Abstract
Eutrophication is a significant issue in aquatic systems that receive wastewater from anthropogenic sources. The reduction of phosphate concentration in wastewater and water bodies is essential to reduce the risk of eutrophication. In this study, biomass obtained from Arthrospira platensis was used to reduce the phosphorus concentration in water. The biomass samples were characterized by spectroscopic and morphological techniques, such as vibrational spectroscopy (FTIR and Raman) and microscopy assay (SEM). Adsorption studies were conducted to evaluate the removal efficacy of the biomass. Phosphate removal capacity was strongly influenced by pH, with the highest effectiveness observed under acidic conditions (88% removal at pH 4.4) and rapid initial adsorption reaching equilibrium. Kinetic modelling showed a maximum removal efficacy (qe = 2.4 mg g−1 and k2 = 0.305 min−1). Isothermal adsorption analysis showed that the Langmuir model described properly experimental results showing physical chemical parameters (qmax = 2.8 mg g−1 and KL = 1.41 L mg−1).
1. Introduction
Anthropogenic alterations to the biogeochemical cycle of phosphorus have a significant impact on water quality in aquatic environments. Excessive phosphate concentrations lead to uncontrolled algal blooms, a primary cause of nutrient pollution and environmental degradation, known as eutrophication [1,2]. Algal blooms limit light penetration, negatively affecting phototrophic and photoheterotrophic organisms. In addition, they contribute to oxygen depletion and pose serious threats to aquatic ecosystems [3,4].
Recently, different technologies and bioremediation strategies have been developed to mitigate phosphate pollution, aiming to reduce phosphate levels in water and minimizing the associated environmental impacts [5]. Among these, chemical treatments are commonly used due to their high efficiency, especially when combined with biological agents. Among all reagents employed in chemical methods, aluminum sulfate is the most commonly used metal salt. This salt reacts with phosphate in solution to form solid precipitates, which are the removed from water through a traditional physical separation method (e.g., filtration, clarification) [6,7]. Furthermore, modifications of coagulants offer a promising alternative for improving phosphate removal from water. Titanium-containing reagents are also used for phosphate remotion [8]. However, chemical methods may produce harmful by-products through reactions with other compounds present in the water matrix, which can further damage the ecosystem [9]. The need of acid of alkali additives during chemical process can also boost sludge production [10,11].
In contrast, biological methods offer a more environmentally friendly alternative, supported by decades of biotechnological advancements. Bioremediation techniques are favored for their minimal ecological impact, though they can face challenges such as nutrient costs, bioreactor design, culture stabilization, and microorganism selection [12]. This technique requires less chemical loads but requires specific microbial and environmental conditions [13].
Biosorption-based techniques have demonstrated effective phosphate removal from water, employing different materials (nanoparticles, nanocomposites, and activated carbon) [14]. This methodology is not expensive compared with traditional methods, and its implementation is easier, offering the possibility of recovering the sorbent material and low quantities of by-products after its implementation [15]. Typical adsorbents employed in phosphorus removal include the following: (i) carbonaceous materials [16], (ii) zeolites [17], (iii) clays [18], (iv) layered double hydroxides [19]. In the last decade, microorganism-based sorbents have represented a promising alternative to complement conventional materials used in adsorption processes [20]. Biomass-based adsorption is gaining attention due to its costs of implementation, low environmental risk, and the availability of biosorbent materials in nature [21]. Different natural sources have been employed to obtain sorbent materials to removal phosphate from water (e.g., wheat straw [22], sawdust [23], eggshell wastes [24], corn straw [25]). These materials offer an green strategy to reuse agricultural and biological waste as an inexpensive and renewable source of sorbent materials [26].
Cyanobacteria, particularly Arthrospira platensis, have shown promise as biosorbents in various studies due to their favorable physicochemical properties, including selectivity and high binding affinity. Several researchers have reported the use of A. platensis to remove several compounds [27]. Mitrogiannis et al. reported its effectiveness in the biosorption of methylene blue [28], while Markou et al. reported the ejection of Cu2+ and Ni2+ on A. platensis biomass [29]. Similarly, Alprol et al. achieved high removal values using A. platensis biomass to remove methyl orange from water [30]. Recently, Diaz et al. reported phosphorus removal from water onto biochar fabricated using a cyanobacteria biomass obtained from a local swamp [31].
Due to the higher amounts of phosphorus delivered to the ambient, along with changes in precipitation around the world, eutrophication is expected to increase during the 21st century [32]. In this context, cyanobacteria biomass could be a very important material to obtain as a sorbent material to remove phosphorus from water. This methodology could solve two problems: (i) higher phosphorous in water, and (ii) bioaccumulation of cyanobacteria in water. However, despite growing interest in the use of A. platensis for biosorption, there is limited research on its kinetic and thermodynamic behavior in phosphate biosorption. Therefore, in this work, we study the implementation of A. platensis biomass for removing phosphorus from aqueous solutions, with a focus on adsorption efficiency, kinetics, and isothermal behavior.
2. Materials and Methods
2.1. A. platensis Cultivation and Biomass Extraction
The cyanobacterium Arthrospira platensis (strain SAG 21.99) was cultured in BG-11 medium. The pH of this medium, composed of macro- and micronutrients, was adjusted to a value of 7.4. Cultivation was carried out at a constant temperature of 303 K.
Cell concentrations were monitored every 48 h using a Neubauer hemocytometer (0.1 mm depth) under a binocular optical microscope. The cell density (cells·mL−1) was calculated, until the culture reached its stationary growth phase, using Equation (1):
where [Cell] denotes cell density, calculated as the total number of cells counted across all chambers of the Neubauer hemocytometer [33]. This data was used to construct the growth curve. After cultivation, approximately 6 L of A. platensis wet biomass was harvested by filtration through 13 μm filter paper. The collected biomass was subsequently dried for 48 h at 60 °C. Finally, the sample was characterized through Fourier transform infrared spectroscopy (FTIR), micro-Raman spectroscopy (model NSR 4500 from Jasco, Hachioji-shi, Tokyo), and scanning electron microscopy (SEM, Phenom Pro X).
2.2. Phosphorus Adsorption Study onto Biomass
Phosphorus concentrations were determined according to the 4500-P Standard Method. The persulfate oxidation method was employed to determine phosphorus concentration. Details are reported in [34].
The adsorption capacity of the biomass for phosphorus remotion was investigated through both kinetic and isothermal studies. In the kinetic study, 500 mg of dried biomass was mixed with P solution (25 mg L−1). The system was stirred at 400 rpm, T = 295 K, at a pH = 5.4 for 30 min. For the isothermal adsorption study, we employed the same conditions of the kinetic study with varying P concentrations (5–25 mg L−1). These mixtures were also stirred at 400 rpm under the same temperature and pH conditions for 30 min. We obtained the phosphate adsorption capacity on biomass as follows (Equation (2)):
where (mg/L) is the initial concentration, (mg/L) is the P concentration in the solution as a function of time, V (L) is the volume of the system, and m (g) is the amount of biomass. The data were used for modeling the kinetic results.
For the isothermal adsorption study, 500 mg of biomass was similarly mixed with 50 mL of [Phosphorus], changing concentrations between 5 and 25 mg·L−1. These mixtures were also stirred at 400 rpm under the same temperature and pH conditions for 30 min [35].
We obtained the phosphate adsorption on biomass according to Equation (3):
where qe is the amount (mg) of phosphate adsorbed (mg/g biomass) at equilibrium and ce is the phosphate concentration at equilibrium biosorption–desorption.
3. Results and Discussions
3.1. Arthrospira Platensis Growth Curve
Figure 1 shows the growth curve of A. platensis obtained in this study. An exponential increase in the cell density was observed up to day 10, corresponding to the period of maximum growth rate. From day 10 to day 15, growth continued but at a gradually decreasing rate, indicating a transition towards the stationary phase. Finally, after day 15, a stabilization of the biomass was observed, suggesting that the algal population reached its load capacity in the culture medium. This growth pattern is consistent with typical microbial growth dynamics, and aligns with previous findings reported for A. platensis cultures [36].
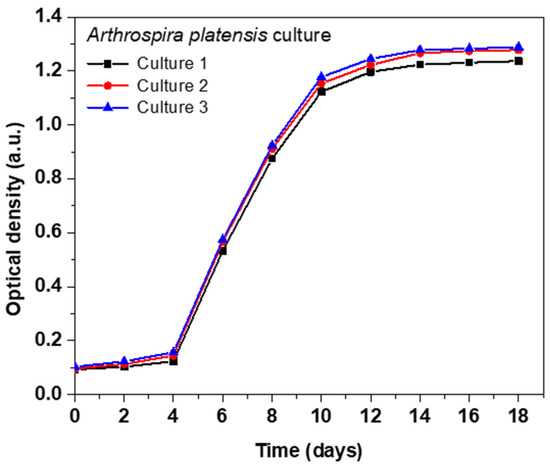
Figure 1.
Growth curve of A. platensis. Cultures were grown in BG-11 medium with sterile distilled water at pH 7.4 and 303 K. Culture 1 is represented by the black line, Culture 2 by the red line, and Culture 3 by the blue line.
3.2. Spectroscopic Characterization
Figure 2 shows the FTIR and Raman spectra obtained from A. platensis biomass. For the FTIR spectrum, several characteristic bands were identified, corresponding to functional groups commonly reported in the literature on microalgae biomass [37]. The broad band located at 3250 cm−1 corresponds to the O–H bond, often associated with hydroxyl groups. In the region of 2800–3200 cm−1, additional signals are observed, which can be linked to symmetric and asymmetric stretching of the –CH3 and –CH2 groups found in hydrocarbon chains. The peak located at 1715 cm−1 is assigned to the C=O stretching, typically associated with the presence of proteins [38], while the peak at 1640 cm−1 corresponds to the N–H vibrations of amine group. Furthermore, peaks located between 1030 and 1110 cm−1 are associated with aromatic C–H bending and symmetric C–O vibrations [39].
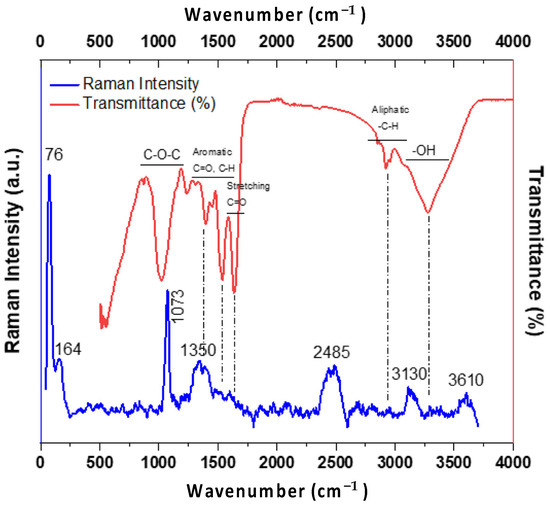
Figure 2.
FTIR (red) and Raman (blue) spectra of biomass obtained from A. platensis.
For the Raman spectrum in Figure 2, a very strong vibration at 1073 cm−1, associated with the C–O–C bond, is observed, indicating the presence of carbohydrates, extracellular polysaccharides, and nucleic acids [40]. In the range of 1200–1500 cm−1, signals correspond to C–H and C–N, including those from Amide III structures, reflecting the presence of proteins and lipids. Although weaker, bands above 3000 cm−1 are also detectable and may correspond to O–H or N–H stretching vibrations, potentially originating from residual moisture, proteins, or polysaccharides in the dry biomass sample [41].
3.3. Morphological Characterization
The images of the A. platensis biomass at different magnifications are shown in Figure 3. In the low-magnification image (Figure 3a), irregularly shaped particles are observed, with a broad range of the sizes (1–10 μm). This result indicates that the sample morphology is heterogeneous in nature, likely exhibiting fractured or eroded surfaces due to the drying process.
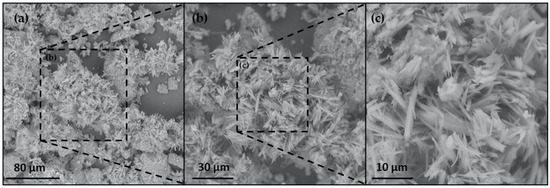
Figure 3.
Scanning electron micrographs of biomass obtained from A. platensis. Magnification: (a) 1000×; (b) 2000×; and (c) 5000×.
At a higher magnification (Figure 3b,c), the biomass surface reveals rod-like structures of varying lengths. This morphological feature is particularly significant, as the presence of nanorods increases the available surface area for phosphate biosorption. A larger contact area enhances the interaction surface of the sorbent and the adsorbate, which is a desirable property for biosorption-based contaminant removal applications.
3.4. Adsorption Study
Before conducting kinetic and isothermal adsorption experiments, the pH value effect on the phosphorus biosorption efficacy was evaluated (see Figure 4). This result shows that phosphorus biosorption is significantly more effective at lower pH values. Specifically, efficiency of the process decreased from 88% at pH 4.4 to just 7.7% at pH 10.7. It is known that pH affects the electrostatic interaction between ions and the surface of the biomass. This is due to the fact that pH can change the charge of the chemical groups located at the biomass surface. At basic pH, the hydroxyl groups are deprotonated, and negative density on the biomass surface increases. At the same time, the negative charge on phosphate reduces the possibility of electrostatic adsorption in the biomass surface. While at an acidic pH, the biomass surface is less negative, assisting the phosphate adsorption on the biomass surface [42].
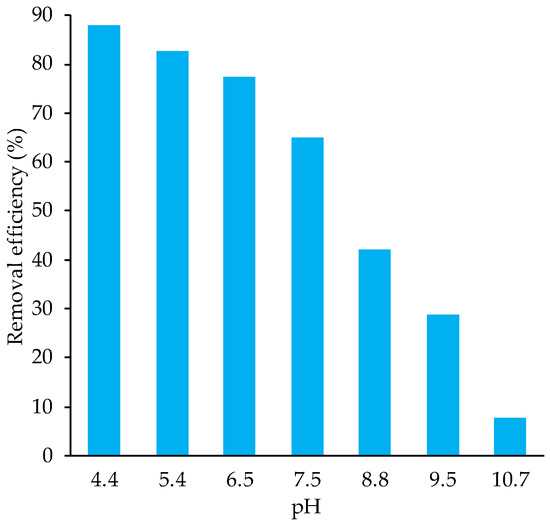
Figure 4.
pH effect on the biosorption efficiency of phosphate onto biomass obtained from A. platensis.
Furthermore, water purification at pH 4.4 can inhibit other biosystems, which means that nitrification/denitrification processes may potentially be attenuated. However, results shown in Figure 4 indicate that the biosorbent remains effective at pH 6.5, where nearly 80% of phosphate is removed. Under this pH condition, the biosystem could survive. Conducting the biosorbent process under neutral conditions is therefore suitable for phosphate removal without adversely affecting other biosystems. Finally, to achieve complete phosphate removal from water, two options can be implemented: (i) renewing the biosorbent material to continue the removal process, or (ii) employing a coagulation process [43].
3.5. Kinetic Study
Figure 5 shows the kinetic adsorption study of phosphate biosorption onto A. platensis biomass. This figure shows that biosorption capacity (qt) increases quickly, reaching saturation of the surface around 15 min and achieving a maximum biosorption capacity (qe) of approximately 2.4 mg g−1. The results show that the saturation point is reached after 20 min, indicating a rapid uptake of phosphate ions.
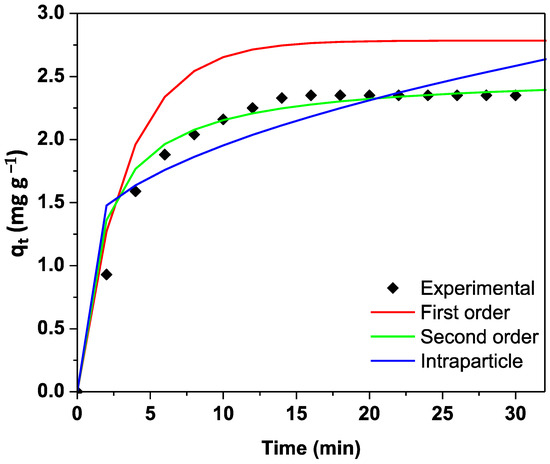
Figure 5.
Kinetic fitting of phosphate adsorption onto biomass obtained from A. platensis.
We employed three models to describe the experimental results, according to the information listed in Table 1 [44]. The PSO was suitable to describe results (Table 2). This model establishes that the biosorption process is controlled by chemical interactions where electrostatic interactions are present during the biosorption process. This implies that chemical interactions (e.g., covalent bonding or electron sharing) between ions and chemical groups on the biomass are present during the removal process. Other authors have reported a PSO model to model the results of the phosphate removal. Manawi et al. reported that phosphorus adsorption on modified biochar followed the pseudo-second order model [45]. Qin et al. reported that phosphorus removal on three different biochar were described by the PSO [46]. This model is frequently employed to fit data results. Table 2 lists some reports when PSO was employed to fit phosphate removal from water.

Table 1.
Mathematical description of adsorption kinetic models employed for fitting the data.

Table 2.
Kinetic fitting of phosphate adsorption data on biomass *.
3.6. Isothermal Study
Figure 6 presents the experimental results of the adsorption isotherm. In this study, three isothermal models were employed to describe experimental results, according to the information listed in Table 3 [47].
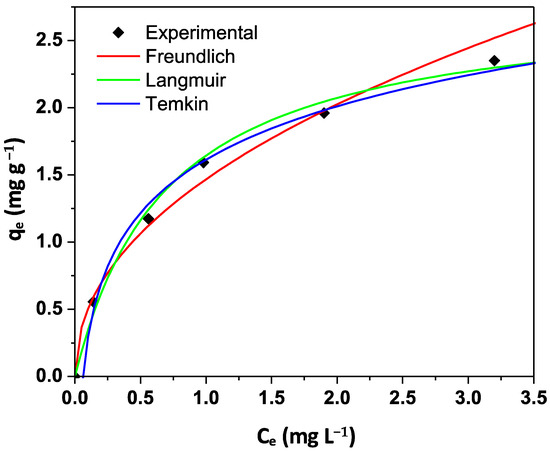
Figure 6.
Isothermal fitting of phosphate adsorption onto biomass obtained from A. platensis.

Table 3.
Mathematical description of isothermal models employed for fitting the data.
Table 4 summarizes the isothermal results for each model. According to Figure 6 and Table 4, the Langmuir model suitably described the isothermal results. Results indicate that phosphate adsorption onto the A. platensis biomass can be explained by a mechanism of monolayer type, in which the process continues until all active sites on the absorbent are fully saturated by ions to form the monolayer. This monolayer model has been applied to describe the phosphorus removal. Liao et al. employed this model to describe the phosphate adsorption on modified pineapple biochar [48]. Other authors have reported that multilayer sorption can describe the phosphate adsorption. Table 5 compares some reports about phosphorus removal from water onto different materials. Others authors applied the Freundlich model to describe phosphorus removal onto biochar [49]. However, due to the fact that the mechanism of phosphate adsorption on biomass can involve more than one step (e.g., surface precipitation, ionic exchange, electrostatic interactions) [50], the type of mechanism in the isothermal sorption process could depend on the sorbent source and the method to obtain sorbent material [51].

Table 4.
Isothermal fitting of phosphate adsorption data on biomass **.
The results listed in Table 5 are comparable to those reported in previous studies on phosphate adsorption employing different adsorbent materials. For example, Jung et al. reported qe = 3.01 mg g−1 in phosphorus adsorption using biochar derived from peanut shells [52]. Similarly, Zhu et al. reported a qe = 3.08 mg g−1 in the phosphate removal using a composite material consisting of Fe2O3/Fe3O4/bamboo biochar [53].

Table 5.
Sorption efficacy for phosphorus remotion from water onto different carbonaceous materials.
Table 5.
Sorption efficacy for phosphorus remotion from water onto different carbonaceous materials.
| Sorbent Material | Adsorption Capacity (mg g−1) 1 | Isothermal Model | Kinetic Model |
|---|---|---|---|
| Biochar cyanobacteria [31] | 5.5 | Langmuir | SS |
| Biochar pineapple [48] | 3.7 | Langmuir | SS 2 |
| Biochar/sewage sludge [54] | 0.7–1.2 | NR 3 | NR |
| Biochar/pine sawdust [55] | 2.0 | NR | NR |
| Fe-biochar modified [56] | 0.56 | NR | NR |
| Iron-loaded tannin gel [57] | 2.7 | Freundlich | NR |
| Cyanobacteria biomass (this work) | 2.8 | Langmuir | SS |
1 Data obtained from reports in literature. 2 Second order. 3 Not reported.
4. Conclusions
In this study, biomass was obtained from A. platensis and evaluated for its potential in phosphate biosorption. Characterization by FTIR, Raman, and SEM revealed a chemical composition and surface morphology conducive to interactions with phosphate ions. The biomass consisted of heterogeneous fragments with fractured, rough surfaces and visible cavities or pores, indicating high surface porosity. The phosphate removal efficiency was strongly influenced by pH, with maximum biosorption observed under acidic conditions and rapid uptake reaching equilibrium within a short time.
The PSO model provided the best fit, indicating that chemical interactions are the driven forces in the sorption process. Furthermore, the Langmuir model was suitable for describing the adsorption behavior, suggesting monolayer coverage on a homogeneous surface. Overall, A. platensis biomass demonstrated promising potential as an effective sorbent for phosphorus removal applications.
Author Contributions
Conceptualization, C.D.-U. and W.V.; methodology, Y.B., C.D.-U., W.V. and E.M.-V.; validation, Y.B.; formal analysis, Y.B., C.D.-U. and W.V.; investigation, Y.B., C.D.-U., W.V. and E.M.-V.; resources, C.D.-U., W.V. and E.M.-V.; data curation, Y.B., C.D.-U. and W.V.; visualization, C.D.-U., W.V., J.E.D. and E.M.-V.; writing—original draft preparation, Y.B., C.D.-U., W.V. and E.M.-V.; writing—review and editing, Y.B., C.D.-U., W.V., J.E.D. and E.M.-V.; supervision, C.D.-U. and W.V.; project administration, C.D.-U., W.V. and E.M.-V.; funding acquisition, C.D.-U., W.V., J.E.D. and E.M.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by the Grant project C.I. 71407.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
C.D.-U.: W.V. would like to thank Universidad del Atlántico. E.M.-V. and J.E.D. thank Engineer J. Betancourt for the SEM and Raman measurements. The authors acknowledge the Universidad del Valle for financial support (Grant project C.I. 71407).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- McDowell, R.W.; Luo, D.; Pletnyakov, P.; Upsdell, M.; Dodds, W.K. Anthropogenic nutrient inputs cause excessive algal growth for nearly half the world’s population. Nat. Commun. 2025, 16, 1830. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.J.; Obenour, D.R.; Morrison, E.S.; Elser, J.J. A critical eutrophication–climate change link. Nat. Sustain. 2025, 8, 222–223. [Google Scholar] [CrossRef]
- Amorim, C.A.; Moura, A. do N. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Varshney, G.; Varshney, V.; Hejase, C.A. Recent Advances in Technologies for Phosphate Removal and Recovery: A Review. ACS Environ. Au 2024, 4, 271. [Google Scholar] [CrossRef]
- Ping, Q.; Zhang, Z.; Guo, W.; Wang, L.; Li, Y. A comprehensive investigation to the fate of phosphorus in full-scale wastewater treatment plants using aluminum salts for enhanced phosphorus removal. Sci. Total Environ. 2024, 913, 169641. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhuang, M.; Xu, H.; Cao, W.; Li, F.; Liao, X.; Zhou, Z.; Wang, D. Investigation of the phosphorus removal performance and mechanism of aluminum-based phosphorus-locking agent in enhanced bioretention facility. Urban Water J. 2025, 77, 108483. [Google Scholar] [CrossRef]
- Kuzin, E. Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes. Inorganics 2025, 13, 9. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Dong, Y. A review of methods, influencing factors and mechanisms for phosphorus recovery from sewage and sludge from municipal wastewater treatment plants. J. Environ. Chem. Eng. 2024, 12, 111657. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C.M. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Kumari, S.; Dong, Y. Alcan-based adsorption: Mitigating phosphate pollution in subsurface drainage water. J. Water Process Eng. 2025, 69, 106781. [Google Scholar] [CrossRef]
- Abdoli, S.; Asgari Lajayer, B.; Dehghanian, Z.; Bagheri, N.; Vafaei, A.H.; Chamani, M.; Rani, S.; Lin, Z.; Shu, W.; Price, G.W. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water 2024, 16, 2507. [Google Scholar] [CrossRef]
- Diaz, R.; Mackey, B.; Chadalavada, S.; Kainthola, J.; Heck, P.; Goel, R. Enhanced Bio-P removal: Past, present, and future—A comprehensive review. Chemosphere 2022, 309, 136518. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.O.; Aturagaba, G.; Ntale, M.; Nyakairu, G.W. A review of adsorption techniques for removal of phosphates from wastewater. Water Sci. Technol. 2022, 86, 3113–3132. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Recepoglu, Y.K.; Goren, A.Y.; Orooji, Y.; Khataee, A. Carbonaceous materials for removal and recovery of phosphate species: Limitations, successes and future improvement. Chemosphere 2022, 287, 132177. [Google Scholar] [CrossRef]
- Pesendorfer, S.; Ellersdorfer, M. Kinetics of simultaneous ammonium and phosphate recovery by natural zeolite. ChemEngineering 2021, 5, 68. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; yan Wang, C.; Chen, Z.; Tao, Q.; Wei, W.; Cho, K.; Yuan, P.; Frost, R.L.; Ni, B.J. Comprehensive review of modified clay minerals for phosphate management and future prospects. J. Clean. Prod. 2024, 447, 141425. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Khataee, A.; Yoon, Y. Layered double hydroxides for removing and recovering phosphate: Recent advances and future directions. Adv. Colloid Interface Sci. 2022, 300, 102598. [Google Scholar] [CrossRef]
- Singh, S.K.; Yadav, N.; Yadav, P.; Shukla, L.; Pradhan, T.; Kumar, M.; Singh, R.; Kumar, A. Cyanobacterial based bioremediation of xenobiotics compounds. Adv. Chem. Pollution, Environ. Manag. Prot. 2025, 12, 507–524. [Google Scholar]
- Diaz-Uribe, C.; Angulo, B.; Patiño, K.; Hernández, V.; Vallejo, W.; Gallego-Cartagena, E.; Romero Bohórquez, A.R.; Zarate, X.; Schott, E. Cyanobacterial Biomass as a Potential Biosorbent for the Removal of Recalcitrant Dyes from Water. Water 2021, 13, 3176. [Google Scholar] [CrossRef]
- Pan, W.; Xie, H.; Zhou, Y.; Wu, Q.; Zhou, J.; Guo, X. Simultaneous adsorption removal of organic and inorganic phosphorus from discharged circulating cooling water on biochar derived from agricultural waste. J. Clean. Prod. 2023, 383, 135496. [Google Scholar] [CrossRef]
- Yang, S.; Katuwal, S.; Zheng, W.; Sharma, B.; Cooke, R. Capture and recover dissolved phosphorous from aqueous solutions by a designer biochar: Mechanism and performance insights. Chemosphere 2021, 274, 129717. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, S.; Zheng, Q.; Huang, B.; Zhang, J.; Fu, H.; Gao, H. Removal and recovery of phosphorus from solution by bifunctional biochar. Inorg. Chem. Commun. 2022, 139, 109341. [Google Scholar] [CrossRef]
- Li, B.; Jing, F.; Hu, Z.; Liu, Y.; Xiao, B.; Guo, D. Simultaneous recovery of nitrogen and phosphorus from biogas slurry by Fe-modified biochar. J. Saudi Chem. Soc. 2021, 25, 101213. [Google Scholar] [CrossRef]
- Kumari, S.; Dong, Y.; Safferman, S.I. Phosphorus adsorption and recovery from waste streams using biochar: Review of mechanisms, modifications, and agricultural applications. Appl. Water Sci. 2025, 15, 162. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Godlewska, K.; Trynda, J.; Marycz, K. Arthrospira (Spirulina) platensis: An effective biosorbent for nutrients. Process Biochem. 2020, 88, 129–137. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Markou, G.; Mitrogiannis, D.; Çelekli, A.; Bozkurt, H.; Georgakakis, D.; Chrysikopoulos, C.V. Biosorption of Cu2+ and Ni2+ by Arthrospira platensis with different biochemical compositions. Chem. Eng. J. 2015, 259, 806–813. [Google Scholar] [CrossRef]
- Alprol, A.E.; Khedawy, M.; Ashour, M.; Thabet, W.M. Arthrospira platensis nanoparticle-based approach for efficient removal of methyl orange dye from aqueous solutions: Isotherm, kinetic, and thermodynamic analysis. Biomass Convers. Biorefinery 2023, 14, 30279–30296. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Monterrosa, F.; Simons, V.; Duran, F.; Florian, V.; Vallejo, W.; Castellanos, K.; Diosa, J.E.; Mosquera-Vargas, E. Phosphorus Removal from Aqueous Solutions Using Biochar Derived from Cyanobacterial Biomass. Water 2025, 17, 1287. [Google Scholar] [CrossRef]
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Fernández, C.; Hervás, D.; Rivas-Sendra, A.; Marín, M.P.; Seguí-Simarro, J.M. Comparison of six different methods to calculate cell densities. Plant Methods 2018, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Walteros, L.; Duran, F.; Vallejo, W.; Romero Bohórquez, A.R. Prosopis juliflora Seed Waste as Biochar for the Removal of Blue Methylene: A Thermodynamic and Kinetic Study. ACS Omega 2022, 7, 42916–42925. [Google Scholar] [CrossRef]
- Sergeeva, Y.E.; Sukhinov, D.V. Influence of Inoculum Age on the Growth and C-Phycocyanin Accumulation in Arthrospira platensis Cyanobacteria. Nanobiotechnol. Rep. 2023, 18, 33–38. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Grace, C.E.E.; Lakshmi, P.K.; Meenakshi, S.; Vaidyanathan, S.; Srisudha, S.; Mary, M.B. Biomolecular transitions and lipid accumulation in green microalgae monitored by FTIR and Raman analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117382. [Google Scholar]
- Ghorbani, E.; Nowruzi, B.; Nezhadali, M.; Hekmat, A. Metal removal capability of two cyanobacterial species in autotrophic and mixotrophic mode of nutrition. BMC Microbiol. 2022, 22, 58. [Google Scholar] [CrossRef]
- Huang, W.E.; Griffiths, R.I.; Thompson, I.P.; Bailey, M.J.; Whiteley, A.S. Raman microscopic analysis of single microbial cells. Anal. Chem. 2004, 76, 4452–4458. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Umapathy, S. Potential of Raman spectroscopic techniques to study proteins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119712. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Kim, S.; Adkins, J. Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars. C 2021, 7, 11. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A review on revolutionary technique for phosphate removal in wastewater using green coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Manawi, Y.; Al-Gaashani, R.; Simson, S.; Tong, Y.; Lawler, J.; Kochkodan, V. Adsorptive removal of phosphate from water with biochar from acacia tree modified with iron and magnesium oxides. Sci. Rep. 2024, 14, 17414. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, X.; Huang, Q.; Beiyuan, J.; Wang, J.; Liu, J.; Yuan, W.; Nie, C.; Wang, H. Phosphate Removal Mechanisms in Aqueous Solutions by Three Different Fe-Modified Biochars. Int. J. Environ. Res. Public Health 2023, 20, 326. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus adsorption by functionalized biochar: A review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- Mari Selvam, S.; Behera, B.; Chauhan, A.; Madaan, A.; Rajamanickam, R.; Akshaya, K.; Selvasembian, R. Sustainable approach of modified biochar based adsorbents towards enhanced phosphorus removal from wastewater. J. Anal. Appl. Pyrolysis 2025, 188, 107020. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Ahn, K.H.; Ok, Y.S. Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, C.P.; Zhu, Y.; Wei, W.; Qin, H. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water. J. Water Process Eng. 2018, 25, 96–104. [Google Scholar] [CrossRef]
- Melia, P.M.; Busquets, R.; Hooda, P.S.; Cundy, A.B.; Sohi, S.P. Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars. Sci. Total Environ. 2019, 675, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Chang, S.X. Pyrolysis temperature and steam activation effects on sorption of phosphate on pine sawdust biochars in aqueous solutions. Chem. Speciat. Bioavailab. 2016, 28, 42–50. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, J.; Chi, T.; Wang, P.; Yang, B. Removing phosphorus from aqueous solutions by using iron-modified corn straw biochar. Front. Environ. Sci. Eng. 2015, 9, 1066–1075. [Google Scholar] [CrossRef]
- Ogata, T.; Morisada, S.; Oinuma, Y.; Seida, Y.; Nakano, Y. Preparation of adsorbent for phosphate recovery from aqueous solutions based on condensed tannin gel. J. Hazard. Mater. 2011, 192, 698–703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).