Can the Cyanobacterium Nostoc commune Exert In Vitro Biocontrol on Fusarium oxysporum, Causal Agent of Wilt in Banana (Musa AAB)?
Abstract
1. Introduction
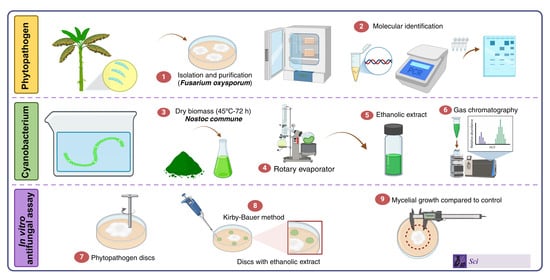
2. Materials and Methods
2.1. Location
2.2. Cultivation and Obtention of Ethanolic Extract of Cyanobacteria (EEC)
2.3. Preliminary Chemical Analysis
2.4. Derivatization of Ethanolic Extract of Cyanobacteria (EEC)
2.5. Gas Chromatographic–Mass-Spectrometric Analysis
2.6. Obtaining the Phytopathogenic Fungus
2.7. Molecular Identification of the Study Microorganisms
2.7.1. Fusarium oxysporum f. sp. Cubense Race 2 (FB-INVEPAR)
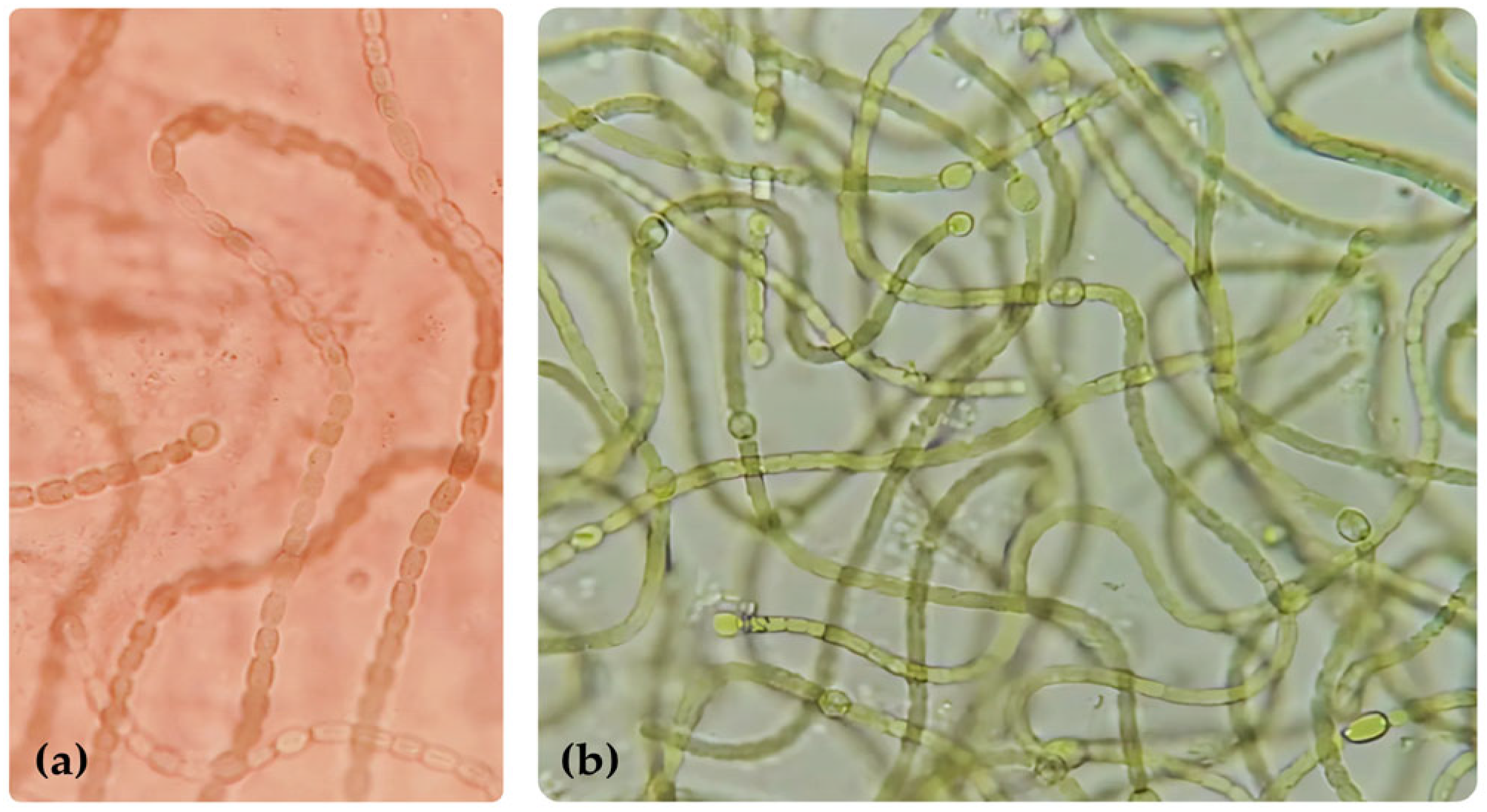
2.7.2. Nostoc commune Strain F56 (GenBank PV865569)
2.8. In Vitro Antifungal Assay
2.9. Statistical Analysis of Data
3. Results
3.1. Preliminary Chemical Analysis
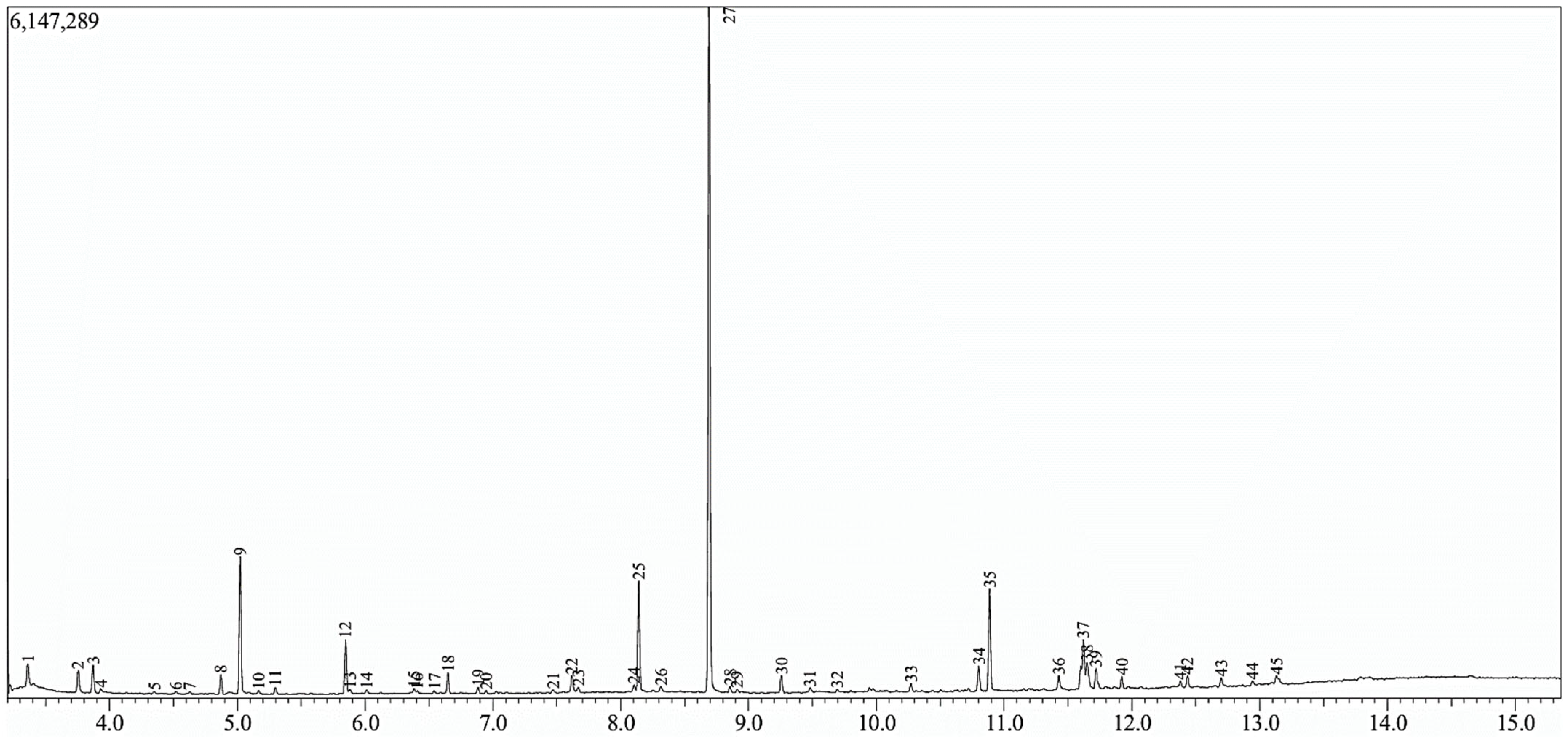
3.2. Gas Chromatography (GC-MS)
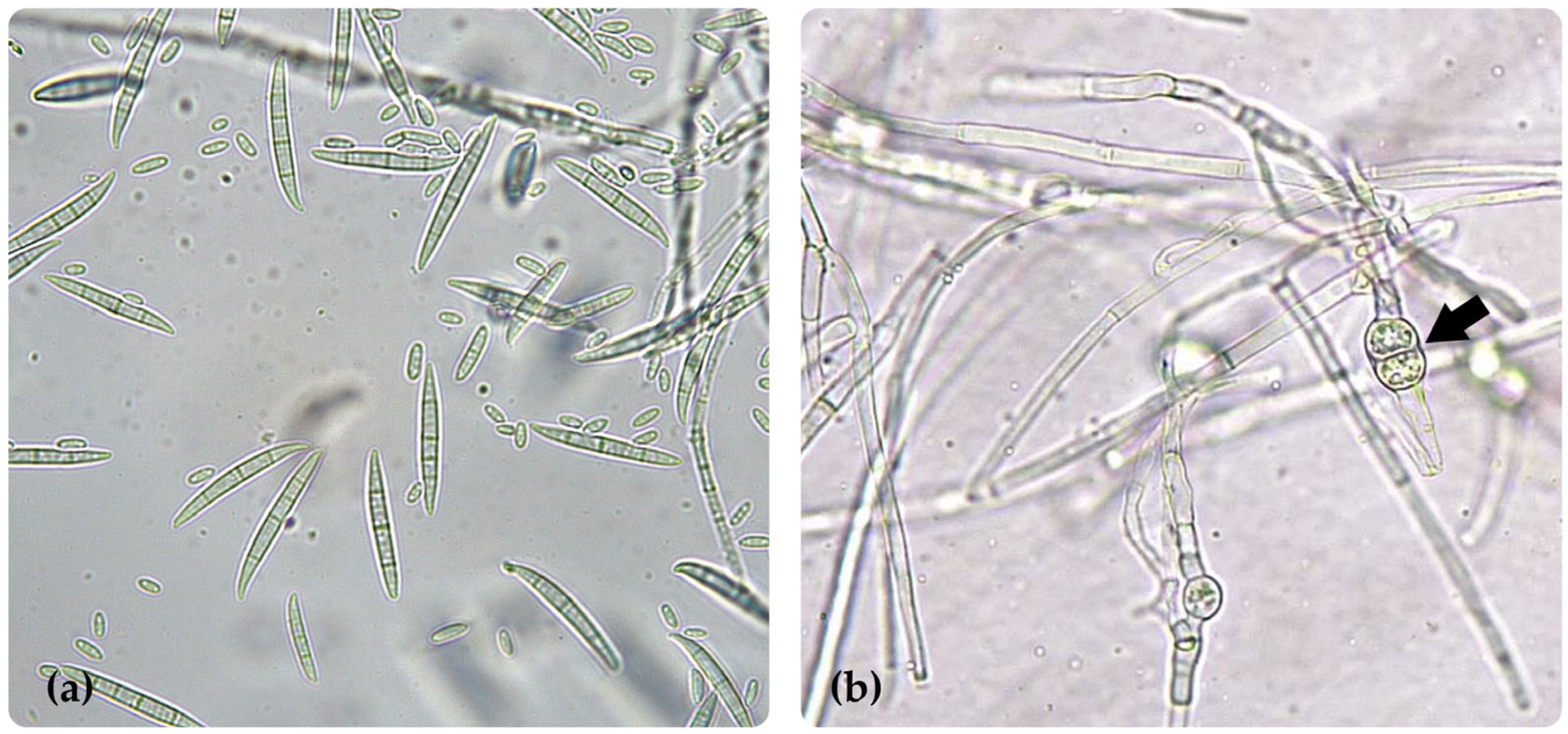
3.3. Identification of the Phytopathogenic Fungus
3.4. Molecular Identification
3.4.1. Fusarium oxysporum f. sp. Cubense Race 2 (FB-INVEPAR)
3.4.2. Nostoc commune Strain F56
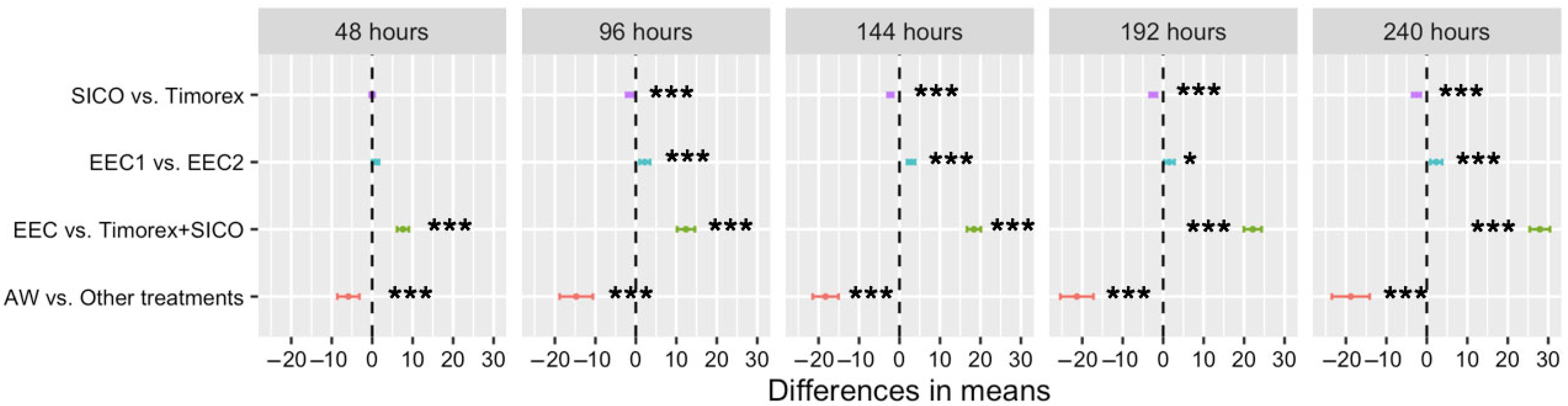
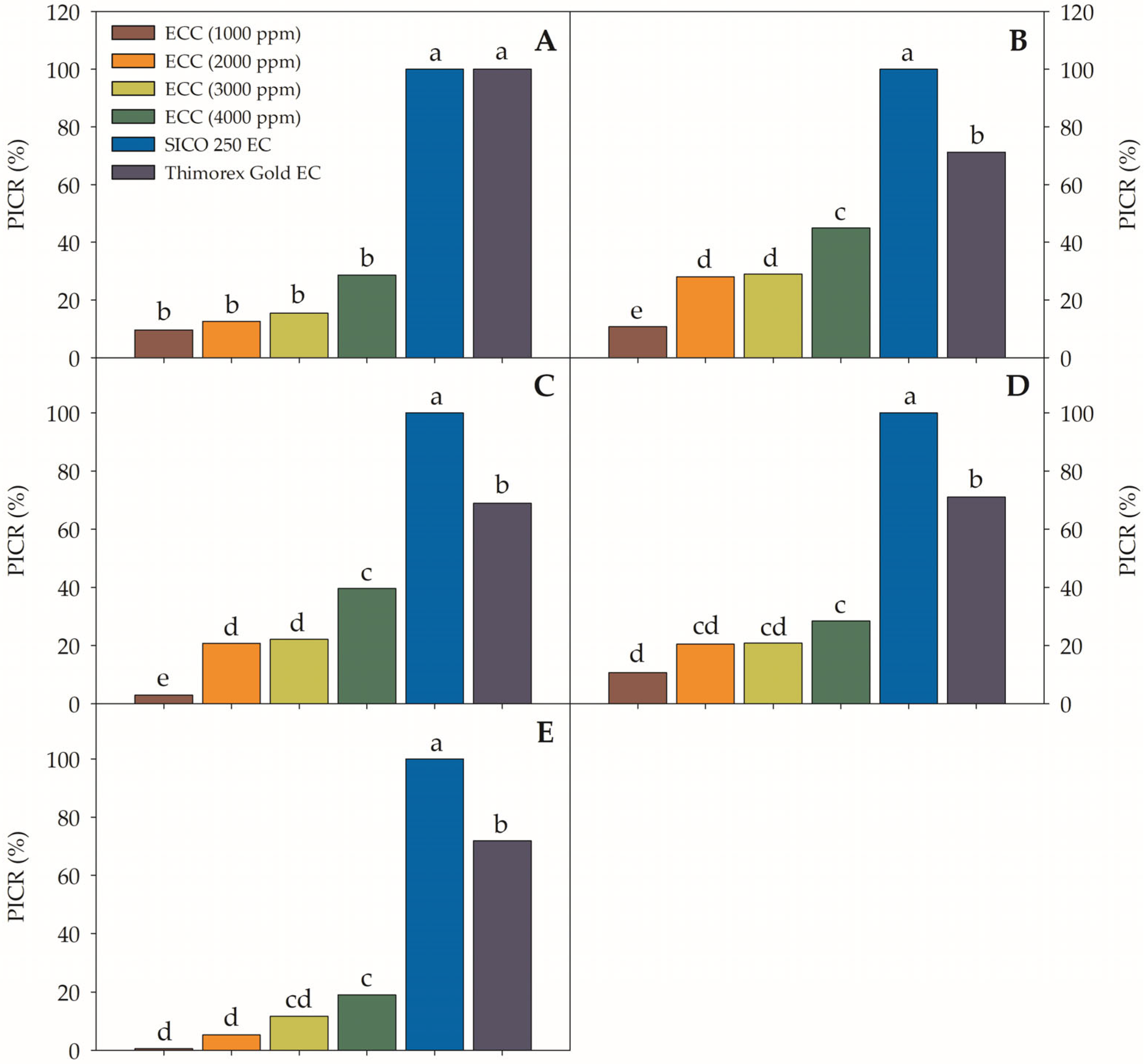
3.5. In Vitro Assays on Fusarium oxysporum
3.5.1. Growth Diameter (cm)
3.5.2. Percentage of Inhibition of Radial Growth (PIRG)
3.5.3. Growth Rate (cm Day−1)
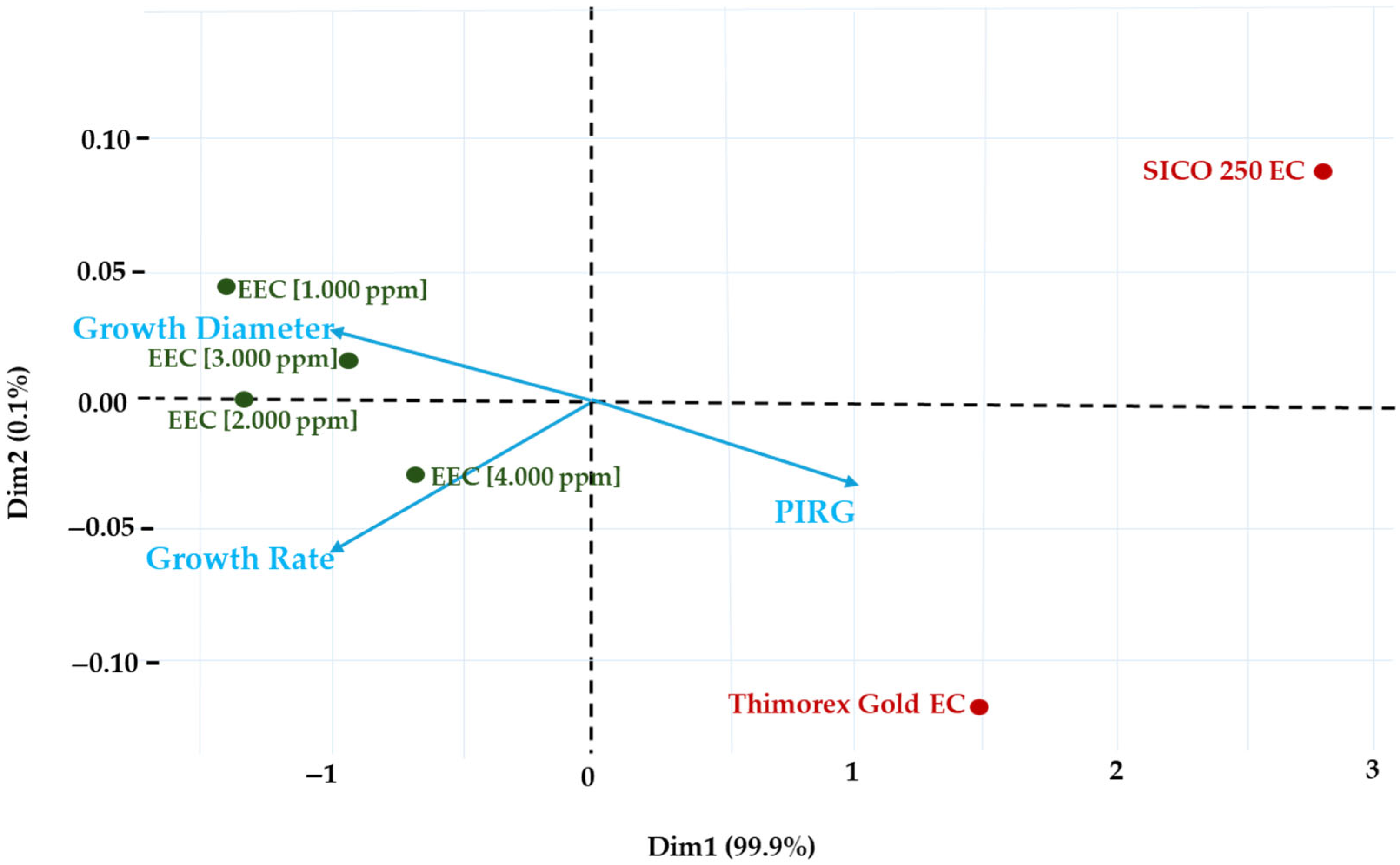
3.5.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Solórzano, G.E.; Rey-Brina, J.C.; Pargas-Pichardo, R.E.; Manzanilla, E.E. Fusarium Wilt Tropical Race 4: Current Status and Presence on the American Continent. Agron. Mesoam. 2019, 31, 259–276. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and Genetic Diversity of the Banana Fusarium Wilt Pathogen Fusarium oxysporum f. sp. cubense in the Indonesian Centre of Origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.M.; Rouard, M.; Lopez-Alvarez, D.; Cenci, A.; Breton, C.; Acuña, R.; Rojas, J.C.; Dita, M.; Cuellar, W.J. Draft Genome Sequence of Fusarium oxysporum f. sp. cubense Tropical Race 4 from Peru, Obtained by Nanopore and Illumina Hybrid Assembly. Microbiol. Resour. Announc. 2022, 11, e00347-22. [Google Scholar] [CrossRef] [PubMed]
- García-Bastidas, F.A.; Quintero-Vargas, J.C.; Ayala-Vasquez, M.; Schermer, T.; Seidl, M.F.; Santos-Paiva, M.; Noguera, A.M.; Aguilera-Galvez, C.; Wittenberg, A.; Hofstede, R.; et al. First Report of Fusarium Wilt Tropical Race 4 in Cavendish Bananas Caused by Fusarium odoratissimum in Colombia. Plant Dis. 2020, 104, 994. [Google Scholar] [CrossRef]
- Dita, M.; Teixeira, L.A.J.; O’Neill, W.; Pattison, A.B.; Weinert, M.P.; Li, C.Y.; Zheng, S.J.; Staver, C.; Thangavelu, R.; Viljoen, A. Current State of Fusarium Wilt of Banana in the Subtropics. Acta Hortic. 2020, 1272, 45–56. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Ploetz, R.C. Fusarium Wilt of Banana Is Caused by Several Pathogens Referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 2006, 96, 653–656. [Google Scholar] [CrossRef]
- Scheerer, L.; Pemsl, D.; Dita, M.; Vicente, L.P.; Staver, C. A Quantified Approach to Projecting Losses Caused by Fusarium Wilt Tropical Race 4. Acta Hortic. 2018, 1196, 211–218. [Google Scholar] [CrossRef]
- Instituto Colombiano Agropecuario (ICA). Actualización de La Condición de Fusarium oxysporum f. sp. cubense Raza 4 Tropical—Foc R4T 2021. Available online: https://www.ica.gov.co/areas/agricola/servicios/epidemiologia-agricola/saf/notificacion-oficial/detalle-notificacion-oficial/actualizacion-de-la-condicion-de-fusarium-oxysporu (accessed on 15 October 2023).
- Quevedo, M. (2021, abril 11). Declaran Emergencia Fitosanitaria En Todo El Territorio Nacional Ante La Presencia de La Plaga Fusarium oxysporum f. sp. cubense Raza 4 Tropical. Diario Oficial del Bicentenario El Peruano. Available online: https://busquedas.elperuano.pe/dispositivo/NL/1942722-1 (accessed on 22 June 2023).
- Instituto Nacional de Salud Agrícola Integral (INSAI). Comunicado Oficial. Available online: https://sigesai.insai.gob.ve/assets/files/fusarium_oxysporum.pdf (accessed on 8 February 2024).
- Olivares, B.O.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium Wilt of Bananas: A Review of Agro-Environmental Factors in the Venezuelan Production System Affecting Its Development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Izquierdo-García, L.F.; Carmona, S.L.; Zuluaga, P.; Rodríguez, G.; Dita, M.; Betancourt, M.; Soto-Suárez, M. Efficacy of Disinfectants against Fusarium oxysporum f. sp. cubense Tropical Race 4 Isolated from La Guajira, Colombia. J. Fungi 2021, 7, 297. [Google Scholar] [CrossRef]
- Van Westerhoven, A.C.; Meijer, H.J.G.; Seidl, M.F.; Kema, G.H.J. Uncontained Spread of Fusarium Wilt of Banana Threatens African Food Security. PLoS Pathog. 2022, 18, e1010769. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura (FAO). FAOSTAT, Cultivos y Productos de Ganadería. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 20 February 2025).
- Instituto Colombiano Agropecuario (ICA). RESOLUCIÓN No.00002081 (11/03/2024). Available online: https://www.ica.gov.co/getattachment/fc57b4b9-ab28-4c1a-83c6-1c1bbbbf2d8f/2024R00002081.aspx (accessed on 16 July 2024).
- García-Bastidas, F.A.; Arango-Isaza, R.; Rodriguez-Cabal, H.A.; Seidl, M.F.; Cappadona, G.; Segura, R.; Salacinas, M.; Kema, G.H.J. Induced Resistance to Fusarium Wilt of Banana Caused by Tropical Race 4 in Cavendish Cv Grand Naine Bananas after Challenging with Avirulent Fusarium spp. PLoS ONE 2022, 17, e0273335. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ryan Penton, C.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the Banana Rhizosphere Microbiome for Biological Control of Panama Disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Dita, M.; Martinuz, A.; Staver, C.; Berg, G. Members of Gammaproteobacteria as Indicator Species of Healthy Banana Plants on Fusarium Wilt-Infested Fields in Central America. Sci. Rep. 2017, 7, 45318. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.; Ruan, Y.; Li, R.; Shen, Q. Suppression of Banana Panama Disease Induced by Soil Microbiome Reconstruction through an Integrated Agricultural Strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Pisciotta, J.M.; Zou, Y.; Baskakov, I.V. Light-Dependent Electrogenic Activity of Cyanobacteria. PLoS ONE 2010, 5, e10821. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A Natural Source for Controlling Agricultural Plant Diseases Caused by Fungi and Oomycetes and Improving Plant Growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Morsy, K.M. Biological Control of Damping-off, Root Rot and Wilt Diseases of Faba Bean by Cyanobacteria (Blue-Green Algal) Culture Filtrate. Egypt J. Phytopathol. 2011, 39, 159–171. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Deyab, M.A.; Hasan, R.S.A.; Ahmed, S.E.A.; Elsadany, A. Biological Controls of Fusarium Tomato-Wilt Disease by the Blue-Green Alga Nostoc spp. Arch. Microbiol. 2022, 204, 116. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant Biostimulants: A Review on the Processing of Macroalgae and Use of Extracts for Crop Management to Reduce Abiotic and Biotic Stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- López-Padrón, I.; Martínez-González, L.; Pérez-Dominguez, G.; Reyes-Guerrero, Y.; Núñez-Vásquez, M.; Cabrera-Rodríguez, J. Algae and Their Uses in Agriculture. An Updated Vision. Cultiv. Trop. 2020, 41, e10. Available online: https://www.redalyc.org/journal/1932/193264539010/ (accessed on 20 February 2025).
- Volk, R.-B.; Furkert, F.H. Antialgal, Antibacterial and Antifungal Activity of Two Metabolites Produced and Excreted by Cyanobacteria during Growth. Microbiol. Res. 2006, 161, 180–186. [Google Scholar] [CrossRef]
- Alwathnani, H.; Perveen, K. Biological Control of Fusarium Wilt of Tomato by Antagonist Fungi and Cyanobacteria. Afr. J. Biotechnol. 2012, 11, 1100–1105. [Google Scholar] [CrossRef]
- Hrouzek, P.; Šimek, M.; Komárek, J. Nitrogenase Activity (Acetylene Reduction Activity) and Diversity of Six Soil Nostoc Strains. Algol. Stud. Für Hydrobiol. Suppl. Vol. 2003, 108, 87–101. [Google Scholar] [CrossRef]
- Rajaniemi, P.; Hrouzek, P.; Kaštovská, K.; Willame, R.; Rantala, A.; Hoffmann, L.; Komárek, J.; Sivonen, K. Phylogenetic and Morphological Evaluation of the Genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int. J. Syst. Evol. Microbiol. 2005, 55, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Šnokhousová, J.; Saraf, A.; Suradkar, A.; Elster, J. Phylogenetic Evaluation of the Genus Nostoc and Description of Nostoc neudorfense sp. Nov., from the Czech Republic. Int. J. Syst. Evol. Microbiol. 2020, 70, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae Metabolites: A Rich Source for Food and Medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Sreenikethanam, A.; Raj, S.; J, R.B.; Gugulothu, P.; Bajhaiya, A.K. Genetic Engineering of Microalgae for Secondary Metabolite Production: Recent Developments, Challenges, and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 836056. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Liu, Y.; Ai, S.; Qin, F.; Li, Z.; Zhang, H.; Huang, Z. Antioxidant and Moisture-Retention Activities of the Polysaccharide from Nostoc Commune. Carbohydr. Polym. 2011, 83, 1821–1827. [Google Scholar] [CrossRef]
- Singh, N.K.; Dhar, D.W.; Tabassum, R. Role of Cyanobacteria in Crop Protection. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an Antifungal Lipopeptide from the Field-Grown Terrestrial Blue-Green Alga Nostoc Commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Hernández-Carlos, B.; Gamboa-Angulo, M.M. Metabolites from Freshwater Aquatic Microalgae and Fungi as Potential Natural Pesticides. Phytochem. Rev. 2011, 10, 261–286. [Google Scholar] [CrossRef]
- Pooja, S.; Niveshika, N. Insight into the Potential Cyanobacterial Metabolites and Their Screening Strategies. Biosci. Biotechnol. Res. Asia 2022, 19, 255–279. [Google Scholar] [CrossRef]
- Poveda, J. Cyanobacteria in Plant Health: Biological Strategy against Abiotic and Biotic Stresses. Crop Prot. 2021, 141, 105450. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, J.-D. Inhibitory Effect of Algal Extracts on Mycelial Growth of the Tomato-Wilt Pathogen, Fusarium oxysporum f. sp. lycopersici. Mycobiology 2008, 36, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-D. Screening of Cyanobacteria (Blue-Green Algae) from Rice Paddy Soil for Anti-Fungal Activity against Plant Pathogenic Fungi. Mycobiology 2006, 34, 138–142. [Google Scholar] [CrossRef]
- Kar, J.; Ramrao, D.P.; Zomuansangi, R.; Lalbiaktluangi, C.; Singh, S.M.; Joshi, N.C.; Kumar, A.; Kaushalendra; Mehta, S.; Yadav, M.K.; et al. Revisiting the Role of Cyanobacteria-Derived Metabolites as Antimicrobial Agent: A 21st Century Perspective. Front. Microbiol. 2022, 13, 1034471. [Google Scholar] [CrossRef]
- Mouga, T.; Pereira, J.; Moreira, V.; Afonso, C. Unveiling the Cultivation of Nostoc sp. under Controlled Laboratory Conditions. Biology 2024, 13, 306. [Google Scholar] [CrossRef]
- Herazo-Cárdenas, D.; Vallejo-Isaza, A.; Vegliante-Arrieta, D.; Pineda-Rodríguez, Y.; Jarma-Orozco, A.; Jaraba-Navas, J.; Ariza-González, A.; Pico-González, A. Cultivo de Cianobacterias: Aspectos Prácticos, 1st ed.; Fondo Editorial Universidad de Córdoba: Montería, Córdoba, 2023; ISBN 978-958-5104-72-3. [Google Scholar]
- García-Bastidas, F.; Pachacama-Gualotuña, S.F.; Jarrín-Escudero, D.A.; Iza-Arteaga, M.L.; Ayala-Vasquez, M.; Ortiz, H.E.; Dix-Luna, O.J.; Echegaray, J.; Farfán, D.; Bartolini, I.; et al. Andean Guide for the Diagnosis of Fusarium Race 4 Tropical (R4T). Fusarium Oxysporum f.Sp. Cubense (Syn. Fusarium Odoratissimum) Causal Agent of Fusarium Wilt in Musaceae (Bananas and Plantains), 1st ed.; General Secretariat of CAN: Andean community: Lima, Perú, 2020; ISBN 978-612-4054-37-2. [Google Scholar]
- Barnett, H.; Hunter, B. Ilustrated Genera of Imperfect Fungi, 4th ed.; APS Press: Saint Paul, MN, USA, 1998. [Google Scholar]
- Pineda-Rodriguez, Y.Y.; Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez, N.V.; Rodriguez-Paez, L.A. A New and Profitable Protocol to DNA Extraction in Limnospira Maxima. Methods Protoc. 2023, 6, 62. [Google Scholar] [CrossRef]
- Benítez, S.; Bentley, J.; Bustamante, P.; Sánchez, L.; Corrales, L. Isolation of Culturable Microorganisms from the Rhizosphere of Ornithogalum Umbellatum and Evaluation of the Potential Biocontrol Effect on Two Soil Pathogens. NOVA Publ. Científica En Cienc. Bioméd. 2007, 5, 147–153. [Google Scholar]
- Astiti, N.P.; Suprapta, D.N. Antifungal Activity of Teak (Tectona grandis L.F) Leaf Extractagainst Arthrinium Phaeospermum (Corda) M.B. Ellis, the Cause of Wood Decay on Albizia falcataria (L.) Fosberg. J. Int. Soc. Southeast Asian Agric. Sci. 2012, 18, 62–69. [Google Scholar]
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sun, E.J.; Su, H.J.; Ko, W.H. Identification of Fusarium oxysporum f. sp. Cubense Race 4 from Soil or Host Tissue by Cultural Characters. Phytopathology 1978, 68, 1672–1673. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Ploetz, R.C.; Gordon, T.R.; Viljoen, A. Current Status of the Taxonomic Position of Fusarium oxysporum Formae Specialis cubense within the Fusarium oxysporum Complex. Infect. Genet. Evol. 2011, 11, 533–542. [Google Scholar] [CrossRef]
- Jaraba-Navas, J.D.D.A.; Pico-González, A.I.; Vargas-Acosta, J.E.A.; Jarma-Orozco, A.J.; Combatt-Caballero, E.A.; Herazo-Cárdenas, D.S.A.; Vallejo-Isaza, A.; Pineda-Rodríguez, Y.Y.A.; Rodríguez-Páez, L.A.A. Fusarium Oxysporum Strain FB INVEPAR 5.8S Ribosomal RNA Gene, Partial Sequence; Internal Transcribed Spacer 2, Complete Sequence; and Large Subunit Ribosomal RNA Gene, Partial Sequence. 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR704561 (accessed on 20 February 2025).
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Grant Burgess, J.; Wright, P.C. Marine Cyanobacteria—A Prolific Source of Natural Products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Biondi, N.; Piccardi, R.; Margheri, M.C.; Rodolfi, L.; Smith, G.D.; Tredici, M.R. Evaluation of Nostoc Strain ATCC 53789 as a Potential Source of Natural Pesticides. Appl. Environ. Microbiol. 2004, 70, 3313–3320. [Google Scholar] [CrossRef]
- Zulpa, G.; Zaccaro, M.C.; Boccazzi, F.; Parada, J.L.; Storni, M. Bioactivity of Intra and Extracellular Substances from Cyanobacteria and Lactic Acid Bacteria on “Wood Blue Stain” Fungi. Biol. Control 2003, 27, 345–348. [Google Scholar] [CrossRef]
- Rodríguez-Palacio, M.C. Laboratorio de Ficología Aplicada, Departamento de Hidrobiología, División de Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana-Iztapalapa Potential Use of Some Species of Microalgae and Cyanobacteria as Erythrocyte Agglutinators and Bactericides. Hidrobiológica 2022, 32, 17–24. [Google Scholar] [CrossRef]
- Asimakis, E.; Shehata, A.A.; Eisenreich, W.; Acheuk, F.; Lasram, S.; Basiouni, S.; Emekci, M.; Ntougias, S.; Taner, G.; May-Simera, H.; et al. Algae and Their Metabolites as Potential Bio-Pesticides. Microorganisms 2022, 10, 307. [Google Scholar] [CrossRef]
- Águila-Carricondo, P.; Román, R.; Marín-Guirao, J.I.; Cantón, Y.; De Cara, M. Native Biocrust Cyanobacteria Strains Showing Antagonism against Three Soilborne Pathogenic Fungi. Pathogens 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of Cyanobacteria in Biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferrinho, S.; Connaris, H.; Mouncey, N.J.; Goss, R.J.M. Compendium of Metabolomic and Genomic Datasets for Cyanobacteria: Mined the Gap. Water Res. 2024, 256, 121492. [Google Scholar] [CrossRef] [PubMed]
- Parambil, F.V.; Yusuf, A. Biochemical Characterization and Phylogenetic Analysis of Nostoc Species Using Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis. Nova Hedwig. 2025, 000, 106615. Available online: https://www.schweizerbart.de/papers/nova_hedwigia/detail/prepub/106615/Biochemical_characterization_and_phylogenetic_analysis_of_Nostoc_species_using_Gas_Chromatography_Mass_Spectroscopy_GC_MS_analysis (accessed on 20 February 2025).
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A Fatty Acid from the Diatom Phaeodactylum tricornutum Is Antibacterial Against Diverse Bacteria Including Multi-Resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Fungicidal Activity of Extracellular Products of Cyanobacteria against Alternaria porri. Eur. J. Phycol. 2015, 50, 239–245. [Google Scholar] [CrossRef]
- Farghl, A.; El-Sheekh, M.; Mousa, A. Extraction and Characterization of Antimicrobial Active Substance from Cyanobacteria Nostoc carneum and Anabaena circinalis. Fresenius Environ. Bull. 2019, 28, 5481–5490. Available online: https://www.researchgate.net/publication/334401092_EXTRACTION_AND_CHARACTERIZATION_OF_ANTIMICROBIAL_ACTIVE_SUBSTANCE_FROM_CYANOBACTERIA_NOSTOC_CARNEUM_AND_ANABAENA_CIRCINALIS (accessed on 20 February 2025).
- Vavilin, D.; Vermaas, W. Continuous Chlorophyll Degradation Accompanied by Chlorophyllide and Phytol Reutilization for Chlorophyll Synthesis in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta BBA Bioenerg. 2007, 1767, 920–929. [Google Scholar] [CrossRef]
- Gupta, D.; Ip, T.; Summers, M.L.; Basu, C. 2-Methyl-3-Buten-2-Ol (MBO) Synthase Expression in Nostoc punctiforme Leads to over Production of Phytols. Bioengineered 2015, 6, 33–41. [Google Scholar] [CrossRef]
- Renganathan, P.; Gaysina, L.A.; Holguín-Peña, R.J.; Sainz-Hernández, J.C.; Ortega-García, J.; Rueda-Puente, E.O. Phycoremediated Microalgae and Cyanobacteria Biomass as Biofertilizer for Sustainable Agriculture: A Holistic Biorefinery Approach to Promote Circular Bioeconomy. Biomass 2024, 4, 1047–1077. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; Sousa, J.M.; Moura, P.; Bandarra, N.M. Microalgae—Source of Natural Bioactive Molecules as Functional Ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Li, Z.; Guo, M. Healthy Efficacy of Nostoc commune Vaucher. Oncotarget 2018, 9, 14669–14679. [Google Scholar] [CrossRef]
- Corrales, M.; Villalobos, A.; Rodríguez, K.; Muñóz, N.; Umaña, R. Identification and Molecular Characterization of Tropical Cyanobacteria of the Genera Nostoc, Calothrix, Tolypothrix and Scytonema (Nostocales: Nostocaceae), with Possible Biotechnological Potential. Res. J. 2017, 9, 280–288. [Google Scholar]
- Lucato, V.; Ihadjadene, Y.; Sut, S.; Dall’Acqua, S.; Sforza, E.; Krujatz, F. Optimization of Nostoc sp. Biomass and Protein Composition in High-Density Cultivator Based on DoE. Algal Res. 2025, 89, 104088. [Google Scholar] [CrossRef]
- Martínez, J. Bioprospecting of the Antimicrobial and Biotoxic Activity of Cyanobacterial and Microalgae Extracts. Master’s Thesis, Ensenada Center for Scientific Research and Higher Education (CICESE), Ensenada, Baja California, México, 2012. [Google Scholar]
- Leão, P.N.; Martins, T.P.; Abt, K.; Reis, J.P.A.; Figueiredo, S.; Castelo-Branco, R.; Freitas, S. Incorporation and Modification of Fatty Acids in Cyanobacterial Natural Products Biosynthesis. Chem. Commun. 2023, 59, 4436–4446. [Google Scholar] [CrossRef] [PubMed]
- López-Arellanes, M.E.; López-Pacheco, L.D.; Elizondo-Luevano, J.H.; González-Meza, G.M. Algae and Cyanobacteria Fatty Acids and Bioactive Metabolites: Natural Antifungal Alternative Against Fusarium sp. Microorganisms 2025, 13, 439. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, Y.; Sun, L.; Deng, Q.; Zhao, J. Fatty Acids and Oxylipins as Antifungal and Anti-Mycotoxin Agents in Food: A Review. Toxins 2021, 13, 852. [Google Scholar] [CrossRef]
- Perveen, K.; Bukhari, N.A.; Al Masoudi, L.M.; Alqahtani, A.N.; Alruways, M.W.; Alkhattaf, F.S. Antifungal Potential, Chemical Composition of Chlorella vulgaris and SEM Analysis of Morphological Changes in Fusarium oxysporum. Saudi J. Biol. Sci. 2022, 29, 2501–2505. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal Polyunsaturated Fatty Acids: Hotspots and Production Techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Valentine, D.L.; Reddy, C.M. Latent Hydrocarbons from Cyanobacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 13434–13435. [Google Scholar] [CrossRef]
- Patrikainen, P.; Carbonell, V.; Thiel, K.; Aro, E.-M.; Kallio, P. Comparison of Orthologous Cyanobacterial Aldehyde Deformylating Oxygenases in the Production of Volatile C3-C7 Alkanes in Engineered E. coli. Metab. Eng. Commun. 2017, 5, 9–18. [Google Scholar] [CrossRef]
- Gheda, S.F.; Ismail, G.A. Natural Products from Some Soil Cyanobacterial Extracts with Potent Antimicrobial, Antioxidant and Cytotoxic Activities. An. Acad. Bras. Ciênc. 2020, 92, e20190934. [Google Scholar] [CrossRef]
- Chu, W.-L. Biotechnological Applications of Microalgae. Int. E-J. Sci. Med. Educ. 2012, 6, S24–S37. [Google Scholar] [CrossRef]
- Shakeel, T.; Fatma, Z.; Fatma, T.; Yazdani, S.S. Heterogeneity of Alkane Chain Length in Freshwater and Marine Cyanobacteria. Front. Bioeng. Biotechnol. 2015, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Heilmann, J.; Sticher, O. New Antibacterial Metabolites from the Cyanobacterium Nostoc commune (EAWAG 122b). J. Nat. Prod. 2000, 63, 1283–1285. [Google Scholar] [CrossRef]
- Ramírez, L.S.; Marin-Castaño, D. Methodologies to Evaluate in Vitro Antibacterial Activity of Plant-Derived Compounds. Sci. Tech. 2009, 15, 263–268. [Google Scholar]
- Rosales-Loaiza, N.; Hassanhi, M.; Morales, E. Biological Activity of Extracts of Two Strains of the Cyanobacterium Nostoc. Biol. Res. Cent. Newsl. 2012, 46, 45–62. [Google Scholar]
- Galal, H.R.M.; Salem, W.M.; Nasr El-De, F. Biological Control of Some Pathogenic Fungi Using Marine Algae Extracts. Res. J. Microbiol. 2011, 6, 645–657. [Google Scholar] [CrossRef]
- Al-Nazwani, M.S.; Aboshosha, S.S.; El-Saedy, M.A.M.; Ghareeb, R.Y.; Komeil, D.A. Antifungal Activities of Chlorella vulgaris Extract on Black Scurf Disease, Growth Performance and Quality of Potato. Arch. Phytopathol. Plant Prot. 2021, 54, 2171–2190. [Google Scholar] [CrossRef]
- Jennifer, S.; Ronny, F.; Echeverría, I. Evaluación de La Actividad Antibacteriana de Extractos de Microalgas Usando Cepas ATCC. Quím. Cent. 2018, 6, 57–66. [Google Scholar] [CrossRef]
- Pawar, S.T.; Puranik, P.R. Screening of Terrestrial and Freshwater Halotolerant Cyanobacteria for Antifungal Activities. World J. Microbiol. Biotechnol. 2008, 24, 1019–1025. [Google Scholar] [CrossRef]
- Marí Parra, M. Optimización de un Método para Evaluar la Capacidad Antifúngica de Extractos de Cianobacterias. Tesis de Licenciatura, Universidad Miguel Hernández, San Juan de Alicante, España, 2018. Available online: https://dspace.umh.es/handle/11000/8533 (accessed on 18 December 2024).
- Toribio Gallardo, A. Aplicación de Cianobacterias como Agentes Biofertilizantes, Bioestimulantes y Bioplaguicidas en Etapas Tempranas del Desarrollo Vegetal. Tesis Doctoral, Universidad de Almería, Almería, España, 2021. Available online: https://repositorio.ual.es/bitstream/handle/10835/12764/01.%20Tesis.pdf?sequence=1 (accessed on 18 December 2023).
- Asghari, S.; Zeinalzadeh, K.; Kheirfam, H.; Habibzadeh Azar, B. The Impact of Cyanobacteria Inoculation on Soil Hydraulic Properties at the Lab-Scale Experiment. Agric. Water Manag. 2022, 272, 107865. [Google Scholar] [CrossRef]
- Massey, M.S.; Davis, J.G. Beyond Soil Inoculation: Cyanobacteria as a Fertilizer Replacement. Nitrogen 2023, 4, 253–262. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental Sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Haque, F.; Banayan, S.; Yee, J.; Chiang, Y.W. Extraction and Applications of Cyanotoxins and Other Cyanobacterial Secondary Metabolites. Chemosphere 2017, 183, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, S.; Saadaoui, I.; Al Jabri, H.; Moheimani, N.R. Techno-Economic Modelling of High-Value Metabolites and Secondary Products from Microalgae Cultivated in Closed Photobioreactors with Supplementary Lighting. Algal Res. 2022, 65, 102733. [Google Scholar] [CrossRef]
- Acea, M. Cyanobacterial Inoculation of Heated Soils: Effect on Microorganisms of C and N Cycles and on Chemical Composition in Soil Surface. Soil Biol. Biochem. 2003, 35, 513–524. [Google Scholar] [CrossRef]
- Rubini, M.; Sivakumar, R. GC-MS Analysis of Ethanol Extract of Leaves and Aerial Parts of Andrographis Stellulata C. B. Clarke. Int. J. Biol. Pharm. Sci. Arch. 2025, 9, 024–029. [Google Scholar] [CrossRef]
- Ahuactzin-Pérez, M.; Tlecuitl-Beristain, S.; García-Dávila, J.; Santacruz-Juárez, E.; González-Pérez, M.; Gutiérrez-Ruíz, M.C.; Sánchez, C. Mineralization of High Concentrations of the Endocrine Disruptor Dibutyl Phthalate by Fusarium culmorum. 3 Biotech 2018, 8, 42. [Google Scholar] [CrossRef]
- González-Márquez, Á.; Ahuactzin-Pérez, M.; Sánchez, C. Lentinula Edodes Grown on Di(2-Ethylhexyl) Phthalate-Containing Media: Mycelial Growth and Enzyme Activities. BioResources 2015, 10, 7898–7906. [Google Scholar] [CrossRef]
- Pace, A.; Vaglica, A.; Maccotta, A.; Savoca, D. The Origin of Phthalates in Algae: Biosynthesis and Environmental Bioaccumulation. Environments 2024, 11, 78. [Google Scholar] [CrossRef]

| Locus | Primers (5′ → 3′) | Amplicon (bp) | Cycling Profile * |
|---|---|---|---|
| ITS rDNA | ITS3 GCATCGATGAAGAACGCAGC/ITS4 TCCTCCGCTTATTGATATGC | ~600 | 95 °C 5 min; 34 × (95 °C 30 s, 54 °C 20 s, 72 °C 90 s); 72 °C 10 min |
| RPB2 (RNA polymerase II β-subunit) | RPB2-5F GAYGAYMGWGATCAYTTYGG/RPB2-7cR CCCATRGCTTGYTTRCCCAT | ~1 020 | 98 °C 3 min; 35 × (98 °C 10 s, 56 °C 30 s, 72 °C 90 s); 72 °C 10 min |
| Metabolite | Reaction/Process Employed | Response 1 |
|---|---|---|
| Alkaloids | Mayer, Valser, Dragendorff | +++ |
| Sterols and terpenoids | Lieberman-Burchard reagent | ++ |
| Flavonoids | Cyanidin (Shinoda test) | − |
| Anthocyanidins | Hydrochloric acid (HCl) | − |
| Naphthoquinones and anthraquinones | Bornträger-Krauss | + |
| Cardiac glycosides, coumarins, terpenic lactones | Vanillin-phosphoric acid reagent | − |
| Tannins | Gelatin-salt | − |
| Saponins | Foam | − |
| PK | RT (min) * | Percentage Area | Compound | Fórmula | N° CAS | Probability of Coincidence |
|---|---|---|---|---|---|---|
| 1 | 3.359 | 1.66 | Butyric Acid, TMS derivative | C7H16O2Si | 16844-99-8 | 88 |
| 2 | 3.754 | 1.65 | 2-Methylbutanoic acid, TMS derivative | C8H18O2Si | 55557-14-7 | 89 |
| 3 | 3.870 | 1.78 | 3-Methylbutanoic acid, TMS derivative | C8H18O2Si | 55557-13-6 | 95 |
| 4 | 3.931 | 0.43 | L-Alanine, TMS derivative | C6H15NO2Si | 0-00-0 | 88 |
| 5 | 4.348 | 0.14 | Ethylene glycol, 2TMS derivative | C8H22O2Si2 | 7381-30-8 | 80 |
| 6 | 4.520 | 0.18 | 1-Dimethylthexylsilyloxypentane | C13H30OSi | 0-00-0 | 72 |
| 7 | 4.629 | 0.15 | 3-Dimethylsilyloxypentadecane | C17H38OSi | 0-00-0 | 70 |
| 8 | 4.870 | 1.23 | 4-Methylvaleric acid, TMS derivative | C9H20O2Si | 959048-10-3 | 93 |
| 9 | 5.022 | 9.13 | Lactic Acid, 2TMS derivative | C9H22O3Si2 | 17596-96-2 | 95 |
| 10 | 5.166 | 0.24 | Glycolic acid-2TMS | C8H20O3Si2 | 33581-77-0 | 78 |
| 11 | 5.296 | 0.41 | L-Valine, TMS derivative | C8H19NO2Si | 7480-78-6 | 90 |
| 12 | 5.846 | 3.29 | 3-Hydroxybutyric acid-2TMS | C10H24O3Si2 | 55133-94-3 | 94 |
| 14 | 6.011 | 0.26 | L-Isoleucine, TMS derivative | C9H21NO2Si | 0-00-0 | 80 |
| 15 | 6.383 | 0.25 | 2-Hydroxyisocaproic acid, 2TMS derivative | C12H28O3Si2 | 54890-08-3 | 88 |
| 16 | 6.409 | 0.14 | Hexanoic acid, 2-[(trimethylsilyl)oxy]-, trimeth | C12H28O3Si2 | 54890-07-2 | 74 |
| 17 | 6.540 | 0.13 | Benzoic acid-TMS | C10H14O2Si | 2078-12-8 | 82 |
| 18 | 6.648 | 1.33 | Glycerol-3TMS | C12H32O3Si3 | 6787-10-6 | 94 |
| 19 | 6.883 | 0.40 | Benzeneacetic acid, TMS derivative | C11H16O2Si | 2078-18-4 | 89 |
| 20 | 6.946 | 0.18 | Butanedioic acid, 2TMS derivative | C10H22O4Si2 | 40309-57-7 | 78 |
| 21 | 7.470 | 0.17 | 4-Pentenoic acid, TMS derivative | C8H16O2Si | 23523-56-0 | 58 |
| 22 | 7.618 | 1.19 | Adipic acid, TMS derivative | C9H18O4Si | 0-00-0 | 91 |
| 23 | 7.667 | 0.35 | Benzenepropanoic acid, TMS derivative | C12H18O2Si | 21273-15-4 | 80 |
| 24 | 8.104 | 0.27 | L-Threitol, 4TMS derivative | C16H42O4Si4 | 0-00-0 | 80 |
| 25 | 8.141 | 6.32 | Adipic acid-2TMS | C12H26O4Si2 | 18105-31-2 | 94 |
| 26 | 8.313 | 0.48 | 2,3,4-Trihydroxybutyric acid tetrakis(trimethylsil | C16H40O5Si4 | 38191-88-7 | 69 |
| 27 | 8.692 | 44.85 | Diethyl Phthalate | C12H14O4 | 84-66-2 | 97 |
| 28 | 8.855 | 0.21 | 4-Hydroxybenzoic acid-2TMS | C13H22O3Si2 | 2078-13-9 | 84 |
| 29 | 8.911 | 0.23 | 4-Hydroxybenzeneacetic acid, 2TMS derivative | C14H24O3Si2 | 27750-57-8 | 78 |
| 30 | 9.257 | 1.12 | Heneicosane | C21H44 | 629-94-7 | 93 |
| 31 | 9.482 | 0.41 | Heptadecane, 7-methyl- | C18H38 | 20959-33-5 | 79 |
| 32 | 9.695 | 0.25 | Terephthalic acid, 2TMS derivative | C14H22O4Si2 | 4147-84-6 | 50 |
| 33 | 10.271 | 0.55 | Pentadecanoic acid, TMS derivative | C18H38O2Si | 74367-22-9 | 80 |
| 34 | 10.801 | 1.84 | Palmitelaidic acid, TMS derivative | C19H38O2Si | 82326-15-6 | 93 |
| 35 | 10.885 | 5.55 | Palmitic acid TMS | C19H40O2Si | 55520-89-3 | 90 |
| 36 | 11.428 | 1.06 | Phytol, TMS derivative | C23H48OSi | 57397-39-4 | 84 |
| 37 | 11.621 | 4.73 | Oleic Acid, (Z)-, TMS derivative | C21H42O2Si | 21556-26-3 | 88 |
| 38 | 11.648 | 1.83 | 11-Octadecenoic acid, (E)-, TMS derivative | C21H42O2Si | 0-00-0 | 91 |
| 39 | 11.719 | 1.50 | Stearic acid-TMS | C21H44O2Si | 18748-91-9 | 89 |
| 40 | 11.920 | 0.67 | Glyceryl-glycoside TMS ether | C27H66O8Si6 | 0-00-0 | 87 |
| 41 | 12.379 | 0.43 | Dehydroabietic acid, TMS derivative | C23H36O2Si | 21414-49-3 | 78 |
| 42 | 12.435 | 0.70 | 1-Triethylsilyloxyheptadecane | C23H50OSi | 0-00-0 | 77 |
| 43 | 12.699 | 0.51 | Sucrose, 8TMS derivative | C36H86O11Si8 | 19159-25-2 | 72 |
| 44 | 12.945 | 0.73 | Sucrose, 8TMS derivative | C36H86O11Si8 | 19159-25-2 | 69 |
| 45 | 13.131 | 0.79 | Sucrose, 8TMS derivative | C36H86O11Si8 | 19159-25-2 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pico-González, A.I.; Jaraba-Navas, J.d.D.; Jarma-Orozco, A.; Pérez-Polo, D.J.; Herazo-Cárdenas, D.S.; Vallejo-Isaza, A.; Angulo-Ortíz, A.A.; Pineda-Rodríguez, Y.Y.; Ariza-González, A.R.; Vegliante Arrieta, D.; et al. Can the Cyanobacterium Nostoc commune Exert In Vitro Biocontrol on Fusarium oxysporum, Causal Agent of Wilt in Banana (Musa AAB)? Sci 2025, 7, 115. https://doi.org/10.3390/sci7030115
Pico-González AI, Jaraba-Navas JdD, Jarma-Orozco A, Pérez-Polo DJ, Herazo-Cárdenas DS, Vallejo-Isaza A, Angulo-Ortíz AA, Pineda-Rodríguez YY, Ariza-González AR, Vegliante Arrieta D, et al. Can the Cyanobacterium Nostoc commune Exert In Vitro Biocontrol on Fusarium oxysporum, Causal Agent of Wilt in Banana (Musa AAB)? Sci. 2025; 7(3):115. https://doi.org/10.3390/sci7030115
Chicago/Turabian StylePico-González, Ana Isabel, Juan de Dios Jaraba-Navas, Alfredo Jarma-Orozco, Dairo Javier Pérez-Polo, Diana Sofia Herazo-Cárdenas, Adriana Vallejo-Isaza, Alberto Antonio Angulo-Ortíz, Yirlis Yadeth Pineda-Rodríguez, Anthony Ricardo Ariza-González, Daniela Vegliante Arrieta, and et al. 2025. "Can the Cyanobacterium Nostoc commune Exert In Vitro Biocontrol on Fusarium oxysporum, Causal Agent of Wilt in Banana (Musa AAB)?" Sci 7, no. 3: 115. https://doi.org/10.3390/sci7030115
APA StylePico-González, A. I., Jaraba-Navas, J. d. D., Jarma-Orozco, A., Pérez-Polo, D. J., Herazo-Cárdenas, D. S., Vallejo-Isaza, A., Angulo-Ortíz, A. A., Pineda-Rodríguez, Y. Y., Ariza-González, A. R., Vegliante Arrieta, D., & Rodríguez-Páez, L. A. (2025). Can the Cyanobacterium Nostoc commune Exert In Vitro Biocontrol on Fusarium oxysporum, Causal Agent of Wilt in Banana (Musa AAB)? Sci, 7(3), 115. https://doi.org/10.3390/sci7030115