Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Biomass Production
2.3. EPS and IAAs Quantification
2.4. Selection of the Strain with the Highest Capacity for Exopolysaccharide (EPS) and Indole-Acetic Acid (IAA) Production Under Different LED Spectra
2.5. Determination of the Effect of Photoperiod and Intensity on EPS and IAA Production
3. Results
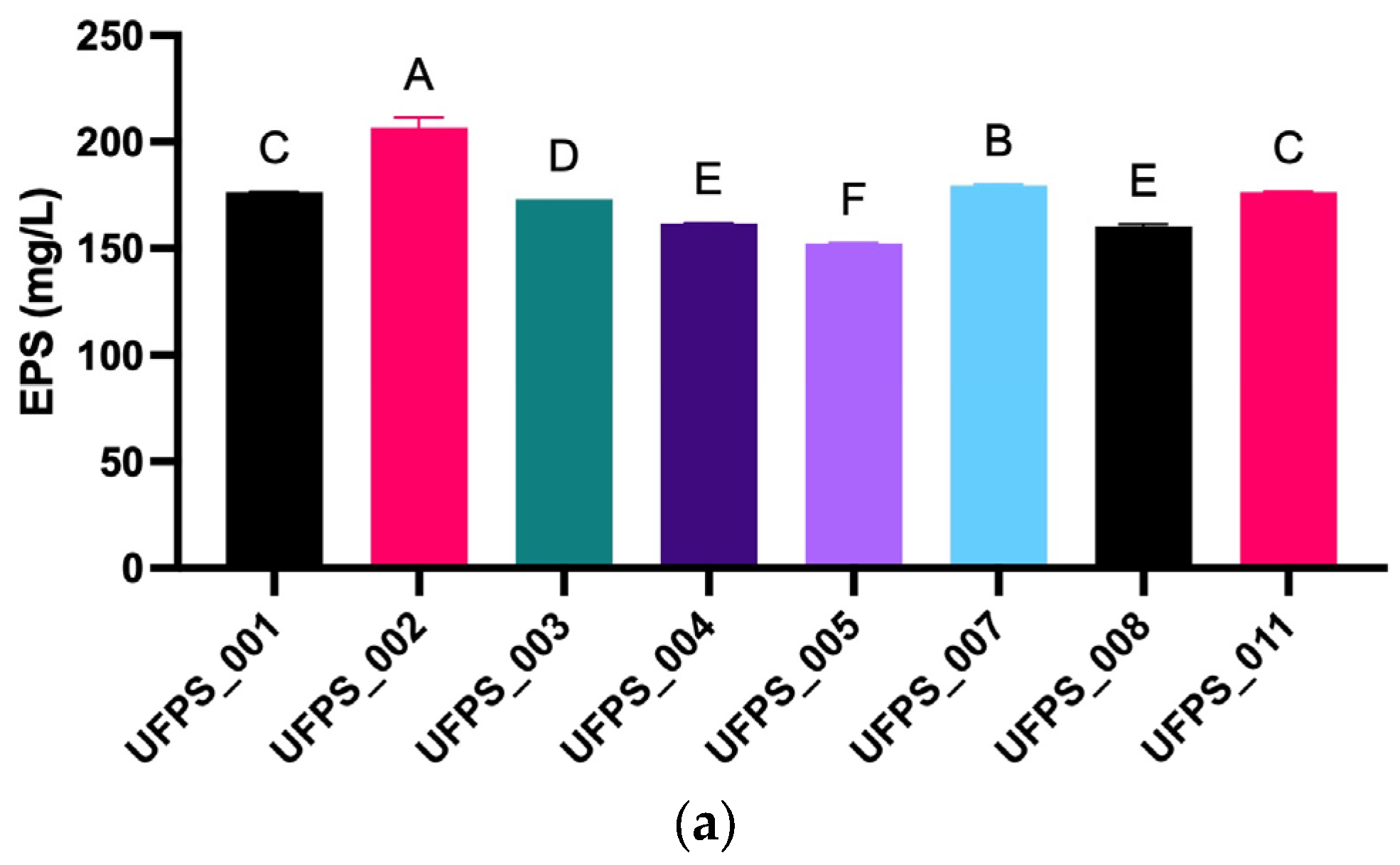
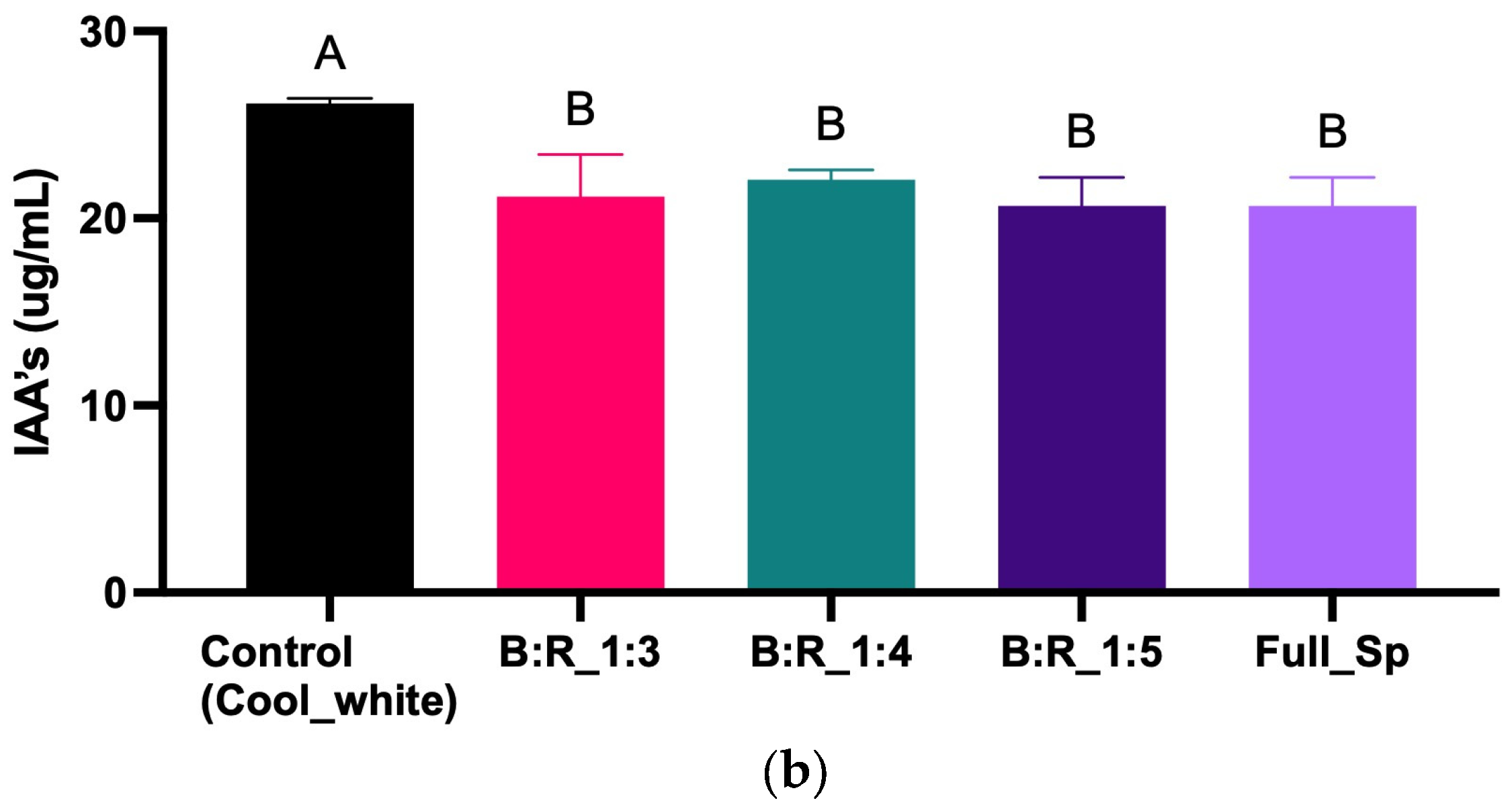
3.1. Strain and LED Spectra Selection
3.2. Effect of Photoperiod and Intensity on EPS and IAAs Production
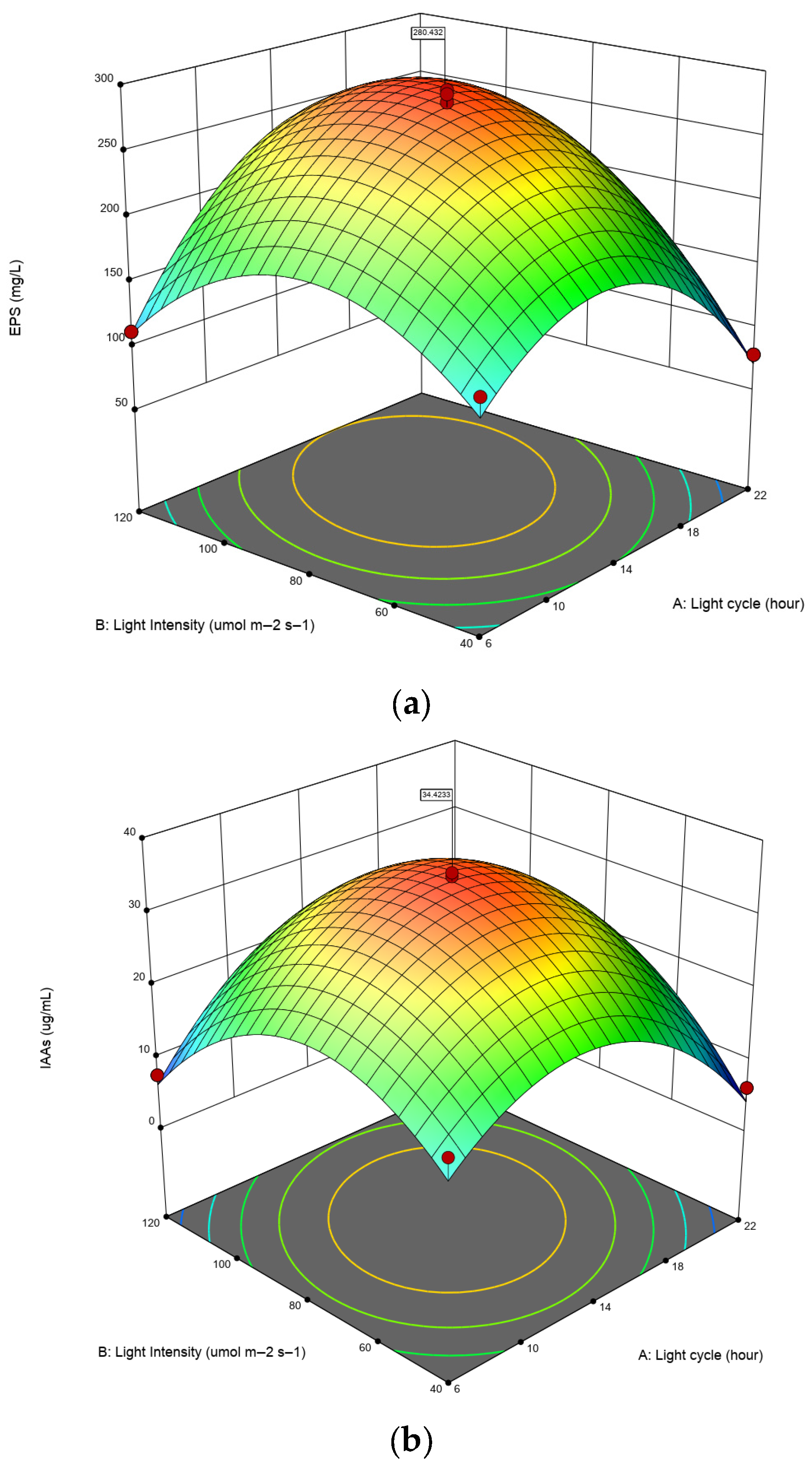
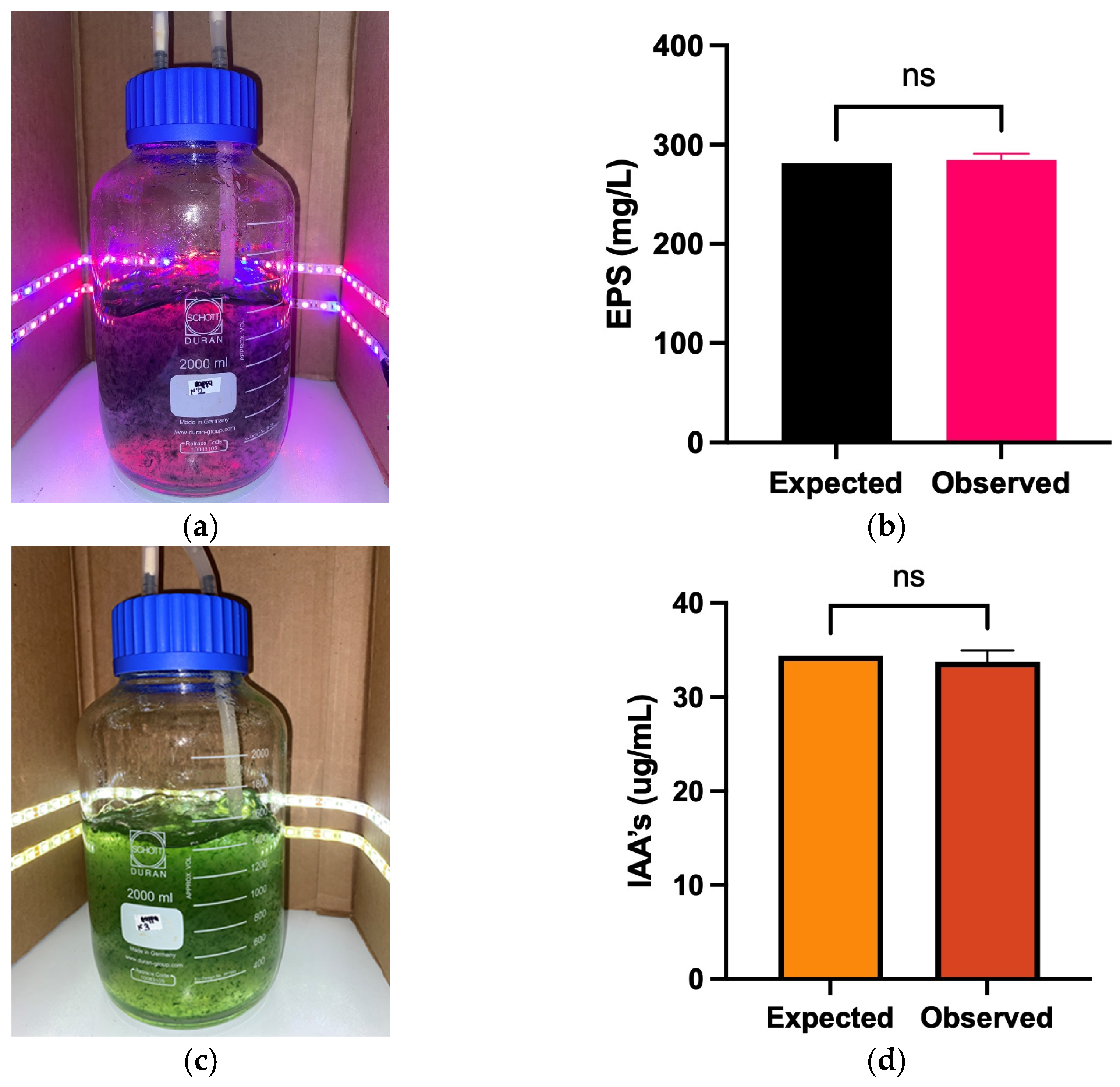
3.3. Optimization of EPS and IAA Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barajas-Solano, A.F. Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes 2022, 10, 836. [Google Scholar] [CrossRef]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Estupiñan, M.; Carrillo-Botello, A.; Rozo-Granados, L.; Becerra-Moreno, D.; García-Martínez, J.; Urbina-Suarez, N.; López-Barrera, G.; Barajas-Solano, A.; Bryan, S.; Zuorro, A. Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water 2022, 14, 558. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Vergel-Suarez, A.H.; García-Martínez, J.B.; López-Barrera, G.L.; Barajas-Solano, A.F.; Zuorro, A. Impact of Biomass Drying Process on the Extraction Efficiency of C-Phycoerythrin. BioTech 2023, 12, 30. [Google Scholar] [CrossRef]
- Vergel-Suarez, A.H.; García-Martínez, J.B.; López-Barrera, G.L.; Urbina-Suarez, N.A.; Barajas-Solano, A.F. Influence of Critical Parameters on the Extraction of Concentrated C-PE from Thermotolerant Cyanobacteria. BioTech 2024, 13, 21. [Google Scholar] [CrossRef]
- Pandey, S.N.; Verma, I.; Kumar, M. Cyanobacteria: Potential Source of Biofertilizer and Synthesizer of Metallic Nanoparticles; INC: Tokyo, Japan, 2020; ISBN 9780128193112. [Google Scholar]
- Roque, J.; Brito, Â.; Rocha, M.; Pissarra, J.; Nunes, T.; Bessa, M.; Vieira, J.; Vieira, C.P.; Melo, P.; Tamagnini, P. Isolation and Characterization of Soil Cyanobacteria and Microalgae and Evaluation of Their Potential as Plant Biostimulants. Plant Soil 2023, 493, 115–136. [Google Scholar] [CrossRef]
- Álvarez, C.; Jiménez-Ríos, L.; Iniesta-Pallarés, M.; Jurado-Flores, A.; Molina-Heredia, F.P.; Ng, C.K.Y.; Mariscal, V. Symbiosis between Cyanobacteria and Plants: From Molecular Studies to Agronomic Applications. J. Exp. Bot. 2023, 74, 6145–6157. [Google Scholar] [CrossRef]
- Wang, J.; Qin, S.; Lin, J.; Wang, Q.; Li, W.; Gao, Y. Phycobiliproteins from Microalgae: Research Progress in Sustainable Production and Extraction Processes. Biotechnol. Biofuels Bioprod. 2023, 16, 170. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Wang, K.; Liu, D.; Dou, S.; Chen, Y.; He, M.; Ma, C. Response of Soil Enzymes to Soil Properties and Seasonal Characteristics of Cyanobacteria-Dominated Crusts in a Dryland Ecosystem. J. Soils Sediments 2023, 23, 2756–2765. [Google Scholar] [CrossRef]
- Reyes-Galvis, M.L.; López-Barrera, G.L.; Urbina-Suarez, N.A.; García-Martínez, J.B.; Barajas-Solano, A.F. Optimizing Cyanobacterial Strain Selection for Antimicrobial Nanoparticle Synthesis: A Comprehensive Analysis. Sci 2024, 6, 83. [Google Scholar] [CrossRef]
- Potnis, A.A.; Raghavan, P.S.; Rajaram, H. Overview on Cyanobacterial Exopolysaccharides and Biofilms: Role in Bioremediation. Rev. Environ. Sci. Biotechnol. 2021, 20, 781–794. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant Biostimulants from Cyanobacteria: An Emerging Strategy to Improve Yields and Sustainability in Agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, M.; Sivasankari, S.; Ahamed, A.K.K.; Alsamhary, K.I.; Al-enazi, N.M.; Abdel-Raouf, N.; Alharbi, R.M.; Govindarajan, R.K.; Ravi, G.; Alarjani, K.M.; et al. Bio-Characterization and Liquid Chromatography–Mass Spectrometry Analysis of Exopolysaccharides in Biofilm-Producing Cyanobacteria Isolated from Soil Crust: Exploring the Potential of Microalgal Biomolecules. Biology 2023, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.N.-D.; Mukhtar, H.; Le, L.-T.; Tran, D.P.-H.; Ngo, M.T.T.; Pham, M.-D.-T.; Nguyen, T.-B.; Vo, T.-K.-Q.; Bui, X.-T. Roles of Microalgae-Based Biofertilizer in Sustainability of Green Agriculture and Food-Water-Energy Security Nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef]
- Sukhikh, S.; Popov, V.; Kashinskikh, E.; Budenkova, E.; Ivanova, S.; Babich, O. Immunomodulatory Properties of Polysaccharide Extract Samples from Cyanobacterium Sp. Rippka B-1200. Sci. Rep. 2024, 14, 30365. [Google Scholar] [CrossRef]
- Zedler, J.A.Z.; Michel, M.; Pohnert, G.; Russo, D.A. Cell Surface Composition, Released Polysaccharides, and Ionic Strength Mediate Fast Sedimentation in the Cyanobacterium Synechococcus Elongatus PCC 7942. Environ. Microbiol. 2023, 25, 1955–1966. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Mondal, A.; Bhunia, B.; Bandyopadhyay, T.K.; Jaladi, P.; Oinam, G.; Indrama, T. Purification, Characterization and Biotechnological Potential of New Exopolysaccharide Polymers Produced by Cyanobacterium Anabaena Sp. CCC 745. Polymer 2019, 178, 121695. [Google Scholar] [CrossRef]
- Cruz, J.D.; Delattre, C.; Felpeto, A.B.; Pereira, H.; Pierre, G.; Morais, J.; Petit, E.; Silva, J.; Azevedo, J.; Elboutachfaiti, R.; et al. Bioprospecting for Industrially Relevant Exopolysaccharide-Producing Cyanobacteria under Portuguese Simulated Climate. Sci. Rep. 2023, 13, 13561. [Google Scholar] [CrossRef]
- Van Camp, C.; Fraikin, C.; Claverie, E.; Onderwater, R.; Wattiez, R. Capsular Polysaccharides and Exopolysaccharides from Gloeothece Verrucosa under Various Nitrogen Regimes and Their Potential Plant Defence Stimulation Activity. Algal Res. 2022, 64, 102680. [Google Scholar] [CrossRef]
- Toribio, A.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Moreno, J. Prospection of Cyanobacteria Producing Bioactive Substances and Their Application as Potential Phytostimulating Agents. Biotechnol. Rep. 2020, 26, e00449. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Yang, H.; Guo, C.; Hong, Z.; Hu, Z.; Wu, Y.; Huang, D.; Wang, C. Effects of High Light Exposure and Heterologous Expression of β-Carotene Ketolase on the Metabolism of Carotenoids in Chlamydomonas Reinhardtii. Front. Bioeng. Biotechnol. 2025, 13, 1533661. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Bacterial Indole-3-Acetic Acid: A Key Regulator for Plant Growth, Plant-Microbe Interactions, and Agricultural Adaptive Resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Renuka, N.; Ratha, S.K.; Kumari, S.; Bux, F. Bioprospecting of Microalgae from Agricultural Fields and Developing Consortia for Sustainable Agriculture. Algal Res. 2024, 78, 103428. [Google Scholar] [CrossRef]
- Debnath, S.; Muthuraj, M.; Bandyopadhyay, T.K.; Bobby, M.N.; Vanitha, K.; Tiwari, O.N.; Bhunia, B. Engineering Strategies and Applications of Cyanobacterial Exopolysaccharides: A Review on Past Achievements and Recent Perspectives. Carbohydr. Polym. 2024, 328, 121686. [Google Scholar] [CrossRef]
- Casero, M.C.; Velázquez, D.; Pereira, A.; Tejedor, M.d.M.; García, L.; Quesada, A.; Cirés, S. Hidden inside Desert Rocks: Salinity Triggers an Increase in Exopolysaccharides from Endolithic Cyanobacteria with Anti-Inflammatory Potential. Algal Res. 2024, 84, 103817. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Torzillo, G.; De Philippis, R. Exopolysaccharide Features Influence Growth Success in Biocrust-Forming Cyanobacteria, Moving from Liquid Culture to Sand Microcosms. Front. Microbiol. 2020, 11, 568224. [Google Scholar] [CrossRef]
- Duan, Z.; Tan, X.; Shi, L.; Zeng, Q.; Ali, I.; Zhu, R.; Chen, H.; Parajuli, K. Phosphorus Accumulation in Extracellular Polymeric Substances (EPS) of Colony-Forming Cyanobacteria Challenges Imbalanced Nutrient Reduction Strategies in Eutrophic Lakes. Environ. Sci. Technol. 2023, 57, 1600–1612. [Google Scholar] [CrossRef]
- Fang, Y.; Lin, G.; Liu, Y.; Zhang, J. Contaminant Removal Performance and Lipid Productivity of a Cyanobacteria-Bacteria Consortium Containing Exogenous Phytohormones during the Treatment of Antibiotic-Polluted Wastewater. Chemosphere 2024, 361, 142473. [Google Scholar] [CrossRef]
- Singh, S.; Kant, C.; Yadav, R.K.; Reddy, Y.P.; Abraham, G. Cyanobacterial Exopolysaccharides: Composition, Biosynthesis, and Biotechnological Applications. In Cyanobacteria; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–358. [Google Scholar]
- Touceda, I.S.; Demay, J.; Duval, C.; Yéprémian, C.; Reinhardt, A.; Marie, B. Light and Temperature Culture Conditions Impact the Metabolome of the Cyanobacterium of Therapeutic Interest Planktothricoides Raciborskii PMC 877.14. Algal Res. 2024, 84, 103738. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.K.; Sadvakasova, A.K.; Sandybayeva, S.K.; Kossalbayev, B.D.; Huang, Z.; Zayadan, B.K.; Akmukhanova, N.R.; Leong, Y.K.; Chang, J.-S.; Allakhverdiev, S.I. Microalgae- and Cyanobacteria-Derived Phytostimulants for Mitigation of Salt Stress and Improved Agriculture. Algal Res. 2024, 82, 103686. [Google Scholar] [CrossRef]
- Gan, L.; Huang, X.; He, Z.; He, T. Exopolysaccharide Production by Salt-Tolerant Bacteria: Recent Advances, Current Challenges, and Future Prospects. Int. J. Biol. Macromol. 2024, 264, 130731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zuo, W.; Xie, Y.; Wang, Y.; Li, C.; Tang, Y.; Wang, Z.; Li, Y. Sodium Bisulfite Boosted Exopolysaccharide Production by Auxenochlorella Protothecoides: Potential Mechanisms Harnessing H2O2 Signaling and Carbon Reallocation. Bioresour. Technol. 2025, 420, 132121. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.A.; Semerdzhiev, S.; Twigg, J.D.; Moss, C.; Bavington, C.D.; Amtmann, A. Environmental Modulation of Exopolysaccharide Production in the Cyanobacterium Synechocystis 6803. Appl. Microbiol. Biotechnol. 2023, 107, 6121–6134. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Ansari, A.A.; Gikonyo, J.G.; Abouhend, A.S.; Park, C. The Coupled Effect of Light and Iron on the Photogranulation Phenomenon. Environ. Sci. Technol. 2023, 57, 9086–9095. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Wang, X.; Tang, Z.; Chen, X.; Chen, Y.; Dai, X.; Chen, H.; Wang, H. Fluidized Bed Photobioreactor Based on Diatomite Powder and High Light Intensity Improved Microalgae Harvesting, Nutrient Removal and Lipid Accumulation: Performance and Microscopic Mechanism. Water Res. 2024, 264, 122172. [Google Scholar] [CrossRef]
- Wu, L.; Quan, L.; Deng, Z.; Vadiveloo, A.; Cheng, Y.; Yang, L.; Zhang, Z.; Saber, A.A.; Lan, S. Performance of a Biocrust Cyanobacteria-Indigenous Bacteria (BCIB) Co-Culture System for Nutrient Capture and Transfer in Municipal Wastewater. Sci. Total Environ. 2023, 888, 164236. [Google Scholar] [CrossRef]
- Contreras-Ropero, J.E.; Barajas-Solano, A.F.; García-Martínez, J.B.; Barajas-Ferrerira, C.; Machuca-Martínez, F. Production of Industrial-Interest Colorants in Microalgae and Cyanobacteria: Leveraging Nutrient Dynamics and Photoperiod Optimization. Ing. Compet. 2024, 26, 1–16. [Google Scholar] [CrossRef]
- de Alvarenga, L.V.; Almeida, A.V.M.; de Castro, N.V.; Oder, J.C.; Esteves-Ferreira, A.; Nunes-Nesi, A.; Araújo, W.L.; Vaz, M.G.M.V. Physiological Responses to Light Intensity and Photoperiod of the Halotolerant Cyanobacterium Desmonostoc Salinum CCM-UFV059. Bioresour. Technol. Rep. 2020, 11, 100443. [Google Scholar] [CrossRef]
- Santos, M.; Pereira, S.B.; Flores, C.; Príncipe, C.; Couto, N.; Karunakaran, E.; Cravo, S.M.; Oliveira, P.; Tamagnini, P. Absence of KpsM (Slr0977) Impairs the Secretion of Extracellular Polymeric Substances (EPS) and Impacts Carbon Fluxes in Synechocystis sp. PCC 6803. mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, T.; Kullmann, E.; Zavřel, T.; Nakielski, A.; Ebenhöh, O.; Červený, J.; Bernát, G.; Matuszyńska, A.B. Shedding Light on Blue-Green Photosynthesis: A Wavelength-Dependent Mathematical Model of Photosynthesis in Synechocystis sp. PCC 6803. PLoS Comput. Biol. 2024, 20, e1012445. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.F.; Salgado, E.M.; Vilar, V.J.P.; Gonçalves, A.L.; Pires, J.C.M. A Growth Phase Analysis on the Influence of Light Intensity on Microalgal Stress and Potential Biofuel Production. Energy Convers. Manag. 2024, 311, 118511. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, L.; Juneau, P.; Chen, Z.; Zheng, X.; Lian, Y.; Li, W.; Huang, P.; Yan, Q.; Chen, X.; et al. The Photosynthetic Toxicity of Nano-Polystyrene to Microcystis Aeruginosa Is Influenced by Surface Modification and Light Intensity. Environ. Pollut. 2024, 356, 124206. [Google Scholar] [CrossRef]
- Moore, V.; Vermaas, W. Functional Consequences of Modification of the Photosystem I/Photosystem II Ratio in the Cyanobacterium Synechocystis sp. PCC 6803. J. Bacteriol. 2024, 206, e00454-23. [Google Scholar] [CrossRef]
- Bellver, M.; Altamira-Algarra, B.; García, J.; Ferrer, I.; Gonzalez-Flo, E. Enhancing Pigment Production with Cyanobacteria-Rich Microbiomes: Effect of Light Quality and Exposure Time. Algal Res. 2024, 83, 103726. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Overexpression of Glucose-6-Phosphate Isomerase in Synechocystis sp. PCC 6803 with Disrupted Glycogen Synthesis Pathway Improves Exopolysaccharides Synthesis. Algal Res. 2021, 57, 102357. [Google Scholar] [CrossRef]
- Werner, A.; Broeckling, C.D.; Prasad, A.; Peebles, C.A.M. A Comprehensive Time-course Metabolite Profiling of the Model Cyanobacterium Synechocystis sp. PCC 6803 under Diurnal Light: Dark Cycles. Plant J. 2019, 99, 379–388. [Google Scholar] [CrossRef]
- Sicora, C.I.; Chiș, I.; Chiș, C.; Sicora, O. Regulation of PSII Function in Cyanothece sp. ATCC 51142 during a Light–Dark Cycle. Photosynth. Res. 2019, 139, 461–473. [Google Scholar] [CrossRef]
- Maity, S.; Tiwari, R.; Mallick, N. Application of Empirical Modelling to Predict the Growth of a Marine Cyanobacterium Leptolyngbya Valderiana BDU 41001 for Production of Bioethanol and C-Phycocyanin under Semi-Outdoor Cultivation. J. Clean. Prod. 2024, 473, 143507. [Google Scholar] [CrossRef]
- Contreras-Ropero, J.E.; Lidueñez-Ballesteros, V.S.; Rodríguez-Bohórquez, A.D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. The Effect of LEDs on Biomass and Phycobiliproteins Production in Thermotolerant Oscillatoria sp. Appl. Sci. 2022, 12, 11664. [Google Scholar] [CrossRef]
- Prasetija, N.; Schneider, S.; Xie, T.; Cao, J. Droplet-Based Microfluidic Photobioreactor as a Growth Optimization Tool for Cyanobacteria and Microalgae. Environments 2024, 11, 255. [Google Scholar] [CrossRef]
- Andersen, R.; Berges, J.; Harrison, P.; Watanabe, M. Recipes for Freshwater and Seawater Media. In Algal Culture Techniques; Academic Press: London, UK, 2005. [Google Scholar]
- Moheimani, N.R.; Borowitzka, M.A.; Isdepsky, A.; Sing, S.F. Standard Methods for Measuring Growth of Algae and Their Composition BT—Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 265–284. ISBN 978-94-007-5479-9. [Google Scholar]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-Acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Ross, M.; Thomas, N.; McNeill, S.; Stanley, M.S. A Rapid and General Method for Measurement of Protein in Micro-Algal Biomass. Bioresour. Technol. 2013, 129, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid Quantification of Microalgal Lipids in Aqueous Medium by a Simple Colorimetric Method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Urbina-Suarez, N.A.; Salcedo-Pabón, C.J.; Contreras-Ropero, J.E.; López-Barrera, G.L.; García-Martínez, J.B.; Barajas-Solano, A.F.; Machuca-Martínez, F. A Phytochemical Approach to the Removal of Contaminants from Industrial Dyeing Wastewater. ChemEngineering 2023, 7, 90. [Google Scholar] [CrossRef]
- Tiwari, O.N.; Bhunia, B.; Mondal, A.; Gopikrishna, K.; Indrama, T. System Metabolic Engineering of Exopolysaccharide-Producing Cyanobacteria in Soil Rehabilitation by Inducing the Formation of Biological Soil Crusts: A Review. J. Clean. Prod. 2019, 211, 70–82. [Google Scholar] [CrossRef]
- Gongi, W.; Gomez Pinchetti, J.L.; Cordeiro, N.; Ouada, H. Ben Extracellular Polymeric Substances Produced by the Thermophilic Cyanobacterium Gloeocapsa Gelatinosa: Characterization and Assessment of Their Antioxidant and Metal-Chelating Activities. Mar. Drugs 2022, 20, 227. [Google Scholar] [CrossRef]
- Sarkar, S.; Cabrera-Barjas, G.; Singh, R.N.; Fabi, J.P.; Breig, S.J.M.; Tapia, J.; Sani, R.K.; Banerjee, A. Unveiling a Novel Exopolysaccharide Produced by Pseudomonas Alcaligenes Med1 Isolated from a Chilean Hot Spring as Biotechnological Additive. Sci. Rep. 2024, 14, 25058. [Google Scholar] [CrossRef]
- Batucan, J.; Susana, S.; Ochoa, M.M.; Salvador-Reyes, L. Biological Activity and Chemical Profiling of Terrestrial and Freshwater Cyanobacteria from the Philippines. Philipp. J. Sci. 2021, 150, 1245–1254. [Google Scholar] [CrossRef]
- Jung, F.; Braune, S.; Jung, C.H.G.; Krüger-Genge, A.; Waldeck, P.; Petrick, I.; Küpper, J.-H. Lipophilic and Hydrophilic Compounds from Arthrospira platensis and Its Effects on Tissue and Blood Cells—An Overview. Life 2022, 12, 1497. [Google Scholar] [CrossRef]
- Han, P.; Sun, Y.; Wu, X.; Yuan, Y.; Dai, Y.; Jia, S. Emulsifying, Flocculating, and Physicochemical Properties of Exopolysaccharide Produced by Cyanobacterium Nostoc Flagelliforme. Appl. Biochem. Biotechnol. 2014, 172, 36–49. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Kanesaki, Y.; Uemura, A.; Yamada, K.; Okajima, M.; Kaneko, T.; Ohki, K. Physiological and Genomic Analysis of Newly-Isolated Polysaccharide Synthesizing Cyanobacterium Chroococcus Sp. FPU101 and Chemical Analysis of the Exopolysaccharide. J. Gen. Appl. Microbiol. 2021, 67, 2021.02.002. [Google Scholar] [CrossRef]
- Ohki, K.; Le, N.Q.T.; Yoshikawa, S.; Kanesaki, Y.; Okajima, M.; Kaneko, T.; Thi, T.H. Exopolysaccharide Production by a Unicellular Freshwater Cyanobacterium Cyanothece sp. Isolated from a Rice Field in Vietnam. J. Appl. Phycol. 2014, 26, 265–272. [Google Scholar] [CrossRef]
- Trabelsi, L.; M’sakni, N.H.; Ben Ouada, H.; Bacha, H.; Roudesli, S. Partial Characterization of Extracellular Polysaccharides Produced by Cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Yustinadiar, N.; Manurung, R.; Suantika, G. Enhanced Biomass Productivity of Microalgae Nannochloropsis Sp. in an Airlift Photobioreactor Using Low-Frequency Flashing Light with Blue LED. Bioresour. Bioprocess. 2020, 7, 43. [Google Scholar] [CrossRef]
- Vásquez-Villalobos, V.; Vergaray, D.; Méndez, J.; Barrios, I.; Baquedano, R.; Caldas, C.; Cruz, J.; Gamboa, J.; Rivera, I. Effect of the Light Emitting Diodes Intensity and Photoperiod in the Optimization of the Spirulina (Arthrospira) Biomass Production. Sci. Agropecu. 2017, 8, 43–55. [Google Scholar] [CrossRef][Green Version]
- Mahesh, R.; Naira, V.R.; Maiti, S.K. Concomitant Production of Fatty Acid Methyl Ester (Biodiesel) and Exopolysaccharides Using Efficient Harvesting Technology in Flat Panel Photobioreactor with Special Sparging System via Scenedesmus Abundans. Bioresour. Technol. 2019, 278, 231–241. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Characterization and Comparison of Porphyridium sordidum and Porphyridium Purpureum Concerning Growth Characteristics and Polysaccharide Production. Algal Res. 2020, 49, 101931. [Google Scholar] [CrossRef]
- Liu, S.; Daigger, G.T.; Kang, J.; Zhang, G. Effects of Light Intensity and Photoperiod on Pigments Production and Corresponding Key Gene Expression of Rhodopseudomonas palustris in a Photobioreactor System. Bioresour. Technol. 2019, 294, 122172. [Google Scholar] [CrossRef]
- Han, P.; Shen, S.; Guo, R.; Zhao, D.; Lin, Y.-H.; Jia, S.; Yan, R.; Wu, Y. ROS Is a Factor Regulating the Increased Polysaccharide Production by Light Quality in the Edible Cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.K.; Ravi, V.; Saravanan, R. Influence of Cyanobacterial Auxin on Sprouting of Taro (Colocasia Esculenta Var. Antiquorum) and Corm Yield. Indian. J. Agric. Sci. 2017, 87, 1437–1444. [Google Scholar] [CrossRef]
- Al-Katib, M.A.; Al-Shaheree, Y.J.; Al-Niemi, A.N. Estimation of Indole Acetic Acid in Some Local Algae and Study the Optimal Conditions for Its Production of Cyanobacteria Gloeocapsa Sp. PCC7428. Tikrit J. Pure Sci. 2023, 22, 63–77. [Google Scholar] [CrossRef]
- Govarthanan, M.; Kamala-Kannan, S.; Selvankumar, T.; Mythili, R.; Srinivasan, P.; Kim, H. Effect of Blue Light on Growth and Exopolysaccharides Production in Phototrophic Rhodobacter sp. BT18 Isolated from Brackish Water. Int. J. Biol. Macromol. 2019, 131, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Baylous, H.R.; Gladfelter, M.F.; Gardner, M.I.; Foley, M.; Wilson, A.E.; Steffen, M.M. Indole-3-Acetic Acid Promotes Growth in Bloom-Forming Microcystis via an Antioxidant Response. Harmful Algae 2024, 133, 102575. [Google Scholar] [CrossRef]
- Duong, T.T.; Nguyen, T.T.L.; Van Dinh, T.H.; Hoang, T.Q.; Vu, T.N.; Doan, T.O.; Dang, T.M.A.; Le, T.P.Q.; Tran, D.T.; Le, V.N.; et al. Auxin Production of the Filamentous Cyanobacterial Planktothricoides Strain Isolated from a Polluted River in Vietnam. Chemosphere 2021, 284, 131242. [Google Scholar] [CrossRef]
- Bispo, R.L.B.; Ceccato-Antonini, S.R.; Takita, M.A.; Rosa-Magri, M.M. Exogenous Indole-3-Acetic Acid Production and Phosphate Solubilization by Chlorella Vulgaris Beijerinck in Heterotrophic Conditions. Fermentation 2023, 9, 116. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Hill, R.T. Microalgal and Bacterial Auxin Biosynthesis: Implications for Algal Biotechnology. Curr. Opin. Biotechnol. 2022, 73, 300–307. [Google Scholar] [CrossRef]
- Zhang, K.; Wen, Y.; Tang, J.; Zhang, Y.; Peng, X.; Ji, Y.; Sun, J.; Liu, X. The Regulation of Photosynthesis and Growth of Rapeseed Seedling by the Interaction of Red and Yellow Lights with Blue Light. Environ. Exp. Bot. 2024, 225, 105869. [Google Scholar] [CrossRef]
- Zournas, A.; Mani, K.; Dismukes, G.C. Cyclic Electron Flow around Photosystem II in Silico: How It Works and Functions in Vivo. Photosynth. Res. 2023, 156, 129–145. [Google Scholar] [CrossRef]
- Chen, S.; Dalla Villa, V.; Kohlen, W.; Kusuma, P.; Offringa, R.; Marcelis, L.F.M.; Heuvelink, E. High Ratio of Blue:Red Light Reduces Fruit Set in Sweet Pepper, Which Is Associated with Low Starch Content and Hormonal Changes. Environ. Exp. Bot. 2024, 225, 105850. [Google Scholar] [CrossRef]
- Pech, R.; Volná, A.; Špunda, V.; Nezval, J. Blue Light as an Important Factor Increasing Plant Tolerance to Acute Photooxidative Stress. Environ. Exp. Bot. 2024, 226, 105923. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, G.; Jiang, L.; Zhang, S.; Miao, L.; Xu, P.; Chen, H.; Chen, L.; Mao, Z.; Guo, T.; et al. Phytochromes A and B Mediate Light Stabilization of BIN2 to Regulate Brassinosteroid Signaling and Photomorphogenesis in Arabidopsis. Front. Plant Sci. 2022, 13, 865019. [Google Scholar] [CrossRef]
- Fukazawa, M.; Miyake, K.; Hoshino, H.; Fushimi, K.; Narikawa, R. Phycocyanobilin Binding and Specific Amino Acid Residues Near the Chromophore Contribute to Orange Light Perception by the Dualchrome Phytochrome Region. Plant Cell Physiol. 2025, 66, 193–203. [Google Scholar] [CrossRef]
- Velmurugan, R.; Kanwal, S.; Incharoensakdi, A. Non-Ionic Surfactant Integrated Extraction of Exopolysaccharides from Engineered Synechocystis sp. PCC 6803 under Fed-Batch Mode Facilitates the Sugar-Rich Syrup Production for Ethanol Fermentation. Algal Res. 2022, 66, 102772. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Wu, S.; Zhang, T.; Yang, G.; Li, Z.; Wang, L.; Chen, W. Function Analysis of RNase III in Response to Oxidative Stress in Synechocystis sp. PCC 6803. Microbiol. Res. 2025, 292, 128045. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, T.; Yuan, X.; Dong, Y.; Liu, C. Biocidal H2O2 Treatment Emphasizes the Crucial Role of Cyanobacterial Extracellular Polysaccharides against External Strong Oxidative Stress. Environ. Sci. Pollut. Res. 2023, 30, 60654–60662. [Google Scholar] [CrossRef] [PubMed]
- Chandra, N.; Mallick, N. Co-production of Bioethanol and Commercially Important Exopolysaccharides from the Marine Cyanobacterium Synechococcus elongatus BDU 10144 in a Novel Low-cost Seawater-fertilizer-based Medium. Int. J. Energy Res. 2022, 46, 13487–13510. [Google Scholar] [CrossRef]
- Uhliariková, I.; Matulová, M.; Košťálová, Z.; Lukavský, J.; Capek, P. Lactylated Acidic Exopolysaccharide Produced by the Cyanobacterium Nostoc Cf. Linckia. Carbohydr. Polym. 2022, 276, 118801. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.; Davis, J.G.; Storteboom, H. Influence of Light on Endogenous Phytohormone Concentrations of a Nitrogen-Fixing Anabaena sp. Cyanobacterium Culture in Open Raceways for Use as Fertilizer for Horticultural Crops. J. Appl. Phycol. 2019, 31, 3371–3384. [Google Scholar] [CrossRef]



| Factor 1 | Factor 2 | |||
|---|---|---|---|---|
| Std | Block | Run | A: Light Cycle | B: Light Intensity |
| hour | µmol m−2 s−1 | |||
| 3 | Block 1 | 1 | 6 | 120 |
| 5 | 2 | 14 | 80 | |
| 4 | 3 | 22 | 120 | |
| 2 | 4 | 22 | 40 | |
| 7 | 5 | 14 | 80 | |
| 6 | 6 | 14 | 80 | |
| 1 | 7 | 6 | 40 | |
| 11 | Block 2 | 8 | 14 | 136.57 |
| 13 | 9 | 14 | 80 | |
| 14 | 10 | 14 | 80 | |
| 10 | 11 | 14 | 23.43 | |
| 9 | 12 | 25.31 | 80 | |
| 12 | 13 | 14 | 80 | |
| 8 | 14 | 2.69 | 80 |
| Metabolite | Source | Sum of Squares | Df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| EPS (mg/mL) | Block | 263.53 | 1 | 263.53 | ||
| Model | 90,909.97 | 5 | 18,181.99 | 136.79 | <0.0001 * | |
| A-Light cycle | 1136.16 | 1 | 1136.16 | 8.55 | 0.0222 * | |
| B-Light Intensity | 7898.44 | 1 | 7898.44 | 59.42 | 0.0001 * | |
| AB | 6172.54 | 1 | 6172.54 | 46.44 | 0.0002 * | |
| A2 | 45,142.74 | 1 | 45,142.74 | 339.64 | <0.0001 * | |
| B2 | 36,343.68 | 1 | 36,343.68 | 273.44 | <0.0001 * | |
| Residual | 930.41 | 7 | 132.92 | |||
| Lack of Fit | 446.62 | 3 | 148.87 | 1.23 | 0.4080 ** | |
| Pure Error | 483.79 | 4 | 120.95 | |||
| Cor Total | 92,103.91 | 13 | ||||
| R2 | 0.9899 | Std. Dev. | 11.53 | |||
| Adjusted R2 | 0.9826 | Mean | 193.18 | |||
| Predicted R2 | 0.9382 | C.V.% | 5.97 | |||
| Adeq Precision | 25.4076 | |||||
| IAA (µg/mL) | Block | 6.90 | 1 | 6.90 | ||
| Model | 2229.75 | 5 | 445.95 | 381.56 | <0.0001 * | |
| A-Light cycle | 1.17 | 1 | 1.17 | 0.9986 | 0.3509 ** | |
| B-Light Intensity | 15.49 | 1 | 15.49 | 13.25 | 0.0083 * | |
| AB | 52.56 | 1 | 52.56 | 44.97 | 0.0003 * | |
| A2 | 1215.85 | 1 | 1215.85 | 1040.29 | <0.0001 * | |
| B2 | 1110.68 | 1 | 1110.68 | 950.31 | <0.0001 * | |
| Residual | 8.18 | 7 | 1.17 | |||
| Lack of Fit | 4.61 | 3 | 1.54 | 1.72 | 0.3004 ** | |
| Pure Error | 3.57 | 4 | 0.8933 | |||
| Cor Total | 2244.83 | 13 | ||||
| R2 | 0.9963 | Std. Dev. | 1.08 | |||
| Adjusted R2 | 0.9937 | Mean | 20.13 | |||
| Predicted R2 | 0.9838 | C.V.% | 5.37 | |||
| Adeq Precision | 38.8906 |
| Coded Name | Response | Light Cycle (h) | Light Intensity (μmol m−2 s−1) | Light Type | Value |
|---|---|---|---|---|---|
| Z1 | EPS (mg/mL) | 14.5 | 85 | Blue/Red 1_5 | 281.4 mg/L |
| Z2 | IAA (µg/mL) | Cool white | 34.4 µg/mL |
| Objective | Strain | LED Spectrum | Intensity | Photoperiod | Metabolite | Reference |
|---|---|---|---|---|---|---|
| μmol m−2 s−1 | h | |||||
| EPS (mg/mL) | Synechococcus elongatus BDU 10144 | White light | 50 | 12 | 280 | [92] |
| Synechocystis sp. LEGE 07367 | Natural Light | 70 | 300 | |||
| Nostoc cf. linckia | White light | 60 | 14 | 5400 | [93] | |
| Synechocystis sp. PCC 6803 | White Light | 75 | 24 | 251 | [49] | |
| P. purpureum | Blue Light | n/a | 12 | 30 | [43] | |
| Orange: Red Light | 90 | |||||
| White Light | 140 | |||||
| P. sordidum | Blue Light | 10 | ||||
| Orange: Red Light | 120 | |||||
| White Light | 100 | |||||
| Hapalosiphon sp. UFPS_002 | Blue/Red 1_5 LED | 85 | 14.5 | 281.4 | This work | |
| IAA (µg/mL) | Anabaena sp. | Natural Light | n/a | 12:12 | 0.189 | [94] |
| Synechococcus elongatus UTEX2973 | Blue LED | 120 | 24 | 45 | [54] | |
| Nostoc sp. | White light | 50–100 | 12:12 | 8.66 | [76] | |
| Planktothricoides raciborskii | Fluorescent Light | n/a | 12:12 | 3,04 | [78] | |
| Planktothricoides raciborskii | Natural Light | 24 | 120 | |||
| Hapalosiphon sp. UFPS_002 | Cool white LED | 85 | 14.5 | 34.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuorro, A.; Lavecchia, R.; Moncada-Jacome, K.A.; García-Martínez, J.B.; Barajas-Solano, A.F. Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria. Sci 2025, 7, 108. https://doi.org/10.3390/sci7030108
Zuorro A, Lavecchia R, Moncada-Jacome KA, García-Martínez JB, Barajas-Solano AF. Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria. Sci. 2025; 7(3):108. https://doi.org/10.3390/sci7030108
Chicago/Turabian StyleZuorro, Antonio, Roberto Lavecchia, Karen A. Moncada-Jacome, Janet B. García-Martínez, and Andrés F. Barajas-Solano. 2025. "Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria" Sci 7, no. 3: 108. https://doi.org/10.3390/sci7030108
APA StyleZuorro, A., Lavecchia, R., Moncada-Jacome, K. A., García-Martínez, J. B., & Barajas-Solano, A. F. (2025). Light-Driven Optimization of Exopolysaccharide and Indole-3-Acetic Acid Production in Thermotolerant Cyanobacteria. Sci, 7(3), 108. https://doi.org/10.3390/sci7030108









