Kinetic and Thermodynamic Study of Methylene Blue Adsorption onto Activated Carbon Obtained from the Peel of musa paradisiaca
Abstract
1. Introduction
2. Materials and Methods
2.1. Activated Carbon Synthesis
2.2. Chemical Modification of Activated Carbon
2.3. Characterization of Activated Carbon
2.4. Isothermal, Kinetic, and Thermodynamic Study
3. Results and Discussion
3.1. Textural Characterization
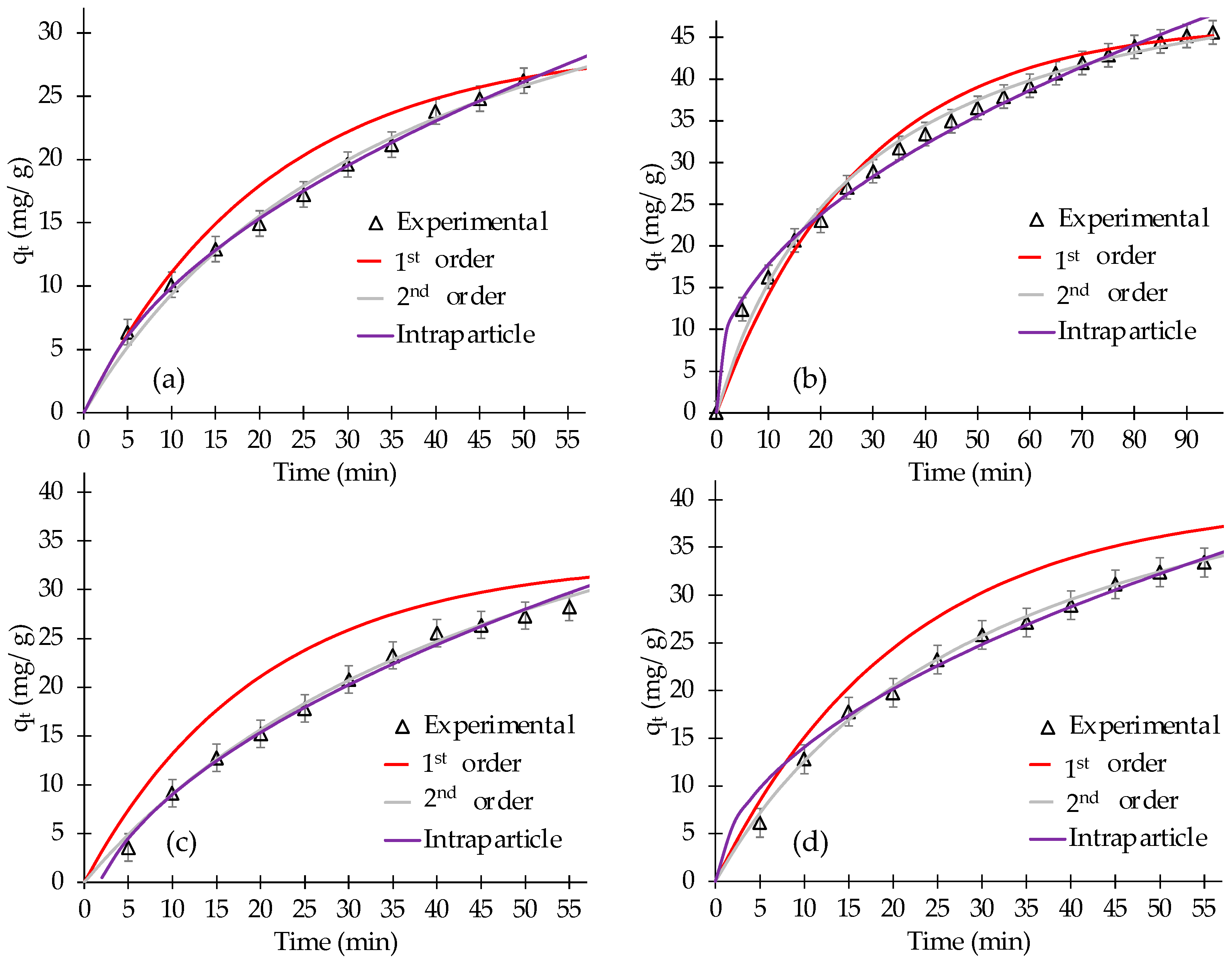
3.2. Kinetic Study
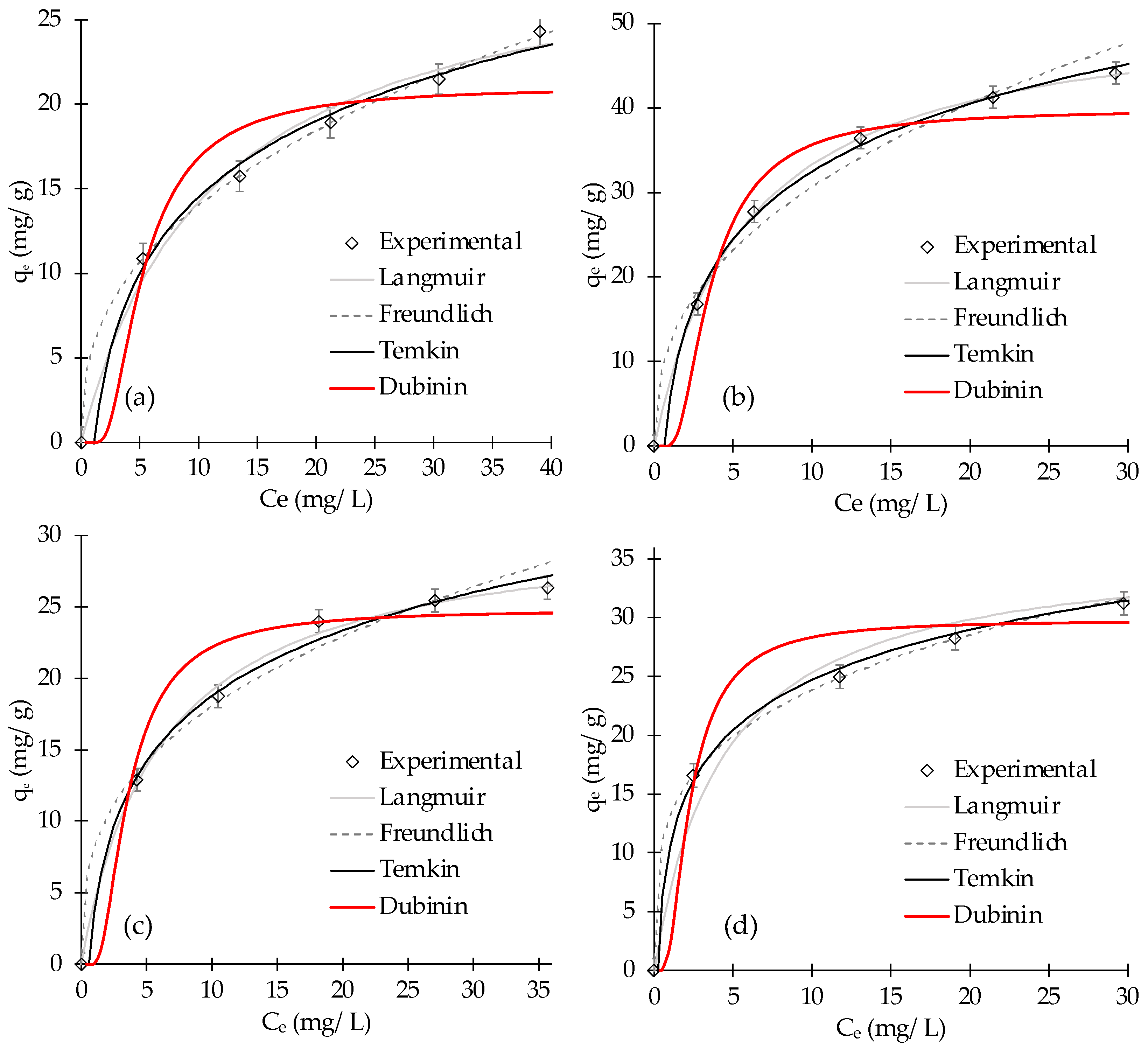
3.3. Isothermal Study
3.4. Thermodynamic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.R.; Bierkens, M.F.P.; van Vliet, M.T.H. Current and future global water scarcity intensifies when accounting for surface water quality. Nat. Clim. Change 2024, 14, 629–635. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Al-Asadi, S.T.; Mussa, Z.H.; Al-Qaim, F.F.; Kamyab, H.; Al-Saedi, H.F.S.; Deyab, I.F.; Kadhim, N.J. A comprehensive review of methylene blue dye adsorption on activated carbon from edible fruit seeds: A case study on kinetics and adsorption models. Carbon Trends 2025, 20, 100507. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Liu, D.; Sheng, C.W.; Casida, J.E.; Wang, C.; Song, P.P.; Chen, Y.M.; Han, Z.J.; Zhao, C.Q. Acute toxicity, bioconcentration, elimination and antioxidant effects of fluralaner in zebrafish, Danio rerio. Environ. Pollut. 2018, 232, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, Y.; Wen, M.; Ji, G. Toxic Effects of Methylene Blue on the Growth, Reproduction and Physiology of Daphnia magna. Toxics 2023, 11, 594. [Google Scholar] [CrossRef]
- Teixeira, Y.N.; de Paula Filho, F.J.; Bacurau, V.P.; Menezes, J.M.C.; Zhong Fan, A.; Melo, R.P.F. Removal of Methylene Blue from a synthetic effluent by ionic flocculation. Heliyon 2022, 8, e10868. [Google Scholar] [CrossRef]
- Atta, D.; Wahab, H.A.; Ibrahim, M.A.; Battisha, I.K. Photocatalytic degradation of methylene blue dye by ZnO nanoparticle thin films, using Sol–gel technique and UV laser irradiation. Sci. Rep. 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Nagraj; Chaurasia, P.K.; Bharati, S.L.; Sharma, N.; Kumar, J.; Sivalingam, A.M. Degradation of dyes by fungi: An overview on recent updates. Microbe 2025, 6, 100232. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Angulo, B.; Patiño, K.; Hernández, V.; Vallejo, W.; Gallego-Cartagena, E.; Romero Bohórquez, A.R.; Zarate, X.; Schott, E. Cyanobacterial Biomass as a Potential Biosorbent for the Removal of Recalcitrant Dyes from Water. Water 2021, 13, 3176. [Google Scholar] [CrossRef]
- Karce, H.E.; Boumessaidia, S.; Bahloul, A.; Lal, B.; Saravanan, A.; Ouakouak, A.; Hosseini-Bandegharaei, A.; Sridevi, C.; Prakash, C. Efficient removal of methylene blue by a biochar from neem tree shell wastes using adsorption technology. Biomass Convers. Biorefinery 2025, 15, 13559–13574. [Google Scholar] [CrossRef]
- Sriram, G.; Dhanabalan, K.; Vishwanath, R.S.; Oh, T.H. Progress in porous carbon-based adsorbents for the removal of dyes from single and binary systems and their adsorption mechanism—A review. Inorg. Chem. Front. 2025, 12, 5563. [Google Scholar] [CrossRef]
- Bichave, M.S.; Kature, A.Y.; Koranne, S.V.; Shinde, R.S.; Gongle, A.S.; Choudhari, V.P.; Topare, N.S.; Raut-Jadhav, S.; Bokil, S.A. Nano-metal oxides-activated carbons for dyes removal: A review. Mater. Today Proc. 2023, 77, 19–30. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Walteros, L.; Duran, F.; Vallejo, W.; Romero Bohórquez, A.R. Prosopis juliflora Seed Waste as Biochar for the Removal of Blue Methylene: A Thermodynamic and Kinetic Study. ACS Omega 2022, 7, 42916–42925. [Google Scholar] [CrossRef] [PubMed]
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-based nanomaterials and their adsorptive removal efficiency for dyes and antibiotics: A review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Huong, D.T.; Ngan, N.N.P.; Anh, D.T.T.; Vinh, N.D.; Xuan, V.T. Durian peel–seed biochar for efficient methylene blue removal from water: Synthesis, characterization, and adsorption performance. RSC Adv. 2025, 15, 33726–33749. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Dey, S. Review on biochar as an adsorbent material for removal of dyes from waterbodies. Int. J. Environ. Sci. Technol. 2023, 20, 9335–9350. [Google Scholar] [CrossRef]
- Kundu, D.; Sharma, P.; Bhattacharya, S.; Gupta, K.; Sengupta, S.; Shang, J. Study of methylene blue dye removal using biochar derived from leaf and stem of Lantana camara L. Carbon Res. 2024, 3, 1–14. [Google Scholar] [CrossRef]
- Kataya, G.; Issa, M.; Badran, A.; Cornu, D.; Bechelany, M.; Jellali, S.; Jeguirim, M.; Hijazi, A. Dynamic removal of methylene blue and methyl orange from water using biochar derived from kitchen waste. Sci. Rep. 2025, 15, 29907. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Farrell, J.J.; Larrea, C.; Luna, E. Banana Prices and Sustainability Sustainable Commodities Marketplace Series Market Overview; International Institute for Sustainable Development: Geneva, Switzerland, 2023. [Google Scholar]
- Neme, I.; Gonfa, G.; Masi, C. Activated carbon from biomass precursors using phosphoric acid: A review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nguyen, T.H.; Nguyen, T.N.; Nghiem, X.S.; Le Minh, T.; Vu, A.T. Preparation of functional materials based on activated carbon from bagasse and its application in Pb2+ adsorption in an aqueous environment. Mater. Today Commun. 2025, 47, 113158. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S.; Chong, K.H. Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption. Carbon 2003, 41, 1979–1986. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Appusamy, A.; Gnanasundaram, N.; Thanabalan, M. Surface modification and characterization of carbonaceous adsorbents for the efficient removal of oil pollutants. J. Hazard. Mater. 2019, 379, 120673. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Khatoon, R.; Attique, S.; Liu, R.; Rauf, S.; Ali, N.; Zhang, L.; Zeng, Y.J.; Guo, Y.; Kaneti, Y.V.; Na, J.; et al. Carbonized waste milk powders as cathodes for stable lithium–sulfur batteries with ultra-large capacity and high initial coulombic efficiency. Green Energy Environ. 2022, 7, 1071–1083. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Yakupova, I. Impact of Chemical and Physical Treatments on the Structural and Surface Properties of Activated Carbon and Hydrochar. ACS Sustain. Chem. Eng. 2025, 13, 2500–2507. [Google Scholar] [CrossRef]
- Nada, A.A.; El Rouby, W.M.A.; Bekheet, M.F.; Antuch, M.; Weber, M.; Miele, P.; Viter, R.; Roualdes, S.; Millet, P.; Bechelany, M. Highly textured boron/nitrogen co-doped TiO2 with honeycomb structure showing enhanced visible-light photoelectrocatalytic activity. Appl. Surf. Sci. 2020, 505, 144419. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Babu, C.M.; Binnemans, K.; Roosen, J. Ethylenediaminetriacetic Acid-Functionalized Activated Carbon for the Adsorption of Rare Earths from Aqueous Solutions. Ind. Eng. Chem. Res. 2018, 57, 1487–1497. [Google Scholar] [CrossRef]
- Wijaya, R.A.; Nakagoe, O.; Sano, H.; Tanabe, S.; Kamada, K. Superior comprehensive performance of modified activated carbon as a hexavalent chromium adsorbent. Heliyon 2024, 10, e35557. [Google Scholar] [CrossRef]
- Sutar, S.; Jadhav, J. A comparative assessment of the methylene blue dye adsorption capacity of natural biochar versus chemically altered activated carbons. Bioresour. Technol. Rep. 2024, 25, 101726. [Google Scholar]
- Huang, L.; Zeng, X.; Fan, C.; Liu, L.; Alam, S.; Zeng, B.; Liu, S.; Huang, W.; Shu, R. In Situ Modification of Activated Carbon Made from Camellia oleifera Shell with Na2EDTA for Enhanced La3+ Recovery. Minerals 2024, 14, 560. [Google Scholar]
- Rashid, U.S.; Bezbaruah, A.N. Citric acid modified granular activated carbon for enhanced defluoridation. Chemosphere 2020, 252, 126639. [Google Scholar] [CrossRef]
- Shao, C.; Fan, F.; Dai, Y. Lead ions removal from water by tartaric acid modified biochar materials: Equilibrium, kinetic studies and mechanism. Desalin. Water Treat. 2024, 320, 100601. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, S.S.A.; Altaf, M.; Hossain, I.; El Sayed, M.E.; Kallel, M.; El-Bahy, Z.M.; Rehman, A.U.; Najam, T.; Nazir, M.A. Activated carbon derived from biomass for wastewater treatment: Synthesis, application and future challenges. J. Anal. Appl. Pyrolysis 2024, 179, 106480. [Google Scholar] [CrossRef]
- Singh, S.; Uppaluri, R. Efficacy of double crucible method for the synthesis of activated carbon from rice husk. Biomass Conv. Bioref. 2025, 15, 20463–20477. [Google Scholar]
- Guo, Y.; Rockstraw, D.A. Activated carbons prepared from rice hull by one-step phosphoric acid activation. Microporous Mesoporous Mater. 2007, 100, 12–19. [Google Scholar] [CrossRef]
- Zikánová, A.; Bülow, M.; Schlodder, H. Intracrystalline diffusion of benzene in ZSM—5 and silicalite. Zeolites 1987, 7, 115–118. [Google Scholar] [CrossRef]
- McKay, G.; Al-Duri, B. Study of the mechanism of pore diffusion in batch adsorption systems. J. Chem. Technol. Biotechnol. 1990, 48, 269–285. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef] [PubMed]
- McKay, G. The adsorption of dyestuffs from aqueous solutions using activated carbon. iii. intraparticle diffusion processes. J. Chem. Technol. Biotechnol. Chem. Technol. 1983, 33, 196–204. [Google Scholar] [CrossRef]
- Zhu, Q.; Moggridge, G.D.; D’Agostino, C. Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 2: Kinetics and diffusion analysis. Chem. Eng. J. 2016, 306, 1223–1233. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Naidoo, E.B.; Modise, S.J. Dynamic studies and pseudo-second order modeling of copper(II) biosorption onto pine cone powder. Desalination 2010, 251, 112–122. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Sawasdee, S.; Watcharabundit, P. Characterization and Adsorption Mechanism of Methylene Blue Dye by Mesoporous Activated Carbon Prepared from Rice Husks. Environ. Nat. Resour. J. 2023, 21, 458–470. [Google Scholar] [CrossRef]
- Castillo-Araiza, C.O.; Che-Galicia, G.; Dutta, A.; Guzmán-González, G.; Martínez-Vera, C.; Ruíz-Martínez, R.S. Effect of diffusion on the conceptual design of a fixed-bed adsorber. Fuel 2015, 149, 100–108. [Google Scholar] [CrossRef]
- Simić, M.; Petrović, J.; Koprivica, M.; Ercegović, M.; Dimitrijević, J.; Vuković, N.S.; Fiol, N. Efficient Adsorption of Lead on Hydro-Pyrochar Synthesized by Two-Step Conversion of Corn Cob in Magnesium Chloride Medium. Toxics 2025, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Matubayasi, N. Understanding Sorption Mechanisms Directly from Isotherms. Langmuir 2023, 39, 6113–6125. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Liu, H. In-situ modification of activated carbon with ethylenediaminetetraacetic acid disodium salt during phosphoric acid activation for enhancement of nickel removal. Powder Technol. 2018, 325, 113–120. [Google Scholar] [CrossRef]
- Kurnia, I.; Karnjanakom, S.; Irkham, I.; Haryono, H.; Situmorang, Y.A.; Indarto, A.; Noviyanti, A.R.; Hartati, Y.W.; Guan, G. Enhanced adsorption capacity of activated carbon over thermal oxidation treatment for methylene blue removal: Kinetics, equilibrium, thermodynamic, and reusability studies. RSC Adv. 2022, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Sanou, I.; Bamogo, H.; Sanou, A.; Ouedraogo, M.; Saadi, L.; Waqif, M.; Millogo, Y. Adsorption of Methylene Blue in Aqueous Medium by Activated Carbon from Peanut Shells. Chem. Afr. 2024, 7, 2777–2794. [Google Scholar] [CrossRef]
- Dalmaz, A.; Özak, S. Methylene blue dye efficient removal using activated carbon developed from waste cigarette butts: Adsorption, thermodynamic and kinetics. Fuel 2024, 372, 132151. [Google Scholar] [CrossRef]
- Alkhabbas, M.; Al-Ma’abreh, A.M.; Edris, G.; Saleh, T.; Alhmood, H. Adsorption of Anionic and Cationic Dyes on Activated Carbon Prepared from Oak Cupules: Kinetics and Thermodynamics Studies. Int. J. Environ. Res. Public Health 2023, 20, 3280. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Plentz Gomes Vasconcelos, L.; Almeida Albuquerque, A.; Roberta Cabral Ribeiro, K.; Beatriz Oliveira Palmeira, M.; Thalis Vaz da Costa Capistrano, R.; Inácio Soletti, J.; Helena Vieira Carvalho, S.; Daltro Bispo, M. Comparison of adsorption potential of methylene blue and 17β-stradiol on biochar, activated biochar and catalytic biochar from lignocellulosic waste. J. Ind. Eng. Chem. 2025, 144, 585–595. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Florez, J.; Vallejo, W.; Duran, F.; Puello, E.; Roa, V.; Schott, E.; Zarate, X. Removal and photocatalytic degradation of methylene blue on ZrO2 thin films modified with Anderson-Polioxometalates (Cr3+, Co3+, Cu2+): An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2024, 454, 115689. [Google Scholar] [CrossRef]
- Chen, X.; Yao, M. Modeling of Experimental Adsorption Isotherm Data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
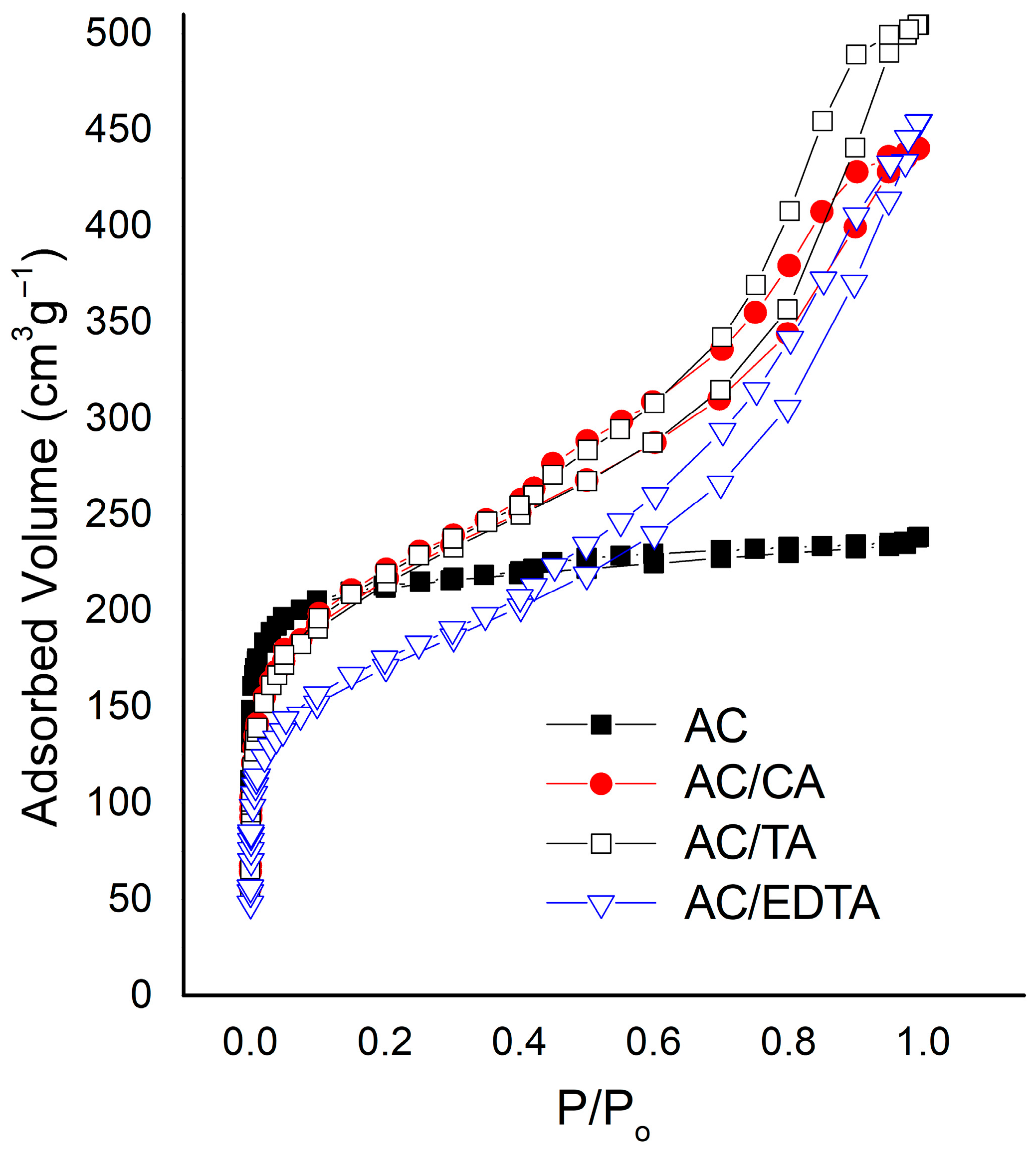
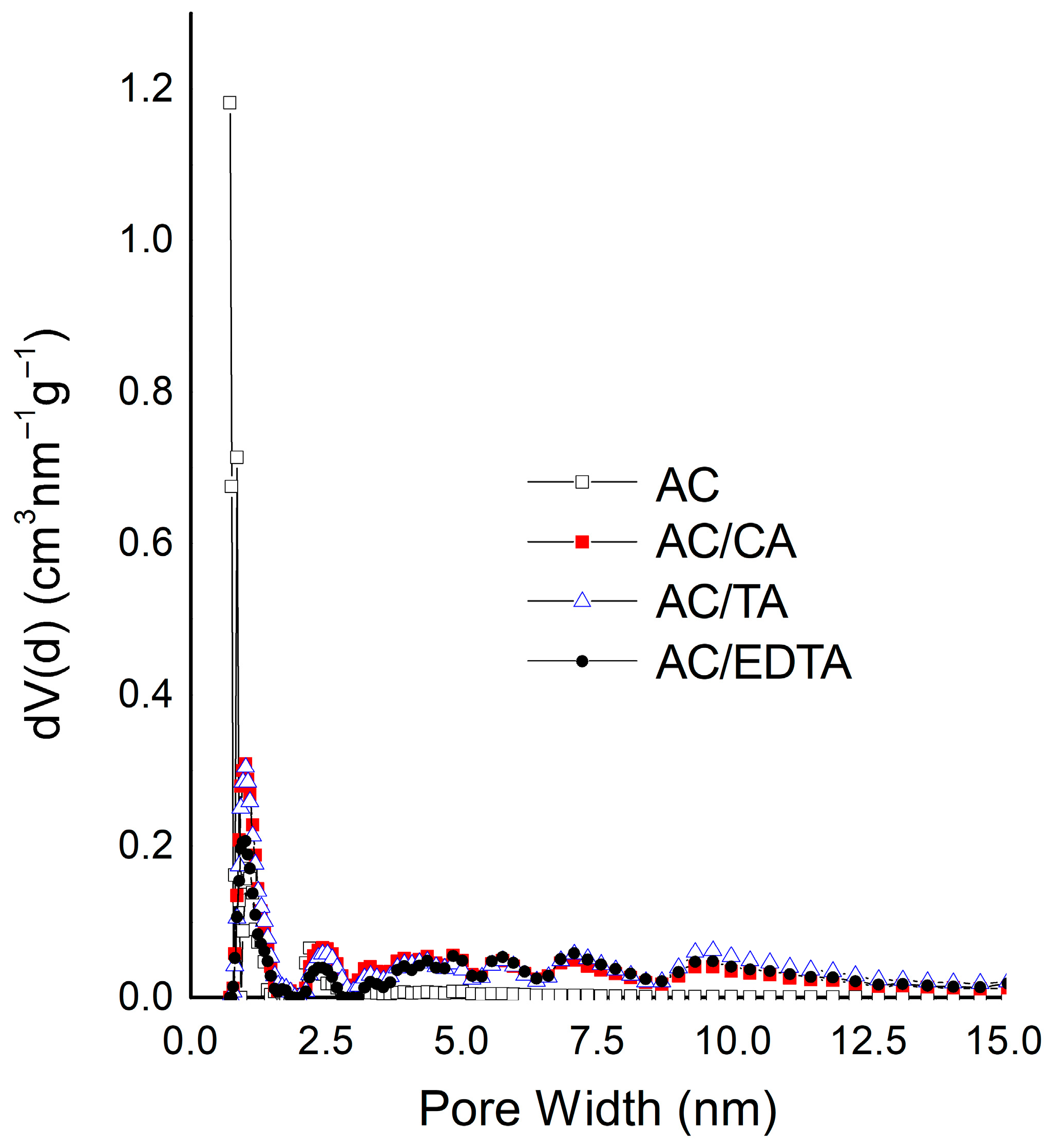
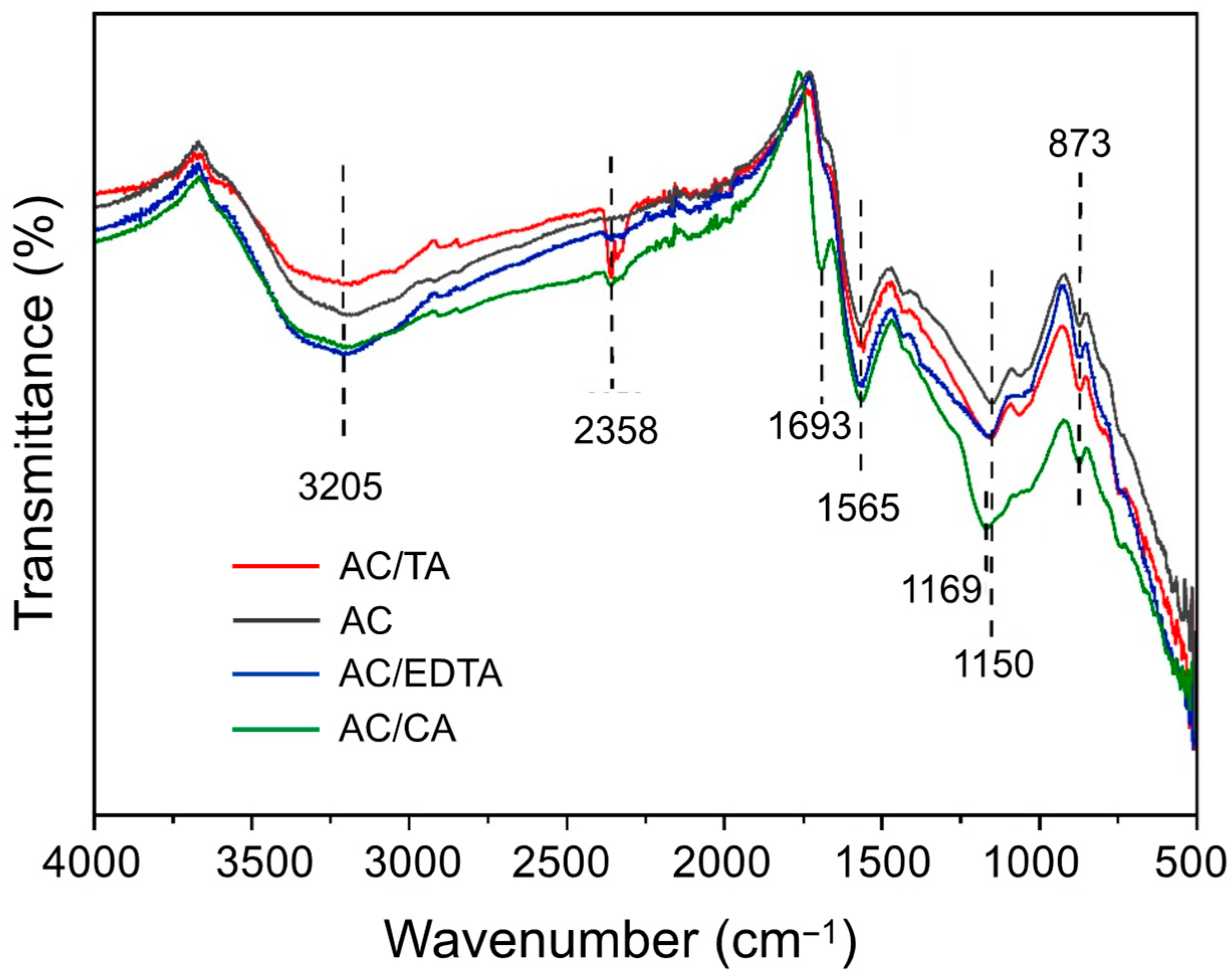
| Sample | SBET (m2 g−1) * | VO (cm3 g−1) | Vmeso (cm3 g−1) | V0.99 (cm3 g−1) |
|---|---|---|---|---|
| AC | 808 | 0.31 | 0.06 | 0.37 |
| AC/CA | 724 | 0.28 | 0.38 | 0.66 |
| AC/EDTA | 749 | 0.28 | 0.40 | 0.68 |
| AC/TA | 731 | 0.28 | 0.50 | 0.78 |
| Sample | * BT (μmol g−1) | ** AT (μmol g−1) | + CG (μmol g−1) | *+ LG (μmol g−1) | ++ PG (μmol g−1) | Pzc (pH) |
|---|---|---|---|---|---|---|
| AC | 70.65 | 241.53 | 48.05 | 102.76 | 90.72 | 5.60 |
| AC/CA | 116.6 | 363.29 | 70.52 | 195.68 | 97.09 | 5.15 |
| AC/EDTA | 102.4 | 389.25 | 94.70 | 185.74 | 108.81 | 4.96 |
| AC/TA | 91.40 | 300.61 | 65.60 | 129.31 | 105.70 | 5.38 |
| Chemical Treatment | Model | Parameters * | R2 | ARE (%) | |
|---|---|---|---|---|---|
| AC | FO | qe (mg/g) | k1 (min−1) | 0.956 | 10.8 |
| 29.1 ± 2.9 | 0.048 ± 0.005 | ||||
| SO | qe (mg/g) | k2 (g mg−1 min−1) | |||
| 46.1 ± 3.1 | 0.0005 ± 0.0001 | 0.976 | 2.9 | ||
| Intraparticle | C (mg/g) | kid (mg g−1 min−1/2) | |||
| −3.2 ± 0.8 | 4.15 ± 0.9 | 0.997 | 1.4 | ||
| AC/EDTA | FO | qe (mg g−1) | k1 (min−1) | 0.967 | 6.2 |
| 46.7 ± 2.7 | 0.036 ± 0.004 | ||||
| SO | qe (mg g−1) | k2 (g mg−1 min−1) | |||
| 57.8 ± 4.4 | 0.0006 ± 0.0001 | 0.987 | 3.7 | ||
| Intraparticle | C (mg/g) | kid (mg g−1 min−1/2) | |||
| 3.4 ± 0.9 | 4.5 ± 0.8 | 0.989 | 2.9 | ||
| AC/TA | FO | qe (mg/g) | k1 (min−1) | 0.979 | 25.9 |
| 33.1 ± 2.5 | 0.051 ± 0.007 | ||||
| SO | qe (mg/g) | k2 (g mg−1 min−1) | |||
| 58.5 ± 3.9 | 0.0003 ± 0.0001 | 0.981 | 2.1 | ||
| Intraparticle | C (mg/g) | kid (mg g−1 min−1/2) | |||
| −6.39 ± 1.1 | 4.86 ± 0.8 | 0.991 | 1.8 | ||
| AC/CA | FO | qe (mg/g) | k1 (min−1) | 0.984 | 16.7 |
| 39.8 ± 2.7 | 0.048 ± 0.007 | ||||
| SO | qe (mg/g) | k2 (g mg−1 min−1) | |||
| 53.5 ± 3.6 | 0.0006 ± 0.0001 | 0.995 | 1.7 | ||
| Intraparticle | C (mg/g) | kid (mg g−1 min−1/2) | |||
| −0.6 ± 0.1 | 4.65 ± 0.7 | 0.991 | 2.7 | ||
| Material | Model | Parameters * | |||
|---|---|---|---|---|---|
| AC | Langmuir | qmax (mg/g) | kL (L/min) | R2 | ARE (%) |
| 30.2 ± 2.8 | 0.09 ± 0.01 | 0.984 | 4.5 | ||
| Freundlich | KF (mg/g) (L/g) 1/n | 1/nf | |||
| 5.6 ± 0.5 | 0.40 ± 0.02 | 0.999 | 0.5 | ||
| Temkin | AT (L/g) | BT (J/mol) | |||
| 5.5 ± 0.5 | 6.2 ± 0.9 | 0.990 | 2.9 | ||
| Dubinin | qs (mg/g) | KD (mol2 kJ2) × 10−6 | 0.843 | 8.5 | |
| 21.0 ± 1.4 | 4 | ||||
| AC/ EDTA | Langmuir | qmax (mg/g) | kL (L/min) | 0.999 | 0.4 |
| 52.6 ± 2.3 | 0.17 ± 0.02 | ||||
| Freundlich | KF (mg/g) (L/g) 1/n | 1/nf | |||
| 12.1 ± 1.1 | 0.40 ± 0.02 | 0.964 | 5.1 | ||
| Temkin | AT (L/g) | BT (J/mol) | |||
| 1.7 ± 0.3 | 11.6 | 0.996 | 1.7 | ||
| Dubinin | qs (mg/g) | KD (mol2 kJ2) × 10−6 | 0.927 | 9.6 | |
| 39.8 ± 3.2 | 2.0 ± 0.1 | ||||
| AC/TA | Langmuir | qmax (mg g−1) | kL (L min−1) | 0.998 | 1.8 |
| 31.1 ± 1.6 | 0.16 ± 0.02 | ||||
| Freundlich | KF (mg g−1) (L g−1) 1/n | 1/nf | |||
| 8.1 ± 0.9 | 0.35 ± 0.02 | 0.966 | 3.4 | ||
| Temkin | AT (L g−1) | BT (J mol−1) | |||
| 1.7 ± 0.3 | 6.6 ± 1.0 | 0.980 | 2.1 | ||
| Dubinin | qs (mg/g) | KD (mol2 kJ2) × 10−6 | 0.912 | 7.0 | |
| 24.8 ± 2.4 | 2 ± 0.1 | ||||
| AC/CA | Langmuir | qmax (mg g−1) | kL (L min−1) | 0.992 | 6.0 |
| 36.4 ± 1.9 | 0.23 | ||||
| Freundlich | KF (mg g−1) (L g−1) 1/n | 1/nf | |||
| 13.1 ± 1.0 | 0.26 ± 0.03 | 0.999 | 0.5 | ||
| Temkin | AT (L g−1) | BT (J mol−1) | |||
| 5.5 ± 0.4 | 6.15 ± 1.1 | 0.980 | 1.8 | ||
| Dubinin | qs (mg/g) | KD (mol2 kJ2) × 10−6 | 0.874 | 6.6 | |
| 29.8 ± 2.3 | 0.9 ± 0.1 | ||||
| Adsorbent Materials | Temperature (K) | Thermodynamic Parameters | ||
|---|---|---|---|---|
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ||
| AC (This work) | 303 | −4.35 | 29.4 | 110.0 |
| 313 | −5.48 | |||
| 323 | −6.97 | |||
| 333 | −7.55 | |||
| AC/EDTA (This work) | 303 | −6.28 | 23.38 | 100.0 |
| 313 | −7.22 | |||
| 323 | −7.98 | |||
| 333 | −9.30 | |||
| AC/TA (This work) | 303 | −5.27 | 27.70 | 110.0 |
| 313 | −5.87 | |||
| 323 | −7.17 | |||
| 333 | −8.47 | |||
| AC/CA (This work) | 303 | −6.48 | 17.60 | 80.0 |
| 313 | −6.95 | |||
| 323 | −7.88 | |||
| 333 | −8.82 | |||
| Activated carbon [57] | 293 | −9.75 | 51.2 | −143 |
| 303 | −6.89 | |||
| 313 | −5.74 | |||
| 323 | −5.45 | |||
| Activated carbon/CS * [53] | 303 | 2.62 | 27.99 | 80.4 |
| 313 | 1.80 | |||
| 323 | 1.23 | |||
| 328 | 0.48 | |||
| ** LC-Biochar [58] | 303 | −11.67 | 17.59 | 100 |
| 313 | −13.19 | |||
| 323 | −13.62 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo, W.; Diaz-Uribe, C.; Duran, F.; Vargas-Delgadillo, D.P.; Barbosa, O. Kinetic and Thermodynamic Study of Methylene Blue Adsorption onto Activated Carbon Obtained from the Peel of musa paradisiaca. Sci 2025, 7, 170. https://doi.org/10.3390/sci7040170
Vallejo W, Diaz-Uribe C, Duran F, Vargas-Delgadillo DP, Barbosa O. Kinetic and Thermodynamic Study of Methylene Blue Adsorption onto Activated Carbon Obtained from the Peel of musa paradisiaca. Sci. 2025; 7(4):170. https://doi.org/10.3390/sci7040170
Chicago/Turabian StyleVallejo, William, Carlos Diaz-Uribe, Freider Duran, Diana P. Vargas-Delgadillo, and Oveimar Barbosa. 2025. "Kinetic and Thermodynamic Study of Methylene Blue Adsorption onto Activated Carbon Obtained from the Peel of musa paradisiaca" Sci 7, no. 4: 170. https://doi.org/10.3390/sci7040170
APA StyleVallejo, W., Diaz-Uribe, C., Duran, F., Vargas-Delgadillo, D. P., & Barbosa, O. (2025). Kinetic and Thermodynamic Study of Methylene Blue Adsorption onto Activated Carbon Obtained from the Peel of musa paradisiaca. Sci, 7(4), 170. https://doi.org/10.3390/sci7040170






