Pesticides in the Environment: Benefits, Harms, and Detection Methods
Abstract
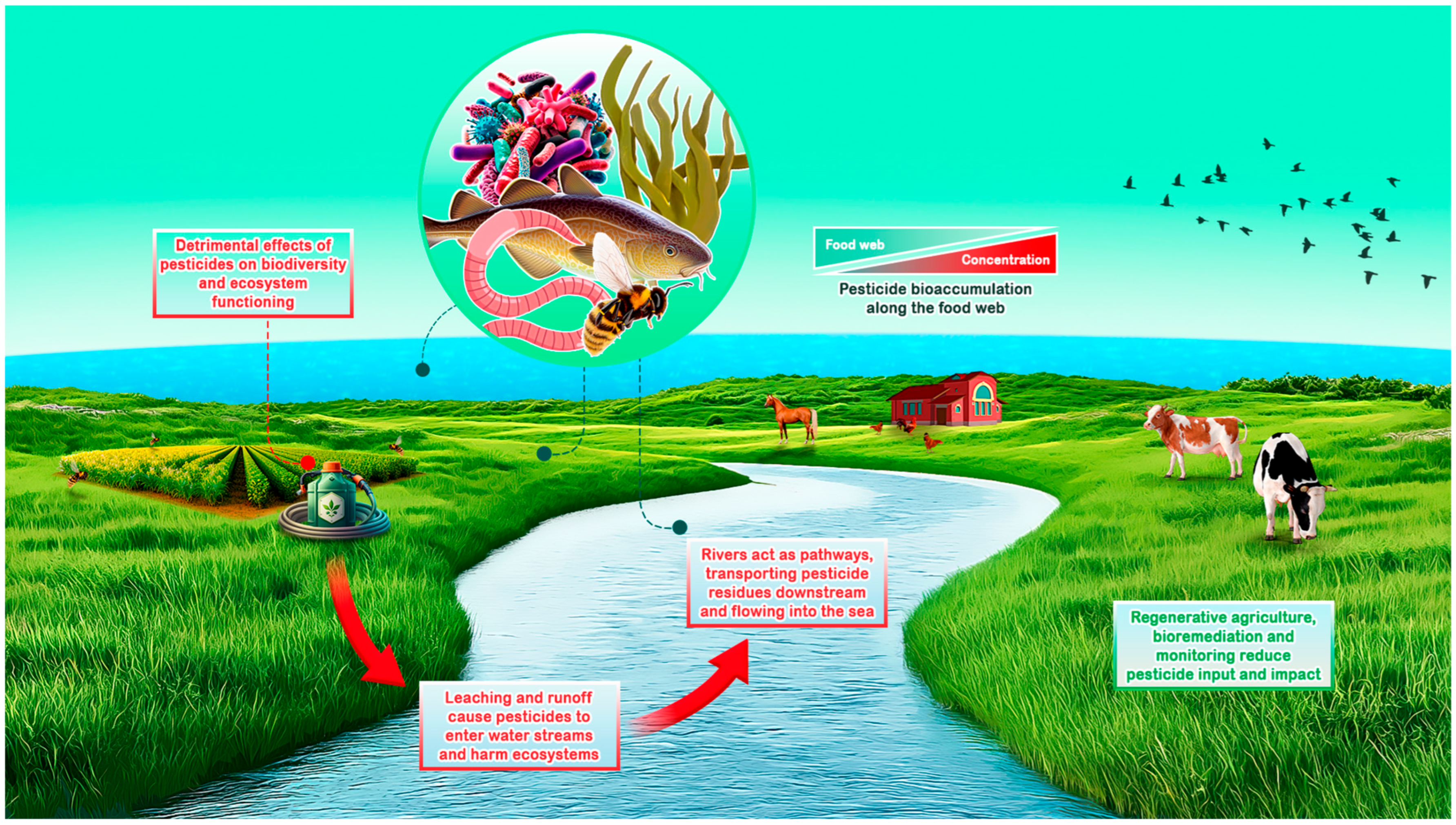
1. Introduction
2. What Are Pesticides?
2.1. Insecticides
2.2. Fungicides
2.3. Herbicides
3. Pesticide Detection
3.1. Analytical Detection Methods
3.2. Biosensor-Based Detection Methods
3.2.1. Electrochemical Biosensors
3.2.2. Fluorescence Biosensors
3.2.3. Colorimetric Biosensors
4. Influential Factors in Pesticide Leaching
5. Pesticide Properties and Weather Conditions
6. Effects of Pesticides on Terrestrial and Marine Life
6.1. Impact on Humans and Animals
6.2. Impact on Soil Microorganisms
| Chemical Category | Contaminated Ecosystem | References |
|---|---|---|
| Organophosphates, viologens, pyrethroids, anticholinesterases, neonicotinoids, triazines | soils, forests harboring fauna (arthropods, reptiles, amphibians) | [89,95,96,97,98,99] |
| Organochlorines, biocides, carbamates, repellants, biopesticides, household pesticides | agricultural lands isolated from and near residential lands, residential lands | [87,88,90,91] |
| Benomyl, metribuzin, imidacloprid | soil microbiome, rhizosphere | [92,93] |
6.3. Impact on Arthropods
6.4. Impact on Reptiles and Amphibians
6.5. Mechanisms of Toxicity
6.6. Impact on Marine Life
7. Mitigation and Management Strategies
8. Alternative Practices and Solutions
9. Conclusions and Research Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, N.F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.Q.; Xin, F.; Scherber, C. The impact of pesticides on non-target organisms. Biol. Sci. 2023. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application, and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Gupta, M.; Garg, N.K.; Srivastava, P.K. Soil water content influence on pesticide persistence and mobility. In Agricultural Water Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 307–327. [Google Scholar] [CrossRef]
- World Health Organization. Pesticide Residues in Food. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/pesticide-residues-in-food (accessed on 8 October 2025).
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Phillips, H.R. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Damalas, C.A.; Georgiou, E.B.; Theodorou, M.G. Pesticide use and safety practices among Greek tobacco farmers: A survey. Int. J. Environ. Health Res. 2006, 16, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C.A. Why farmers continue to use pesticides despite environmental health and sustainability costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Klein, K.; Dubey, A.; Donley, N. Pesticides and soil invertebrates: A hazard assessment. Front. Environ. Sci. 2021, 9, 643847. [Google Scholar] [CrossRef]
- Salingay, M.L.B.; Giesen, D.; Zevenbergen, C. Pesticide Assessment Using Passive Samplers in Two River System of Cagayan de Oro River Basin, Philippines; Pre-Print in Research Square, 2020. Available online: https://www.researchsquare.com/article/rs-98094/v1 (accessed on 8 October 2025).
- Hernández, A.F. Food safety: Pesticides. In Encyclopedia of Human Nutrition, 4th ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 1–4. [Google Scholar] [CrossRef]
- Browning, D.L.; Winter, C.K. Agricultural chemicals. In Foodborne Disease Handbook, Revised and Expanded: Volume 4: Seafood and Environmental Toxins, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Bolognesi, C.; Holland, N. CHAPTER 30: Pesticide Exposure and Its Effects on Micronucleus Frequency. In Issues in Toxicology; Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Jepson, P.C. Pesticides, Uses and Effects of. In Encyclopedia of Biodiversity, 3rd ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 1–7. [Google Scholar] [CrossRef]
- Mangan, R.L. Priorities in formulation and activity of adulticidal insecticide bait sprays for fruit flies. In Trapping And The Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Matthews, G.A. Pesticides: Health, Safety and the Environment; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Sargent, R.D.; Carrillo, J.; Kremen, C. Common pesticides disrupt critical ecological interactions. Trends Ecol. Evol. 2023, 38, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.A.; Bakir, M.A.; Khan, H.A. Microbial remediation of pesticides. In Pesticides: Evaluation of Environmental Pollution; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Hayes, T.B.; Hansen, M. From silent spring to silent night: Agrochemicals and the anthropocene. Elem. Sci. Anthr. 2017, 5, 57. [Google Scholar] [CrossRef]
- Colosio, C.; Rubino, F.M.; Moretto, A. Pesticides. In International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Pretty, J. The pesticide detox: Towards a more sustainable agriculture. In The Pesticide Detox: Towards a More Sustainable Agriculture; Routledge: London, UK, 2012. [Google Scholar] [CrossRef]
- Singh, S.; Datta, P. The Role of Cyanobacteria in the Biodegradation of Agrochemical Waste. In Environmental Waste Management; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Gupta, R.C.; Miller Mukherjee, I.R.; Malik, J.K.; Doss, R.B.; Dettbarn, W.-D.; Milatovic, D. Insecticides. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Gupta, R.C. Toxicity of pesticides. In Lu’s Basic Toxicology: Fundamentals, Target Organs, and Risk Assessment, 7th ed.; Routledge: London, UK, 2017; Available online: https://extension.psu.edu/toxicity-of-pesticides (accessed on 8 October 2025).
- Arya, S.; Kumar, R.; Prakash, O.; Rawat, A.; Pant, A.K. Impact of Insecticides on Soil and Environment and Their Management Strategies. In Agrochemicals in Soil and Environment: Impacts and Remediation; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Matsumura, F. Insecticides. In Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Gupta, R.C.; Milatovic, D. Insecticides. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Pant, M.; Dubey, S.; Patanjali, P.K. Recent advancements in bio-botanical pesticide formulation technology development. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. In Integrated Pest Management; Rainforest Alliance: New York, NY, USA, 2009; Volume 1. [Google Scholar] [CrossRef]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Soberón, M.; Bravo, A.; Blanco, C.A. Strategies to Reduce Insecticide Use in Agricultural Production. In Sustainable Food Science—A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1–4. [Google Scholar] [CrossRef]
- Barathi, S.; Sabapathi, N.; Kandasamy, S.; Lee, J. Present status of insecticide impacts and eco-friendly approaches for remediation-a review. Environ. Res. 2024, 240, 117432. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Bioremediation of Fungicide-contaminated Environment. In Biofungicides: Eco-Safety and Future Trends: Volume 2: Novel Sources and Mechanisms; Routledge: London, UK, 2023. [Google Scholar] [CrossRef]
- Preeti, S.; Aksha, S.; Nakuleshwar, J.D.; Nidhi, S.; Suresh, J.C. A review on toxicological effects of fungicides. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 348–360. [Google Scholar]
- Chauhan, J.; Sharma, A.K.; Bhattacharya, G. Comparative X-ray crystallographic studies of systemic Fungicide hexaconozole and tricyclazole. J. Phys. Conf. Ser. 2012, 365, 012012. [Google Scholar] [CrossRef]
- Thind, T.S. Role of fungicides in crop health management: Prospects and challenges. In Developments in Fungal Biology and Applied Mycology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Rodríguez, A. Fungicide residues. In Handbook of Food Analysis Second Edition: Residues and Other Food Component Analysis; CRC Press: Boca Raton, FL, USA, 2004; Volume 2. [Google Scholar]
- Pérez-Rodríguez, P.; Soto-Gómez, D.; de la Calle, I. Fungicides: Perspectives, Resistance Management and Risk Assessment; Nova Science Pub Inc: New York, NY, USA, 2018. [Google Scholar]
- Kenarova, A.; Boteva, S. Fungicides in agriculture and their side effects on soil enzyme activities: A review. Bulg. J. Agric. Sci. 2023, 29, 33–42. [Google Scholar]
- Mir, S.A.; Padhiary, A.; Ekka, N.J.; Baitharu, I.; Nayak, B. Environmental impacts of synthetic and biofungicides. In Current Developments in Biotechnology and Bioengineering: Pesticides: Human Health, Environmental Impacts and Management; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Leadbeater, A. Recent developments and challenges in chemical disease control. Plant Prot. Sci. 2015, 51, 163–169. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.A. Pesticides and Herbicides: Types, Uses, and Determination of Herbicides. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar] [CrossRef]
- Martínez, S.S.; Sánchez, J.V. Herbicides: Applications, degradation, and environmental impact. In Herbicides: Properties, Crop Protection and Environmental Hazards; Nova Science Pub Inc: New York, NY, USA, 2011. [Google Scholar]
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as Weed Control Agents: State of the Art: I. Weed Control Research and Safener Technology: The Path to Modern Agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef]
- Dewhurst, I. Pesticide Residues: Herbicides. In Encyclopedia of Food Safety; Academic Press: Cambridge, MA, USA, 2014; Volume 3. [Google Scholar] [CrossRef]
- Anand, T.P.; Shanthini, C.F.; Chellaram, C. Screening for herbicidal and growth promotor activities in marine bacteria. Int. J. Pharma Bio Sci. 2012, 3, 659–668. [Google Scholar]
- Hutchinson, J.T.; MacDonald, G.E.; Langeland, K.A. The potential for herbicide resistance in non-native plants in Florida’s natural areas. Nat. Areas J. 2007, 27, 258–263. [Google Scholar] [CrossRef]
- Székács, A. Herbicide mode of action. In Herbicides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–86. [Google Scholar] [CrossRef]
- Parven, A.; Meftaul, I.M.; Venkateswarlu, K.; Megharaj, M. Herbicides in modern sustainable agriculture: Environmental fate, ecological implications, and human health concerns. Int. J. Environ. Sci. Technol. 2024, 22, 1181–1202. [Google Scholar] [CrossRef]
- Lika, E.; Sutherland, C.; Gleim, S.; Smyth, S.J. Quantifying changes in the environmental impact of in-crop herbicide use in Saskatchewan, Canada. Weed Technol. 2024, 38, e28. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Cancho-Grande, B.; Rial-Otero, R.; Simal-Gándara, J. The dissipation rates of cyprodinil, fludioxonil, procymidone and vinclozoline during storage of grape juice. Food Control 2006, 17, 1012–1017. [Google Scholar] [CrossRef]
- Gilevska, T.; Wiegert, C.; Droz, B.; Junginger, T.; Prieto-Espinoza, M.; Borreca, A.; Imfeld, G. Simple extraction methods for pesticide compound-specific isotope analysis from environmental samples. MethodsX 2022, 9, 101880. [Google Scholar] [CrossRef] [PubMed]
- Farina, Y.; Abdullah, M.P.; Bibi, N.; Khalik, W.M.A.W.M. Determination of pesticide residues in leafy vegetables at parts per billion levels by a chemometric study using GC-ECD in Cameron Highlands, Malaysia. Food Chem. 2017, 224, 55–61. [Google Scholar] [CrossRef]
- Rashidi Nodeh, H.; Wan Ibrahim, W.A.; Kamboh, M.A.; Sanagi, M.M. New magnetic graphene-based inorganic–organic sol-gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction. Chemosphere 2017, 166, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Verette, E. Chromatography Automation. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 343–352. [Google Scholar] [CrossRef]
- Balsebre, A.; Báez, M.E.; Martínez, J.; Fuentes, E. Matrix solid-phase dispersion associated to gas chromatography for the assessment in honey bee of a group of pesticides of concern in the apicultural field. J. Chromatogr. A 2018, 1567, 47–54. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Mastovska, K.; Dorweiler, K.J.; Leepipatpiboon, N. Extension of the QuEChERS Method for Pesticide Residues in Cereals to Flaxseeds, Peanuts, and Doughs. J. Agric. Food Chem. 2010, 58, 5950–5958. [Google Scholar] [CrossRef]
- Kaewsuya, P.; Brewer, W.E.; Wong, J.; Morgan, S.L. Automated QuEChERS Tips for Analysis of Pesticide Residues in Fruits and Vegetables by GC-MS. J. Agric. Food Chem. 2013, 61, 2299–2314. [Google Scholar] [CrossRef]
- Sack, C.; Vonderbrink, J.; Smoker, M.; Smith, R.E. Determination of Acid Herbicides Using Modified QuEChERS with Fast Switching ESI+/ESI− LC-MS/MS. J. Agric. Food Chem. 2015, 63, 9657–9665. [Google Scholar] [CrossRef]
- Moawed, E.A.; Radwan, A.M. Application of acid modified polyurethane foam surface for detection and removing of organochlorine pesticides from wastewater. J. Chromatogr. B 2017, 1044–1045, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lan, T.; Li, X.; Chen, Y.; Yang, Q.; Qu, B.; Zhang, Y.; Pan, C. A comparison of the determination of multiple pesticide residues in fruits, vegetables, and edible fungi using gas chromatography combined with filtration purification and solid-phase extraction. RSC Adv. 2024, 14, 16898–16911. [Google Scholar] [CrossRef]
- Xiao, Z.; He, M.; Chen, B.; Hu, B. Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to gas chromatography-flame photometric detection for the determination of organophosphorus pesticides in environmental water samples. Talanta 2016, 156–157, 126–133. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Li, D.; Kang, L.; Zhou, C.; Zhang, J.; Pan, C. Multi-residue analytical method development and risk assessment of 56 pesticides and their metabolites in tea by chromatography tandem mass spectroscopy. Food Chem. 2022, 375, 131819. [Google Scholar] [CrossRef]
- Cotton, J.; Leroux, F.; Broudin, S.; Poirel, M.; Corman, B.; Junot, C.; Ducruix, C. Development and validation of a multiresidue method for the analysis of more than 500 pesticides and drugs in water based on on-line and liquid chromatography coupled to high resolution mass spectrometry. Water Res. 2016, 104, 20–27. [Google Scholar] [CrossRef]
- Schwanz, T.G.; Carpilovsky, C.K.; Weis, G.C.C.; Costabeber, I.H. Validation of a multi-residue method and estimation of measurement uncertainty of pesticides in drinking water using gas chromatography–mass spectrometry and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1585, 10–18. [Google Scholar] [CrossRef]
- López-Vázquez, J.; Pérez-Mayán, L.; Fernández-Fernández, V.; Cela, R.; Rodríguez, I. Direct, automated and sensitive determination of glyphosate and related anionic pesticides in environmental water samples using solid-phase extraction on-line combined with liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2023, 1687, 463697. [Google Scholar] [CrossRef]
- Ma, J.; Fan, S.; Yang, L.; He, L.; Zhai, H.; Ren, X.; Li, Q.; Zhang, Y. Rapid screening of 420 pesticide residues in fruits and vegetables using ultra high-performance liquid chromatography combined with quadrupole-time of flight mass spectrometry. Food Sci. Hum. Wellness 2023, 12, 1064–1070. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Zhang, Y.; Zhou, K. Simultaneous Determination of Seven Carbamate Pesticide Residues in Vegetable by Capillary Electrophoresis with Solid Phase Microextraction. Int. J. Electrochem. Sci. 2021, 16, 210652. [Google Scholar] [CrossRef]
- Qian, G.; Wang, L.; Wu, Y.; Zhang, Q.; Sun, Q.; Liu, Y.; Liu, F. A monoclonal antibody-based sensitive enzyme-linked immunosorbent assay ELISA for the analysis of the organophosphorous pesticides chlorpyrifos-methyl in real samples. Food Chem. 2009, 117, 364–370. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Gai, T.; Nie, J.; Ding, Z.; Wu, W.; Liu, X. Progress of rapid detection of pesticides in fruits and vegetables. Front. Food Sci. Technol. 2023, 3, 1253227. [Google Scholar] [CrossRef]
- Çevik, S.; Timur, S.; Anik, Ü. Polyallylamine hydrochloride Functionalized Multiwalled Carbon Nanotube Modified Carbon Paste Electrode as Acetylcholinesterase Biosensor Transducer. Electroanalysis 2013, 25, 2377–2383. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, B.; Zhang, Y.; Zhao, G.; Zhao, B. A multi-residue electrochemical biosensor based on graphene/chitosan/parathion for sensitive organophosphorus pesticides detection. Chem. Phys. Lett. 2021, 767, 138355. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, H.; Zhao, Y.; Wang, Y.; Li, F. Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens. Bioelectron. 2022, 199, 113906. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Lin, L.; Guo, H.; Yang, F. A color-changed fluorescence sensor for pesticide triclopyr 2-butoxyethyl ester based on naphthalimide Schiff-base. J. Photochem. Photobiol. A Chem. 2024, 457, 115894. [Google Scholar] [CrossRef]
- Sahu, B.; Kurrey, R.; Khalkho, B.R.; Deb, M.K. α-Cyclodextrin functionalized silver nanoparticles as colorimetric sensor for micro extraction and trace level detection of chlorpyrifos pesticide in fruits and vegetables. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 129947. [Google Scholar] [CrossRef]
- Tai, S.; Wang, J.; Sun, F.; Pan, Q.; Peng, C.; Wang, Z. A colorimetric sensor array based on nanoceria crosslinked and heteroatom-doped graphene oxide nanoribbons for the detection and discrimination of multiple pesticides. Anal. Chim. Acta 2023, 1283, 341929. [Google Scholar] [CrossRef] [PubMed]
- Nolan, B.; Dubus, I.; Surdyk, N.; Fowler, H.; Burton, A.; Hollis, J.; Reichenberger, S.; Jarvis, N. Identification of key climatic factors regulating the transport of pesticides in leaching and to tile drains. Pest Manag. Sci. 2008, 64, 933–944. [Google Scholar] [CrossRef]
- Lammoglia, S.; Moeys, J.; Barriuso, E.; Larsbo, M.; Marín-Benito, J.; Justes, E.; Alletto, L.; Ubertosi, M.; Nicolardot, B.; Munier-Jolain, N.; et al. Sequential use of the STICS crop model and of the MACRO pesticide fate model to simulate pesticides leaching in cropping systems. Environ. Sci. Pollut. Res. 2017, 24, 6895–6909. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lucas, G.; Vela, N.; Aatik, A.; Navarro, S. Environmental Risk of Groundwater Pollution by Pesticide Leaching through the Soil Profile. In Pesticides—Use and Misuse and Their Impact in the Environment; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Renaud, F.; Brown, C.; Fryer, C.; Walker, A. A lysimeter experiment to investigate temporal changes in the availability of pesticide residues for leaching. Environ. Pollut. 2004, 131, 81–91. [Google Scholar] [CrossRef]
- Nicholls, P. Factors influencing entry of pesticides into soil water. Pestic. Sci. 1988, 22, 123–137. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Vryzas, Z. Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Curr. Opin. Environ. Sci. Health 2018, 4, 5–9. [Google Scholar] [CrossRef]
- Lu, J.L. Knowledge, attitudes, and practices on pesticide among farmers in the Philippines. Acta Medica Philipp. 2022, 56, 29–36. [Google Scholar] [CrossRef]
- Lu, J.L.; Salas, E.K. Occupational Risk Exposures and Adverse Health Findings Among Farmers in Southern Philippines. Acta Medica Philipp. 2021, 55, 621–631. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhang, Y.M.; Li, Z.W.; Xing, X.J. Effect of organophosphorus pesticide pollution on soil animals. J. Environ. Sci. 2000, 1, 49–58. [Google Scholar]
- Agrawal, A.N.J.U.; Sharma, B. Pesticides induced oxidative stress in mammalian systems. Int. J. Biol. Med. Res. 2010, 1, 90–104. [Google Scholar]
- Lu, J.L.; Cosca, K.Z.; Del Mundo, J. Trends of pesticide exposure and related cases in the Philippines. J. Rural. Med. 2010, 5, 153–164. [Google Scholar] [CrossRef]
- Paprah, S.; Addo-Fordjour, P.; Fei-Baffoe, B.; Boampong, K.; Avicor, S.; Damsere-Derry, J. Effects of Pesticide Application on Soil Bacteria Community Structure as Revealed by Pacbio Sequencing. Available online: https://ssrn.com/abstract=4617341 (accessed on 8 October 2025).
- Streletskii, R.; Astaykina, A.; Cheptsov, V.; Belov, A.; Gorbatov, V. Effects of the Pesticides Benomyl, Metribuzin and Imidacloprid on Soil Microbial Communities in the Field. Agriculture 2023, 13, 1330. [Google Scholar] [CrossRef]
- Drocco, C.; Coors, A.; Devers, M.; Spor, A.; Martin, F.; Rouard, N. Evaluating the Effects of Environmental Disturbances and Pesticide Mixtures on Soil Microbial Endpoints. Peer Community J. 2025, 5, e33. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Indirect effect of pesticides on insects and other arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Wanger, T.C.; Brook, B.W.; Evans, T.; Tscharntke, T. Pesticides reduce tropical amphibian and reptile diversity in agricultural landscapes in Indonesia. PeerJ 2023, 11, e15046. [Google Scholar] [CrossRef]
- Khan, M.Z.; Law, F.C. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: A review. Proc. Pak. Acad. Sci. USA 2005, 42, 315–323. [Google Scholar]
- Gasso, V.Y.; Yermolenko, S.V.; Bobyliov, Y.P.; Hahut, A.M.; Huslystyi, A.O.; Hasso, I.A.; Petrushevskyi, V.B. Biomarkers of the influence of pyrethroids and neonicotinoids on amphibian larvae. Ecol. Noospherology 2020, 31, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.A.; McDaniel, T.V.; de Solla, S.R. Atrazine in the environment and its implications for amphibians and reptiles. Ecotoxicol. Amphib. Reptiles 2010, 2, 227–259. [Google Scholar]
- Coman, G.; Farcas, A.; Matei, A.V.; Florian, C. Pesticides Mechanisms of action in living organisms. In NATO Science for Peace and Security Ser. C Environmental Security; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–184. [Google Scholar] [CrossRef]
- Saratovskikh, E.A. Molecular mechanisms of the damage effect of pesticides of various structures on target organisms. Russ. J. Phys. Chem. B 2017, 11, 652–662. [Google Scholar] [CrossRef]
- Mileson, B.E.; Chambers, J.E.; Chen, W.L.; Dettbarn, W.; Ehrich, M.; Eldefrawi, A.T.; Gaylor, D.W.; Hamernik, K.; Hodgson, E.; Karczmar, A.G.; et al. Common mechanism of toxicity: A case study of organophosphorus pesticides. Toxicol. Sci. 1998, 41, 8–20. [Google Scholar] [CrossRef]
- Marutescu, L.; Chifiriuc, M.C. Molecular mechanisms of pesticides toxicity. In New Pesticides and Soil Sensors; Academic Press: Cambridge, MA, USA, 2017; pp. 393–435. [Google Scholar]
- Katagi, T.; Ose, K. Toxicity, bioaccumulation and metabolism of pesticides in the earthworm. J. Pestic. Sci. 2015, 40, 69–81. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mathew, B.T.; Aljasmi, A.N.; Alhammadi, H.S.; Salem, H.M.; El-Mageed, T.a.A.; et al. Ecological impacts and management strategies of pesticide pollution on aquatic life and human beings. Mar. Pollut. Bull. 2024, 206, 116613. [Google Scholar] [CrossRef]
- Stemmler, I.; Lammel, G. Cycling of DDT in the global environment 1950–2002: World ocean returns the pollutant. Geophys. Res. Lett. 2009, 36, 24. [Google Scholar] [CrossRef]
- Stockholm Convention on Persistent Organic Pollutants. UNEP: Persistent Organic Pollutants. 2001. Available online: http://www.pops.int/ (accessed on 8 October 2025).
- Fontanals, N.; Marce, R.M. Analytical Methods for Environmental Contaminants of Emerging Concern; John Wiley Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Kim, S. Trophic transfer of organochlorine pesticides through food-chain in coastal marine ecosystem. Environ. Eng. Res. 2019, 25, 43–51. [Google Scholar] [CrossRef]
- Sundhar, S.; Shakila, R.J.; Jeyasekaran, G.; Aanand, S.; Shalini, R.; Arisekar, U.; Surya, T.; Malini, N.a.H.; Boda, S. Risk assessment of organochlorine pesticides in seaweeds along the Gulf of Mannar, Southeast India. Mar. Pollut. Bull. 2020, 161, 111709. [Google Scholar] [CrossRef]
- Hook, S.E.; Doan, H.; Gonzago, D.; Musson, D.; Du, J.; Kookana, R.; Sellars, M.J.; Kumar, A. The impacts of modern-use pesticides on shrimp aquaculture: An assessment for north eastern Australia. Ecotoxicol. Environ. Saf. 2018, 148, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Jia, J.; Liu, A.; Yu, Z.; Zhao, Z. Pollution levels of banned and non-banned pesticides in surface sediments from the East China Sea. Mar. Pollut. Bull. 2019, 139, 332–338. [Google Scholar] [CrossRef]
- Charles, L. Marine Environments: Diversity, Threats and Conservation; Nova Science Publishers: New York, NY, USA, 2020. [Google Scholar]
- Peters, E.C.; Gassman, N.J.; Firman, J.C.; Richmond, R.H.; Power, E.A. Ecotoxicology of tropical marine ecosystems. Environ. Toxicol. Chem. 1997, 16, 12–40. [Google Scholar] [CrossRef]
- Glynn, P.W.; Szmant, A.M.; Corcoran, E.F.; Cofer-Shabica, S.V. Condition of coral reef cnidarians from the northern Florida reef tract: Pesticides, heavy metals, and histopathological examination. Mar. Pollut. Bull. 1989, 20, 568–576. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Jones, R.; Muller, J.; Haynes, D.; Schreiber, U. Effects of herbicides diuron and atrazine on corals of the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2003, 251, 153–167. [Google Scholar] [CrossRef]
- Brodie, J.; Landos, M. Pesticides in Queensland and Great Barrier Reef waterways—potential impacts on aquatic ecosystems and the failure of national management. Estuar. Coast. Shelf Sci. 2019, 230, 106447. [Google Scholar] [CrossRef]
- Tulcan, R.X.S.; Ouyang, W.; Gu, X.; Lin, C.; Tysklind, M.; Wang, B. Typical herbicide residues, trophic transfer, bioconcentration, and health risk of marine organisms. Environ. Int. 2021, 152, 106500. [Google Scholar] [CrossRef]
- Islam, M.S.; Tanaka, M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef]
- Matsunaka, S.; Hutson, D.H.; Murphy, S.D. Mode of Action, Metabolism and Toxicology: Pesticide Chemistry: Human Welfare and the Environment; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Osuna-Flores, I.; Pérez-Morales, A.; Olivos-Ortiz, A.; Álvarez-González, C.A. Effect of organophosphorus pesticides in juveniles of Litopenaeus vannamei: Alteration of glycogen, triglycerides, and proteins. Ecotoxicology 2019, 28, 698–706. [Google Scholar] [CrossRef]
- Valdés-Castro, V.; Fernandez, C. Effect of three pesticides used in salmon farming on ammonium uptake in Central-Southern and Northern Patagonia, Chile. Front. Mar. Sci. 2021, 7, 602002. [Google Scholar] [CrossRef]
- Häder, D.; Helbling, E.W.; Villafañe, V.E. Anthropogenic Pollution of Aquatic Ecosystems; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Halstead, N.T.; Civitello, D.J.; Rohr, J.R. Comparative toxicities of organophosphate and pyrethroid insecticides to aquatic macroarthropods. Chemosphere 2015, 135, 265–271. [Google Scholar] [CrossRef]
- Brain, R.A.; Anderson, J.C.; Hanson, M.L. Toxicity of Atrazine to Marine Invertebrates Under Flow-Through Conditions—Eastern Oyster (Crassostrea virginica) and Mysid Shrimp (Americamysis bahia). Water Air Soil Pollut. 2021, 232, 142. [Google Scholar] [CrossRef]
- Yoon, D.; Park, J.C.; Park, H.G.; Lee, J.; Han, J. Effects of atrazine on life parameters, oxidative stress, and ecdysteroid biosynthetic pathway in the marine copepod Tigriopus japonicus. Aquat. Toxicol. 2019, 213, 105213. [Google Scholar] [CrossRef]
- Francolino, B.Y.; Valdes, Y.; De Luna, C.A.; De França, F.J.L.; Moens, T.; Santos, G.a.P.D. Short-term lethal and sublethal atrazine effects on Litoditis marina: Towards a nematode model for marine toxicity assessment? Ecol. Indic. 2021, 126, 107642. [Google Scholar] [CrossRef]
- Ying, G.G. Remediation and mitigation strategies. In Integrated Analytical Approaches for Pesticide Management; Academic Press: Cambridge, MA, USA, 2018; pp. 207–217. [Google Scholar]
- Berame, J.; Mariano, M.; Lascano, J.; Sariana, L.; Macasinag, L.; Alam, Z. Environmental biomonitoring of terrestrial ecosystems in the Philippines: A critical assessment and evaluation. AMURE Int. J. Ecol. Conserv. 2020, 32, 1–24. [Google Scholar]
- Trocio, D.Y.C.; Paguntalan, D.P. Review on the Use of Arbuscular Mycorrhizal Fungi in Bioremediation of Heavy Metal Contaminated Soils in the Philippines. Philipp. J. Sci. 2023, 152, 1139–1159. [Google Scholar] [CrossRef]
- Rose, S.; Carter, A. Agrochemical Leaching and Water Contamination. In Conservation Agriculture; Springer: Dordrecht, Netherlands, 2003; pp. 417–424. [Google Scholar] [CrossRef]
- Lammoglia, S.; Makowski, D.; Moeys, J.; Justes, E.; Barriuso, E.; Mamy, L. Sensitivity analysis of the STICS-MACRO model to identify cropping practices reducing pesticides losses. Sci. Total Environ. 2017, 580, 117–129. [Google Scholar] [CrossRef]
- Lewan, E.; Kreuger, J.; Jarvis, N. Implications of precipitation patterns and antecedent soil water content for leaching of pesticides from arable land. Agric. Water Manag. 2009, 96, 1633–1640. [Google Scholar] [CrossRef]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants 2017, 3, 17008. [Google Scholar] [CrossRef]
- Chèze, B.; David, M.; Martinet, V. Understanding farmers’ reluctance to reduce pesticide use: A choice experiment. Ecol. Econ. 2020, 167, 106349. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, X. Farm Machine Use and Pesticide Expenditure in Maize Production: Health and Environment Implications. Int. J. Environ. Res. Public Health 2019, 16, 1808. [Google Scholar] [CrossRef]
- Muneret, L.; Mitchell, M.; Seufert, V.; Aviron, S.; Djoudi, E.; Pétillon, J.; Plantegenest, M.; Thiéry, D.; Rusch, A. Evidence that organic farming promotes pest control. Nat. Sustain. 2018, 1, 361–368. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Sattler, C.; Schrader, J.; Flor, R.; Keo, M.; Chhun, S.; Choun, S.; Hadi, B.; Settele, J. Reducing Pesticides and Increasing Crop Diversification Offer Ecological and Economic Benefits for Farmers—A Case Study in Cambodian Rice Fields. Insects 2021, 12, 267. [Google Scholar] [CrossRef]
- Abdollahzadeh, G.; Sharifzadeh, M.S.; Damalas, C.A. Perceptions of the beneficial and harmful effects of pesticides among Iranian rice farmers influence the adoption of biological control. Crop Prot. 2015, 75, 124–131. [Google Scholar] [CrossRef]
- Brühl, C.A.; Zaller, J.G. Indirect herbicide effects on biodiversity, ecosystem functions, and interactions with global changes. In Herbicides: Chemistry, Efficacy, Toxicology, and Environmental Impacts; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Prudente, M.S.; Malarvannan, G.; Tanabe, S. Chapter 12 Persistent Toxic Substances in the Philippine environment. In Developments in Environmental Science; Elsevier: Amsterdam, The Netherlands, 2007; pp. 559–585. [Google Scholar] [CrossRef]
- Leung, K.M.; Yeung, K.W.; You, J.; Choi, K.; Zhang, X.; Smith, R.; Zhou, G.; Yung, M.M.; Arias-Barreiro, C.; An, Y.; et al. Toward Sustainable Environmental Quality: Priority Research Questions for Asia. Environ. Toxicol. Chem. 2020, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Melchor-Martínez, E.M.; Macías-Garbett, R.; Alvarado-Ramírez, L.; Araújo, R.G.; Sosa-Hernández, J.E.; Ramírez-Gamboa, D.; Parra-Arroyo, L.; Alvarez, A.G.; Monteverde, R.P.B.; Cazares, K.a.S.; et al. Towards a Circular Economy of Plastics: An evaluation of the systematic transition to a new generation of bioplastics. Polymers 2022, 14, 1203. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Muñoz-Mira, S.; Durán-Patrón, R.; Aleu, J. Marine-derived fungi as biocatalysts. Frontiers in Microbiology 2023, 14, 1125639. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P.; Villeneuve, J.; Cattini, C.; Bajet, C.M.; Navarro-Calingacion, M. Chlorinated hydrocarbons in sediments from Manila Bay, the Philippines. Int. J. Environ. Stud. 2010, 67, 493–504. [Google Scholar] [CrossRef]
| Pesticide Group | Chemical Category | Chemical Name | Molecular Formula |
|---|---|---|---|
| Insecticides | Organochlorine | Aldrin | C12H8Cl6 |
| Organochlorine | Chlordane | C10H6Cl8 | |
| Organochlorine | DDT (Dichloro-diphenyl-trichloroethane) | C14H9Cl5 | |
| Organochlorine | Dieldrin | C12H8Cl6O | |
| Organochlorine | Endosulfan | C9H6Cl6O3S | |
| Organochlorine | Endrin | C12H8Cl6O | |
| Organochlorine | Heptachlor | C10H5Cl7 | |
| Organochlorine | Hexachlorobenzene (HCB) | C6Cl6 | |
| Organochlorine | Hexachlorocyclohexane (HCH) | C6H6Cl6 | |
| Organochlorine | Lindane | C6H6Cl6 | |
| Organochlorine | Methoxychlor | C16H15Cl3O2 | |
| Organochlorine | Mirex | C10Cl12 | |
| Organochlorine | Nonachlor | C10H5Cl9 | |
| Organochlorine | Pentachlorophenol | C6Cl5OH | |
| Organophosphate | Chlorpyrifos | C9H11Cl3NO3PS | |
| Organophosphate | Iprobenfos | C13H21O3PS | |
| Organophosphate | Temephos | C16H20O6P2S3 | |
| Organophosphate | Malathion | C10H19O6PS2 | |
| Carbamate | Carbaryl | C12H11NO2 | |
| Carbamate | Aldicarb | C7H14N2O2S | |
| Pyrethroid | Permethrin | C21H20Cl2O3 | |
| Pyrethroid | Bifenthrin | C23H22ClF3O2 | |
| Neonicotinoid | Acetamiprid | C10H11ClN4 | |
| Neonicotinoid | Imidacloprid | C9H10ClN5O2 | |
| Neonicotinoid | Thiacloprid | C10H9ClN4S | |
| Neonicotinoid | Thiamethoxam | C8H10ClN5O3S | |
| Fungicides | Acylalanine | Metalaxyl | C15H21NO4 |
| Anilinopyrimidine | Pyrimethanil | C12H13N3 | |
| Azole (Triazole) | Propiconazole | C15H17Cl2N3O2 | |
| Azole (Triazole) | Tebuconazole | C16H22ClN3O | |
| Benzimidazole | Carbendazim | C9H9N3O2 | |
| Dithiocarbamate | Mancozeb | C4H6MnN2S4·Zn | |
| Dithiocarbamate | Ziram | C6H12N2S4 | |
| Cyanopyrrole | Fludioxonil | C12H6F2N2O2 | |
| Dicarboximide | Iprodione | C13H13Cl2N3O3 | |
| Morpholine | Fenpropimorph | C20H33NO | |
| N-trihalomethylthio | Folpet | C9H4Cl3NO2S | |
| Organophosphorus | Fosetyl-Al | C6H18AlO9P3 | |
| Pyrimidine | Fenarimol | C17H12Cl2N2O | |
| Pyrimidinyl Carbinol | Fenhexamid | C14H17Cl2NO2 | |
| Carboxylic Acid Amide (CAA) | Mandipropamid | C23H22ClNO4 | |
| Carboxylic Acid Amide (CAA) | Dimethomorph | C21H22ClNO4 | |
| Herbicides | Triazine | Atrazine | C8H14ClN5 |
| Triazine | Simazine | C7H12ClN5 | |
| Organophosphonate | Glyphosate | C3H8NO5P | |
| Organophosphonate | Glufosinate | C5H12NO4P | |
| Phenoxyacetic acid | 2,4-D | C8H6Cl2O3 | |
| Phenylurea | Diuron | C9H10Cl2N2O | |
| Chloroacetamide | Alachlor | C14H20ClNO2 | |
| Bipyridyl | Paraquat | C12H14N2Cl2 | |
| Bipyridyl | Diquat | C12H12N2 |
| Representative Chemicals | Benefits | Harms (Environmental/Health) | Biota Impacts |
|---|---|---|---|
| Insecticide | |||
| Chlorinated hydrocarbons | Effective in eliminating or managing insect pests that threaten human health, agricultural productivity, and environmental stability [23,24,25,26] | Persistence in the environment; potential contamination [27,28] | Mortality of non-target organisms; disruption of natural pest control mechanisms; contamination of food chains [30,31,32] |
| Organophosphorus compounds | Inhibit critical enzymes in the insect nervous system [24,27,28] | Potential environmental hazards [27,28] | Toxicity to non-target organisms; ecosystem imbalances [30,31,32] |
| Carbamates | Inhibit critical enzymes in the insect nervous system [24,27,28] | Potential environmental hazards [27,28] | Toxicity to non-target organisms; ecosystem imbalances [30,31,32] |
| Pyrethroids | Interfere with insect nerve impulses [24,27,28] | Potential environmental hazards [27,28] | Resistance development in insect populations [33] |
| Neonicotinoids | Interfere with receptor function in the insect nervous system [24,27,28] | Potential environmental hazards [27,28] | Resistance development in insect populations [33] |
| Biological insecticides (microbial, biochemical, botanicals) | More eco-friendly with greater target specificity; considered safer and more sustainable than synthetic insecticides [29] | Effectiveness in large-scale agriculture remains under study [27] | Generally lower impact on non-target organisms [29] |
| Fungicide | |||
| Acylalanine, Anilinopyrimidine, Azole, Benzimidazole, and others | Prevention and eradication of fungal infections in plants and seeds; ensure optimum crop yields, particularly in vulnerable crops such as potatoes, melons, and grapes [34,35,36,37] | Overuse can lead to fungicide-resistant strains of pathogens, requiring higher dosages or new chemicals, increasing costs and environmental risks [34,37] | Disrupt local ecosystems; affect non-target organisms; contribute to ecological imbalances [34,37,38] |
| Systemic fungicides (absorbed and distributed within plants) | Provide comprehensive protection by eradicating established infections and preventing new ones [36] | Persistence and residues in food, soil, air, and water; pollution from production processes [39] | Reduce soil microbial enzyme activity, lowering soil fertility and ecosystem health; accumulation disrupts ecological functions and biodiversity [40,41] |
| General fungicide use (various families) | Vital for managing fungal pathogens by inhibiting their growth and spread [34,35,36] | Environmental persistence; regulatory challenges related to resistance and ecological risks [38,39,42] | Residues accumulate in the environment, posing risks to biodiversity and human health [39,40,41] |
| Herbicide | |||
| General herbicides (chemical substances targeting weeds) | Enhance food production by reducing weed competition; allow more efficient farming practices such as reduced tillage and earlier planting dates [43,44]; improve crop quantity and quality; economical weed control solution [38,45] | Environmental, ecological, and human health risks associated with widespread use [44]; persistence of residues in food raises human exposure concerns [46] | Soil degradation, water contamination, and harm to non-target plant species, reducing biodiversity and disrupting ecosystem balance [44] |
| Targeted herbicide actions (e.g., ALS inhibitors, oxidative stress inducers) | Provide precise weed population control; ensure sustainable crop productivity [47,48,49] | Development of herbicide-resistant weed species increases environmental impact and production costs [44] | Long-term ecological imbalance due to resistant weed species and reliance on more potent chemicals [44] |
| Systemic and pre-emergence herbicides | Effective in controlling weeds before or during germination; improve crop productivity [47,48,49] | Environmental persistence threatens biodiversity, environmental health, and food safety [50] | Persistence leads to degradation of non-target plant species and disruption of ecological balance [50] |
| Chemical Category | Contaminated Ecosystem | References |
|---|---|---|
| Organochlorine | ||
| 2,4-Dichlorophenoxyacetic acid | mangrove forest | [114] |
| Aldrin | estuary, marine sediment, seaweed bed | [110,115] |
| Chlordane | marine sediment, coral reef | [114,115,116] |
| dicholor-diethy-tricholorethane (DDT) | marine sediment, mangrove forest, seagrass meadow, seaweed bed, coral reef | [110,114,115,116] |
| Dieldrin | marine sediment, coral reef | [114,115,116] |
| Endosulfan | estuary, seaweed bed, coral reef | [114,115,116] |
| Endrin | marine sediment, seaweed bed, coral reef | [114,115,116] |
| Heptachlor | marine sediment, seaweed bed, coral reef | [110,114,116] |
| Hexochlorobenzene (HCB) | marine sediment | [116] |
| Hexocholorcyclohexane (HCH) | marine sediment, seaweed bed | [110,116] |
| Lindane | marine sediment, seagrass meadow, coral reef | [110,114,115,116] |
| Methoxychlor | seaweed bed, coral reef | [110,114,115] |
| Mirex | coral reef | [115] |
| Nonachlor | marine sediment | [116] |
| Pentachlorophenol | seagrass meadow | [114] |
| Organophosphate | ||
| Chlorpyrifos | estuary | [114] |
| Iprobenfos | marine sediment | [112] |
| Temephos | mangrove forest | [114] |
| Pyrethroid | ||
| Bifenthrin | estuary | [111] |
| Triazine | ||
| Atrazine | mangrove forest, seagrass meadow, coral reef | [114,117,118,119] |
| Carbamate | ||
| Carbaryl | seagrass meadow | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdadero, F.X.D.; Agarap, A.Z.; Macatingrao, C.N.E.; Ordonez, I.A., Jr.; Tavu, L.E.J.; Pires, D.; Balendres, M.A.O. Pesticides in the Environment: Benefits, Harms, and Detection Methods. Sci 2025, 7, 171. https://doi.org/10.3390/sci7040171
Verdadero FXD, Agarap AZ, Macatingrao CNE, Ordonez IA Jr., Tavu LEJ, Pires D, Balendres MAO. Pesticides in the Environment: Benefits, Harms, and Detection Methods. Sci. 2025; 7(4):171. https://doi.org/10.3390/sci7040171
Chicago/Turabian StyleVerdadero, Francis Xavier D., Alfred Z. Agarap, Czarina Nicole E. Macatingrao, Isagani A. Ordonez, Jr., Lady Edlenill J. Tavu, David Pires, and Mark Angelo O. Balendres. 2025. "Pesticides in the Environment: Benefits, Harms, and Detection Methods" Sci 7, no. 4: 171. https://doi.org/10.3390/sci7040171
APA StyleVerdadero, F. X. D., Agarap, A. Z., Macatingrao, C. N. E., Ordonez, I. A., Jr., Tavu, L. E. J., Pires, D., & Balendres, M. A. O. (2025). Pesticides in the Environment: Benefits, Harms, and Detection Methods. Sci, 7(4), 171. https://doi.org/10.3390/sci7040171








