 | 2 | 1 | 6 |
| World Name | No OT Modulation | OT Increases Social Salience | OT Decreases Social Salience |
|---|---|---|---|
| Easy | E0 | E+ | E− |
| Challenging | C0 | C+ | C− |
| Super Challenging | S0 | S+ | S− |
| Social Salience Direction/touchingIntensity Direction | Easy | Challenging | Super Challenging |
|---|---|---|---|
| None/Direct | E0D | C0D | S0D |
| None/Indirect | E0I | C0I | S0I |
| Increase/Direct | E+D | C+D | S+D |
| Increase/Inverse | E+I | C+I | S+I |
| Decrease/Direct | E−D | C−D | S−D |
| Decrease/Inverse | E−I | C−I | S−I |
| Experiment Condition | Touch Intensity % | World Condition | Social Salience Direction | Viability Indicator | Result | p-Value vs. Control | p-Value vs. + Condition | p-Value vs. − Condition |
|---|---|---|---|---|---|---|---|---|
| E0 (Control) | All | Easy | None | Life Length | 100% | - | n/a | n/a |
| E+ | All | Easy | Increase | Life Length | 100% | n/a | - | n/a |
| E− | All | Easy | Decrease | Life Length | 100% | n/a | n/a | - |
| C0 (Control) | ⩽20% | Challenging | None | Life Length | 66% | - | 0.006528 * | 0.00001 * |
| C+ | ⩽20% | Challenging | Increase | Life Length | 70% | 0.006528 * | - | 0.00001 * |
| C− | ⩽20% | Challenging | Decrease | Life Length | 57% | 0.00001 * | 0.00001 * | - |
| C0 (Control) | >20% | Challenging | None | Life Length | 69% | - | 0.692435 | 0.740763 |
| C+ | >20% | Challenging | Increase | Life Length | 67% | 0.692435 | - | 0.73957 |
| C− | >20% | Challenging | Decrease | Life Length | 70% | 0.740763 | 0.73957 | - |
| SC0 (Control) | ⩽20% | Super Challenging | None | Life Length | 42% | - | 0.098236 | 0.00001 * |
| SC+ | ⩽20% | Super Challenging | Increase | Life Length | 42% | 0.098236 | - | 0.00001 * |
| SC− | ⩽20% | Super Challenging | Decrease | Life Length | 33% | 0.00001 * | 0.00001 * | - |
| SC0 (Control) | >20% | Super Challenging | None | Life Length | 43% | - | 0.801502 | 0.012161 * |
| SC+ | >20% | Super Challenging | Increase | Life Length | 43% | 0.801502 | - | 0.021922 * |
| SC− | >20% | Super Challenging | Decrease | Life Length | 42% | 0.012161 * | 0.021922 * | - |
| E0 (Control) | All | Easy | None | Comfort | 0.93 | - | 0.00001 * | 0.00001 * |
| E+ | All | Easy | Increase | Comfort | 0.94 | 0.00001 * | - | 0.00001 * |
| E− | All | Easy | Decrease | Comfort | 0.8 | 0.00001 * | 0.00001 * | - |
| C0 (Control) | ⩽20% | Challenging | None | Comfort | 0.67 | - | 0.000977 * | 0.00001 * |
| C+ | ⩽20% | Challenging | Increase | Comfort | 0.7 | 0.000977 * | - | 0.00001 * |
| C− | ⩽20% | Challenging | Decrease | Comfort | 0.65 | 0.00001 * | 0.00001 * | - |
| C0 (Control) | >20% | Challenging | None | Comfort | 0.72 | - | 0.00001 * | 0.00001 * |
| C+ | >20% | Challenging | Increase | Comfort | 0.73 | 0.00001 * | - | 0.00001 * |
| C− | >20% | Challenging | Decrease | Comfort | 0.71 | 0.00001 * | 0.00001 * | - |
| SC0 (Control) | ⩽20% | Super Challenging | None | Comfort | 0.64 | - | 0.007487 * | 0.00001 * |
| SC+ | ⩽20% | Super Challenging | Increase | Comfort | 0.66 | 0.007487 * | - | .00001. |
| SC− | ⩽20% | Super Challenging | Decrease | Comfort | 0.59 | 0.00001 * | 0.00001 * | - |
| SC0 (Control) | >20% | Super Challenging | None | Comfort | 0.67 | - | 0.118938 | 0.00001 * |
| SC+ | >20% | Super Challenging | Increase | Comfort | 0.67 | 0.118938 | - | 0.00001 * |
| SC− | >20% | Super Challenging | Decrease | Comfort | 0.65 | 0.00001 * | 0.00001 * | - |
| Experiment Condition | Oxytocin Touch Modulation Direction | World Condition | Social Salience Direction | Viability Indicator | Result | p-Value vs. Control | p-Value vs. + Condition | p-Value vs. − Condition |
|---|---|---|---|---|---|---|---|---|
| E0D (Control) | Direct | Easy | None | Life Length | 100% | - | n/a | n/a |
| E+D | Direct | Easy | Increase | Life Length | 100% | n/a | - | n/a |
| E−D | Direct | Easy | Decrease | Life Length | 100% | n/a | n/a | - |
| C0D (Control) | Direct | Challenging | None | Life Length | 70% | - | 0.648132 | 0.00001 * |
| C+D | Direct | Challenging | Increase | Life Length | 70% | 0.648132 | - | 0.00001 * |
| C−D | Direct | Challenging | Decrease | Life Length | 63% | 0.00001 * | 0.00001 * | - |
| S0D (Control) | Direct | Super Challenging | None | Life Length | 43% | - | 0.701904 | 0.00001 * |
| S+D | Direct | Super Challenging | Increase | Life Length | 42% | 0.701904 | - | 0.00001 * |
| S−D | Direct | Super Challenging | Decrease | Life Length | 39% | 0.00001 * | 0.00001 * | - |
| E0D (Control) | Direct | Easy | None | Comfort | 0.937 | - | 0.00001 * | 0.00001 * |
| E+D | Direct | Easy | Increase | Comfort | 0.941 | 0.00001 * | - | 0.00001 * |
| E−D | Direct | Easy | Decrease | Comfort | 0.92 | 0.00001 * | 0.00001 * | - |
| C0D (Control) | Direct | Challenging | None | Comfort | 0.71 | - | 0.001468 * | 0.00001 * |
| C+D | Direct | Challenging | Increase | Comfort | 0.72 | 0.001468 * | - | 0.00001 * |
| C−D | Direct | Challenging | Decrease | Comfort | 0.68 | 0.00001 * | 0.00001 * | - |
| S0D (Control) | Direct | Super Challenging | None | Comfort | 0.665 | - | 0.028883 * | 0.00001 * |
| S+D | Direct | Super Challenging | Increase | Comfort | 0.671 | 0.028883 * | - | 0.00001 * |
| S−D | Direct | Super Challenging | Decrease | Comfort | 0.628 | 0.00001 * | 0.00001 * | - |
| E0I (Control) | Inverse | Easy | None | Life Length | 100% | - | n/a | n/a |
| E+I | Inverse | Easy | Increase | Life Length | 100% | n/a | - | n/a |
| E−I | Inverse | Easy | Decrease | Life Length | 100% | n/a | n/a | - |
| C0I (Control) | Inverse | Challenging | None | Life Length | 70% | - | 0.00001 * | 0.00001 * |
| C+I | Inverse | Challenging | Increase | Life Length | 57% | 0.00001 * | - | 0.197257 |
| C−I | Inverse | Challenging | Decrease | Life Length | 58% | 0.00001 * | 0.197257 | - |
| S0I (Control) | Inverse | Super Challenging | None | Life Length | 22% | - | 0.00001 * | 0.00001 * |
| S+I | Inverse | Super Challenging | Increase | Life Length | 34% | 0.00001 * | - | 0.598223 |
| S−I | Inverse | Super Challenging | Decrease | Life Length | 34% | 0.00001 * | 0.598223 | - |
| E0I (Control) | Inverse | Easy | None | Comfort | 0.64 | - | 0.00001 * | 0.00001 * |
| E+I | Inverse | Easy | Increase | Comfort | 0.79 | 0.00001 * | - | 0.249459 |
| E−I | Inverse | Easy | Decrease | Comfort | 0.8 | 0.00001 * | 0.249459 | - |
| C0I (Control) | Inverse | Challenging | None | Comfort | 0.71 | - | 0.00001 * | 0.00001 * |
| C+I | Inverse | Challenging | Increase | Comfort | 0.63 | 0.00001 * | - | 0.411145 |
| C−I | Inverse | Challenging | Decrease | Comfort | 0.62 | 0.00001 * | 0.411145 | - |
| S0I (Control) | Inverse | Super Challenging | None | Comfort | 0.47 | - | 0.00001 * | 0.00001 * |
| S+I | Inverse | Super Challenging | Increase | Comfort | 0.6 | 0.00001 * | - | 0.259483 |
| S−I | Inverse | Super Challenging | Decrease | Comfort | 0.59 | 0.00001 * | 0.259483 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Cañamero, L. Modelling Adaptation through Social Allostasis: Modulating the Effects of Social Touch with Oxytocin in Embodied Agents. Multimodal Technol. Interact. 2018, 2, 67. https://doi.org/10.3390/mti2040067
Khan I, Cañamero L. Modelling Adaptation through Social Allostasis: Modulating the Effects of Social Touch with Oxytocin in Embodied Agents. Multimodal Technologies and Interaction. 2018; 2(4):67. https://doi.org/10.3390/mti2040067
Chicago/Turabian StyleKhan, Imran, and Lola Cañamero. 2018. "Modelling Adaptation through Social Allostasis: Modulating the Effects of Social Touch with Oxytocin in Embodied Agents" Multimodal Technologies and Interaction 2, no. 4: 67. https://doi.org/10.3390/mti2040067
APA StyleKhan, I., & Cañamero, L. (2018). Modelling Adaptation through Social Allostasis: Modulating the Effects of Social Touch with Oxytocin in Embodied Agents. Multimodal Technologies and Interaction, 2(4), 67. https://doi.org/10.3390/mti2040067





