Toxicity of Recreational Drugs and Medications During Lactation: A Systematic Review
Abstract
1. Introduction
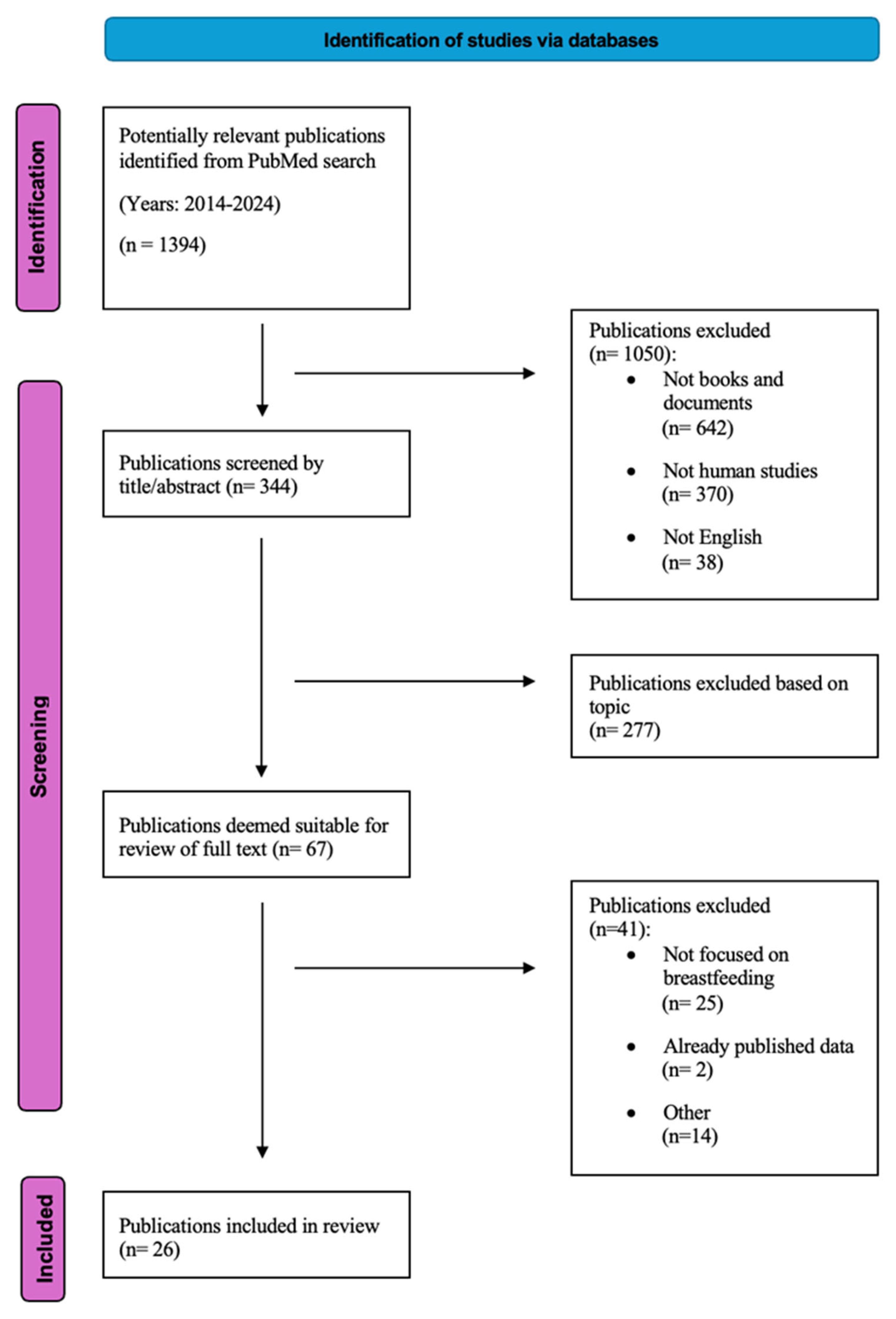
2. Methods
3. Results
3.1. Opioids
3.2. Antidepressants and Antipsychotics
3.3. Cannabinoids
3.4. Stimulants
3.5. Alcohol
3.6. Sedatives and Benzodiazepines
3.7. Antiepileptics and Anticonvulsants
3.8. Immunomodulatory and Dermatologic Drugs
3.9. Summary Tables and Figures
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14. [Google Scholar] [CrossRef]
- Westerfield, K.L.; Koenig, K.; Oh, R. Breastfeeding: Common Questions and Answers. Am. Fam. Physician 2018, 98, 368–376. [Google Scholar]
- Forray, A.; Foster, D. Substance Use in the Perinatal Period. Curr. Psychiatry Rep. 2015, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- European Union Drugs Agency. Available online: https://www.euda.europa.eu/index_en (accessed on 27 May 2025).
- Louw, K.-A. Substance Use in Pregnancy: The Medical Challenge. Obs. Med. 2018, 11, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Vaiano, F.; Dimitrova, A.; Vullo, C.; Croce, E.B.; Rossi, R.; Arena, V.; Strano Rossi, S.; Campuzano, O.; Brugada, R.; et al. Fatal Intoxications and Inherited Cardiac Disorders in the Young: Where to Draw the Line? Int. J. Leg. Med. 2025, 139, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Spence, A.R.; Abenhaim, H.A. National SIDS Trends in the United States From 2000 to 2019: A Population-Based Study on 80 Million Live Births. Clin. Pediatr. 2024, 63, 1216–1224. [Google Scholar] [CrossRef]
- Madadi, P.; Kelly, L.E.; Ross, C.J.; Kepron, C.; Edwards, J.N.; Koren, G. Forensic Investigation of Methadone Concentrations in Deceased Breastfed Infants. J. Forensic Sci. 2016, 61, 576–580. [Google Scholar] [CrossRef]
- Soussan, C.; Gouraud, A.; Portolan, G.; Jean-Pastor, M.-J.; Pecriaux, C.; Montastruc, J.-L.; Damase-Michel, C.; Lacroix, I. Drug-Induced Adverse Reactions via Breastfeeding: A Descriptive Study in the French Pharmacovigilance Database. Eur. J. Clin. Pharmacol. 2014, 70, 1361–1366. [Google Scholar] [CrossRef]
- Cheuk Kiu Chow, G.K. Sedating Drugs and Breastfeeding. Can. Fam. Physician 2015, 61, 241–243. [Google Scholar]
- Verstegen, R.H.J.; Anderson, P.O.; Ito, S. Infant Drug Exposure via Breast Milk. Br. J. Clin. Pharmacol. 2022, 88, 4311–4327. [Google Scholar] [CrossRef]
- Newton, E.R.; Hale, T.W. Drugs in Breast Milk. Clin. Obs. Gynecol. 2015, 58, 868–884. [Google Scholar] [CrossRef]
- Payne, J.L. Psychopharmacology in Pregnancy and Breastfeeding. Psychiatr. Clin. N. Am. 2017, 40, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, G.; Zandonella Callegher, R.; Butera, R.; De Santis, M.; Cavaliere, A.F.; Vecchio, S.; Lanzi, C.; Davanzo, R.; Mangili, G.; Bondi, E.; et al. Consensus Panel Recommendations for the Pharmacological Management of Breastfeeding Women with Postpartum Depression. Int. J. Environ. Res. Public Health 2024, 21, 551. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.; Wisner, K.L.; Bogen, D.L. Pharmacotherapy for Depression and Bipolar Disorder during Lactation: A Framework to Aid Decision Making. Semin. Perinatol. 2020, 44, 151224. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.; Desai, G.; Chandra, P. Antipsychotics in Pregnancy and Lactation. Indian J. Psychiatry 2015, 57, 303. [Google Scholar] [CrossRef]
- Mehta, T.M.; Van Lieshout, R.J. A Review of the Safety of Clozapine during Pregnancy and Lactation. Arch. Women’s Ment. Health 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Hanan, N.J.; Honerkamp-Smith, G.; Best, B.M.; Chambers, C.D. Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics 2018, 142, e20181076. [Google Scholar] [CrossRef]
- Aryana, A.; Williams, M.A. Marijuana as a Trigger of Cardiovascular Events: Speculation or Scientific Certainty? Int. J. Cardiol. 2007, 118, 141–144. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Gasparyan, A.; Austrich-Olivares, A.; Femenía, T.; Manzanares, J. Cannabis Use in Pregnant and Breastfeeding Women: Behavioral and Neurobiological Consequences. Front. Psychiatry 2020, 11, 586447. [Google Scholar] [CrossRef]
- Chomchai, C.; Chomchai, S.; Kitsommart, R. Transfer of Methamphetamine (MA) into Breast Milk and Urine of Postpartum Women Who Smoked MA Tablets During Pregnancy. J. Hum. Lact. 2016, 32, 333–339. [Google Scholar] [CrossRef]
- Anderson, P.O. Alcohol Use During Breastfeeding. Breastfeed. Med. 2018, 13, 315–317. [Google Scholar] [CrossRef]
- May, P.A.; Hasken, J.M.; Blankenship, J.; Marais, A.-S.; Joubert, B.; Cloete, M.; de Vries, M.M.; Barnard, R.; Botha, I.; Roux, S.; et al. Breastfeeding and Maternal Alcohol Use: Prevalence and Effects on Child Outcomes and Fetal Alcohol Spectrum Disorders. Reprod. Toxicol. 2016, 63, 13–21. [Google Scholar] [CrossRef]
- Haastrup, M.B.; Pottegård, A.; Damkier, P. Alcohol and Breastfeeding. Basic Clin. Pharmacol. Toxicol. 2014, 114, 168–173. [Google Scholar] [CrossRef]
- Ter Horst, P.G.J.; Madjunkov, M.; Chaudry, S. Alcohol: A Pharmaceutical and Pharmacological Point of View During Lactation. J. Popul. Ther. Clin. Pharmacol. 2016, 23, 145–150. [Google Scholar]
- Oei, J.L. Alcohol Use in Pregnancy and Its Impact on the Mother and Child. Addiction 2020, 115, 2148–2163. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.E.; Poon, S.; Madadi, P.; Koren, G. Neonatal Benzodiazepines Exposure during Breastfeeding. J. Pediatr. 2012, 161, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Shawahna, R.; Zaid, L. Concentrations of Antiseizure Medications in Breast Milk of Lactating Women with Epilepsy: A Systematic Review with Qualitative Synthesis. Seizure 2022, 98, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hope, O.A.; Harris, K.M. Management of Epilepsy during Pregnancy and Lactation. BMJ 2023, 13, e074630. [Google Scholar] [CrossRef]
- Siegel, C.H.; Sammaritano, L.R. Safety of Medications Used to Treat Autoimmune Rheumatic Diseases During Pregnancy and Lactation. JCR J. Clin. Rheumatol. 2024, 30, S25–S33. [Google Scholar] [CrossRef]
- Yaghi, M.; McMullan, P.; Truong, T.M.; Rothe, M.; Murase, J.; Grant-Kels, J.M. Safety of Dermatologic Medications in Pregnancy and Lactation: An Update—Part II: Lactation. J. Am. Acad. Dermatol. 2024, 91, 651–668. [Google Scholar] [CrossRef]
- Is It Compatible with Breastfeeding? Available online: https://www.e-lactancia.org/ (accessed on 27 May 2025).
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human Breast Milk: A Review on Its Composition and Bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Verduci, E.; Scaglioni, S.; Vecchi, F.; Contarini, G.; Giovannini, M.; Koletzko, B.; Agostoni, C. Breast Milk Composition and Infant Nutrient Intakes during the First 12 Months of Life. Eur. J. Clin. Nutr. 2016, 70, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Pons, G.; Rey, E.; Matheson, I. Excretion of Psychoactive Drugs into Breast Milk. Clin. Pharmacokinet. 1994, 27, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Reece-Stremtan, S.; Marinelli, K.A. ABM Clinical Protocol #21: Guidelines for Breastfeeding and Substance Use or Substance Use Disorder, Revised 2015. Breastfeed. Med. 2015, 10, 135–141. [Google Scholar] [CrossRef]
- Schoretsanitis, G.; Augustin, M.; Saßmannshausen, H.; Franz, C.; Gründer, G.; Paulzen, M. Antidepressants in Breast Milk; Comparative Analysis of Excretion Ratios. Arch. Women’s Ment. Health 2019, 22, 383–390. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, Y.; Li, B.; He, M. Rapid Detection of Cocaine Using Aptamer-Based Biosensor on an Evanescent Wave Fibre Platform. R. Soc. Open Sci. 2018, 5, 180821. [Google Scholar] [CrossRef]
- D’Avila, F.B.; Limberger, R.P.; Fröehlich, P.E. Cocaine and Crack Cocaine Abuse by Pregnant or Lactating Mothers and Analysis of Its Biomarkers in Meconium and Breast Milk by LC–MS—A Review. Clin. Biochem. 2016, 49, 1096–1103. [Google Scholar] [CrossRef]
- Winecker, R.; Goldberger, B.; Tebbett, I.; Behnke, M.; Eyler, F.; Karlix, J.; Wobie, K.; Conlon, M.; Phillips, D.; Bertholf, R. Detection of Cocaine and Its Metabolites in Breast Milk. J. Forensic Sci. 2001, 46, 1221–1223. [Google Scholar] [CrossRef]
- Binkiewicz, A.; Robinson, M.J.; Senior, B. Pseudo-Cushing Syndrome Caused by Alcohol in Breast Milk. J. Pediatr. 1978, 93, 965–967. [Google Scholar] [CrossRef]
- Madadi, P.; Ross, C.; Hayden, M.; Carleton, B.; Gaedigk, A.; Leeder, J.; Koren, G. Pharmacogenetics of Neonatal Opioid Toxicity Following Maternal Use of Codeine During Breastfeeding: A Case–Control Study. Clin. Pharmacol. Ther. 2009, 85, 31–35. [Google Scholar] [CrossRef]
- Pjevac, M.; Kokalj Palandacic, A.; Pregelj, P. Non-fatal gamma hydroxy butyrate intoxication with unusually high blood level. Psychiatr. Danub. 2024, 36, 126–129. [Google Scholar] [CrossRef]
- Wolfson, P.; Cole, R.; Lynch, K.; Yun, C.; Wallach, J.; Andries, J.; Whippo, M. The Pharmacokinetics of Ketamine in the Breast Milk of Lactating Women: Quantification of Ketamine and Metabolites. J. Psychoact. Drugs 2023, 55, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Ramnarine, R.S.; Poklis, J.L.; Wolf, C.E. Determination of Cannabinoids in Breast Milk Using QuEChERS and Ultra-Performance Liquid Chromatography and Tandem Mass Spectrometry. J. Anal. Toxicol. 2019, 43, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Ito, S. Drugs in Lactation. J. Obstet. Gynaecol. Res. 2019, 45, 522–531. [Google Scholar] [CrossRef] [PubMed]
- López-García, E.; Mastroianni, N.; Postigo, C.; Valcárcel, Y.; González-Alonso, S.; Barceló, D.; López de Alda, M. Simultaneous LC–MS/MS Determination of 40 Legal and Illegal Psychoactive Drugs in Breast and Bovine Milk. Food Chem. 2018, 245, 159–167. [Google Scholar] [CrossRef]
- Marchei, E.; Escuder, D.; Pallas, C.R.; Garcia-Algar, O.; Gómez, A.; Friguls, B.; Pellegrini, M.; Pichini, S. Simultaneous Analysis of Frequently Used Licit and Illicit Psychoactive Drugs in Breast Milk by Liquid Chromatography Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 309–316. [Google Scholar] [CrossRef]
- Kandall, S.R.; Gaines, J.; Habel, L.; Davidson, G.; Jessop, D. Relationship of Maternal Substance Abuse to Subsequent Sudden Infant Death Syndrome in Offspring. J. Pediatr. 1993, 123, 120–126. [Google Scholar] [CrossRef]
- Chang, G.Y.-S.; VanSteelandt, A.; McKenzie, K.; Kouyoumdjian, F. Accidental Substance-Related Acute Toxicity Deaths among Youth in Canada: A Descriptive Analysis of a National Chart Review Study of Coroner and Medical Examiner Data. Health Promot. Chronic Dis. Prev. Can. 2024, 44, 77–88. [Google Scholar] [CrossRef]
- Makarious, L.; Teng, A.; Oei, J.L. SIDS Is Associated with Prenatal Drug Use: A Meta-Analysis and Systematic Review of 4 238 685 Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 617–623. [Google Scholar] [CrossRef]
- Pritham, U.A. Breastfeeding Promotion for Management of Neonatal Abstinence Syndrome. J. Obstet. Gynecol. Neonatal Nurs. 2013, 42, 517–526. [Google Scholar] [CrossRef]
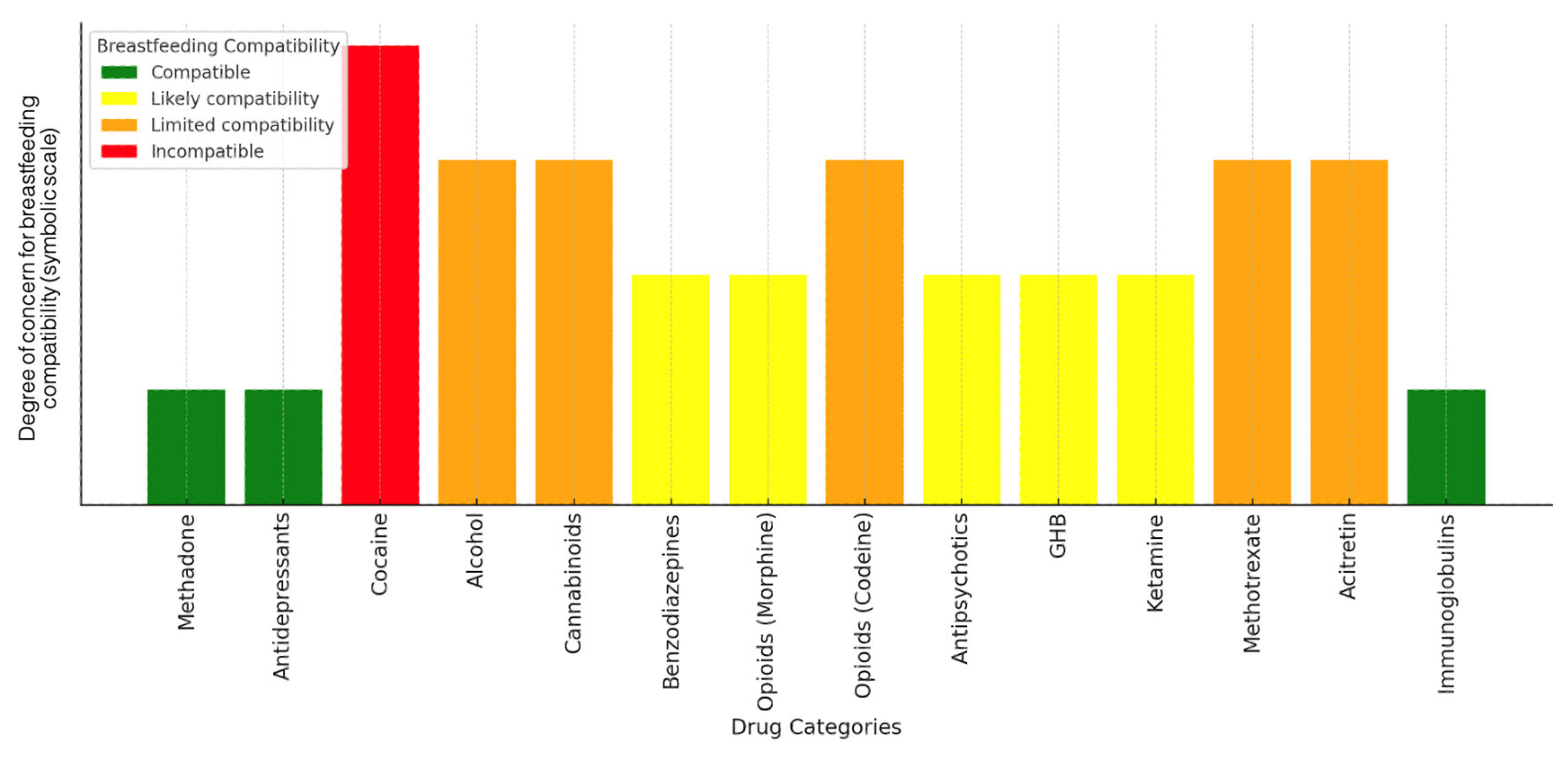
| Drug | Characteristics and Milk Transmission | Effects on the Infant | Recommendations | References |
|---|---|---|---|---|
| Methadone | Lipophilic, higher in mature milk and hindmilk. Reduces NAS (neonatal abstinence syndrome). | Possible delayed motor development. | Infant monitoring required. | [8] |
| Antidepressants (Sertraline, Citalopram, Venlafaxine) | Higher concentrations in hindmilk. Sertraline has low penetration; citalopram and venlafaxine have moderate–high penetration with accumulation risks. | Irritability, lethargy, long-term neurological effects. | Infant monitoring; sertraline preferred. | [37] |
| Cocaine | Highly lipophilic, accumulates in foremilk and hindmilk. Transfers 13.8 µg/100 mL, with 8.3 µg absorbed. | Risk of seizures, hypertonia, tachycardia. | Contraindicated; avoid breastfeeding after use. | [38,39,40] |
| Alcohol | Not highly lipophilic, mirrors plasma levels, peaks at 60 min. | Irritability, reduced deep sleep, developmental delays. | Avoid breastfeeding for at least 2–3 h after intake. | [22,23,25,26,41] |
| Cannabinoids (THC, CBD, CBN) | THC is highly lipophilic, detected up to 6 days. CBD/CBN lower or undetectable. | Potential neurotoxic effects. | Avoid cannabis use while breastfeeding. | [18] |
| Benzodiazepines (Diazepam, Lorazepam) | Pass into breast milk via diffusion; long half-life compounds accumulate more. | Sedation, lethargy, reduced sucking reflex. | Prefer short-acting lorazepam when needed; monitor closely. | [10] |
| Opioids (Morphine, Codeine) | Codeine metabolized into morphine, risk of accumulation in hindmilk. | Respiratory depression, sedation. | Avoid codeine; morphine can be used with caution. | [42] |
| Antipsychotics (Clozapine, Olanzapine) | Clozapine penetrates significantly, olanzapine moderately. | Clozapine: sedation, hypotonia, neutropenia. Olanzapine: lower risk but requires monitoring. | Clozapine not recommended; olanzapine used with monitoring. | [17] |
| GHB | Milk levels 71–80% lower than blood, return to endogenous levels after 5 h. | Low risks if waiting period is followed. | Safe after 5 h post-dose. | [43] |
| Ketamine | Low oral bioavailability, minimal secretion into milk. | Minimal neonatal exposure. | Breastfeeding can resume after 12 h. | [44] |
| Other (Methotrexate, Acitretin) | May accumulate in neonatal tissues. | High toxicity. | Contraindicated. | [31] |
| Immunoglobulins | Transfer into milk but no adverse effects. | No known risks. | Safe for the infant. | [30] |
| THC: 9—tetrahydrocannabinol; CBD: Cannabidiol; CBN: Cannabinol; GHB: Gamma Hydroxybutyric | ||||
| Categories | Aspects | Key Points | Notes |
|---|---|---|---|
| factors related to infants | Dynamic Nature | Changes in infant needs | Adapts nutritionally and immunologically |
| factors related to breastfeeding mothers | Maternal Diet | Affects fatty acid composition | Nutrition impacts milk |
| Maternal Factors | Body weight, age, and ethnicity matter | Genetic influences | |
| Hormonal Control | Prolactin and oxytocin regulate milk flow | Key lactation hormones | |
| External Influences | Stress and medications alter milk | Emotional and medical factors | |
| Maternal Health | Infections and inflammation impact permeability | Health affects composition | |
| factors related to breast milk | Colostrum | High in antibodies, proteins, and growth factors | Essential immune protection |
| Transitional Milk | Increases in volume and fat | Intermediate stage | |
| Mature Milk | Balanced nutrients: macronutrients, vitamins, and minerals | Supports steady growth | |
| Foremilk | Watery and hydrates infant | Early feed | |
| Hindmilk | High in fat and energy-rich | Late feed | |
| Daily Variations | Fat peaks in the morning and drops at night | Diurnal changes | |
| factors related to breastfeeding | Lactation Duration | Protein decreases and fat is stable | Long-term adaptation |
| miscellaneous | Influencing Factors | Lactation stage, feeding patterns, and maternal traits | Time, diet, health |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barranco, R.; Grassi, S.; Dimitrova, A.; Caristo, I.; Costantino, A.; Vaiano, F.; Pinchi, V.; Ventura, F. Toxicity of Recreational Drugs and Medications During Lactation: A Systematic Review. Sci 2025, 7, 144. https://doi.org/10.3390/sci7040144
Barranco R, Grassi S, Dimitrova A, Caristo I, Costantino A, Vaiano F, Pinchi V, Ventura F. Toxicity of Recreational Drugs and Medications During Lactation: A Systematic Review. Sci. 2025; 7(4):144. https://doi.org/10.3390/sci7040144
Chicago/Turabian StyleBarranco, Rosario, Simone Grassi, Alexandra Dimitrova, Isabella Caristo, Andrea Costantino, Fabio Vaiano, Vilma Pinchi, and Francesco Ventura. 2025. "Toxicity of Recreational Drugs and Medications During Lactation: A Systematic Review" Sci 7, no. 4: 144. https://doi.org/10.3390/sci7040144
APA StyleBarranco, R., Grassi, S., Dimitrova, A., Caristo, I., Costantino, A., Vaiano, F., Pinchi, V., & Ventura, F. (2025). Toxicity of Recreational Drugs and Medications During Lactation: A Systematic Review. Sci, 7(4), 144. https://doi.org/10.3390/sci7040144









