Physicochemical, Granulometric, Morphological, and Surface Characterization of Dried Yellow Pitaya Powder as a Potential Diluent for Immediate-Release Quercetin Tablets
Abstract
1. Introduction
2. Materials and Methods
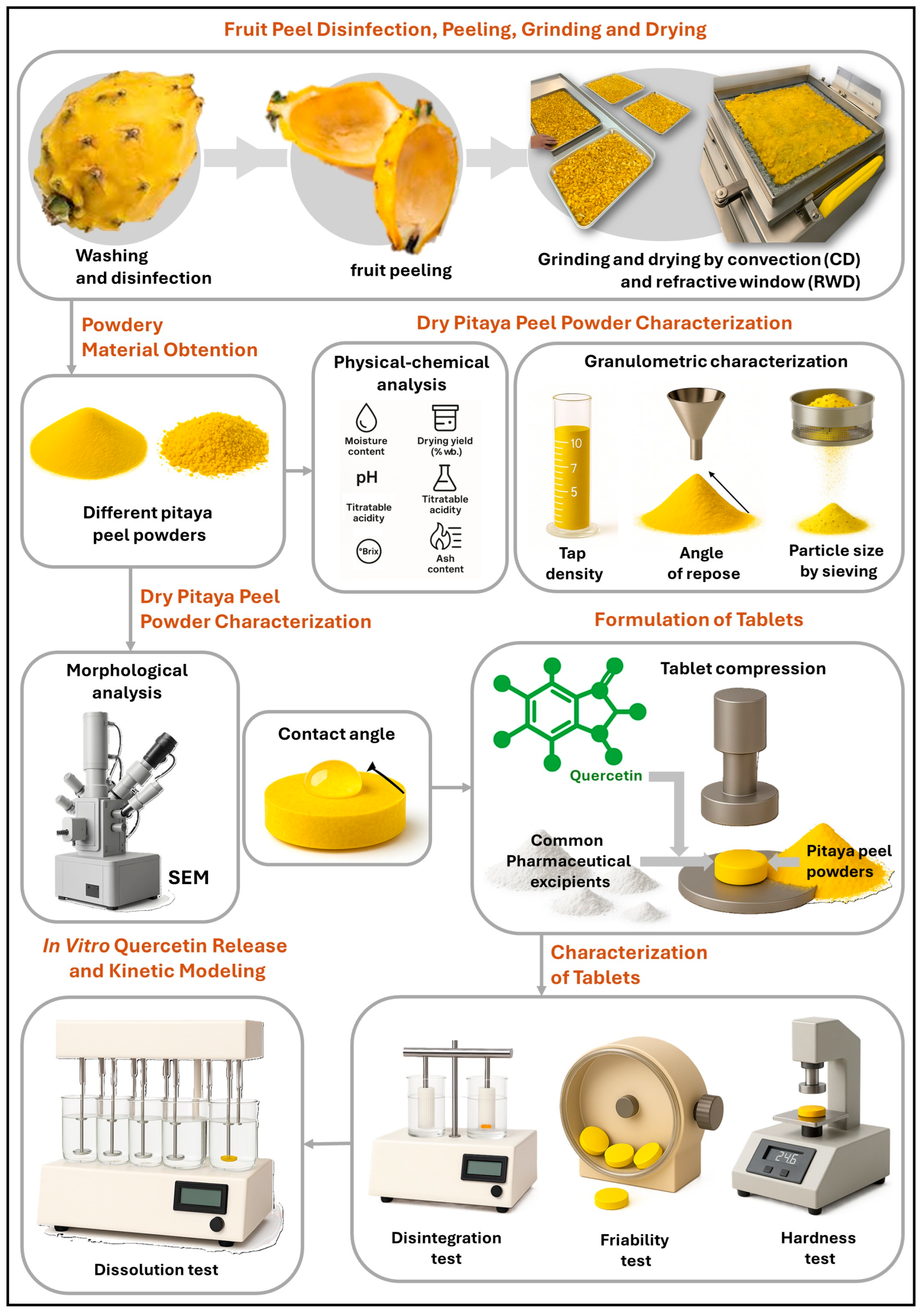
2.1. Obtaining the Powdered Material
2.2. Physicochemical Properties
2.3. Granulometric Properties
2.4. Morphological Characterization by Scanning Electron Microscopy (SEM)
2.5. Contact Angle Measurements
2.6. Formulation of Quercetin Tablets
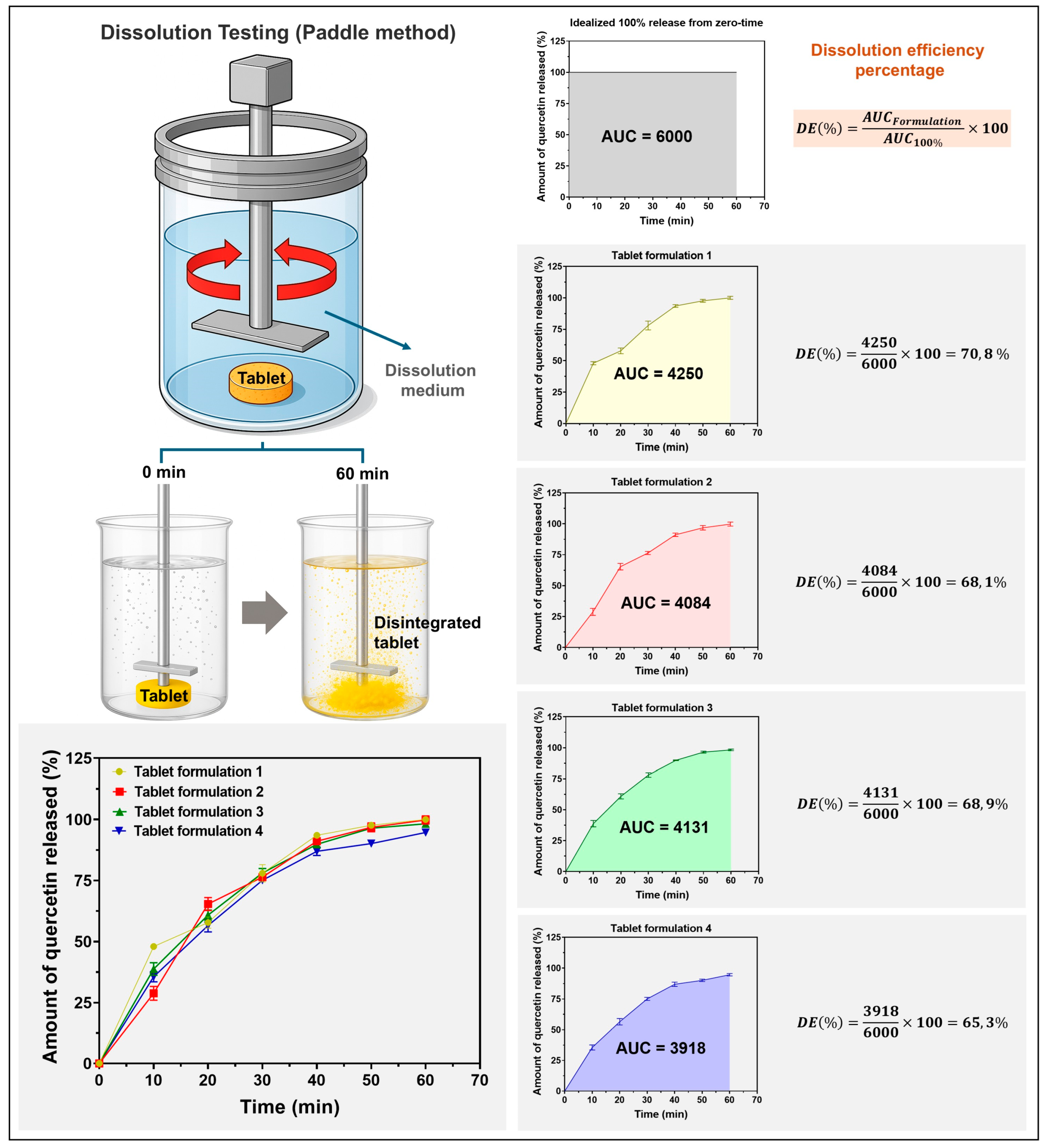
2.7. In Vitro Quercetin Release and Kinetic Modeling
2.8. Data Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Granulometric Properties
3.3. Morphological Characterization by SEM
3.4. Formulation of Quercetin Tablets
3.4.1. Physical–Mechanical Characterization
3.4.2. Determination of the Contact Angle
3.5. In Vitro Quercetin Release and Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAD: | Diode Array Detector |
| DE%: | Dissolution Efficiency (percentage) |
| HCl: | Hydrochloric Acid |
| HPLC: | High-Performance Liquid Chromatography |
| HR: | Hausner Ratio |
| ISO: | International Organization for Standardization |
| MeOH: | Methanol |
| NaOH | Sodium Hydroxide |
| PBS: | Phosphate-Buffered Saline |
| PPCS | Partially Pregelatinized Corn Starch |
| QCT: | Quercetin |
| RWDP: | Refractance Window Dried Pitaya Peel Powder |
| SCE: | Specular Component Excluded (colorimetric mode) |
| SCI: | Specular Component Included (colorimetric mode) |
| SDLM: | Spray-Dried Lactose Monohydrate |
| TRL: | Technology Readiness Level |
| USP: | United States Pharmacopeia |
References
- Grand View Research Nutraceuticals Market Size and Share: Industry Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market (accessed on 29 June 2025).
- Singh, P.; Mishra, G.; Dinda, S.C. Correction to: Natural Excipients in Pharmaceutical Formulations (Evidence Based Validation of Traditional Medicines). In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Olaniyan, O.T.; Anani, O.A.; Bodunrinde, R.E.; Osemwegie, O.O.; Ubi, B.E. Integrated Processes for Production of Pharmaceutical Products from Agro-Wastes. In Biomass, Biofuels, Biochemicals: Circular Bioeconomy: Technologies for Biofuels and Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 439–461. [Google Scholar] [CrossRef]
- Lahiri, A.; Daniel, S.; Kanthapazham, R.; Vanaraj, R.; Thambidurai, A.; Peter, L.S. A Critical Review on Food Waste Management for the Production of Materials and Biofuel. J. Hazard. Mater. Adv. 2023, 10, 100266. [Google Scholar] [CrossRef]
- Marquez Molina, O.; Domínguez-Avila, J.A.; Lopez-Martínez, L.X.; Pareek, S.; Madera Santana, T.J.; González Aguilar, G.A. Valorization of Tropical Fruit Peel Powders: Physicochemical Composition, Techno-Functional Properties, and in Vitro Antioxidant and Antidiabetic Activities. Emir. J. Food Agric. 2023, 35, 3105. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion. Foods 2023, 12, 2700. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Mucilage from Yellow Pitahaya (Selenicereus megalanthus) Fruit Peel: Extraction, Proximal Analysis, and Molecular Characterization. Molecules 2023, 28, 786. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Koschella, A. Carboxymethyl Ethers of Cellulose and Starch—A Review. Macromol. Symp. 2005, 223, 13–40. [Google Scholar] [CrossRef]
- Garg, T.; Arora, S.; Pahwa, R. Cellulose and Its Derivatives: Structure, Modification, and Application in Controlled Drug Delivery. Futur. J. Pharm. Sci. 2025, 11, 76. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, H.; Fang, Y.; Shen, L.; Zhao, L. Lactose in Tablets: Functionality, Critical Material Attributes, Applications, Modifications and Co-Processed Excipients. Drug Discov. Today 2023, 28, 103696. [Google Scholar] [CrossRef]
- Shibly, M.A.H.; Islam, M.I.; Hoque, M.M.U.; Sabit, M.; Rahman, M.M.; Islam, Z.; Rashid, M.J. Hylocereus undatus Plant’s Stem Agro-Waste: A Potential Source of Natural Cellulosic Fiber for Polymer Composites. Sustain. Chem. Pharm. 2024, 41, 101692. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Fathollahi, S.; Dickhoff, B.H.J.; Frijlink, H.W. Critical Review on the Role of Excipient Properties in Pharmaceutical Powder-to-Tablet Continuous Manufacturing. Expert. Opin. Drug Deliv. 2024, 21, 1069–1079. [Google Scholar] [CrossRef]
- Anwar, M.A.; Galal, D.; Khalifa, I.; Zahran, H.A.; Capanoglu, E.; Farag, M.A. Metabolomics: A Compilation of Applications for Enhancing Agricultural Traits, Disease Resistance, Biotic Interaction, Byproducts Valorization, and Quality Control Purposes of Olive. Trends Food Sci. Technol. 2024, 143, 104311. [Google Scholar] [CrossRef]
- Zayed, A.; Zahran, H.A.; Li, Z.; Khalifa, I.; Serag, A.; Fayek, N.M.; Nicolescu, A.; Mocan, A.; Capanoglu, E.; Farag, M.A. Olive Solid Wastes: UHPLC-MS/MS-Based Biochemometric Approach for Investigating the Effect of Conventional versus Modern Extraction Methods on in Vitro Antioxidant, α-Glucosidase, and Lipase Actions. Food Biosci. 2024, 62, 105496. [Google Scholar] [CrossRef]
- Fayek, N.M.; Zayed, A.; Zahran, H.A.; Ramadan, N.S.; Capanoglu, E.; Li, Z.; Fang, Y.; Khalifa, I.; Farag, M.A. Effect of Cultivar Type, Ontogeny and Extraction Methods as Determinant Factors of Olive Leaf Metabolome: A Case Study in 8 Egyptian Cultivars as Analyzed Using LC/MS-Based Metabolomics. Ind. Crops Prod. 2024, 222, 120085. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ayaz, Q.; Mir, S.A.; Singh, A.; Goksen, G.; Majid, D.; Makroo, H.A.; Dar, B.N. Fruit Peel Valorization, Phytochemical Profile, Biological Activity, and Applications in Food and Packaging Industries: Comprehensive Review. Curr. Food Sci. Technol. Rep. 2023, 1, 63–79. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Megías-Pérez, R.; Ferreira-Lazarte, A.; Villamiel, M. Valorization of Grape Pomace as a Renewable Source of Techno-Functional and Antioxidant Pectins. Antioxidants 2023, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of Food Waste per Product Group along the Food Supply Chain in the European Union: A Mass Flow Analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Arslan, A.; Alibaş, İ. Assessing the Effects of Different Drying Methods and Minimal Processing on the Sustainability of the Organic Food Quality. Innov. Food Sci. Emerg. Technol. 2024, 94, 103681. [Google Scholar] [CrossRef]
- Raghavi, L.M.; Moses, J.A.; Anandharamakrishnan, C. Refractance Window Drying of Foods: A Review. J. Food Eng. 2018, 222, 267–275. [Google Scholar] [CrossRef]
- Waghmare, R. Refractance Window Drying: A Cohort Review on Quality Characteristics. Trends Food Sci. Technol. 2021, 110, 652–662. [Google Scholar] [CrossRef]
- Shende, D.; Datta, A.K. Refractance Window Drying of Fruits and Vegetables: A Review. J. Sci. Food Agric. 2019, 99, 1449–1456. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Chakraborty, S.K.; Sudhakar, A.; Verma, D.K.; Shankar, S.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Srivastav, P.P. Refractance WindowTM-Drying vs. Other Drying Methods and Effect of Different Process Parameters on Quality of Foods: A Comprehensive Review of Trends and Technological Developments. Future Foods 2021, 3, 100024. [Google Scholar] [CrossRef]
- Chew, Y.M.; King, V.A.-E. Microwave Drying of Pitaya (Hylocereus) Peel and the Effects Compared with Hot-Air and Freeze-Drying. Trans. ASABE 2019, 62, 919–928. [Google Scholar] [CrossRef]
- Amorim, T.A.; dos Santos Lima, M.; de Souza, M.E.A.O.; Albuquerque, N.M.; da Silva Figueiredo, L.; da Silva, A.B.M.; de Oliveira Vilar, S.B.; de Brito Araújo Carvalho, A.J. Drying Kinetics, Extraction Kinetics and Microencapsulation of Antioxidant Bioactive Compounds of Pitaya (Hylocereus undatus) Peel. J. Food Meas. Charact. 2023, 17, 4073–4085. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Update EJOURNAL 2007, 2, 39851. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- NTC 5400; Good Agricultural Practices for Fresh Fruits, Culinary Aromatic Herbs, and Vegetables. Colombian Institute of Technical Standards and Certification (ICONTEC): Bogotá, Colombia, 12 December 2012.
- Dadhaneeya, H.; Kesavan, R.K.; Inbaraj, B.S.; Sharma, M.; Kamma, S.; Nayak, P.K.; Sridhar, K. Impact of Different Drying Methods on the Phenolic Composition, In Vitro Antioxidant Activity, and Quality Attributes of Dragon Fruit Slices and Pulp. Foods 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- AOAC International Moisture in Dried Fruits. In Official Methods of Analysis of AOAC International; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 1. [Google Scholar]
- AOAC International Acidity (Titratable) of Fruit Products. In Official Methods of Analysis of AOAC International; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 1. [Google Scholar]
- International Organization for Standardization (ISO). Animal Feeding Stuffs—Determination of Crude Ash; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- AOAC International Total Dietary Fiber in Foods—Non-Enzymatic Gravimetric Method. In Official Methods of Analysis of AOAC International; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; Volume 2. [Google Scholar]
- Liao, H.; Zhu, W.; Zhong, K.; Liu, Y. Evaluation of Colour Stability of Clear Red Pitaya Juice Treated by Thermosonication. LWT Food Sci. Technol. 2020, 121, 108997. [Google Scholar] [CrossRef]
- Prior, B.A. Measurement of Water Activity in Foods: A Review. J. Food Prot. 1979, 42, 668–674. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.-W.; Zhang, Z. Methods for Measuring Water Activity (Aw) of Foods and Its Applications to Moisture Sorption Isotherm Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1052–1058. [Google Scholar] [CrossRef]
- Birch, H.; Redman, A.D.; Letinski, D.J.; Lyon, D.Y.; Mayer, P. Determining the Water Solubility of Difficult-to-Test Substances: A Tutorial Review. Anal. Chim. Acta 2019, 1086, 16–28. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention (USP). General Chapter <616>: Bulk Density and Tapped Density of Powders. In United States Pharmacopeia and National Formulary (USP 41–NF 36); USP Expert Committee, Ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2018; Volume USP 41/NF 36. [Google Scholar]
- García-Triñanes, P.; Luding, S.; Shi, H. Tensile Strength of Cohesive Powders. Adv. Powder Technol. 2019, 30, 2868–2880. [Google Scholar] [CrossRef]
- Valverde, J.M.; Ramos, A.; Castellanos, A.; Watson, P.K. The Tensile Strength of Cohesive Powders and Its Relationship to Consolidation, Free Volume and Cohesivity. Powder Technol. 1998, 97, 237–245. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van Der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Butt, H.J.; Liu, J.; Koynov, K.; Straub, B.; Hinduja, C.; Roismann, I.; Berger, R.; Li, X.; Vollmer, D.; Steffen, W.; et al. Contact Angle Hysteresis. Curr. Opin. Colloid. Interface Sci. 2022, 59, 101574. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.M.Z. Techniques for Determining Contact Angle and Wettability of Powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Ghourichay, M.P.; Kiaie, S.H.; Nokhodchi, A.; Javadzadeh, Y. Formulation and Quality Control of Orally Disintegrating Tablets (ODTs): Recent Advances and Perspectives. Biomed. Res. Int. 2021, 2021, 6618934. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention (USP). General Chapter <701>: Disintegration. In United States Pharmacopeia and National Formulary (USP 41–NF 36); USP Expert Committee, Ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2018; Volume USP 41/NF 36. [Google Scholar]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid. Interface Sci. 1998, 81, 167249. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile Drop Method: Critical Analysis and Optimization for Measuring the Contact Angle of an Ion-Exchange Membrane Surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention (USP). General Chapter <711>: Dissolution. In United States Pharmacopeia and National Formulary (USP 41–NF 36); USP Expert Committee, Ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2018; Volume USP 41/NF 36. [Google Scholar]
- United States Pharmacopeial Convention (USP). Dietary Supplement Monographs: Quercetin. In United States Pharmacopeia and National Formulary (USP-NF); USP Expert Committee, Ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2024; Volume USP 47/NF 42. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.H.; Bauer, M.; Boussac, N.; Khan-Malek, R.; Munden, P.; Sardaro, M. An Evaluation of Fit Factors and Dissolution Efficiency for the Comparison of in Vitro Dissolution Profiles. J. Pharm. Biomed. Anal. 1998, 17, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Podczeck, F. Comparison of in Vitro Dissolution Profiles by Calculating Mean Dissolution Time (MDT) or Mean Residence Time (MRT). Int. J. Pharm. 1993, 97, 93–100. [Google Scholar] [CrossRef]
- Qalbi, R.; Giovani, S.; Guo, Q.; Adelina, N.M. Effect of Drying Time on Physicochemical Characteristics of Dragon Fruit Peels Powder (Hylocereus polyrhizus). J. Agri-Food Sci. Technol. 2024, 4, 81–96. [Google Scholar] [CrossRef]
- Moreira Morais, D.; Alves, V.; Asquieri, E.; Souza, A.; Damiani, C. Physical, Chemical, Nutritional and Antinutritional Characterization of Fresh Peels of Yellow Pitaya (Selenicereus megalanthus) and Red Pitaya (Hylocereus costaricensis) and Their Flours. Rev. Ciência Agronômica 2021, 52, e20207289. [Google Scholar] [CrossRef]
- Villafán-González, D.; Betancur, D.; Gallegos, S. Freeze-Dried Pulp and Peel from Pitahaya (Selenicereus undatus): Physicochemical Properties and Potential Source of Fructooligosaccharides. Rev. Colomb. De Investig. Agroindustriales 2024, 11, 80–94. [Google Scholar] [CrossRef]
- Quirino, D.F.; Palma, M.N.N.; Franco, M.O.; Detmann, E. Variations in Methods for Quantification of Crude Ash in Animal Feeds. J. AOAC Int. 2022, 106, 6–13. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of Grape Skins: Significance of Plant Cell-Wall Structural Components and Extraction Techniques for Phenol Release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Britton, G. Functions of Intact Carotenoids. In Carotenoids, Vol. 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Springer: Basel, Switzerland, 2008; Volume 4, pp. 189–212. ISBN 978-3-7643-7498-3. [Google Scholar]
- Chakraborty, S.; Bala, N.N.; Das, S. Development, Evaluation and in Vitro Release Kinetics Study of Immediate Release Tablets of Quercetin Isolated from Bauhinia acuminata Leaves Extract. Indian. J. Sci. Technol. 2022, 15, 2534–2541. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Wei, X.; Huang, C.; Zheng, J.; Ou, S.; Yang, T.; Liu, F. Preparation and Characterization of a Novel Natural Quercetin Self-Stabilizing Pickering Emulsion. Foods 2023, 12, 1415. [Google Scholar] [CrossRef]
- Zhang, J.; Ebbens, S.; Chen, X.; Jin, Z.; Luk, S.; Madden, C.; Patel, N.; Roberts, C.J. Determination of the Surface Free Energy of Crystalline and Amorphous Lactose by Atomic Force Microscopy Adhesion Measurement. Pharm. Res. 2006, 23, 401–407. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Dissolution Testing and Acceptance Criteria for Immediate-Release Solid Oral Dosage Form Drug Products Containing High Solubility Drug Substances: Guidance for Industry. In FDA Guidance Documents; Center for Drug Evaluation and Research (CDER), Ed.; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2018; pp. 1–12. [Google Scholar]
- Dash, S.; Murthy, P.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas NA, S. Mechanisms of Solute Release from Porous Hydrophilic Polymer. Int J Pharm 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, V.K.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative Statistical Analysis of the Release Kinetics Models for Nanoprecipitated Drug Delivery Systems Based on Poly(Lactic-Co-Glycolic Acid). PLoS ONE 2022, 17, e0264825. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.G.; Yusof, Y.A.; Blanchard, C.; Saifullah, M.; Farahnaky, A.; Scheiling, G. Material Properties and Tableting of Fruit Powders. Food Eng. Rev. 2018, 10, 66–80. [Google Scholar] [CrossRef]

| Formulation Ingredient | Ingredient Amount Per Tablet (mg) | % w/w | ||||
|---|---|---|---|---|---|---|
| Tablet Formulation 1 | Tablet Formulation 2 | Tablet Formulation 3 | Tablet Formulation 4 | |||
| Active ingredient | Quercetin | 250 | 50 | 50 | 50 | 50 |
| Diluent | PPCS | 245 | 49 | -- | -- | -- |
| SDLM | -- | 49 | -- | -- | ||
| CDP | -- | -- | 49 | -- | ||
| RWDP | -- | -- | -- | 49 | ||
| Lubricant | Colloidal silicon dioxide | 2.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Glidant | Magnesium stearate | 2.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Parameter | CDP | WDP | |
|---|---|---|---|
| Moisture content (%) | 4.00 ± 0.05 | 6.00 ± 0.15 | |
| Water activity () | 0.214 ± 0.088 | 0.364 ± 0.005 | |
| Water solubility (%) | 8.00 ± 0.003 | 12.00 ± 0.006 | |
| Drying yield (% w.b.) | 14.00 ± 0.01 | 12.00 ± 0.23 | |
| Powder retained < 250 µm (% of batch) | 38.00 ± 0.02 | 34.00 ± 0.12 | |
| pH | 4.95 ± 0.008 | 4.49 ± 0.11 | |
| Titratable acidity (% citric acid) | 1.28 ± 0.12 | 1.47 ± 0.12 | |
| °Brix | 5.00 | 5.00 | |
| Total dietary fiber (g/100 g b.s.) | 63.4 | 64.0 | |
| Ash content (g/100 g b.s.) | 14.98 | 16.70 | |
| Colorimetric parameters | a* (redness) | 5.34 ± 0.0430 | 8.65 ± 0.0170 |
| b* (yellowness) | 33.68 ± 0.0400 | 38.16 ± 0.0190 | |
| L* (lightness) | 72.89 ± 0.0490 | 64.63 ± 0.0550 | |
| Parameter | CDP | RWDP |
|---|---|---|
| Carr Index (%) | 22.9 ± 0.95 | 20.07 ± 0.76 |
| Hausner Ratio | 1.30 ± 0.08 | 1.26 ± 0.03 |
| Angle of repose (°) | 45.42 ± 0.52 | 40.82 ± 0.53 |
| System | Tablet Parameter | |||
|---|---|---|---|---|
| Hardness (kp) | Friability (%) | Weight Variation | Disintegration Time (min:s ± s) at 37 °C | |
| Tablet Formulation 1 (QCT + PPCS) | 9.54 ± 1.34 | 0.3253 | 500.3 ± 0.05 | 3:03 ± 0 |
| Tablet Formulation 2 (QCT + SDLM) | 13.84 ± 1.64 | 0.4773 | 500.1 ± 0.05 | 4:33 ± 2 |
| Tablet Formulation 3 (QCT + CDP) | 11.84 ± 1.06 | 0.6136 | 500.0 ± 0.05 | 3:58 ± 5 |
| Tablet Formulation 4 (QCT + RWDP) | 12.26 ± 0.84 | 0.3324 | 500.0 ± 0.01 | 4:03 ± 7 |
| Surface System | Static Contact Angle (°) | |
|---|---|---|
| Pure Active ingredient | Quercetin | 103.2 ± 0.6 |
| Pure Diluent | PPCS | 51.9 ± 0.4 |
| SDLM | 40.0 ± 0.4 | |
| CDP | 75.4 ± 0.6 | |
| RWDP | 90.30 ± 0.3 | |
| Tablet Formulations | Tablet Formulation 1 (QCT + PPCS) | 67.3 ± 1.9 |
| Tablet Formulation 2 (QCT + SDLM) | 72.5 ± 3.0 | |
| Tablet Formulation 3 (QCT + CDP) | 74.8 ± 2.4 | |
| Tablet Formulation 4 (QCT + RWDP) | 76.4. ± 8.9 | |
| Model Type | Model Name/ | Equation | P(s) | Tablet Formulation | |||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | ||||
| Classical | Zero-order | 1.61 | 1.61 | 1.57 | 1.53 | ||
| 0.967 | 0.958 | 0.961 | 0.950 | ||||
| First-order | 0.054 | 0.060 | 0.056 | 0.052 | |||
| 0.985 | 0.990 | 0.983 | 0.972 | ||||
| Higuchi | 12.48 | 12.17 | 11.99 | 11.62 | |||
| 0.973 | 0.964 | 0.968 | 0.956 | ||||
| Hixson–Crowell | −0.023 | −0.024 | −0.025 | −0.027 | |||
| 0.957 | 0.953 | 0.960 | 0.946 | ||||
| Korsmeyer–Peppas | 1.13/ | 1.21 | 1.18 | 1.09 | |||
| n | 0.91 | 0.89 | 0.88 | 0.86 | |||
| 0.990 | 0.986 | 0.984 | 0.975 | ||||
| Heuristic | Weibull | a | 22.6 | 21.9 | 23.2 | 24.8 | |
| b | 1.72 | 1.70 | 1.65 | 1.59 | |||
| 0.996 | 0.995 | 0.994 | 0.991 | ||||
| Logistic | 0.16 | 0.18 | 0.17 | 0.15 | |||
| 27.5 | 26.8 | 28.2 | 29.4 | ||||
| 0.993 | 0.994 | 0.992 | 0.989 | ||||
| Gompertz | 1.22 | 1.29 | 1.21 | 1.14 | |||
| 25.1 | 24.4 | 25.9 | 26.9 | ||||
| A | 11.8 | 11.2 | 12.1 | 12.6 | |||
| 0.992 | 0.993 | 0.990 | 0.987 | ||||
| Modified Hill | n | 3.42 | 3.58 | 3.39 | 3.11 | ||
| k | 21.1 | 20.5 | 22.0 | 23.4 | |||
| 0.994 | 0.993 | 0.992 | 0.988 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesa, A.; Leyva, M.; Gonzáles, J.G.; Oñate-Garzón, J.; Salamanca, C.H. Physicochemical, Granulometric, Morphological, and Surface Characterization of Dried Yellow Pitaya Powder as a Potential Diluent for Immediate-Release Quercetin Tablets. Sci 2025, 7, 126. https://doi.org/10.3390/sci7030126
Mesa A, Leyva M, Gonzáles JG, Oñate-Garzón J, Salamanca CH. Physicochemical, Granulometric, Morphological, and Surface Characterization of Dried Yellow Pitaya Powder as a Potential Diluent for Immediate-Release Quercetin Tablets. Sci. 2025; 7(3):126. https://doi.org/10.3390/sci7030126
Chicago/Turabian StyleMesa, Alejandra, Melanie Leyva, Jesús Gil Gonzáles, José Oñate-Garzón, and Constain H. Salamanca. 2025. "Physicochemical, Granulometric, Morphological, and Surface Characterization of Dried Yellow Pitaya Powder as a Potential Diluent for Immediate-Release Quercetin Tablets" Sci 7, no. 3: 126. https://doi.org/10.3390/sci7030126
APA StyleMesa, A., Leyva, M., Gonzáles, J. G., Oñate-Garzón, J., & Salamanca, C. H. (2025). Physicochemical, Granulometric, Morphological, and Surface Characterization of Dried Yellow Pitaya Powder as a Potential Diluent for Immediate-Release Quercetin Tablets. Sci, 7(3), 126. https://doi.org/10.3390/sci7030126









