Valorization of Grape Pomace Through Integration in Chocolate: A Functional Strategy to Enhance Antioxidants and Fiber Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Pomace Flour Composition
2.2. Chocolate Samples Preparation
2.3. Chemical Analysis
2.3.1. Protein
2.3.2. Fat
2.3.3. Total Sugars
2.3.4. Total Dietary Fiber
2.3.5. Preparation of Methanolic Extracts for TPC (2.3.6), DPPH (2.3.7), and ORAC (2.3.8)
2.3.6. Total Phenolic Content (TPC)
2.3.7. DPPH (2,2-Diphenyl-1-picrylhydrazyl)
2.3.8. ORAC (Oxygen-Radical Absorbance Capacity)
2.4. Colorimetric Analysis
2.5. Texture Analysis
2.6. Sensory Evaluation
2.7. Statistical Analysis and Hierarchical Clustering
3. Results
3.1. Chemical Composition
3.1.1. Protein, Fat, Total Sugars, and Total Dietary Fiber
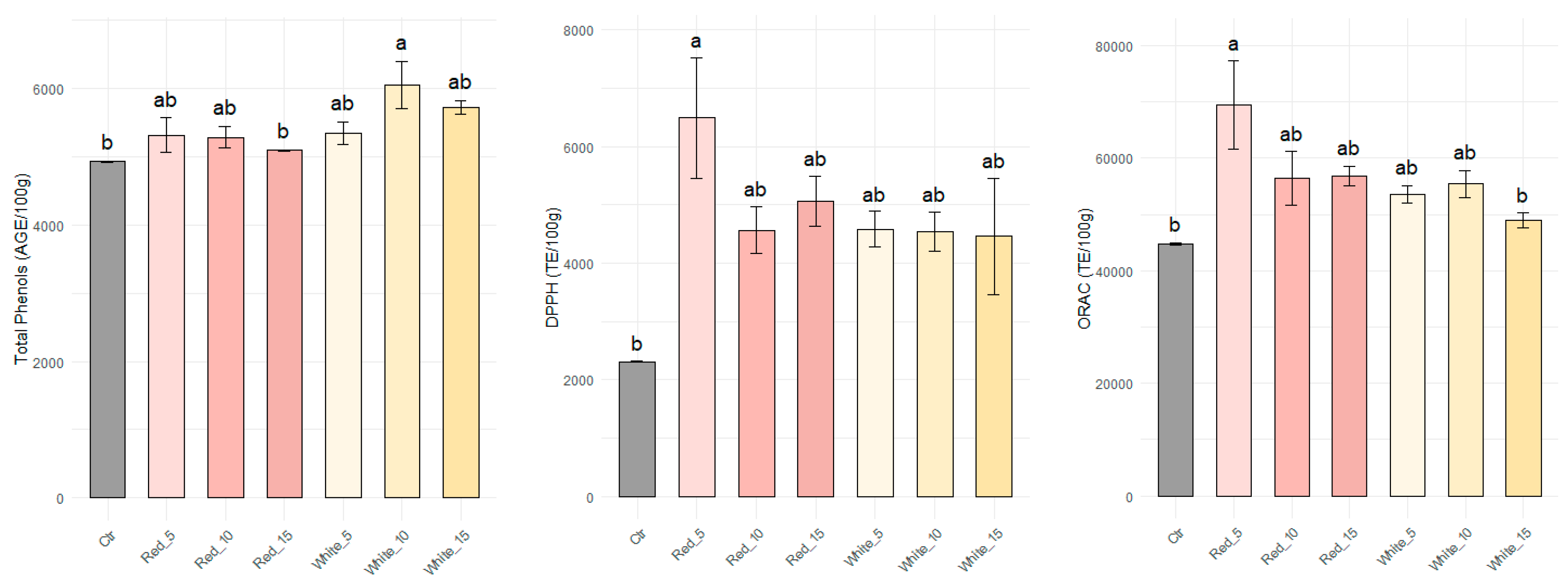
3.1.2. Total Phenolic Content, DPPH Activity, and ORAC
3.2. Colorimetric Assessment
3.3. Texture Assessment
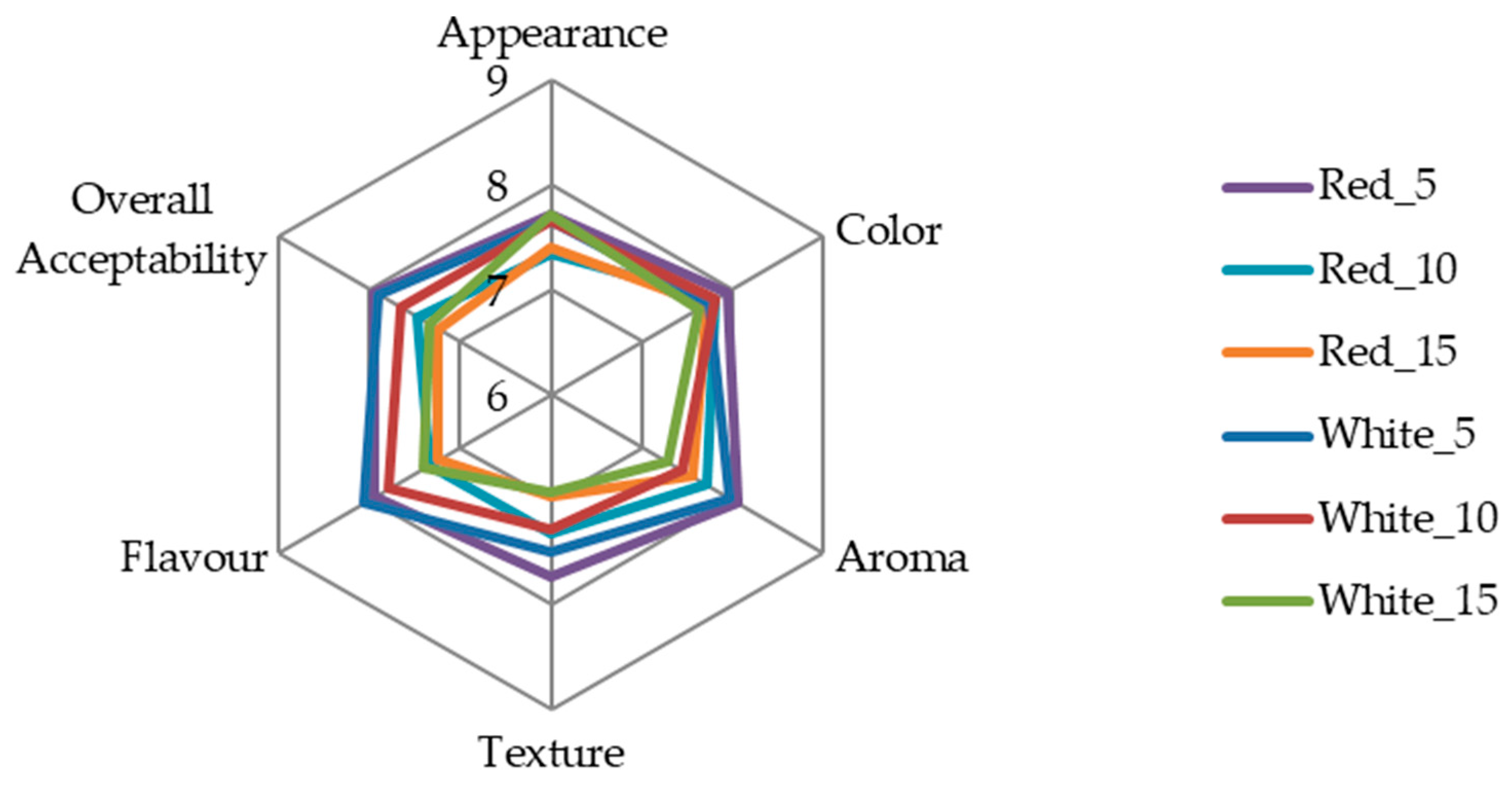
3.4. Sensory Analysis
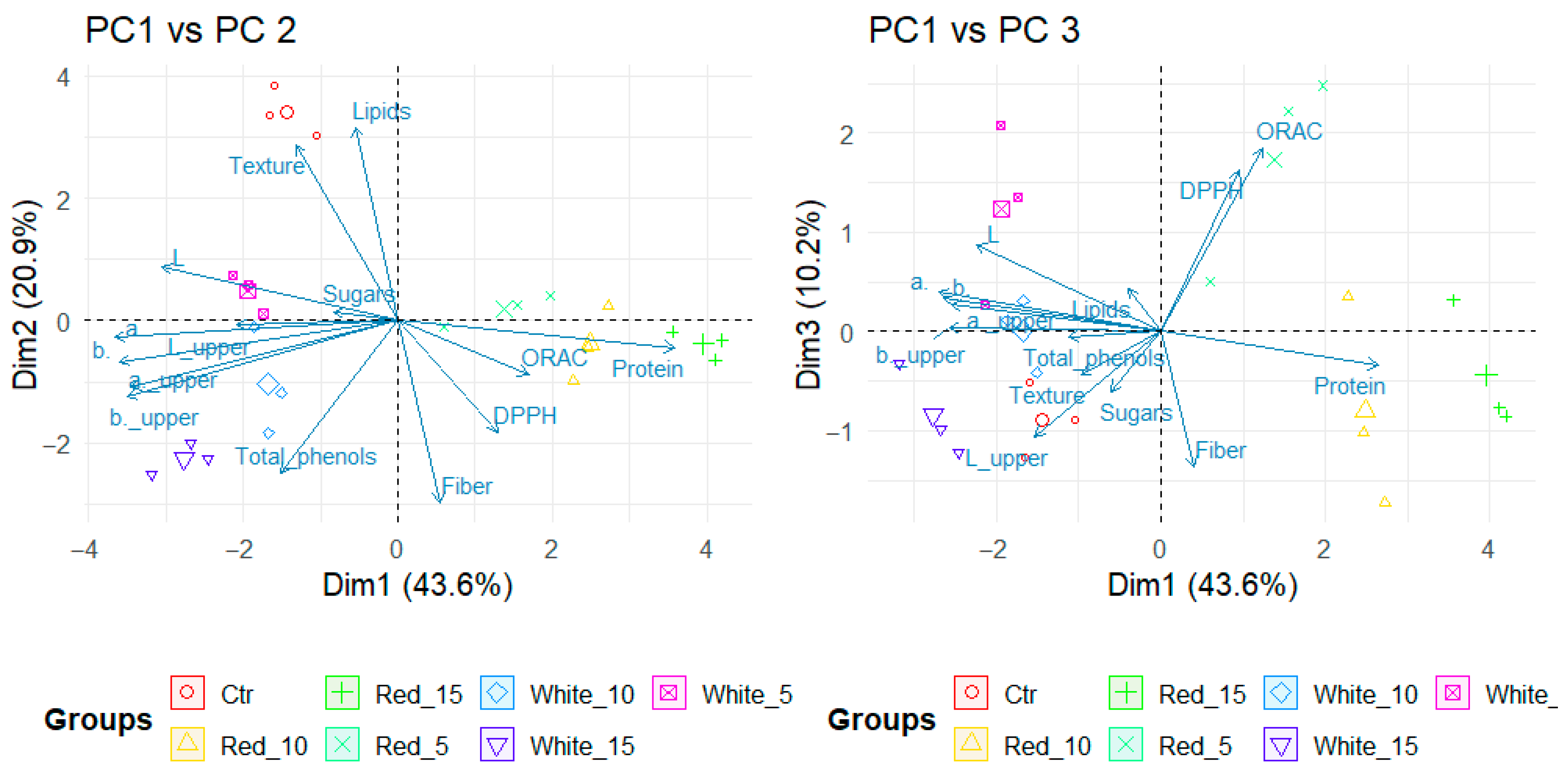
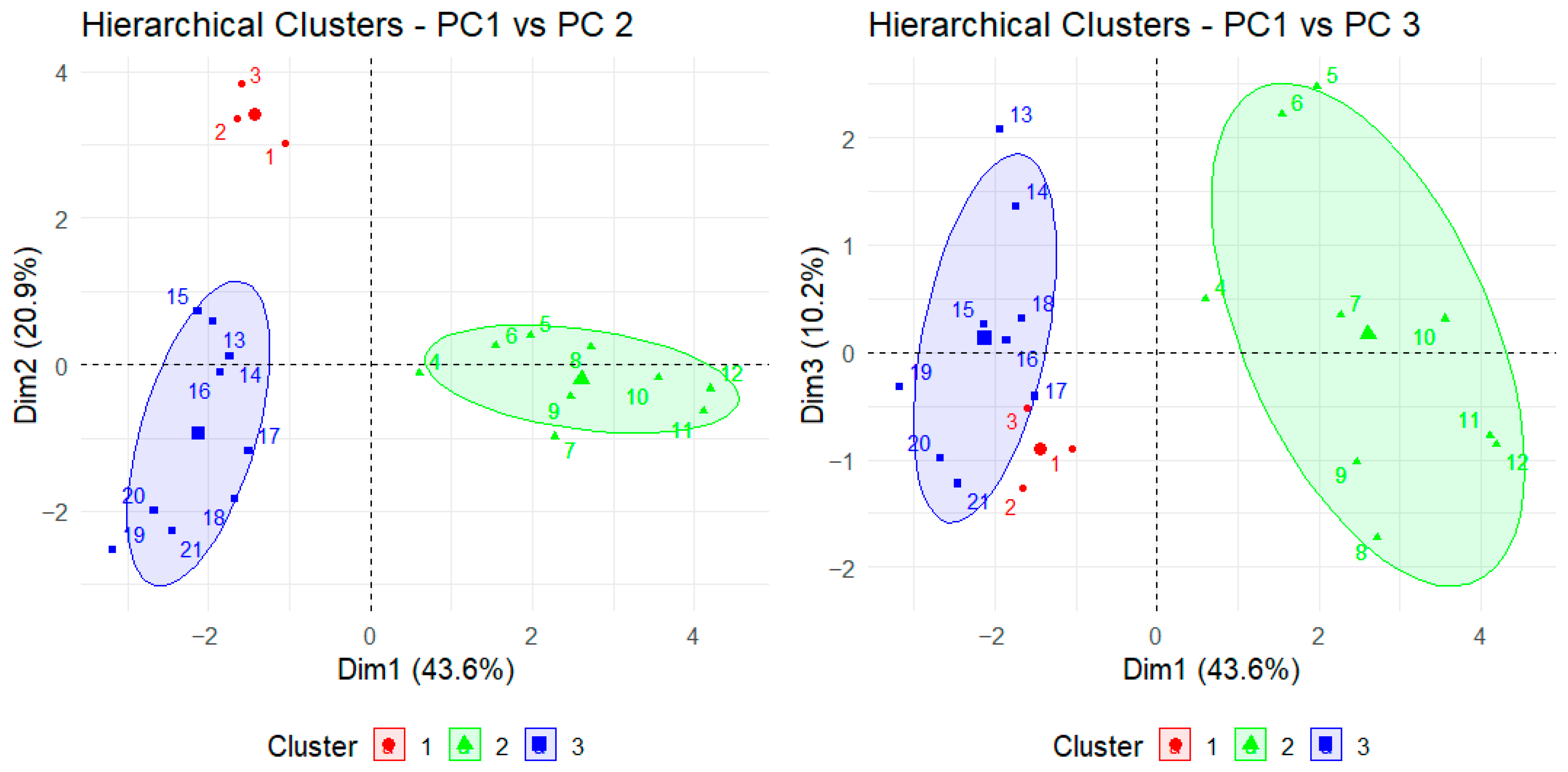
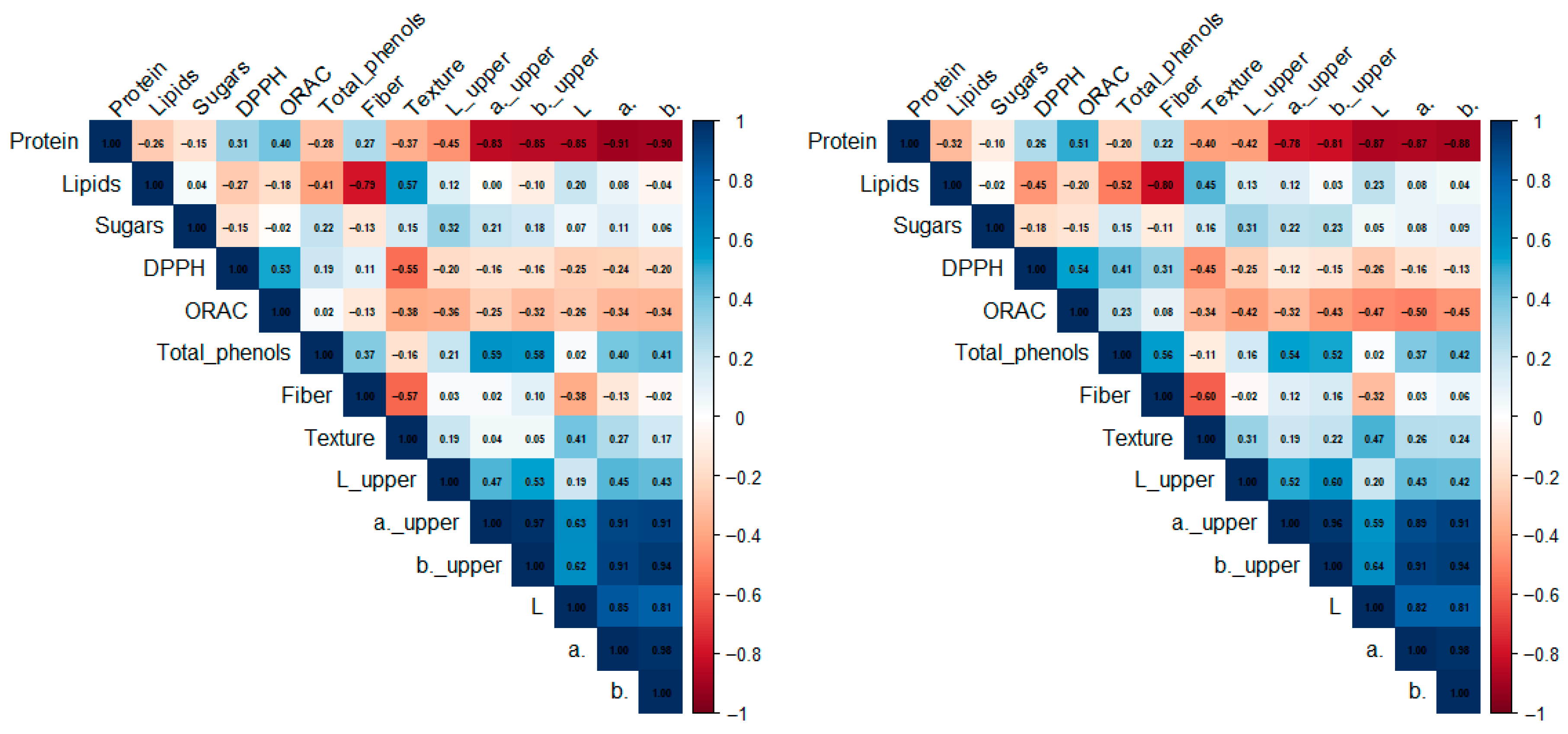
3.5. Principal Component Analysis and Hierarchical Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity–a review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Cao, X.; Wang, J. Preparation and modification of high dietary fiber flour: A review. Food Res. Int. 2018, 113, 24–35. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Balaha, M.; Acquaviva, A.; Ferrante, C.; Cataldi, A.; Menghini, L.; Rapino, M.; Orlando, G.; Brunetti, L.; Leone, S.; et al. Phenolic Characterization and Neuroprotective Properties of Grape Pomace Extracts. Molecules 2021, 26, 6216. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-Lopez, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.; Martínez-Ávila, G.C.G. Grape Pomace—Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef]
- Tikhonova, A.; Ageeva, N.; Globa, E. Grape pomace as a promising source of biologically valuable components. BIO Web Conf. 2021, 34, 06002. [Google Scholar] [CrossRef]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. Int. J. Food Sci. Technol. 2018, 54, 952–958. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadstick fortification with red grape pomace: Effect on nutritional, technological and sensory properties. J. Sci. Food Agric. 2022, 102, 2545–2552. [Google Scholar] [CrossRef]
- Hayta, M.; Özuğur, G.; Etgü, H.; Şeker, İ.T. Effect of Grape (Vitis vinifera L.) Pomace on the Quality, Total Phenolic Content and Anti-Radical Activity of Bread. J. Food Process. Preserv. 2014, 38, 980–986. [Google Scholar] [CrossRef]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 3, 251–263. [Google Scholar] [CrossRef]
- Hoye, C.J.; Ross, C.F. Total phenolic content, consumer acceptance, and instrumental analysis of bread made with grape seed flour. J. Food Sci. 2011, 76, S428–S436. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of grape pomace powder addition on chemical, nutritional and technological properties of cakes. LWT Food Sci. Technol. 2020, 134, 109950. [Google Scholar] [CrossRef]
- Aivalioti, M.; Cossu, R.; Gidarakos, E. New opportunities in industrial waste management. Waste Manag. 2014, 34, 1737–1738. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Rimbach, G.; Melchin, M.; Moehring, J.; Wagner, A.E. Polyphenols from Cocoa and Vascular Health—A Critical Review. Int. J. Mol. Sci. 2009, 10, 4290–4309. [Google Scholar] [CrossRef] [PubMed]
- Ackar, D.; Lendi, K.; Valek, M.; Subari, D.; Borislav, M.; Babic, J.; Nedic, I. Review Article: Cocoa Polyphenols: Can We Consider Cocoa and Chocolate as Potential Functional Food? J. Chem. 2013, 2013, 289392. [Google Scholar] [CrossRef]
- Khan, N.; Khymenets, O.; Urpí-Sardà, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef]
- Efraim, P.; Alves, A.B.; Jardim, D. Review: Polyphenols in cocoa and derivatives: Factors of variation and health effects. Braz. J. Food Technol. 2011, 14, 181–201. [Google Scholar] [CrossRef]
- Vinson, J.A.; Proch, J.; Zubik, L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999, 47, 4821–4824. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Acan, B.G.; Kılıclı, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of grape pomace usage in chocolate spread formulation on textural, rheological and digestibility properties. LWT Food Sci. Technol. 2021, 138, 110451. [Google Scholar] [CrossRef]
- Bursa, K.; Kilicli, M.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Kian-Pour, N.; Konar, N. Formulating and studying compound chocolate with adding dried grape pomace as a bulking agent. J. Food Sci. Technol. 2022, 59, 1704–1714. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC Inc.: Arlington, VA, USA, 1990; Volume I and II, ISBN 0-935584-42-0. [Google Scholar]
- Instituto Português da Qualidade. NP 1419:1987-Determinação dos Açúcares Totais; IPQ: Caparica, Portugal, 1987. [Google Scholar]
- Coelho, A.R.F.; Pessoa, C.C.; Marques, A.C.; Luís, I.C.; Daccak, D.; Silva, M.M.; Simões, M.; Reboredo, F.H.; Pessoa, M.F.; Legoinha, P.; et al. Natural Mineral Enrichment in Solanumtuberosum L. cv. Agria: Accumulation of Ca and Interaction with Other Nutrients by XRF Analysis. Biol. Life Sci. Forum 2021, 4, 77. [Google Scholar] [CrossRef]
- ISO 11136; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area, 1st ed. International Organization of Standardization: Geneva, Switzerland, 2014.
- Lopes, J.d.C.; Madureira, J.; Margaça, F.M.A.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Zanini, M.; Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Valorization of Wastes Generated in Organic Grape Processing. Braz. Arch. Biol. Technol. 2024, 67, e24230183. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Mosele, J.; Souza da Costa, B.; Bobadilla, S.; Motilva, M.J. Phenolic Composition of Red and White Wine Byproducts from Different Grapevine Cultivars from La Rioja (Spain) and How This Is Affected by the Winemaking Process. J. Agric. Food Chem. 2023, 71, 18746–18757. [Google Scholar] [CrossRef] [PubMed]
- Živković, N.; Čakar, U.; Petrović, A. Effects of spontaneous and inoculated fermentation on the total phenolic content and antioxidant activity of Cabernet Sauvignon wines and fermented pomace. Food Feed. Res. 2024, 51, 119–129. [Google Scholar] [CrossRef]
- Chire-Fajardo, G.; Arrunategui, R.; Orihuela-Rivera, C.; Ureña, M. Assessment of physical and physicochemical quality of main chocolates traded in Peru. Acta Agron. 2017, 66, 164–171. [Google Scholar] [CrossRef]
- Rezende, N.; Benassi, M.; Vissotto, F.; Augusto, P.; Grossmann, M. Effects of fat replacement and fibre addition on the texture, sensory acceptance and structure of sucrose-free chocolate. Int. J. Food Sci. Technol. 2015, 50, 1413–1420. [Google Scholar] [CrossRef]

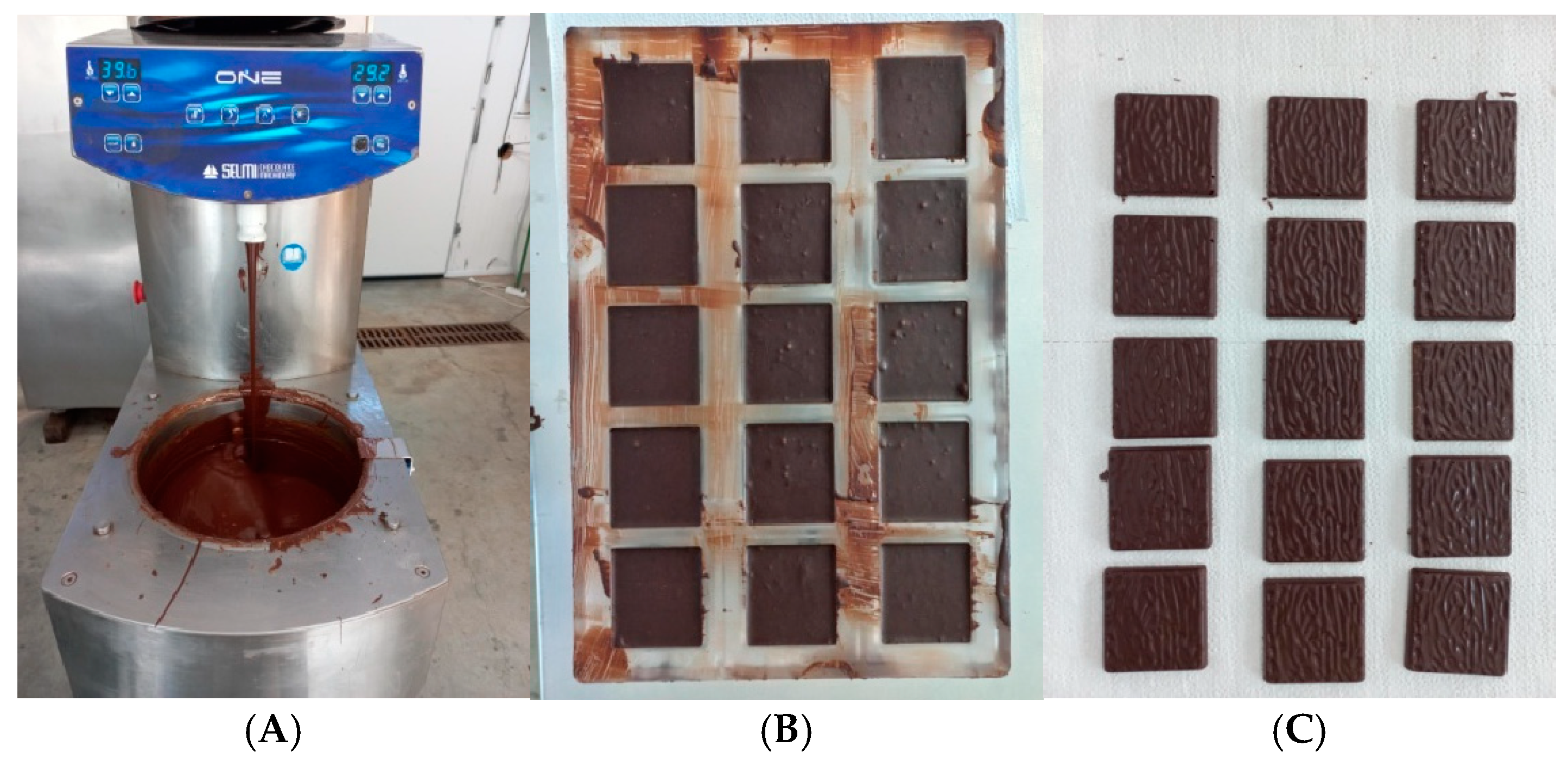




| Composition | Red Pomace | White Pomace |
|---|---|---|
| Protein (% w/w) | 11.1 ± 0.1 | 7.4 ± 0.2 |
| Total sugars (% w/w) | 3.2 ± 0.2 | 31.7 ± 0.1 |
| Fat (% w/w) | 7.5 ± 0.1 | 8.7 ± 0.1 |
| Phenolics (mg GAE/100 g) | 8986 ± 10 | 8395 ± 7 |
| ORAC (µmol TE/100 g) | 79,820 ± 419 | 84,565 ± 383 |
| DPPH (µmol TE/100 g) | 30,200 ± 465 | 30,990 ± 522 |
| Fiber (% w/w) | 58.6 ± 0.5 | 46.2 ± 0.3 |
| Sample | Upper Part of the Chocolate | Lower Part of the Chocolate | ||||
|---|---|---|---|---|---|---|
| L | a* | b* | L | a* | b* | |
| Control | 26.3 ± 0.14 a | 4.88 ± 0.041 b | −1.06 ± 0.017 c | 26.6 ± 0.049 a | 5.29 ± 0.073 bc | −0.86 ± 0.082 cd |
| Red_5 | 26.1 ± 0.043 a | 4.80 ± 0.033 b | −1.19 ± 0.034 d | 26.4 ± 0.038 ab | 5.02 ± 0.025 cd | −1.06 ± 0.026 de |
| Red_10 | 26.1 ± 0.084 a | 4.69 ± 0.027 b | −1.27 ± 0.018 d | 26.0 ± 0.112 bc | 4.75 ± 0.094 de | −1.24 ± 0.047 ef |
| Red_15 | 25.9 ± 0.085 a | 4.43 ± 0.061 c | −1.42 ± 0.014 e | 25.8 ± 0.124 c | 4.58 ± 0.087 e | −1.33 ± 0.062 f |
| White_5 | 26.1 ± 0.10 a | 5.20 ± 0.058 a | −0.808 ± 0.026 b | 26.7 ± 0.058 a | 5.49 ± 0.064 ab | −0.546 ± 0.021 ab |
| White_10 | 26.3 ± 0.038 a | 5.30 ± 0.031 a | −0.698 ± 0.001 ab | 26.2 ± 0.139 abc | 5.38 ± 0.018 ab | −0.698 ± 0.049 bc |
| White_15 | 26.4 ± 0.20 a | 5.33 ± 0.050 a | −0.606 ± 0.045 a | 26.6 ± 0.163 a | 5.62 ± 0.037 a | −0.424 ± 0.024 a |
| Variables | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Protein | 0.951 | −0.116 | −0.123 | −0.058 |
| Lipids | −0.146 | 0.837 | 0.158 | −0.139 |
| Sugars | −0.221 | 0.040 | −0.218 | −0.836 |
| DPPH | 0.346 | −0.482 | 0.585 | −0.146 |
| ORAC | 0.448 | −0.234 | 0.667 | −0.313 |
| Total_phenols | −0.404 | −0.662 | −0.018 | −0.291 |
| Fiber | 0.147 | −0.789 | −0.494 | 0.241 |
| Texture | −0.350 | 0.759 | −0.156 | −0.067 |
| L_upper | −0.552 | −0.020 | −0.381 | −0.360 |
| a*_upper | −0.915 | −0.286 | 0.098 | −0.070 |
| b*_upper | −0.927 | −0.328 | 0.014 | −0.017 |
| L (lower) | −0.807 | 0.232 | 0.316 | 0.215 |
| a* (lower) | −0.970 | −0.070 | 0.142 | 0.090 |
| b* (lower) | −0.953 | −0.177 | 0.119 | 0.160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, D.; Coelho, A.R.F.; Dias, J.; Floro, M.; Marques, A.C.; Ribeiro, C.; Simões, M.; Amaral, O. Valorization of Grape Pomace Through Integration in Chocolate: A Functional Strategy to Enhance Antioxidants and Fiber Content. Sci 2025, 7, 125. https://doi.org/10.3390/sci7030125
Freitas D, Coelho ARF, Dias J, Floro M, Marques AC, Ribeiro C, Simões M, Amaral O. Valorization of Grape Pomace Through Integration in Chocolate: A Functional Strategy to Enhance Antioxidants and Fiber Content. Sci. 2025; 7(3):125. https://doi.org/10.3390/sci7030125
Chicago/Turabian StyleFreitas, Daniela, Ana Rita F. Coelho, João Dias, Miguel Floro, Ana Coelho Marques, Carlos Ribeiro, Manuela Simões, and Olga Amaral. 2025. "Valorization of Grape Pomace Through Integration in Chocolate: A Functional Strategy to Enhance Antioxidants and Fiber Content" Sci 7, no. 3: 125. https://doi.org/10.3390/sci7030125
APA StyleFreitas, D., Coelho, A. R. F., Dias, J., Floro, M., Marques, A. C., Ribeiro, C., Simões, M., & Amaral, O. (2025). Valorization of Grape Pomace Through Integration in Chocolate: A Functional Strategy to Enhance Antioxidants and Fiber Content. Sci, 7(3), 125. https://doi.org/10.3390/sci7030125








