Neighborhood Effects on Acute Pediatric Asthma: Race, Greenspace, and PM2.5
Abstract
1. Introduction
2. Data and Methods
2.1. Data
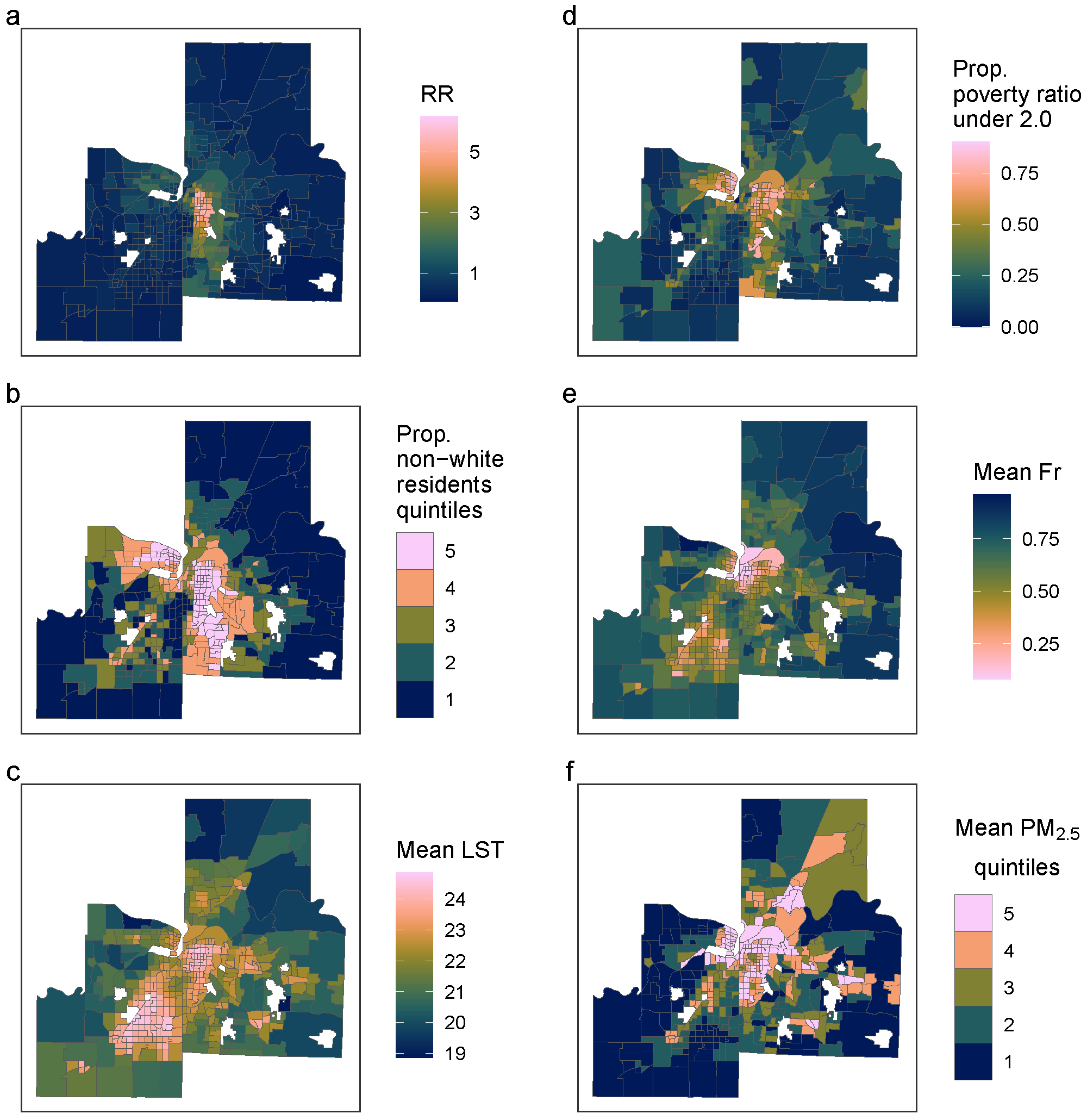
2.1.1. Study Area
2.1.2. Pediatric Asthma
2.1.3. Environmental Data
2.1.4. Climatic Data
2.1.5. Socioeconomic Data
2.1.6. Data Processing
2.2. Analysis Methods
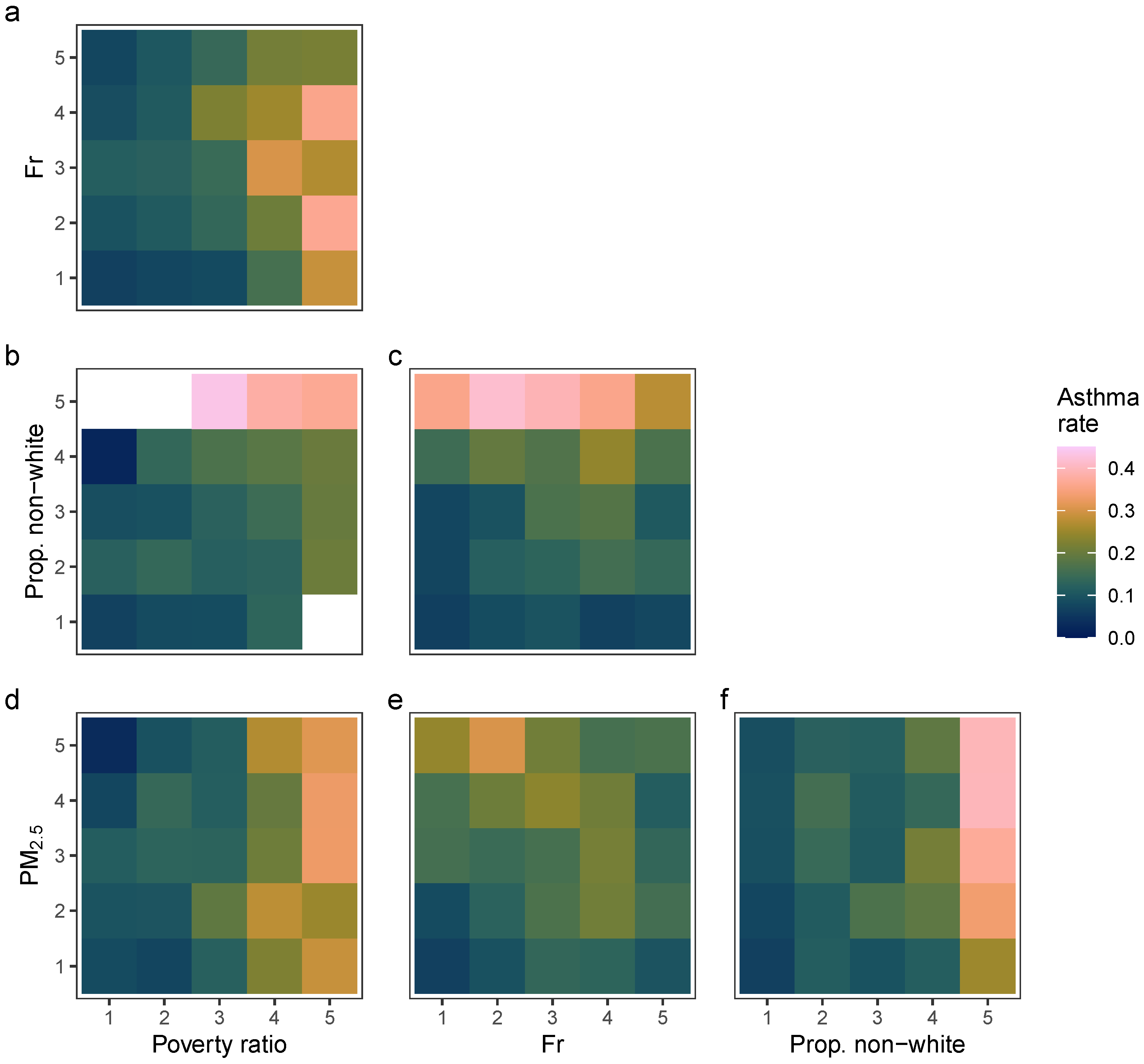
2.2.1. Descriptive Analysis
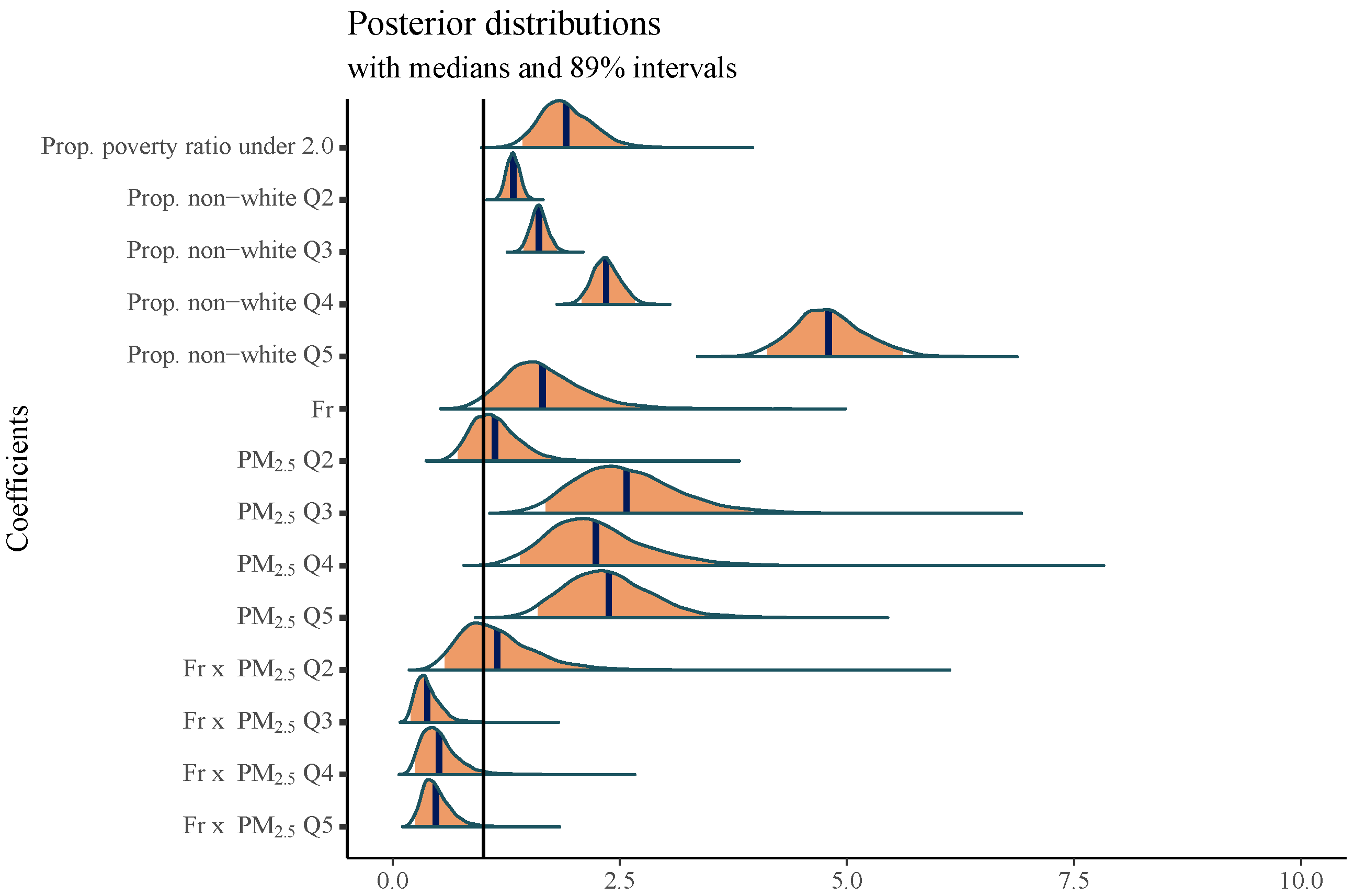
2.2.2. Statistical Modelling
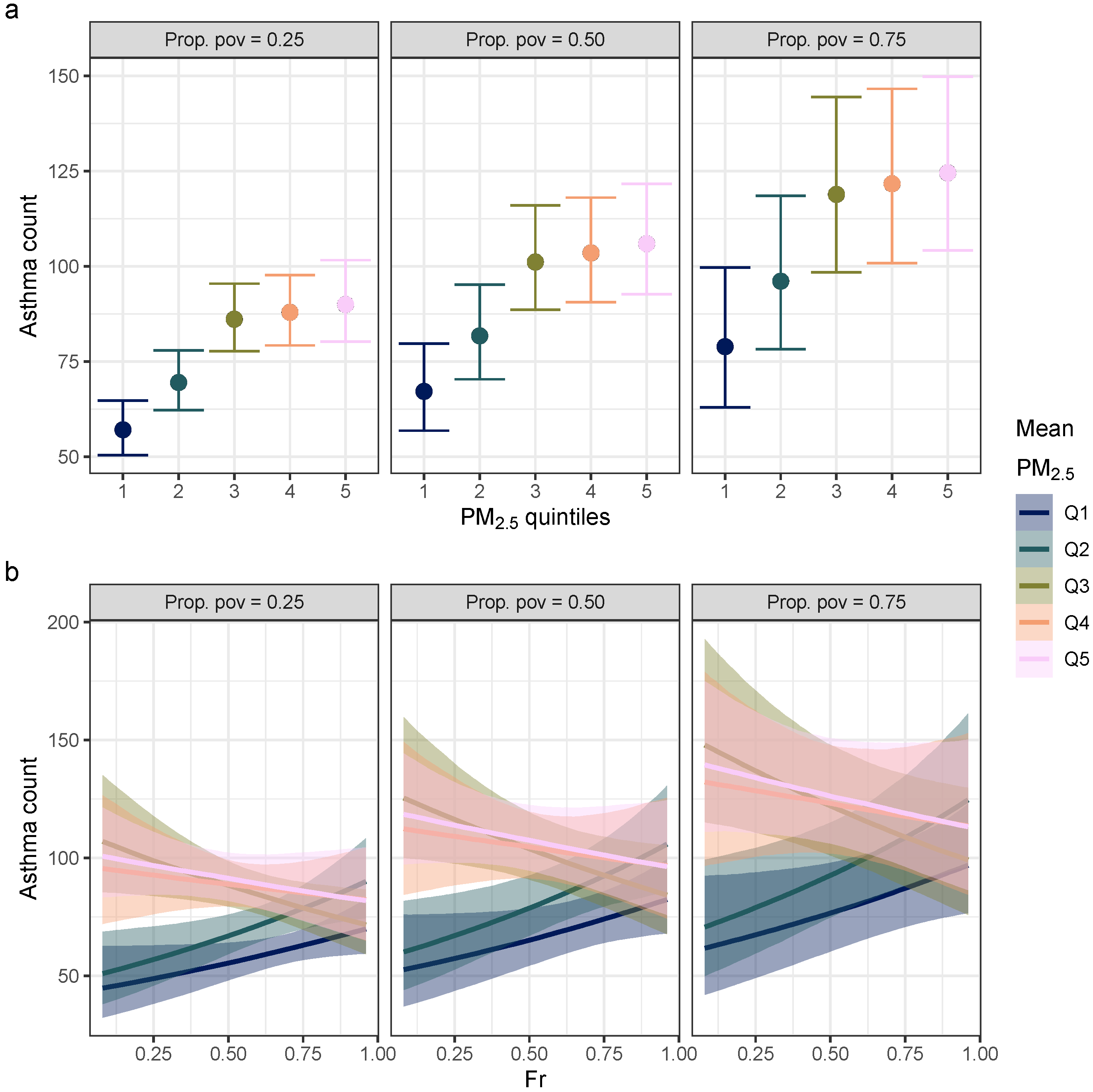
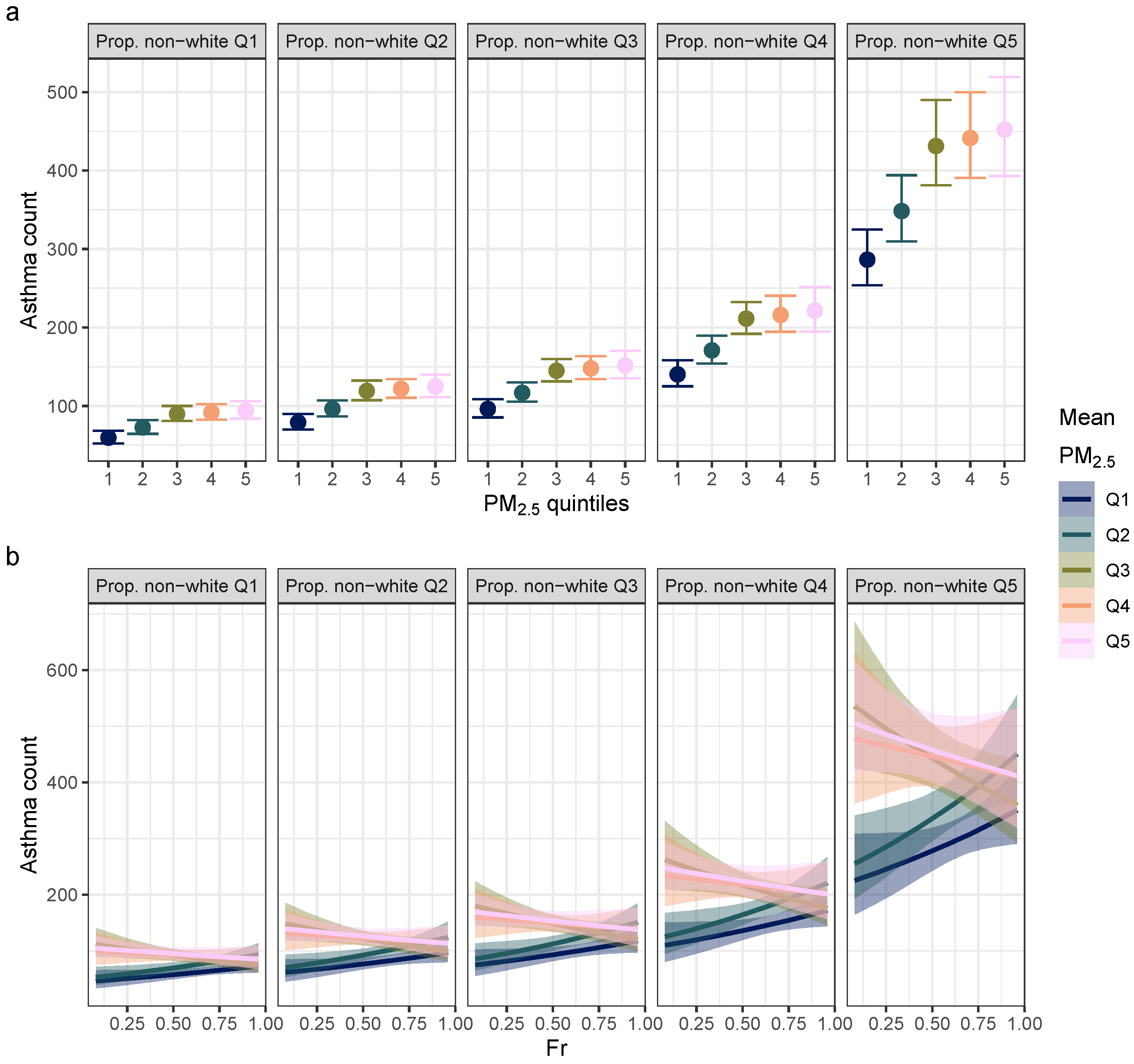
3. Results
4. Discussion
4.1. Limitations
4.2. Strengths
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, E.S.; Mannino, D.M. Considerations Regarding the Epidemiology and Public Health Burden of Asthma. In Asthma, Health and Society: A Public Health Perspective; Harver, A., Kotses, H., Eds.; Springer: Boston, MA, USA, 2010; pp. 3–17. [Google Scholar] [CrossRef]
- Won, Y.K.; Hwang, T.H.; Roh, E.J.; Chung, E.H. Seasonal Patterns of Asthma in Children and Adolescents Presenting at Emergency Departments in Korea. Allergy Asthma Immunol. Res. 2016, 8, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trivedi, M.; Denton, E. Asthma in Children and Adults—What Are the Differences and What Can They Tell Us About Asthma? Front. Pediatr. 2019, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Milligan, K.L.; Matsui, E.; Sharma, H. Asthma in Urban Children: Epidemiology, Environmental Risk Factors, and the Public Health Domain. Curr. Allergy Asthma Rep. 2016, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L.J.; Simon, A.E.; Rossen, L.M. Changing Trends in Asthma Prevalence Among Children. Pediatrics 2016, 137, e20152354. [Google Scholar] [CrossRef]
- Delfino, R.J.; Wu, J.; Tjoa, T.; Gullesserian, S.K.; Nickerson, B.; Gillen, D.L. Asthma Morbidity and Ambient Air Pollution: Effect Modification by Residential Traffic-Related Air Pollution. Epidemiology 2014, 25, 48–57. [Google Scholar] [CrossRef]
- Chang, H.H.; Pan, A.; Lary, D.J.; Waller, L.A.; Zhang, L.; Brackin, B.T.; Finley, R.W.; Faruque, F.S. Time-Series Analysis of Satellite-Derived Fine Particulate Matter Pollution and Asthma Morbidity in Jackson, MS. Environ. Monit. Assess. 2019, 191, 280. [Google Scholar] [CrossRef]
- Delamater, P.L.; Finley, A.O.; Banerjee, S. An Analysis of Asthma Hospitalizations, Air Pollution, and Weather Conditions in Los Angeles County, California. Sci. Total. Environ. 2012, 425, 110–118. [Google Scholar] [CrossRef]
- Buteau, S.; Geng, X.; Labelle, R.; Smargiassi, A. Review of the Effect of Air Pollution Exposure from Industrial Point Sources on Asthma-Related Effects in Childhood. Environ. Epidemiol. 2019, 3, e077. [Google Scholar] [CrossRef]
- Pollock, J.; Shi, L.; Gimbel, R.W. Outdoor Environment and Pediatric Asthma: An Update on the Evidence from North America. Can. Respir. J. 2017, 2017, 8921917. [Google Scholar] [CrossRef]
- Chen, L.; Mengersen, K.; Tong, S. Spatiotemporal Relationship between Particle Air Pollution and Respiratory Emergency Hospital Admissions in Brisbane, Australia. Sci. Total. Environ. 2007, 373, 57–67. [Google Scholar] [CrossRef]
- Kane, N. Revealing the Racial and Spatial Disparity in Pediatric Asthma: A Kansas City Case Study. Soc. Sci. Med. 2022, 292, 114543. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Patton, A.; Atkinson, R.; Brook, J.; Chang, H.; Crouse, D.; Fussell, J.; Hoek, G.; Hoffmann, B.; Kappeler, R.; et al. Long-Term Exposure to Traffic-Related Air Pollution and Selected Health Outcomes: A Systematic Review and Meta-Analysis. Environ. Int. 2022, 164, 107262. [Google Scholar] [CrossRef] [PubMed]
- Oke, T.R. The Energetic Basis of the Urban Heat Island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Wesley, E.J.; Brunsell, N.A. Greenspace Pattern and the Surface Urban Heat Island: A Biophysically-Based Approach to Investigating the Effects of Urban Landscape Configuration. Remote. Sens. 2019, 11, 2322. [Google Scholar] [CrossRef]
- Anderson, G.B.; Dominici, F.; Wang, Y.; McCormack, M.C.; Bell, M.L.; Peng, R.D. Heat-Related Emergency Hospitalizations for Respiratory Diseases in the Medicare Population. Am. J. Respir. Crit. Care Med. 2013, 187, 1098–1103. [Google Scholar] [CrossRef]
- Kim, J.; Lim, Y.; Kim, H. Outdoor Temperature Changes and Emergency Department Visits for Asthma in Seoul, Korea: A Time-Series Study. Environ. Res. 2014, 135, 15–20. [Google Scholar] [CrossRef]
- Lam, H.C.y.; Li, A.M.; Chan, E.Y.y.; Goggins, W.B. The Short-Term Association between Asthma Hospitalisations, Ambient Temperature, Other Meteorological Factors and Air Pollutants in Hong Kong: A Time-Series Study. Thorax 2016, 71, 1097–1109. [Google Scholar] [CrossRef]
- O’Lenick, C.R.; Winquist, A.; Chang, H.H.; Kramer, M.R.; Mulholland, J.A.; Grundstein, A.; Sarnat, S.E. Evaluation of Individual and Area-Level Factors as Modifiers of the Association between Warm-Season Temperature and Pediatric Asthma Morbidity in Atlanta, GA. Environ. Res. 2017, 156, 132–144. [Google Scholar] [CrossRef]
- Soneja, S.; Jiang, C.; Fisher, J.; Upperman, C.R.; Mitchell, C.; Sapkota, A. Exposure to Extreme Heat and Precipitation Events Associated with Increased Risk of Hospitalization for Asthma in Maryland, U.S.A. Environ. Health 2016, 15, 57. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air Pollution Removal by Urban Trees and Shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Janhäll, S. Review on Urban Vegetation and Particle Air Pollution – Deposition and Dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Alcock, I.; White, M.; Cherrie, M.; Wheeler, B.; Taylor, J.; Mcinnes, R.; Otte Im Kampe, E.; Vardoulakis, S.; Sarran, C.; Soyiri, I.; et al. Land Cover and Air Pollution Are Associated with Asthma Hospitalisations: A Cross-Sectional Study. Environ. Int. 2017, 109, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ayres-Sampaio, D.; Teodoro, A.C.; Sillero, N.; Santos, C.; Fonseca, J.; Freitas, A. An Investigation of the Environmental Determinants of Asthma Hospitalizations: An Applied Spatial Approach. Appl. Geogr. 2014, 47, 10–19. [Google Scholar] [CrossRef]
- Feng, X.; Astell-Burt, T. Is Neighborhood Green Space Protective against Associations between Child Asthma, Neighborhood Traffic Volume and Perceived Lack of Area Safety? Multilevel Analysis of 4447 Australian Children. Int. J. Environ. Res. Public Health 2017, 14, 543. [Google Scholar] [CrossRef]
- Brewer, M.; Kimbro, R.T.; Denney, J.T.; Osiecki, K.M.; Moffett, B.; Lopez, K. Does Neighborhood Social and Environmental Context Impact Race/Ethnic Disparities in Childhood Asthma? Health Place 2017, 44, 86–93. [Google Scholar] [CrossRef]
- Castillo, M.D.; Kinney, P.L.; Southerland, V.; Arno, C.A.; Crawford, K.; van Donkelaar, A.; Hammer, M.; Martin, R.V.; Anenberg, S.C. Estimating Intra-Urban Inequities in PM 2.5-Attributable Health Impacts: A Case Study for Washington, DC. GeoHealth 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Hicken, M.T. Invited Commentary: Fundamental Causes, Social Context, and Modifiable Risk Factors in the Racial/Ethnic Inequalities in Blood Pressure and Hypertension. Am. J. Epidemiol. 2015, 182, 354–357. [Google Scholar] [CrossRef][Green Version]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Nilon, C.; Pouyat, R.; Zipperer, W.C.; Costanza, R. Urban Ecological Sysytems: Linking Terrestrial Ecological, Physical, and Socioeconomic Components of Metropolitan Areas. Annu. Rev. Ecol. Syst. 2001, 32, 127–157. [Google Scholar] [CrossRef]
- Stone, B.; Hess, J.J.; Frumkin, H. Urban Form and Extreme Heat Events: Are Sprawling Cities More Vulnerable to Climate Change than Compact Cities? Environ. Health Perspect. 2010, 118, 1425–1428. [Google Scholar] [CrossRef]
- Ji, W. Landscape Effects of Urban Sprawl: Spatial and Temporal Analyses Using Remote Sensing Images and Landscape Metrics. Int. Arch. Photogramm. Remote. Sens. Spat. Inf. Sci. 2008, XXXVII, 1691–1694. [Google Scholar]
- Gotham, K.F. Race, Real Estate, and Uneven Development: The Kansas City Experience, 1900–2010, 2nd ed.; State University of New York Press: Albany, NY, USA, 2014. [Google Scholar]
- Mid-America Regional Council. Fair Housing and Equity Assessment for the Greater Kansas City Region; Technical Report; Mid-America Regional Council: Kansas City, MO, USA, 2014. [Google Scholar]
- U.S. Housing and Urban Development. Affirmatively Furthering Fair Housing Data and Mapping Tool (AFFH-T) Data Documentation; Technical Report; U.S. Housing and Urban Development, Office of Policy Development & Research: Washington, DC, USA, 2020. [Google Scholar]
- The Urban Institute. The Cost of Segregation: National Trends and the Case of Chicago, 1990–2010. 2017, p. 78. Available online: https://www.urban.org/sites/default/files/publication/89201/the_cost_of_segregation_final.pdf (accessed on 16 October 2024).
- Kane, N.J. An Interdisciplinary Health Disparities Research and Intervention Strategy Applied to the Problem of Pediatric Asthma in Kansas City. Ph.D. Thesis, University of Missouri–Kansas City, Kansas City, MO, USA, 2020. [Google Scholar]
- Aybar, C. Rgee: R Bindings for Calling the ’earth Engine’ API. J. Open Source Softw. 2020, 5, 2272. [Google Scholar] [CrossRef]
- Carlson, T.N.; Ripley, D.A. On the Relation between NDVI, Fractional Vegetation Cover, and Leaf Area Index. Remote. Sens. Environ. 1997, 62, 241–252. [Google Scholar] [CrossRef]
- Gillies, R.R.; Carlson, T.N. Thermal Remote Sensing of Surface Soil Water Content with Partial Vegetation Cover for Incorporation into Climate Models. J. Appl. Meteorol. 1995, 34, 745–756. [Google Scholar] [CrossRef]
- Kim, S.Y.; Bechle, M.; Hankey, S.; Sheppard, L.; Szpiro, A.A.; Marshall, J.D. Concentrations of Criteria Pollutants in the Contiguous U.S., 1979–2015: Role of Prediction Model Parsimony in Integrated Empirical Geographic Regression. PLoS ONE 2020, 15, e0228535. [Google Scholar] [CrossRef]
- Walker, K.; Herman, M. Tidycensus: Load US Census Boundary and Attribute Data as ‘Tidyverse’ and ‘Sf’-Ready Data Frames. 2022. Available online: https://walker-data.com/tidycensus/ (accessed on 16 October 2024).
- Social Explorer. Ratio of Income in 1999 to Poverty Level; Technical Report; Social Explorer: New York, NY, USA, 2022. [Google Scholar]
- Social Explorer. Age-Cumulative (Less); Technical Report; Social Explorer: New York, NY, USA, 2022. [Google Scholar]
- Schulz, A.J.; Mentz, G.B.; Sampson, N.; Ward, M.; Anderson, R.; de Majo, R.; Israel, B.A.; Lewis, T.C.; Wilkins, D. Race and the Distribution of Social and Physical Environmental Risk. Bois Rev. Soc. Sci. Res. Race 2016, 13, 285–304. [Google Scholar] [CrossRef]
- Bullard, R.D. Dismantling Environmental Racism in the USA. Local Environ. 1999, 4, 5–19. [Google Scholar] [CrossRef]
- Schlosberg, D. Theorising Environmental Justice: The Expanding Sphere of a Discourse. Environ. Politics 2013, 22, 37–55. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Bürkner, P.C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J.; Vehtari, A. Regression and Other Stories; Cambridge University Press: New York, NY, USA, 2021. [Google Scholar]
- Kruschke, J.K.; Liddell, T.M. Bayesian Data Analysis for Newcomers. Psychon. Bull. Rev. 2018, 25, 155–177. [Google Scholar] [CrossRef]
- Lynch, S.M. Introduction to Applied Bayesian Statistics and Estimation for Social Scientists; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lemoine, N.P. Moving beyond Noninformative Priors: Why and How to Choose Weakly Informative Priors in Bayesian Analyses. Oikos 2019, 128, 912–928. [Google Scholar] [CrossRef]
- Gabry, J.; Simpson, D.; Vehtari, A.; Betancourt, M.; Gelman, A. Visualization in Bayesian Workflow. J. R. Stat. Soc. Ser. (Stat. Soc.) 2019, 182, 389–402. [Google Scholar] [CrossRef]
- Kruschke, J.K. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Kumar, P.; Druckman, A.; Gallagher, J.; Gatersleben, B.; Allison, S.; Eisenman, T.S.; Hoang, U.; Hama, S.; Tiwari, A.; Sharma, A.; et al. The Nexus between Air Pollution, Green Infrastructure and Human Health. Environ. Int. 2019, 133, 105181. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Sternthal, M.; Wright, R.J. Social Determinants: Taking the Social Context of Asthma Seriously. Pediatrics 2009, 123, S174–S184. [Google Scholar] [CrossRef]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural Racism and Health Inequities in the USA: Evidence and Interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Wolch, J.R.; Byrne, J.; Newell, J.P. Urban Green Space, Public Health, and Environmental Justice: The Challenge of Making Cities ‘Just Green Enough’. Landsc. Urban Plan. 2014, 125, 234–244. [Google Scholar] [CrossRef]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasència, A.; Nieuwenhuijsen, M.J. Residential Green Spaces and Mortality: A Systematic Review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef]
- Dadvand, P.; Villanueva, C.M.; Font-Ribera, L.; Martinez, D.; Basagaña, X.; Belmonte, J.; Vrijheid, M.; Gražulevičienė, R.; Kogevinas, M.; Nieuwenhuijsen, M.J. Risks and Benefits of Green Spaces for Children: A Cross-Sectional Study of Associations with Sedentary Behavior, Obesity, Asthma, and Allergy. Environ. Health Perspect. 2014, 122, 1329–1335. [Google Scholar] [CrossRef]
- Macintyre, S.; Ellaway, A.; Cummins, S. Place Effects on Health: How Can We Conceptualise, Operationalise and Measure Them? Soc. Sci. Med. 2002, 55, 125–139. [Google Scholar] [CrossRef]



| Category | Attributes | Population | Percent |
|---|---|---|---|
| Total | Total population | 1,999,718 | |
| Age | Pop. under 18 | 515,653 | 25.8 |
| Race | White Alone | 1,594,663 | 79.7 |
| Black or African American Alone | 246,536 | 12.3 | |
| American Indian and Alaska Native Alone | 9010 | 0.5 | |
| Asian Alone | 44,589 | 2.2 | |
| Native Hawaiian and Other Pacific Islander Alone | 2415 | 0.1 | |
| Some Other Race Alone | 50,012 | 2.5 | |
| Two or More Races | 52,493 | 2.6 | |
| Ratio of income-to-poverty level | Pop. for whom poverty status is determined | 1,967,280 | |
| Under 1.00 (Doing Poorly) | 217,606 | 11.1 | |
| 1.00 to 1.99 (Struggling) | 307,623 | 15.6 | |
| Under 2.00 (Poor or Struggling) | 525,229 | 26.7 | |
| 2.00 and Over (Doing Ok) | 1,442,051 | 73.3 |
| Category | Attributes |
|---|---|
| Diagnosis | Date of admission |
| ICD-9 code | |
| Event account number | |
| Patient medical record number (MRN) | |
| Patient residential address | |
| Demographics | Birthdate |
| Sex | |
| Race | |
| Ethnicity | |
| Visit characteristics | Payment type |
| Patient class |
| Asthma Rate (Quintiles) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Mean proportion poverty–income ratio below 2.0 | 0.12 | 0.18 | 0.29 | 0.42 | 0.57 |
| Mean proportion non-White residents | 0.10 | 0.14 | 0.20 | 0.38 | 0.72 |
| Mean Fr | 0.63 | 0.58 | 0.55 | 0.57 | 0.46 |
| Mean PM2.5 | 10.42 | 10.72 | 10.88 | 11.00 | 11.14 |
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Intercept | 0.06 | 0.05 | 0.05 | 0.04 |
| [0.06, 0.07] | [0.05, 0.06] | [0.04, 0.06] | [0.03, 0.05] | |
| Prop. poverty ratio under 2.0 | 3.42 | 2.14 | 2.15 | 1.91 |
| [2.63, 4.44] | [1.63, 2.83] | [1.62, 2.85] | [1.43, 2.56] | |
| Prop. non-White Q2 | 1.32 | 1.29 | 1.29 | 1.33 |
| [1.19, 1.47] | [1.17, 1.44] | [1.16, 1.44] | [1.19, 1.48] | |
| Prop. non-White Q3 | 1.60 | 1.57 | 1.57 | 1.61 |
| [1.43, 1.79] | [1.41, 1.74] | [1.41, 1.76] | [1.44, 1.80] | |
| Prop. non-White Q4 | 2.25 | 2.32 | 2.32 | 2.35 |
| [1.98, 2.55] | [2.05, 2.62] | [2.05, 2.63] | [2.08, 2.66] | |
| Prop. non-White Q5 | 4.33 | 4.66 | 4.65 | 4.80 |
| [3.71, 5.07] | [4.01, 5.43] | [4.00, 5.43] | [4.12, 5.61] | |
| Fr | 1.00 | 1.65 | ||
| [0.81, 1.24] | [1.00, 2.73] | |||
| PM2.5 Q2 | 1.20 | 1.19 | 1.13 | |
| [1.08, 1.33] | [1.08, 1.32] | [0.72, 1.77] | ||
| PM2.5 Q3 | 1.41 | 1.40 | 2.57 | |
| [1.26, 1.57] | [1.26, 1.56] | [1.68, 3.94] | ||
| PM2.5 Q4 | 1.44 | 1.43 | 2.24 | |
| [1.28, 1.61] | [1.28, 1.60] | [1.40, 3.57] | ||
| PM2.5 Q5 | 1.50 | 1.49 | 2.38 | |
| [1.33, 1.69] | [1.32, 1.69] | [1.60, 3.56] | ||
| Fr × PM2.5 Q2 | 1.15 | |||
| [0.58, 2.27] | ||||
| Fr × PM2.5 Q3 | 0.38 | |||
| [0.20, 0.74] | ||||
| Fr × PM2.5 Q4 | 0.51 | |||
| [0.25, 1.07] | ||||
| Fr × PMV Q5 | 0.48 | |||
| [0.25, 0.91] | ||||
| Bayes’ R2 | 0.759 | 0.770 | 0.769 | 0.767 |
| WAIC | 4598.5 | 4567.9 | 4570.0 | 4566.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesley, E.J.; Brunsell, N.A.; Rahn, D.R.; Saint Onge, J.M.; Kane, N.J.; Kennedy, K.F. Neighborhood Effects on Acute Pediatric Asthma: Race, Greenspace, and PM2.5. Urban Sci. 2024, 8, 176. https://doi.org/10.3390/urbansci8040176
Wesley EJ, Brunsell NA, Rahn DR, Saint Onge JM, Kane NJ, Kennedy KF. Neighborhood Effects on Acute Pediatric Asthma: Race, Greenspace, and PM2.5. Urban Science. 2024; 8(4):176. https://doi.org/10.3390/urbansci8040176
Chicago/Turabian StyleWesley, Elizabeth J., Nathaniel A. Brunsell, David R. Rahn, Jarron M. Saint Onge, Natalie J. Kane, and Kevin F. Kennedy. 2024. "Neighborhood Effects on Acute Pediatric Asthma: Race, Greenspace, and PM2.5" Urban Science 8, no. 4: 176. https://doi.org/10.3390/urbansci8040176
APA StyleWesley, E. J., Brunsell, N. A., Rahn, D. R., Saint Onge, J. M., Kane, N. J., & Kennedy, K. F. (2024). Neighborhood Effects on Acute Pediatric Asthma: Race, Greenspace, and PM2.5. Urban Science, 8(4), 176. https://doi.org/10.3390/urbansci8040176






