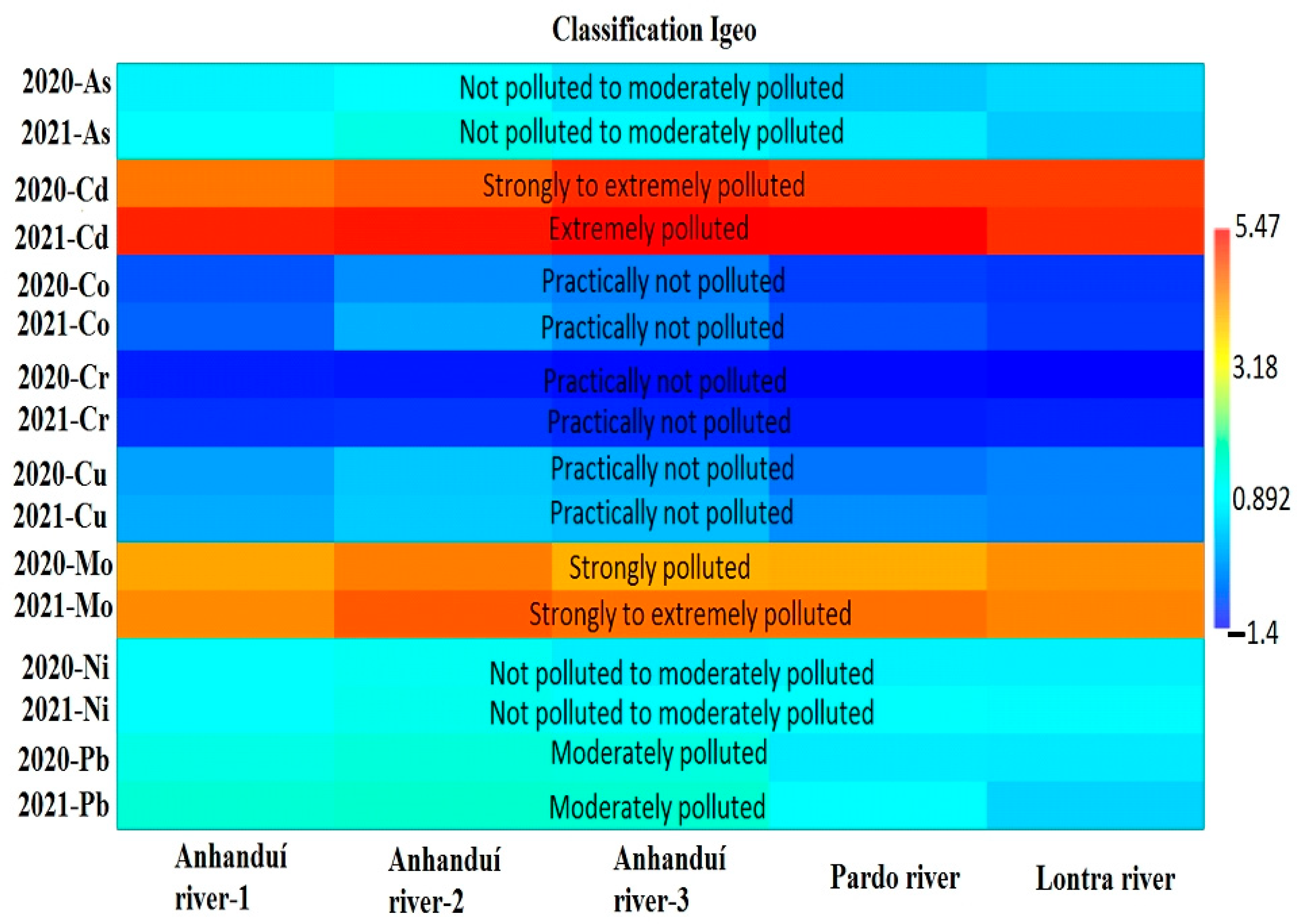
3.1. Metal(loid) Concentration in the River Waters
The values of metal(loid) concentrations in river water samples from different locations are shown in
Table 3. According to the results of the Kruskal–Wallis test, there are significant differences between the median values of concentrations of elements in the respective locations (
p = 1.5 × 10
−7). However, to understand the association of water samples from the five different sampling sites, depending on metal(loid) content, principal component analysis was applied. According to PCA results, the first two principal components explain 95.68% of the total variance of the results, that is, with PC1 contributing 75.47%, and the factor loads of Cu, Cr, Ni, and Pb are all higher than 0.2 (
Figure 2), and with PC2 contributing 20.21% (
Figure 2), and the factor loads of Cu, Cr, Ni, and Pb are all higher than 0.15. Finally, according to
Figure 2, the factor loads of As, Co, Cd, Hg, and Mo for PC1 and PC2 are less than 0.1. In addition, the PCA showed a spatial distribution pattern with a greater tendency for elements in Anhanduí River-1 and Anhanduí River-2 (
Figure 2). There is a greater trend in the concentrations of metal(loid)s such as Cu (2020–2021), Ni (2021), Co (2020–2021), As (2020), Hg (2020), and Al (2020–2021) in Anhanduí River-1. On the other hand, a greater trend was found for elements such as Cr (2021–2021) and Pb (2020–2021) in Anhanduí River-2. According to PCA, the concentrations values of As, Co, Cd, Cr, Cu, Hg, Mo, Ni, and Pb in water contributed to a lesser extent to Anhanduí River-3, Pardo River, and Lontra River.
The Al concentrations (
Table 3) are below the value established by Conama for freshwater (0.1 mg/kg). The concentrations of As, Cd, Cr, and Hg in the waters of the Anhanduí River (sites 1, 2, and 3) are above these permitted limit values for heavy metal ions in drinking water established by the World Health Organization (WHO) (As 0.05 mg/kg; Cd 0.003 mg/kg; Cr 0.05 mg/kg; and Hg 0.001 mg/kg) [
44]. In addition, the concentrations of Cu in the waters of the Anhanduí River-2 are above those established by the WHO (2 mg/kg). The Ni concentrations in rivers are above those established by the WHO for Ni (0.07 mg/kg). The concentrations of Pb in
Table 3 are above those values established by the WHO for Pb (0.05 mg/kg) [
44].
Taking into account that the level of Cd, Co, Cr, Hg, Cu, Ni, and Pb in the river water samples obtained (
Table 3) significantly exceeded the WHO criterion concentration limits, this indicates that the water from these rivers may not be safe for drinking and/or cooking by riverside residents or for use in crop irrigation [
1]. In fact, the ingestion of heavy metals such as Cd, Co, Cr, Hg, Cu, Ni, and Pb in water poses significant potential health risks, with no safe levels established for many of these elements. Heavy metals are toxic even at low concentrations, leading to severe health issues, including cancer and neurological disorders [
4,
9]. Studies indicate that average concentrations of these metals often exceed permissible limits set by organizations like the WHO [
10].
The Anhanduí River rises from the urban area of the city of Campo Grande (MS), being exposed to vehicle traffic and illegal discharge of contaminated substances from residences or gas stations. In addition, this river crosses large cattle ranches and agricultural activities. All these factors can significantly contribute to increasing the concentration of elements such as Al, As, Ni, Pb, Hg, Co, Cu, Cr, and Cd in the water [
1,
2,
4]. In fact, vehicles release oil, fuel residues, brake fluid, and wear-and-tear particles from tire fragments and brake pads, which contain heavy metals such as Pb, Cu, Cr, Al, and Cd. During rainstorms, these contaminants are washed off roads and highways into storm drains, which often discharge directly into rivers without treatment. In addition, the use of agricultural pesticides is associated with the presence of heavy metals such as cadmium (Cd), lead (Pb), copper (Cu), and zinc (Zn), which also contaminate rivers [
4,
6], mainly at location 2 (Anhanduí River-2). Thus, our results presented in
Table 3 corroborate those obtained by Mohammad et al. (2022) [
1], in which the presence of elements such as As, Cr, Cd, and Pb in urban rivers is due to industrialization and the influence of agricultural activity [
4]. However, the non-industrialized areas are also contaminated, suggesting the dispersal of heavy metals along the rivers. Therefore, the contamination of Pardo and Lontra rivers by Al, As, Ni, Pb, Hg, Co, Cu, Cr, and Cd throughout the years is a significant concern in both urban and rural areas, despite the distance from the urban center of the city of Campo Grande. In fact, various anthropogenic and natural factors contribute to this issue, affecting water quality and the ecosystem health of these rivers.
The concentration of metal(loid)s in rivers is significantly influenced by seasonal variations, particularly between dry and rainy seasons. However, although our study was carried out during the dry season in July 2020 and July 2021, there was a small variation in the metal concentration values in the waters of these rivers. In addition, there is a possibility that the concentration of certain heavy metals may be higher during the dry season, while others are higher in the rainy season [
45,
46]. Recent studies have shown that higher water volume can dilute some heavy metal concentrations, leading to variable results depending on the location and intensity of rainfall [
47], while lower water flow reduces dilution capacity, potentially increasing the concentration of heavy metals in the water [
48].
3.2. Metal(loid) Concentration in the Sediments
Concentrations of metal(loid)s quantified in sediments are shown in
Table 4 and are compared with maximum limits established by the Brazilian regulation [
39]. The concentration of metal(loid)s in sediments (non-parametric Kruskal–Wallis test) differed among locations (Anhanduí River-2 and Anhanduí River-3) for Cu (
p = 7.7 × 10
−5). According to PCA, there is a greater trend in metal concentrations in locations Anhanduí River-1, Anhanduí River-2, and Anhanduí River-3 (
Figure 3). In this case, the highest element concentration trend is observed in Anhanduí River-2.
Only the concentration of As in the sediments of the Pardo River in 2020 is below the value established by Conama [
39] (
Table 4). Moreover, with the exception of Lontra River in 2021, the concentrations of Ni and Hg in river sediments were above the values established by Conama. Only the Pardo River, in 2020 and 2021, and the Lontra River, in 2020 and 2021, presented Pb concentrations below the values established by Conama. The Pardo River in 2020 and the Lontra River in the period 2020–2021 both presented Cu concentrations below those established by Conama. Similarly, Cr concentrations in the sediments of rivers were lower than those values established by Conama. In contrast, Mo concentrations in the period studied were above those of Conama.
The values of Al concentrations (
Table 4) were below those found in sediments (125.47 mg/kg) from the Strzegomka River in southwestern Poland [
49], which has highest aluminium concentrations that vary across different rivers in that country. In addition, Mo concentrations were below river sediments near the molybdenite mining region (13.2 mg/kg) in China [
50], which has critical pollution levels. Cobalt concentration in Anhanduí River-2 was higher than the Chinese sediment value (17.38 mg/kg) [
51]. On the other hand, the mean concentration of Cr, Ni, Cu, Cd, and Pb in river sediments (
Table 4) was higher in relation to values of study by Poland for Cr 165.1 mg/kg, Ni 8.21 mg/kg, Cu 10.92 mg/kg, Cd 0.168, and Pb 18.0 mg/kg in river sediments [
52].
The dry and rainy seasons also influence the concentration and distribution of heavy metals in river sediments. Heavy metal concentrations often peak due to reduced water flow and increased sedimentation, which can lead to higher levels of metals like Pb and Cr. According to studies, the concentrations of metals such as Cu were lower in the dry season compared to the rainy season [
53,
54]. However, the concentration values of elements such as Al, As, Ni, Pb, Hg, Co, Cu, Cr, Cd, and Mo in sediment samples collected from rivers during a seasonal dry period vary between the year 2020 and 2021 (
Table 4). Future studies should consider the rainy season to understand the influence of rainfall and other factors on these rivers. These variations are driven by processes like erosion, sediment deposition, resuspension, and organic matter changes, which differ between seasons.
3.6. Concentration of Metal(loid)s Quantified in Prochilodus lineatus and Pimelodus maculatus
For a better understanding of our results, metal(loid) concentration in
Prochilodus lineatus muscle (
Table 7) and
Pimelodus maculatus muscle (
Table 8) were compared to the maximum limit for human consumption established by Anvisa/Brazil and values of permissible limits of metals in fishes recommended by the FAO. In addition, the metal(loid) concentration values obtained in muscle samples from
Prochilodus lineatus (
Table 7) and
Pimelodus maculatus (
Table 8) were compared with those available in the literature. The concentration of Al was the highest in
P. lineatus (
Table 7) and
P. maculatus (
Table 8) in relation to the Cr, Cu, Cd, Hg, Ni, As, Pb, Mo, and Co analyzed in this study. Furthermore, in both fishes there was an increase in the concentration found in the second collection (2021), up to 3.5 mg/kg, with minimum values of 4.05 ± 0.86 and 3.88 ± 0.73 mg/kg in the first collection (2020) and maximum values of 12.763 ± 2.50 and 9.98 ± 1.0 mg/kg in the second collection (2021) of
P. lineatus and
P. maculatus, respectively. The levels of Pb in the
P. lineatus muscle was greater than 0.3 mg/kg in the first collection (2020) in Anhanduí River-2 and Pardo River and in the second collection (2021) in Anhanduí River-1 and Pardo River. The concentration of this element in
P. maculatus was also higher than 0.3 mg/kg in Anhanduí River-1, Anhanduí River-2, and Anhanduí River-3 in both collections (2020–2021).
Our study corroborates with Meche et al. (2010) [
58], who obtained high levels of Al when analyzing 16 fish species in the Piracicaba River, state of São Paulo (SP), Brazil. The average concentrations among all samples ranged from 8.38 to 24.9 mg/kg found in
Geophagus brasiliensis. According to the authors [
58], the soil in the region is rich in Al and this element can be released from the soil by acid rain. Furthermore, the high concentration of Al can be attributed to the burning of sugar cane, which is common in that region. There is no limit established by Anvisa/Brazil and the FAO for Al in fishes. However, Al concentration in fish muscles varies significantly across species and environmental conditions. Studies indicate that aluminium bioaccumulation occurs in fish tissues, with muscle concentrations generally lower than in organs like gills and liver. In commercially important fish from the Caspian Sea in Iran, Al concentrations in muscle of the
Cyprinus carpio ranged from 0.89 to 4.63 μg/g, influenced by seasonal variation [
13]. Therefore, with the exception of the Al concentration in Pardo River in 2020 and 2021 (
Table 7 and
Table 8) and Lontra River in 2020 (
Table 8), the concentrations of Al are above those quantified in the Caspian Sea [
13]. Histological changes in gill tissues, such as alterations in morphology and increased reactive oxygen species (ROS), have been observed using zebrafish as an experimental model [
59].
The average levels of As found in
P. lineatus and
P. maculatus in the five collection sites were lower than that established by Anvisa/Brazil, which provides for the Mercosur Technical Regulation on Maximum Limits of Inorganic Contaminants in Food [
60]. According to the results published by Sheikhzadeh et al. (2021) [
7], As concentrations in the muscles of some fish species from the Persian Gulf are high when compared to other countries [
7]. In fact, the mean concentration of As in the muscle tissue of
Pomadasys spp. (1 mg/kg) from the northern region of the Persian Gulf [
7] was higher than in
P. lineatus (
Table 7) and
P. maculatus (
Table 8) in the five collection sites. Studies on various fish species, including rohu carp and zebrafish, reveal that arsenic leads to deformities, behavioral changes, and biochemical disruptions [
61,
62].
On the other hand, the concentration of Cd with minimum values of 0.66 ± 0.074 (2021) to 0.59 ± 0.14 mg/kg (2020) and maximum values of 1.56 ± 0.31 (2021) to 2.24 ± 0.69 mg/kg (2021) in
P. lineatus and
P. maculatus was higher than the limit established by Anvisa/Brazil (0.05 mg/kg), respectively. Mean concentrations of Cd in the muscle tissue of
P. lineatus (
Table 7) and
P. maculatus (
Table 8) were higher than
Otolithes ruber (0.33 mg/kg) and
Scomberomorus guttatus (0.21 mg/kg) from Khark Island in the Persian Gulf and
Otolithes ruber (0.23 mg/kg) from Bushehr Port in Iran [
7]. The results in
Table 7 and
Table 8, when compared with other studies, reveal that the concentrations of metals such as Cd in samples of
Prochilodus lineatus muscle (
Table 7) and
Pimelodus maculatus muscle (
Table 8) are higher than those obtained in Iran for fish species
Liza aurata (0.04 mg/kg) and
Rutilus frisii kutum (0.17 mg/kg) [
13]. The accumulation of Cd varies across fish species and tissues, leading to diverse toxic effects, including damage to the immune and reproductive systems [
63]. That is, reproductive capabilities are compromised, as seen in rare minnows where Cd exposure resulted in reduced spawning success and altered gonadal health [
64].
Cobalt concentrations in muscle tissue of the species
Prochilodus lineatus (0.049 ± 0.02–0.124 ± 0.056 mg/kg) and
Pimelodus maculatus (0.022 ± 0.010–0.06 ± 0.008 mg/kg) are lower than the values established by the FAO (0.26 mg/kg) [
65] and those obtained in muscle tissue of
Euryglossa orientalis (3.21 mg/kg) and
Otolithes ruber (1.94 mg/kg) from the mouth of the Arvand River and northwestern coastal waters of the Persian Gulf [
7]. There are no Co values established by Anvisa/Brazil for fishes. Cobalt exposure in fishes has been shown to induce various toxic effects, impacting growth, biochemical parameters, and overall health. Studies indicate that cobalt chloride and other cobalt compounds can lead to significant physiological alterations in species such as rainbow trout
Oncorhynchus mykiss, Mozambique tilapia
Oreochromis mossambicus, and zebrafish [
66,
67,
68].
The mean concentrations of Cr were 1.22 (±0.29)–3.47 (±0.78) mg/kg (
Table 7) in
Prochilodus lineatus and 1.02 (±0.1)–2.97 (±0.30) mg/kg in
Pimelodus maculatus (
Table 8), which are above the values of permissible limits of metals in fish recommended by the FAO (1 mg/kg). Moreover, the concentration of Cr quantified in
P. lineatus from the rivers in the state of Mato Grosso do Sul were higher than that found by Meche et al. (2010) [
58], who studied
P. lineatus (0.76 mg/kg) in the Piracicaba (SP), Brazil. However, our findings are below those found in the muscle tissue of
Liza subviridis from the Persian Gulf (4.54–8 mg/kg) [
7]. Chromium poses significant toxic effects on fishes; this element leads to various physiological and biochemical alterations, including hematological changes, organ damage, and impaired immune responses. According to studies, Cr can enter fish through the gills, skin, and digestive tracts, accumulating in vital organs and disrupting oxidative homeostasis [
69,
70].
Table 7.
Metal(loid) concentration in Prochilodus lineatus muscle (mg/kg) compared to the maximum limit established by Anvisa/Brazil and values of permissible limits of metals in fish recommended by the FAO.
Table 7.
Metal(loid) concentration in Prochilodus lineatus muscle (mg/kg) compared to the maximum limit established by Anvisa/Brazil and values of permissible limits of metals in fish recommended by the FAO.
| Element | Year of Collection | Anhanduí River-1 | Anhanduí River-2 | Anhanduí River-3 | Pardo River | Lontra River | Maximum Limit * |
|---|
| Al | 2020 | 7.49 ± 0.66 | 9.26 ± 1.78 | 6.78 ± 0.62 | 4.05 ± 0.86 | 5.44 ± 0.50 | NE |
| 2021 | 9.34 ± 1.20 | 12.763 ± 2.5 | 8.359 ± 0.90 | 5.312 ± 1.12 | 6.256 ± 1.07 |
| As | 2020 | 0.15 ± 0.05 | 0.601 ± 0.12 | 0.346 ± 0.051 | 0.155 ± 0.032 | 0.167 ± 0.054 | 1.0 * |
| 2021 | 0.22 ± 0.04 | 0.66 ± 0.06 | 0.39 ± 0.05 | 0.204 ± 0.047 | 0.134 ± 0.050 |
| Cd | 2020 | 1.04 ± 0.06 | 1.32 ± 0.36 | 1.07 ± 0.15 | 0.68 ± 0.096 | 1.05 ± 0.06 | 0.05 * |
| 2021 | 1.10 ± 0.06 | 1.56 ± 0.31 | 1.17± 0.30 | 0.66 ± 0.074 | 0.856 ± 0.096 |
| Co | 2020 | 0.049 ± 0.02 | 0.071 ± 0.01 | 0.083 ± 0.010 | 0.055 ± 0.011 | 0.067 ± 0.010 | 0.26 ** |
| 2021 | 0.050 ± 0.01 | 0.086 ± 0.05 | 0.124 ± 0.056 | 0.053 ± 0.011 | 0.080 ± 0.015 |
| Cr | 2020 | 2.5 ± 0.46 | 2.87 ± 0.70 | 2.36 ± 0.55 | 1.22 ± 0.29 | 1.52 ± 0.43 | 1.0 ** |
| 2021 | 3.12 ± 0.47 | 3.47 ± 0.78 | 2.41 ± 0.31 | 1.37 ± 0.27 | 1.65 ± 0.37 |
| Cu | 2020 | 1.37 ± 0.14 | 2.14 ± 0.18 | 1.09 ± 0.16 | 0.450 ± 0.09 | 0.134 ± 0.05 | 30.0 ** |
| 2021 | 1.29 ± 0.30 | 3.13 ± 0.55 | 0.991 ± 0.11 | 0.201 ± 0.06 | 0.122 ± 0.02 |
| Hg | 2020 | 0.956 ± 0.03 | 1.13 ± 0.12 | 0.801 ± 0.01 | 0.722 ± 0.016 | 0.652 ± 0.042 | 1.0 *** |
| 2021 | 1.00 ± 0.02 | 1.27 ± 0.27 | 1.319 ± 0.55 | 0.913 ± 0.065 | 0.882 ± 0.102 |
| Mo | 2020 | 0.39 ± 0.09 | 0.046 ± 0.009 | 0.04 ± 0.017 | 0.123 ± 0.024 | 0.035 ± 0.008 | NE |
| 2021 | 0.62 ±0.11 | 0.272 ± 0.074 | 0.081 ± 0.013 | 0.272 ± 0.074 | 0.060 ± 0.02 |
| Ni | 2020 | 0.427 ± 0.04 | 0.836 ± 0.044 | 0.73 ± 0.033 | 0.230 ± 0.032 | 0.503 ± 0.047 | 80.0 ** |
| 2021 | 0.495 ± 0.10 | 0.90 ± 0.18 | 1.04 ± 0.16 | 0.467 ± 0.138 | 0.659 ± 0.05 |
| Pb | 2020 | 0.209 ± 0.06 | 0.318 ± 0.064 | 0.212 ± 0.028 | 0.310 ± 0.069 | 0.198 ± 0.040 | 0.30 * |
| 2021 | 0.317 ± 0.011 | 0.392 ± 0.034 | 0.238 ± 0.035 | 0.403± 0.047 | 0.248 ± 0.039 |
Table 8.
Metal(loid) concentration in Pimelodus maculatus muscle (mg/kg) compared to the maximum limit established by Anvisa/Brazil and values of permissible limits of metals in fish recommended by the FAO.
Table 8.
Metal(loid) concentration in Pimelodus maculatus muscle (mg/kg) compared to the maximum limit established by Anvisa/Brazil and values of permissible limits of metals in fish recommended by the FAO.
| Element | Year of Collection | Anhanduí River-1 | Anhanduí River-2 | Anhanduí River-3 | Pardo River | Lontra River | Maximum Limit * |
|---|
| Al | 2020 | 5.40 ± 0.54 | 6.36 ± 1.15 | 4.58 ± 0.85 | 3.88 ± 0.73 | 4.01 ± 0.51 | NE |
| 2021 | 7.11 ± 0.17 | 9.98 ± 1.0 | 5.04 ± 0.96 | 4.91 ± 0.22 | 4.63 ± 0.55 |
| As | 2020 | 0.105 ± 0.017 | 0.230 ± 0.05 | 0.132 ± 0.20 | 0.011 ± 0.018 | 0.119 ± 0.30 | 1.00 * |
| 2021 | 0.239 ± 0.011 | 0.367 ± 0.074 | 0.191 ± 0.011 | 0.032 ± 0.012 | 0.119 ± 0.020 |
| Cd | 2020 | 1.66 ± 0.51 | 1.52 ± 0.60 | 1.46 ± 0.38 | 0.59 ± 0.14 | 1.20 ± 0.45 | 0.05 * |
| 2021 | 2.24 ± 0.69 | 1.86 ± 0.70 | 1.59 ± 0.58 | 0.628 ± 0.14 | 1.30 ± 0.47 |
| Co | 2020 | 0.034 ± 0.012 | 0.054 ± 0.012 | 0.015± 0.004 | 0.033 ± 0.010 | 0.050 ± 0.004 | 0.26 ** |
| 2021 | 0.048 ± 0.005 | 0.06 ± 0.008 | 0.022 ± 0.010 | 0.040 ± 0.013 | 0.048 ± 0.019 |
| Cr | 2020 | 2.55 ± 0.58 | 3.02 ± 0.46 | 2.32 ± 0.57 | 1.37 ± 0.23 | 1.02 ± 0.1 | 1.0 ** |
| 2021 | 2.97 ± 0.30 | 2.95 ± 0.50 | 2.81 ± 0.47 | 1.20 ± 0.26 | 1.10 ± 0.30 |
| Cu | 2020 | 1.08 ± 0.28 | 1.95 ± 0.70 | 0.99 ± 0.12 | 0.215 ± 0.056 | 0.081 ± 0.025 | 30.0 ** |
| 2021 | 1.90 ± 0.120 | 2.20 ± 0.95 | 1.0 ± 0.13 | 0.42 ± 0.072 | 1.4 ± 0.05 |
| Hg | 2020 | 0.742 ± 0.13 | 1.05 ± 0.08 | 0.599 ± 0.013 | 0.493 ± 0.030 | 0.50 ± 0.11 | 1.0 *** |
| 2021 | 0.96 ± 0.06 | 1.50 ± 0.46 | 0.560 ± 0.230 | 0.60 ± 0.30 | 0.46 ± 0.20 |
| Mo | 2020 | 0.17 ± 0.06 | 0.027 ± 0.011 | 0.027 ± 0.09 | 0.12 ± 0.024 | 0.016 ± 0.03 | NE |
| 2021 | 0.290 ± 0.02 | 0.12 ± 0.03 | 0.034 ± 0.06 | 0.32 ± 0.09 | 0.031 ± 0.09 |
| Ni | 2020 | 0.216 ± 0.073 | 0.437 ± 0.028 | 0.459 ± 0.038 | 0.187 ± 0.054 | 0.197 ± 0.039 | 80.0 ** |
| 2021 | 0.201 ± 0.052 | 0.560 ± 0.049 | 0.450 ± 0.046 | 0.236 ± 0.03 | 0.303 ± 0.043 |
| Pb | 2020 | 0.457 ± 0.053 | 0.560 ± 0.054 | 0.413 ± 0.069 | 0.11 ± 0.013 | 0.13 ± 0.007 | 0.3 * |
| 2021 | 0.590 ± 0.031 | 0.742 ± 0.084 | 0.449 ± 0.063 | 0.140 ± 0.078 | 0.169 ± 0.038 |
The mean concentrations of Cu in fish samples from
Table 7 ranged between 0.991 (±0.11) and 3.13 (±0.55) mg/kg in
Prochilodus lineatus muscle, and 0.081 (±0.025)–2.20 (±0.95) mg/kg in
Pimelodus maculatus muscle (
Table 8). All values of Cu concentration were below the permissible limits of Cu in fish recommended by the FAO (30 mg/kg). Meche et al. (2010) in Brazil [
58] obtained concentration values of 1.80 mg/kg in their study using muscle samples from
Prochilodus lineatus, and 0.76 mg/kg in
Pimelodus maculatus muscle. Thus, the concentrations of Cu values in Anhanduí River-2 in 2020–2021 (
Table 7 and
Table 8) are above the results obtained by Meche et al. (2010) [
58] for both species. It can be seen from the results obtained in
Table 7 and
Table 8 and comparisons with other studies that the same or different species of fish within a country have different variations in concentration values of metal(loid)s. In fact, the mean Cu concentration in the muscle tissue of
Otolithes ruber from Mahshahr Port in Khozestan Province in Iran (25.3 mg/kg) is higher than the all values in
Table 7 and
Table 8. Copper exhibits both essential and toxic effects on fish, depending on its concentration and form. Excessive exposure can lead to significant physiological and biochemical disruptions, while high concentrations of copper sulfate can cause skin irritation, gill damage, and even mortality in fishes [
71,
72].
According to
Table 7 and 8, Hg concentrations range from 0.652 (±0.042) to 1.319 (±0.55) mg/kg in
Prochilodus lineatus muscle tissue and 0.46 (±0.20)–1.50 (±0.46) mg/kg in
Pimelodus maculatus muscle tissue. However, only the concentration of Hg in muscle samples of
Prochilodus lineatus from Anhanduí-2 River (year 2020–2021) and Anhanduí-3 River (2021), and Hg in the muscle of
Pimelodus maculatus from Rio Anhanduí-2 (2021) are higher than the concentrations stipulated by Anvisa for predatory fish (1.0 mg/kg). However, the Hg concentration values in the fish muscle samples presented in
Table 7 and
Table 8 are below the concentration values obtained for other species, such as
Anodontostoma chacunda (2.04 mg/kg),
Johnius belangerii (4.40 mg/kg), and
Cynoglossurs arel (5.82 mg/kg) from the Musa Estuary and Mahshahr Port in the Persian Gulf [
7]. Mercury exposure in fish leads to significant toxic effects, impacting their health and development. Histological examinations revealed severe tissue damage in the gills, liver, and stomach of
Clarias batrachus [
73]. Furthermore, chronic exposure in silver carp larvae resulted in decreased antioxidant capacity and neurotoxic changes [
74]. These findings underscore the urgent need for monitoring mercury levels in aquatic ecosystems to protect fish health and, by extension, human health through the food chain.
In
Table 7, Mo concentration was 0.035 (±0.008)–0.62 (±0.11) mg/kg in
Prochilodus lineatus muscle, and 0.016 (±0.03)–0.290 (±0.02) mg/kg in
Pimelodus maculatus muscle (
Table 8). There are no permitted levels defined by Anvisa/Brazil and the FAO for Mo in fishes. However, some values of Mo in
Table 7 and
Table 8 are above those obtained by other Brazilian studies, which quantified 0.05 mg/kg of this element in samples of
Pimelodus maculatus muscle [
58]. According to study using wild rainbow trout captured from Zayandeh-Rood River in Chaharmahal-va-Baghtiari province, Iran, concentration of Mo in tissues of wild rainbow trout was 0.121 (±0.046) mg/kg [
75]. After comparison, it was found that the concentration of Mo in the muscle of
Prochilodus lineatus and
Pimelodus maculatus from Anhanduí River-1 and Pardo River during the years 2020–2021 and from Anhanduí River-2 in 2021 are above those obtained in Iran. Molybdenum in high concentrations is a toxic element for fishes. Studies indicate that molybdenum can be acutely lethal to various fish species, including rainbow trout [
76]. Furthermore, it has been shown to adversely affect fish embryos and spermatogenesis, raising concerns about its ecological impact [
77]. According to Wang et al. (2022) [
57], the toxicity of molybdenum is influenced by its chemical form, with ammonium molybdate exhibiting the highest toxicity in aquatic environments. Additionally, current water quality guidelines for molybdenum may not accurately reflect its potential toxicity, suggesting a need for reevaluation [
78].
The mean contents of Ni in fish species sampled in 2020 and 2021 from the river waters of the city of Campo Grande range from 0.230 (±0.032) to 1.04 (±0.16) in
Prochilodus lineatus muscle, and from 0.187 (±0.054) to 0.560 (±0.049) in
Pimelodus maculatus muscle. All mean concentrations of Ni found in the muscle tissue of
Prochilodus lineatus and
Pimelodus maculatus from the rivers are below the 80.0 mg/kg acceptable limit recommended by the FAO. Meche et al. (2010) [
58], who studied
Pimelodus maculatus and
Prochilodus lineatus in Brazilian rivers, obtained 0.34 mg/kg and 1.53 mg/kg in muscle samples of those fishes. Therefore, the highest values of Ni concentrations in the samples in
Table 7 and
Table 8 are above the values obtained by Meche et al. (2010) [
58]. Ni concentration in
Prochilodus lineatus muscle (
Table 7) and
Pimelodus maculatus muscle (
Table 8), depending on the year of collection, are above or below the levels of Ni in fish species from Caspian Sea (0.52, 0.12
Rutilus frisii kutum) and 0.44 μg/g of wet weight in
Cyprinus carpio [
13]. Nickel poses toxicity risks to fish, primarily through bioaccumulation and oxidative stress mechanisms. Studies indicate that nickel can enter fish through the gills, skin, and ingestion of contaminated food, leading to various physiological and histopathological effects [
79]. Chronic exposure results in oxidative stress, organ damage, and altered gene expression, affecting growth and reproductive capabilities [
79,
80]. Furthermore, nickel’s presence in aquatic ecosystems disrupts ecological balance, causing cellular dysfunction in some species [
81].
In
Table 7, the Ni concentration in
Prochilodus lineatus muscle ranged from 0.198 (±0.040) to 0.403 (±0.047) mg/kg, and in
Table 8, the concentration in
Pimelodus maculatus muscle ranged from 0.11 (±0.013) to 0.742 (±0.084) mg/kg. All values of concentrations of Ni in
Table 7 and
Table 8 are notably lower than those established by the FAO (80.0 mg/kg) and one Brazilian study using fish from the Piracicaba River
Pimelodus maculatus (3.25 mg/kg) and
Prochilodus lineatus (0.57 mg/kg). On the other hand, Pb concentrations in samples of
Prochilodus lineatus muscle from the rivers Anhanduí River-1 (2020), Anhanduí River-3 (2020–2021), and Lontra River (2020–2021) in
Table 7, as well as
Pimelodus maculatus muscle in Pardo River (2020–2021) and Lontra River (2020–2021) were lower than those obtained fish species from the Caspian Sea (0.38 mg/kg of wet weight in
Rutilus frisii kutum and
Cyprinus carpio) [
13]. Exposure to lead can lead to genotoxicity, hepatotoxicity, and reproductive impairments, affecting fish health and populations [
82].
As seen above, the variation in metal(loid) levels in fishes across different studies can be attributed to geographical, geological, and climatic factors. Research indicates that metal concentrations in fish tissues vary significantly based on location, species, and environmental conditions [
13].
For instance, in the trans-Himalayan ecosystem, chemical elements like Cu and Zn showed substantial differences in concentration across seasons and sites in
Cyprinus carpio muscle, highlighting the influence of local pollution sources [
83]. In the Gulf of California, mining activities contributed to elevated metal levels in fishes, demonstrating the impact of anthropogenic factors on metal accumulation [
84]. Furthermore, the study of Sheikhzadeh and Hamidian (2021) [
7] showed that fishes from different aquatic ecosystems, particularly the Persian Gulf and Caspian Sea, exhibit varying levels of metal and metalloid accumulation, influenced by factors such as species habitat and feeding habits [
7]. These findings underscore the complex interplay of environmental factors in determining metal levels in fishes, necessitating ongoing monitoring and assessment.