Competition and Environmental Stress Impacts on Trophic Performance of Three Sympatric Insectivorous Lizard Species in Eastern Spain
Abstract
1. Introduction
2. Material and Methods
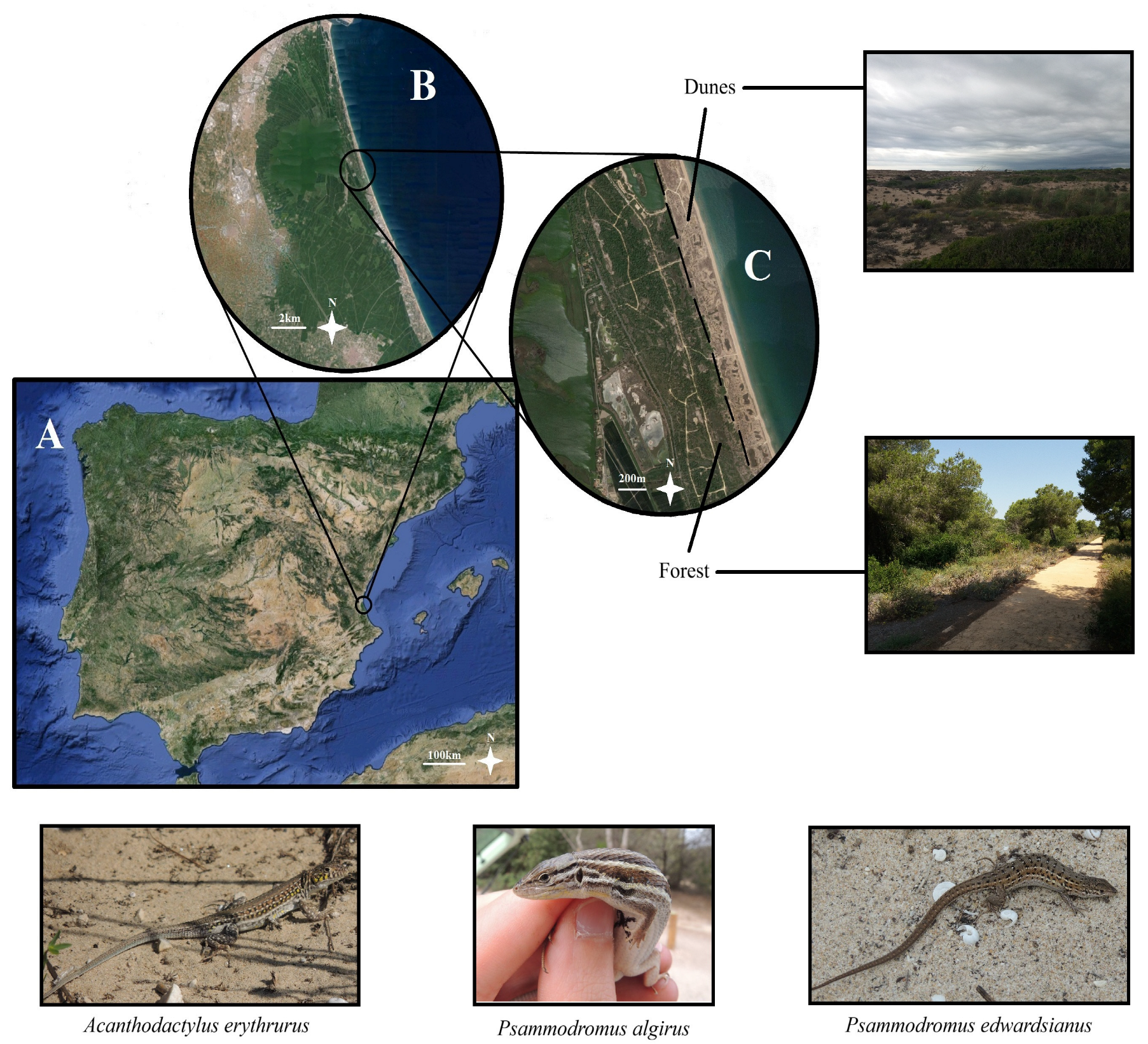
2.1. Study Area
2.2. Fieldwork
2.3. Fecal Sample Processing and Data Analysis
3. Results
3.1. Comparison of Diet Between Species
3.2. Descriptive Diet Analysis by Species
3.3. Niche Breadth and Overlap
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitt, L.J.; Zani, P.A.; de Barros, A.A.M. Ecological variation among populations of the gekkonid lizard Gonatodes humeralis in the Amazon Basin. Copeia 1997, 1997, 32–43. [Google Scholar] [CrossRef]
- Best, T.L.; Pfaffenberger, G.S. Age and sexual variation in the diet of collared lizards (Crotaphytus collaris). Southwest. Nat. 1987, 32, 415–426. [Google Scholar] [CrossRef]
- Perry, G. The evolution of sexual dimorphism in the lizard Anolis polylepis (Iguania): Evidence from intraspecific variation in foraging behavior and diet. Can. J. Zool. 1996, 74, 1238–1245. [Google Scholar] [CrossRef]
- Verwaijen, D.; Van Damme, R.; Herrel, A. Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Funct. Ecol. 2002, 16, 842–850. [Google Scholar] [CrossRef]
- Wright, S.J. Competition between insectivorous lizards and birds in central Panama. Am. Zool. 1979, 19, 1145–1156. [Google Scholar] [CrossRef]
- Costa-Pereira, R.; Araújo, M.S.; Souza, F.L.; Ingram, T. Competition and resource breadth shape niche variation and overlap in multiple trophic dimensions. Proc. R. Soc. B 2019, 286, 20190369. [Google Scholar] [CrossRef] [PubMed]
- Vitt, L.J.; Pianka, E.R. Lizard Ecology: Historical and Experimental Perspectives; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Luiselli, L. Do lizard communities partition the trophic niche? A worldwide meta-analysis using null models. Oikos 2008, 117, 321–330. [Google Scholar] [CrossRef]
- Arnold, E.N. Resource partition among lacertid lizards in southern Europe. J. Zool. 1987, 1, 739–782. [Google Scholar] [CrossRef]
- Rouag, R.; Djilali, H.; Gueraiche, H.; Luiselli, L. Resource partitioning patterns between two sympatric lizard species from Algeria. J. Arid Environ. 2007, 69, 158–168. [Google Scholar] [CrossRef]
- Carretero, M.A. From set menu to a la carte. Linking issues in trophic ecology of Mediterranean lacertids. Ital. J. Zool. 2004, 71, 121–133. [Google Scholar] [CrossRef]
- Ortiz, P.R.; Jenssen, T.A. Interspecific aggression between lizard competitors, Anolis cooki and Anolis cristatellus. Z. Tierpsychol. 1982, 60, 227–238. [Google Scholar] [CrossRef]
- Pacala, S.; Roughgarden, J. Resource partitioning and interspecific competition in two two-species insular Anolis lizard communities. Science 1982, 217, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Busack, S.D.; Jaksic, F.M. Autecological observations of Acanthodactylus erythrurus (Sauria: Lacertidae) in southern Spain. Amphib.-Reptil. 1982, 3, 237–255. [Google Scholar] [CrossRef]
- Pérez Mellado, V. Estructura en una taxocenosis de Lacertidae (Sauria, Reptilia) del sistema central. Mediterránea. Ser. Estud. Biol. 1982, 6, 39–64. [Google Scholar] [CrossRef]
- Carretero, M.A.; Llórente, G.A. Alimentación de Psammodromus hispanicus en un arenal costero del nordeste ibérico. Rev. Esp. Herp. 1991, 6, 31–44. [Google Scholar] [CrossRef]
- Seva, E. Reparto de recursos en dos especies psammófilas de saurios: Acanthodactylus erythrurus y Psammodromus algirus, en un arenal costero de Alicante. Mediterránea 1984, 4, 133–162. [Google Scholar]
- Pollo, C.J.; Pérez-Mellado, V. An analysis of a Mediterranean assemblage of three small lacertid lizards in central Spain. Acta Oecol. 1991, 12, 655–671. [Google Scholar]
- Belliure, J.; Carrascal, L.M.; Diaz, J.A. Covariation of thermal biology and foraging mode in two Mediterranean lacertid lizards. Ecology 1996, 77, 1163–1173. [Google Scholar] [CrossRef]
- Verwaijen, D.; Van Damme, R. Correlated evolution of thermal characteristics and foraging strategy in lacertid lizards. J. Therm. Biol. 2007, 32, 388–395. [Google Scholar] [CrossRef]
- Drechsler, R.M.; Monrós, J.S. Body growth and its implications in population dynamics of Acanthodactylus erythrurus (Schinz, 1834) in the Eastern Iberian peninsula. Amphib.-Reptil. 2019, 40, 305–312. [Google Scholar] [CrossRef]
- Comas, M.; Reguera, S.; Zamora-Camacho, F.J.; Moreno-Rueda, G. Age structure of a lizard along an elevational gradient reveals nonlinear lifespan patterns with altitude. Curr. Zool. 2020, 66, 373–382. [Google Scholar] [CrossRef]
- Llorca, A.B.; Tortosa, F.S.; Guerrero-Casado, J. Arboreal behavior of Psammodromus algirus (Squamata: Lacertidae) in olive groves. Herp. Cons. Biol. 2023, 18, 155–160. [Google Scholar]
- Fitze, P.S. Edward’s Sand Racer—Psammodromus edwardsianus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2012; Available online: https://www.vertebradosibericos.org/reptiles/psaedw.html (accessed on 27 June 2022).
- Guillén-Salazar, F.; Font, E.; Desfilis, E. Comportamiento de homing en la lagartija colirroja (Acanthodactylus erythrurus). Rev. Esp. Herp. 2007, 21, 119–129. [Google Scholar]
- Renet, J.; Dokhelar, T.; Tortosa, T.; Monnet, C. Spring home range and spatiotemporal activity of Edward’s Sand Racer (Psammodromus edwarsianus) in a protected natural area of southern France. Ecol. Mediterr. 2023, 49, 25–36. [Google Scholar] [CrossRef]
- Melic, A. Curso Practico de Entomología. In Manuals de la Universitat Autònoma de Barcelona. 41. Entomologia; Barrientos, J.A., Ed.; Asociación Española de Entomología, CIBIO-Centro Iberoamericano de Biodiversidad & Universitat Autònoma de Barcelona: Barcelona, Spain, 2005; ISBN 84-490-2383-1. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wolda, H. Similarity indices, sample size and diversity. Oecologia 1981, 50, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Somerfield, P.J. Identification of the Bray-Curtis similarity index: Comment on Yoshioka (2008). Mar. Ecol. Prog. Ser. 2008, 372, 303–306. [Google Scholar] [CrossRef]
- Sasa, M.; Monrós, J.S. Dietary analysis of helmeted basilisks, Corytophanes (Reptilia: Corytophanidae). Southwest. Nat. 2000, 45, 358–361. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations (No. 2); Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar] [CrossRef]
- Serafini, P.; Lovari, S. Food habits and trophic niche overlap of the red fox and the stone marten in a Mediterranean rural area. Acta Theriol. 1993, 38, 233. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Lehsten, V.; Harmand, P. Null models for species co-occurrence patterns: Assessing bias and minimum iteration number for the sequential swap. Ecography 2006, 29, 786–792. [Google Scholar] [CrossRef]
- Lawlor, L.R. Structure and stability in natural and randomly constructed competitive communities. Am. Nat. 1980, 116, 394–408. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 3.4.1; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 11 November 2019).
- Belliure, J. Lagartija colirroja—Acanthodactylus erythrurus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2015; Available online: https://www.vertebradosibericos.org/reptiles/acaery.html (accessed on 27 June 2022).
- Mamou, R.; Marniche, F.; Amroun, M.; Herrel, A. Trophic ecology of two sympatric lizard species: The Algerian sand lizard and the wall lizard in Djurdjura, northern Algeria. Zool. Ecol. 2016, 26, 256–264. [Google Scholar] [CrossRef]
- Salvador, A. Lagartija colilarga—Psammodromus algirus. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Marco, A., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2011; Available online: https://www.vertebradosibericos.org/reptiles/psaalg.html (accessed on 27 June 2022).
- Albornà, P.X.; Mateos, J.; Carretero, M.A. Depredación ocasional de juveniles de Acanthodactylus erythrurus por adultos de Psammodromus algirus. Boletín De La Asoc. Herpetológica Española 2004, 15, 33–34. [Google Scholar]
- Duré, M.I.; Kehr, A.I. Influence of microhabitat on the trophic ecology of two leptodactylids from northeastern Argentina. Herpetologica 2004, 60, 295–303. [Google Scholar] [CrossRef]
- Verwaijen, D.; Van Damme, R. Does foraging mode mould morphology in lacertid lizards? J. Evol. Biol. 2007, 20, 1950–1961. [Google Scholar] [CrossRef]
- McLachlan, A. Ecology of coastal dune fauna. J. Arid Environ. 1991, 21, 229–243. [Google Scholar] [CrossRef]
- Liu, R.; Wasserstrom, H.; Meller, R.; Steinberger, Y. Ground-active arthropod response to distance from the Mediterranean seashore in coastal sand areas of western Israel. Arid Land Res. Manag. 2018, 32, 316–336. [Google Scholar] [CrossRef]
- Van Damme, R.; Bauwens, D.; Verheyen, R.F. The thermal dependence of feeding behaviour, food consumption and gut-passage time in the lizard Lacerta vivipara Jacquin. Funct. Ecol. 1991, 5, 507–517. [Google Scholar] [CrossRef]
- Pough, F.H.; Busack, S.D. Metabolism and activity of the Spanish fringe-toed lizard (Lacertidae: Acanthodactylus erythrurus). J. Therm. Biol. 1978, 3, 203–205. [Google Scholar] [CrossRef]
- Patterson, J.W.; Davies, P.M.C. The influence of temperature, sexual condition, and season on the metabolic rate of the lizard Psammodromus hispanicus. J. Comp. Physiol. B 1984, 154, 311–316. [Google Scholar] [CrossRef]
- Carrascal, L.M.; Díaz, J.A. Thermal ecology and spatio-temporal distribution of the Mediterranean lizard Psammodromus algirus. Ecography 1989, 12, 137–143. [Google Scholar] [CrossRef]
- Weiperth, A.; Gaebele, T.; Potyó, I.; Puky, M. A global overview on the diet of the dice snake (Natrix tessellata) from a geographical perspective: Foraging in atypical habitats and feeding spectrum widening helps colonisation and survival under suboptimal conditions for a piscivorous snake. Zool. Stud. 2014, 53, 42. [Google Scholar] [CrossRef]
- Hernández, M.; Hereira-Pacheco, S.; Alberdi, A.; Díaz de la Vega-Pérez, A.H.; Estrada-Torres, A.; Ancona, S.; Navarro-Noya, Y.E. DNA metabarcoding reveals seasonal changes in diet composition across four arthropod-eating lizard species (Phrynosomatidae: Sceloporus). Integr. Zool. 2024, 19, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Verwaijen, D.; Van Damme, R. Foraging mode and its flexibility in lacertid lizards from Europe. J. Herp. 2008, 42, 124–134. [Google Scholar] [CrossRef]
- Hawlena, D.; Pérez-Mellado, V. Change your diet or die: Predator-induced shifts in insectivorous lizard feeding ecology. Oecologia 2009, 161, 411–419. [Google Scholar] [CrossRef]
 A. erythrurus;
A. erythrurus;  P. algirus;
P. algirus;  P. edwardsianus). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.001 (***).
P. edwardsianus). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.001 (***).
 A. erythrurus;
A. erythrurus;  P. algirus;
P. algirus;  P. edwardsianus). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.001 (***).
P. edwardsianus). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.001 (***).
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  30–39;
30–39;  40–49;
40–49;  50–59;
50–59;  60–69;
60–69;  70–79), habitat types ((C)
70–79), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  30–39;
30–39;  40–49;
40–49;  50–59;
50–59;  60–69;
60–69;  70–79), habitat types ((C)
70–79), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  30–39;
30–39;  40–49;
40–49;  50–59;
50–59;  60–69;
60–69;  70–79), habitat types ((C)
70–79), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  30–39;
30–39;  40–49;
40–49;  50–59;
50–59;  60–69;
60–69;  70–79), habitat types ((C)
70–79), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).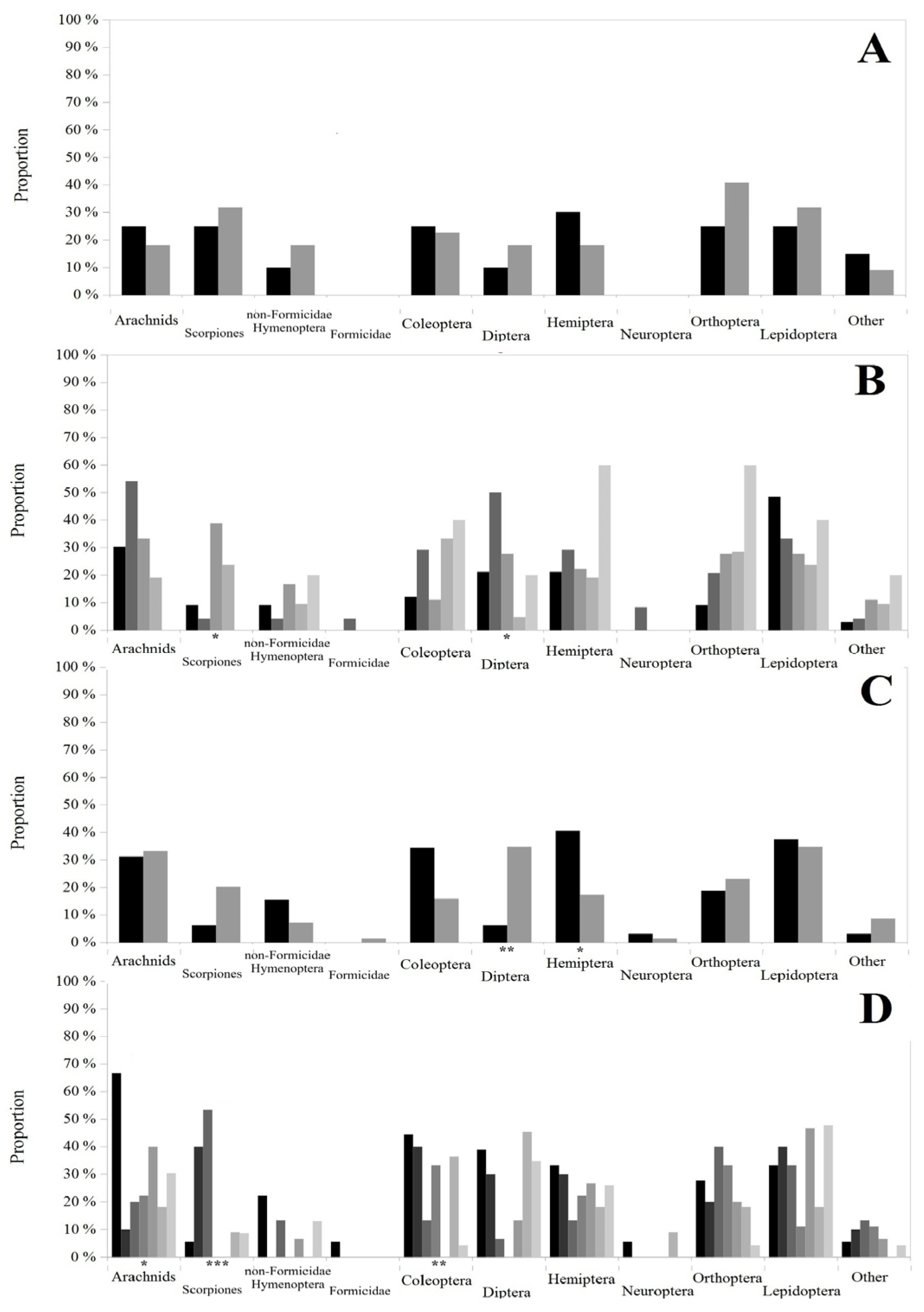
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  <35;
<35;  35–39;
35–39;  40–44;
40–44;  >44), habitat types ((C)
>44), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
 females;
females;  males), SVL ranges in mm ((B)
males), SVL ranges in mm ((B)  <35;
<35;  35–39;
35–39;  40–44;
40–44;  >44), habitat types ((C)
>44), habitat types ((C)  forest;
forest;  dunes) and months ((D)
dunes) and months ((D)  April;
April;  May;
May;  June;
June;  July;
July;  August;
August;  September;
September;  October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
October). Asterisks below the bars show the significance level of the Kruskal–Wallis test: p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
| n | Bs | |||
|---|---|---|---|---|
| A. erythrurus | 246 | 0.34 | ||
| P. algirus | 101 | 0.41 | ||
| P. edwardsianus | 138 | 0.34 | ||
| Sex | A. erythrurus | F | 37 | 0.31 |
| M | 139 | 0.35 | ||
| P. algirus | F | 20 | 0.44 | |
| M | 22 | 0.4 | ||
| P. edwardsianus | F | 22 | 0.33 | |
| M | 40 | 0.35 | ||
| Habitat | A. erythrurus | forest | 142 | 0.33 |
| dunes | 104 | 0.34 | ||
| P. algirus | forest | 32 | 0.33 | |
| dunes | 69 | 0.39 | ||
| P. edwardsianus | forest | 82 | 0.25 | |
| dunes | 56 | 0.42 | ||
| Overall Data | A. erythrurus | P. algirus | P. edwardsianus |
|---|---|---|---|
| A. erythrurus | - | 0.61 | 36.83 | 0.82 | 54.94 |
| P. algirus | 0.41 ± 0.14 | - | 0.81 | 69.07 |
| P. edwardsianus | 0.37 ± 0.15 | 0.40 ± 0.14 | - |
| forest | |||
| A. erythrurus | - | 0.78 | 32.39 | 0.83 | 65.22 |
| P. algirus | 0.35 ± 0.16 | - | 0.85 | 50.43 |
| P. edwardsianus | 0.32 ± 0.16 | 0.31 ± 0.17 | - |
| dunes | |||
| A. erythrurus | - | 0.50 | 36.40 | 0.78 | 38.77 |
| P. algirus | 0.39 ± 0.15 | - | 0.84 | 67.73 |
| P. edwardsianus | 0.40 ± 0.15 | 0.43 ± 0.14 | - |
| forest vs. dunes | |||
| Pianka | B-C | 0.96 | 79.89 | 0.82 | 62.00 | 0.84 | 66.67 |
| Monte Carlo | 0.36 ± 0.16 | 0.37 ± 0.15 | 0.36 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drechsler, R.M.; Monrós, J.S. Competition and Environmental Stress Impacts on Trophic Performance of Three Sympatric Insectivorous Lizard Species in Eastern Spain. Sci 2025, 7, 146. https://doi.org/10.3390/sci7040146
Drechsler RM, Monrós JS. Competition and Environmental Stress Impacts on Trophic Performance of Three Sympatric Insectivorous Lizard Species in Eastern Spain. Sci. 2025; 7(4):146. https://doi.org/10.3390/sci7040146
Chicago/Turabian StyleDrechsler, Robby M., and Juan S. Monrós. 2025. "Competition and Environmental Stress Impacts on Trophic Performance of Three Sympatric Insectivorous Lizard Species in Eastern Spain" Sci 7, no. 4: 146. https://doi.org/10.3390/sci7040146
APA StyleDrechsler, R. M., & Monrós, J. S. (2025). Competition and Environmental Stress Impacts on Trophic Performance of Three Sympatric Insectivorous Lizard Species in Eastern Spain. Sci, 7(4), 146. https://doi.org/10.3390/sci7040146







