The Role of Additive Manufacturing in Dental Implant Production—A Narrative Literature Review
Abstract
1. Introduction
2. Theoretical Background
3. Materials Utilized in the Manufacture of Dental Implants
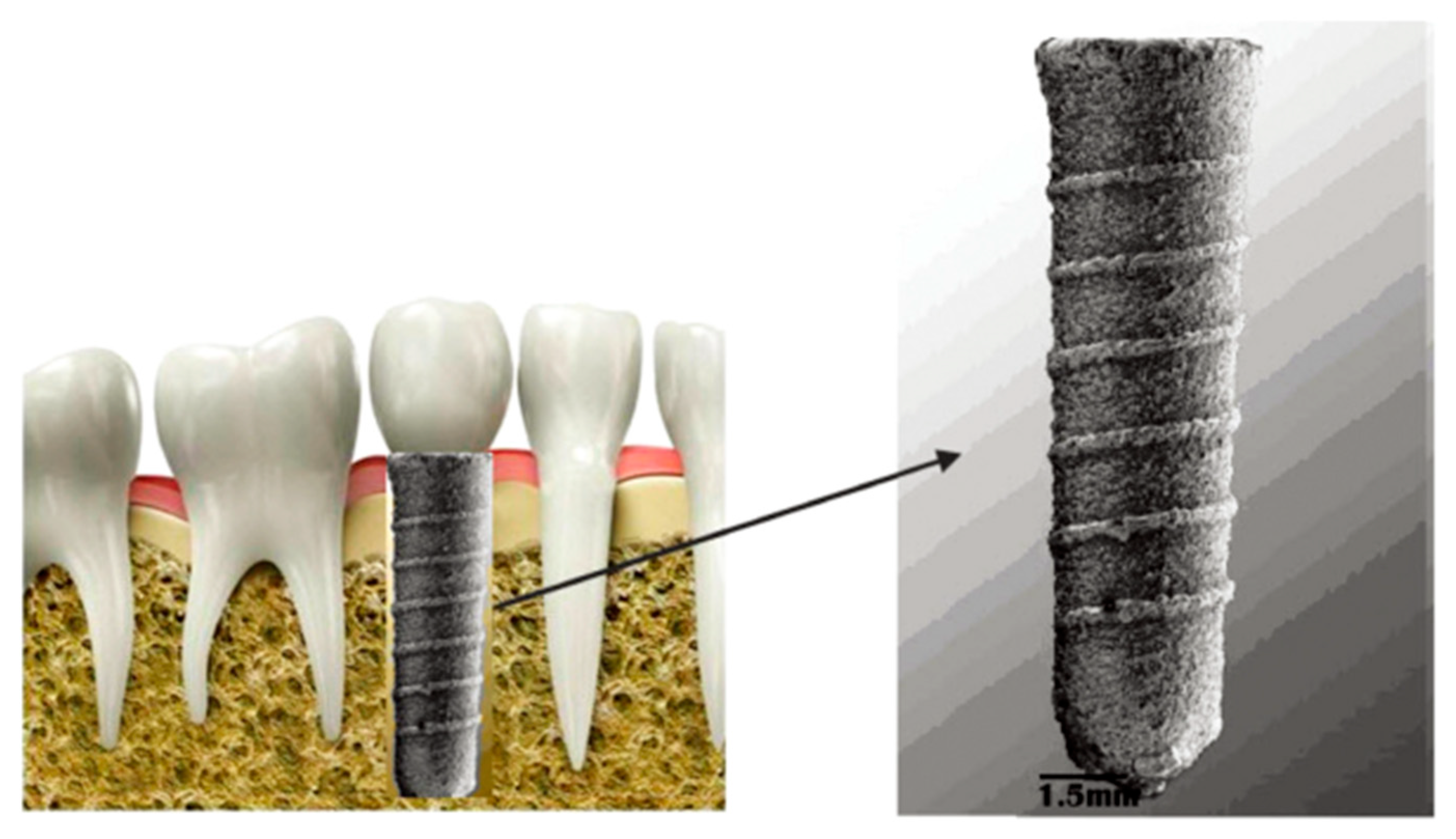
3.1. Porous Dental Implants
3.2. Trabecular Metal Implants
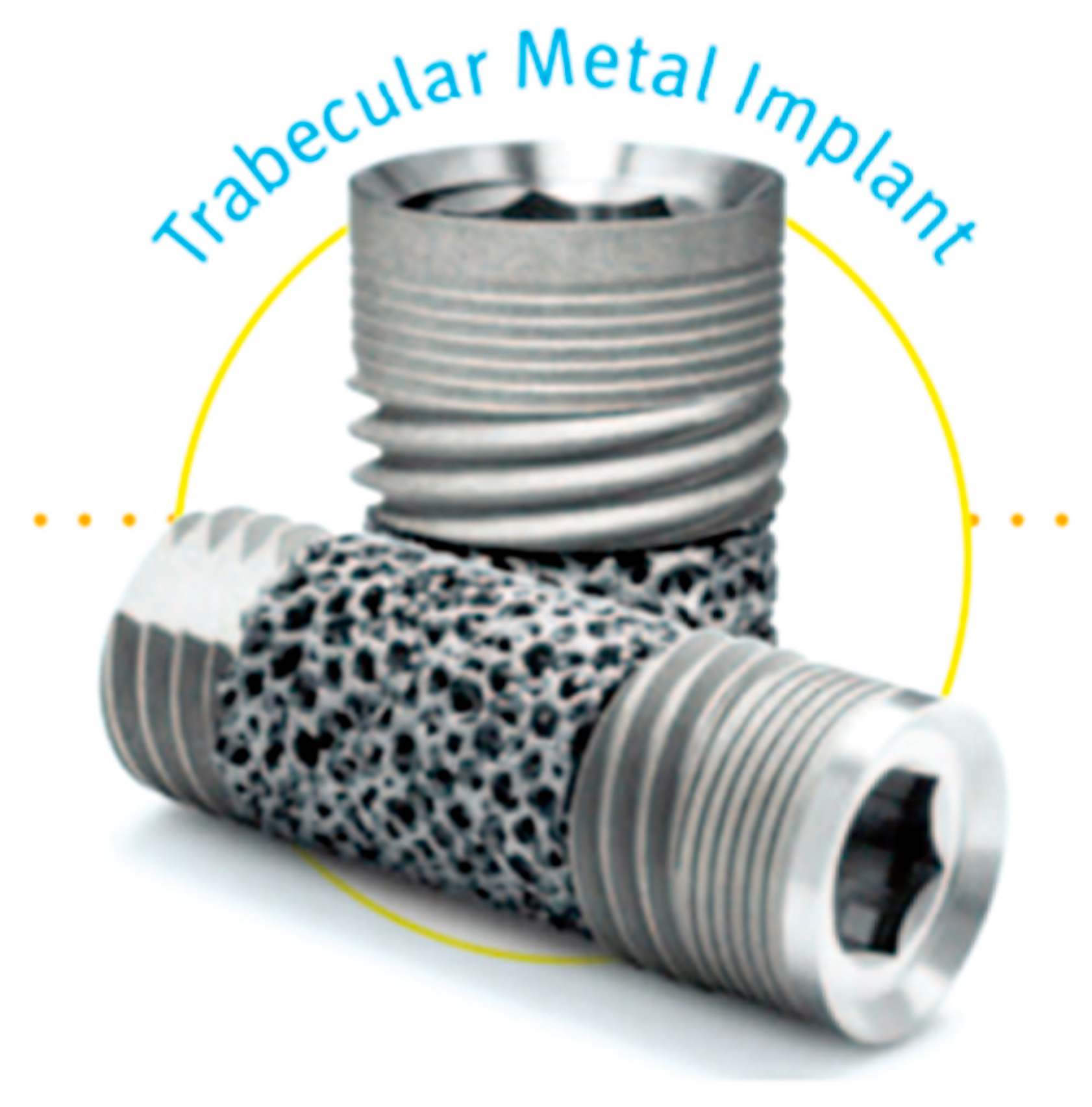
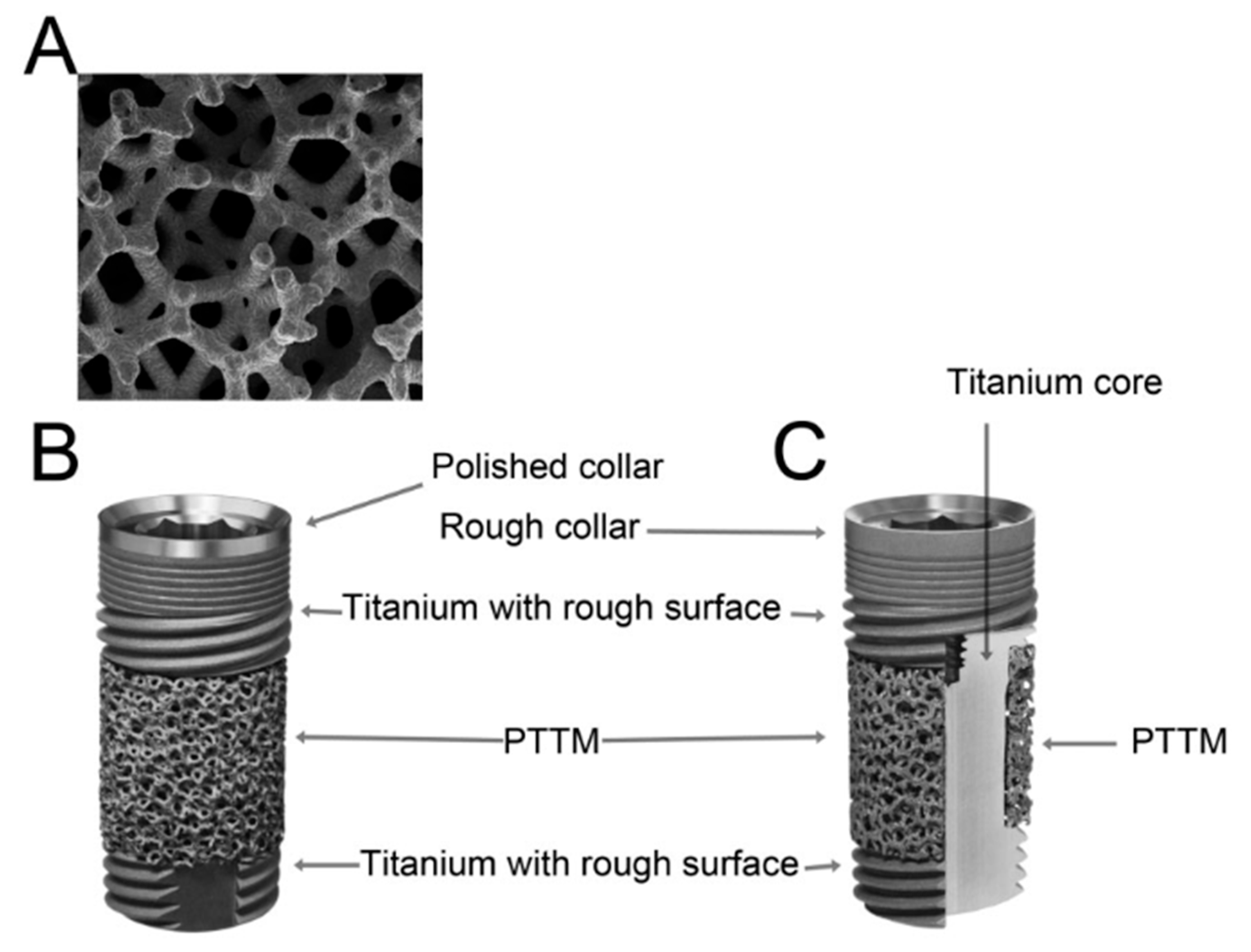
3.3. Zirconia Implants
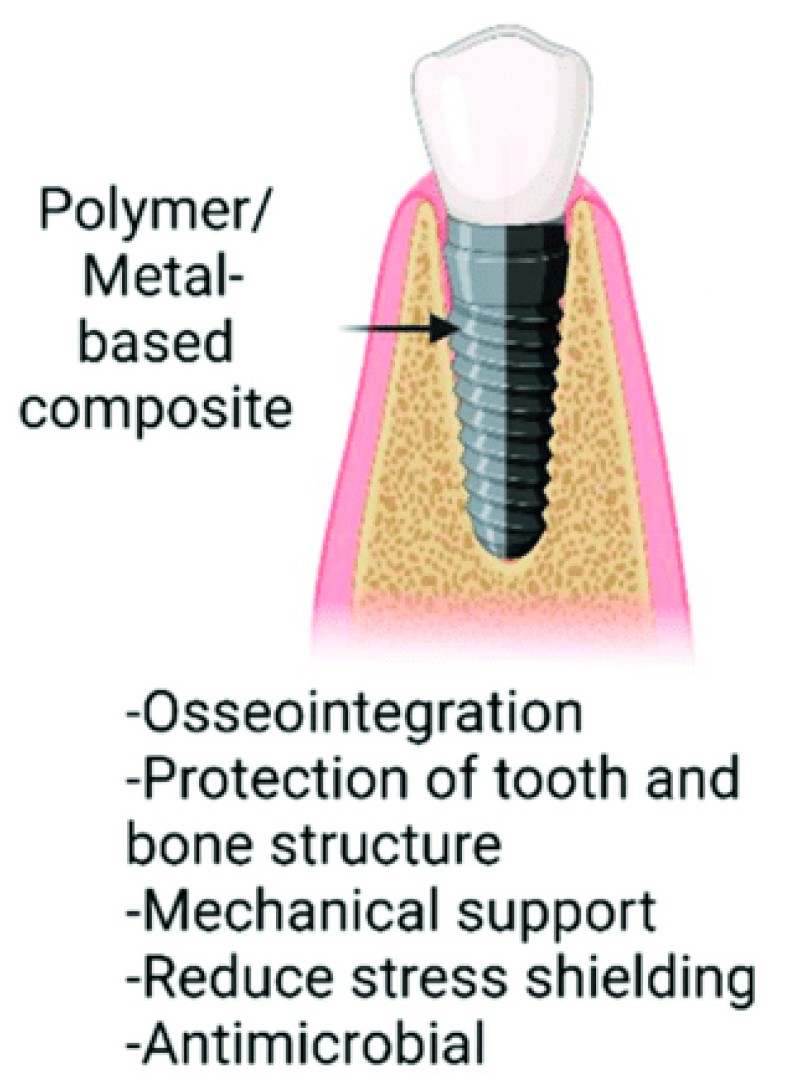
3.4. Polymers and Composite Materials
3.5. Evaluation of the Properties of Selected Materials
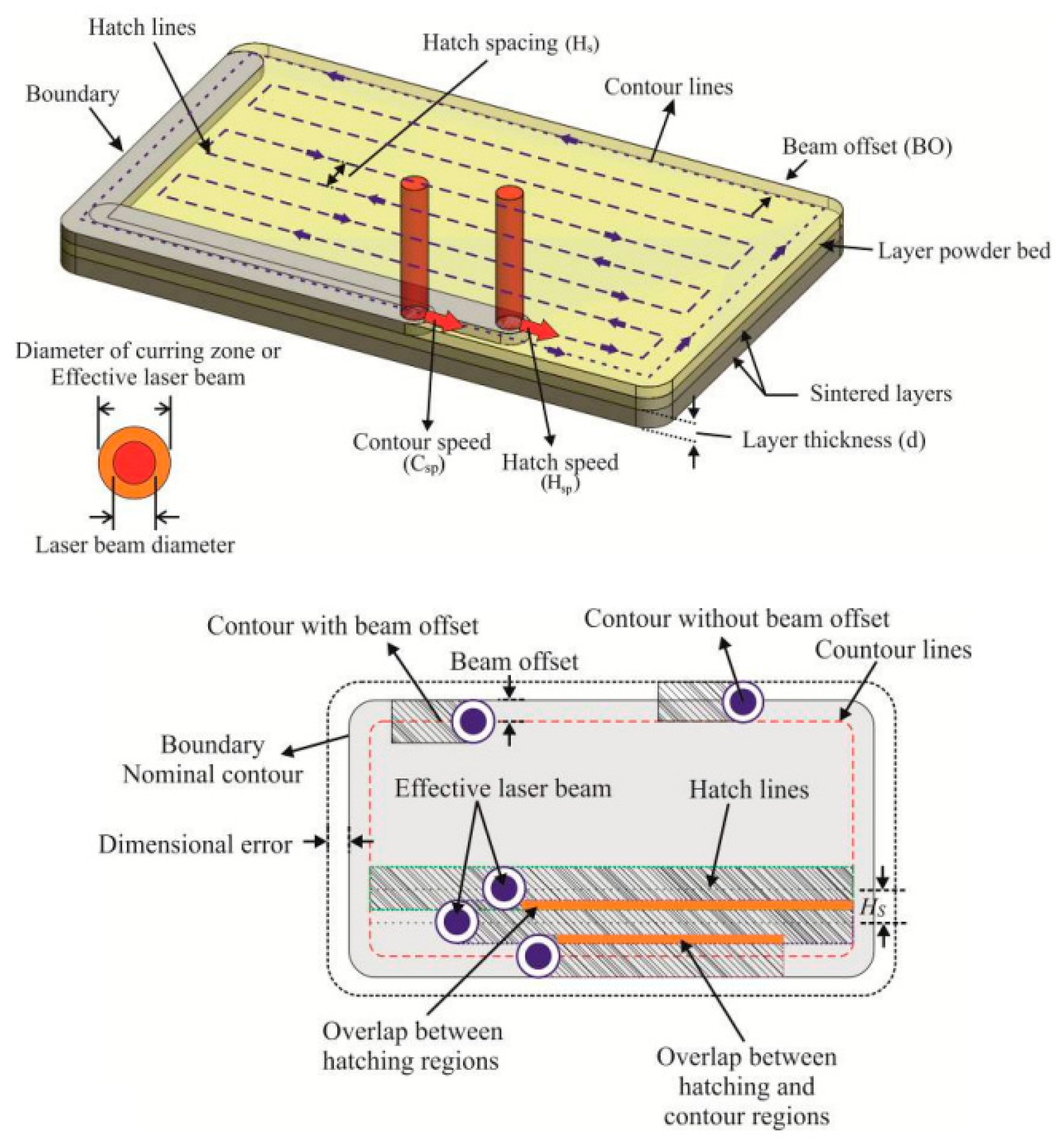
4. Additive Technologies Utilized in the Manufacture of Dental Implants
- Stereolithography (SL);
- Laminated object manufacturing (LOM);
- Laser cladding (LCF);
- Selective laser sintering (SLS);
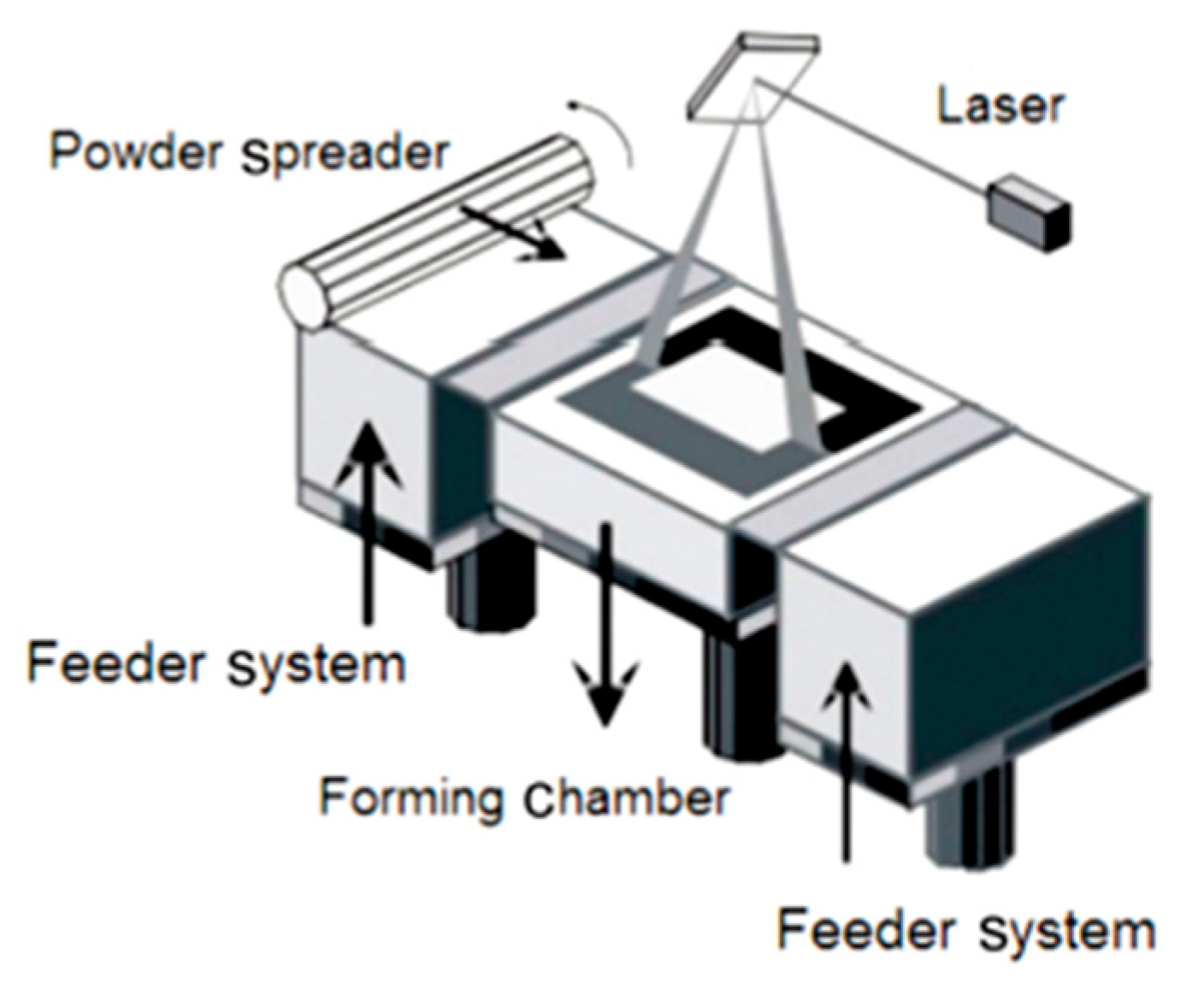
- Selective laser melting (SLM);
- Electron beam melting (EBM).
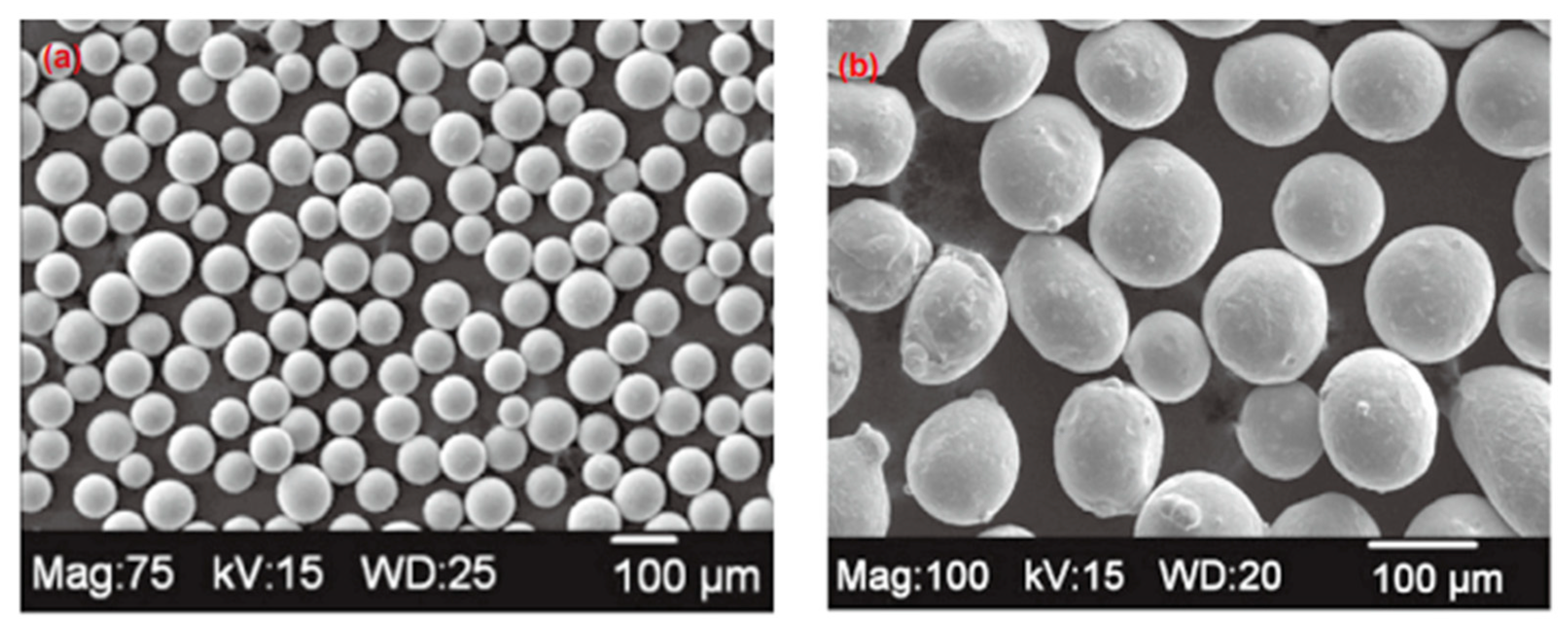
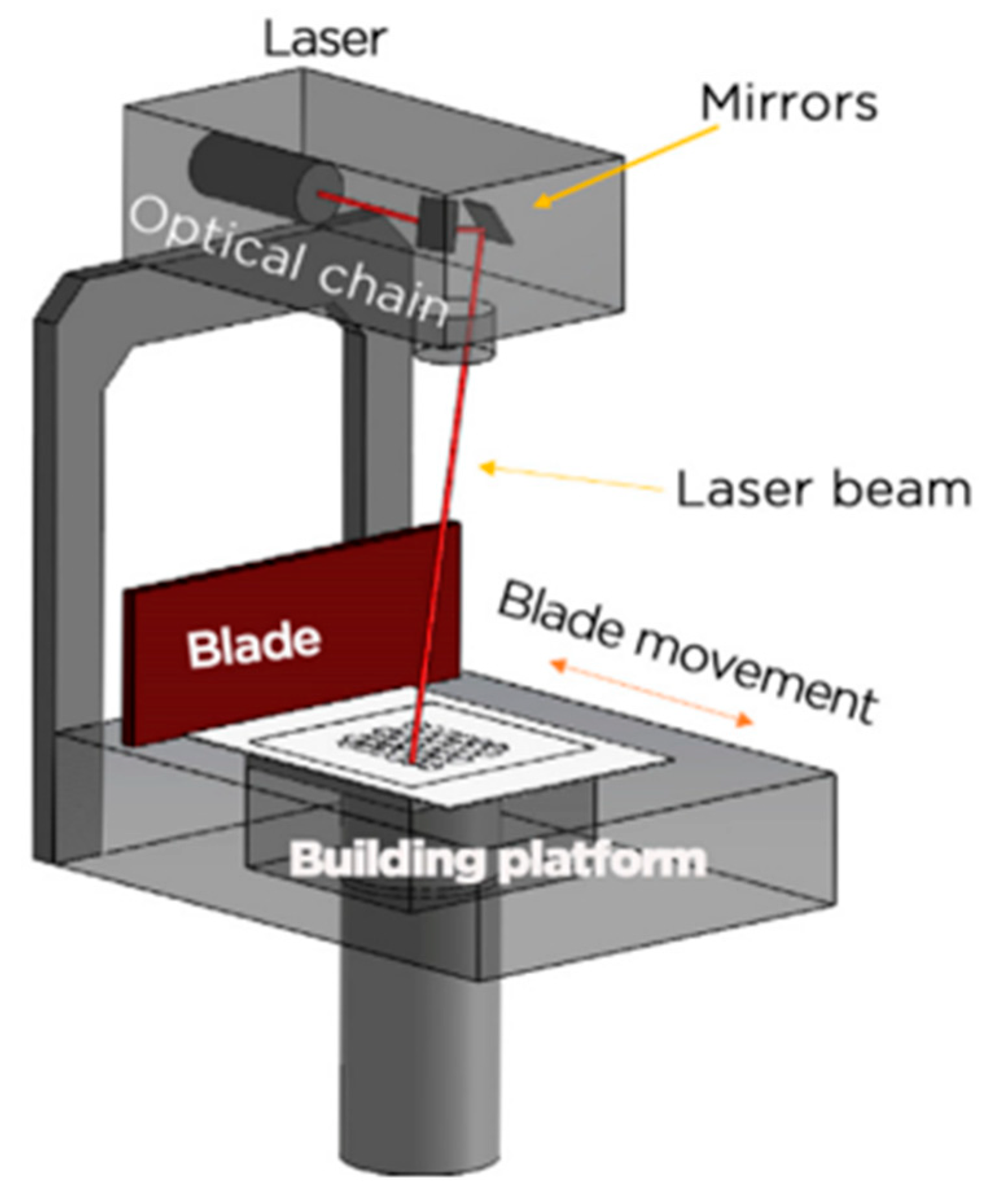
4.1. SLM and EBM
- Higher forming accuracy due to smaller laser beam diameter and finer powder particle size.
- Cheaper equipment and more accessible technology.
- Disadvantage: lower production speed.
- Significantly higher production speed of up to 80 cm3/h, which is four to five times faster than SLM, because of the high energy output (up to 3000 W—ten times the power of SLM).
- Using larger powder particles in this technology results in lower molding precision compared to SLM.
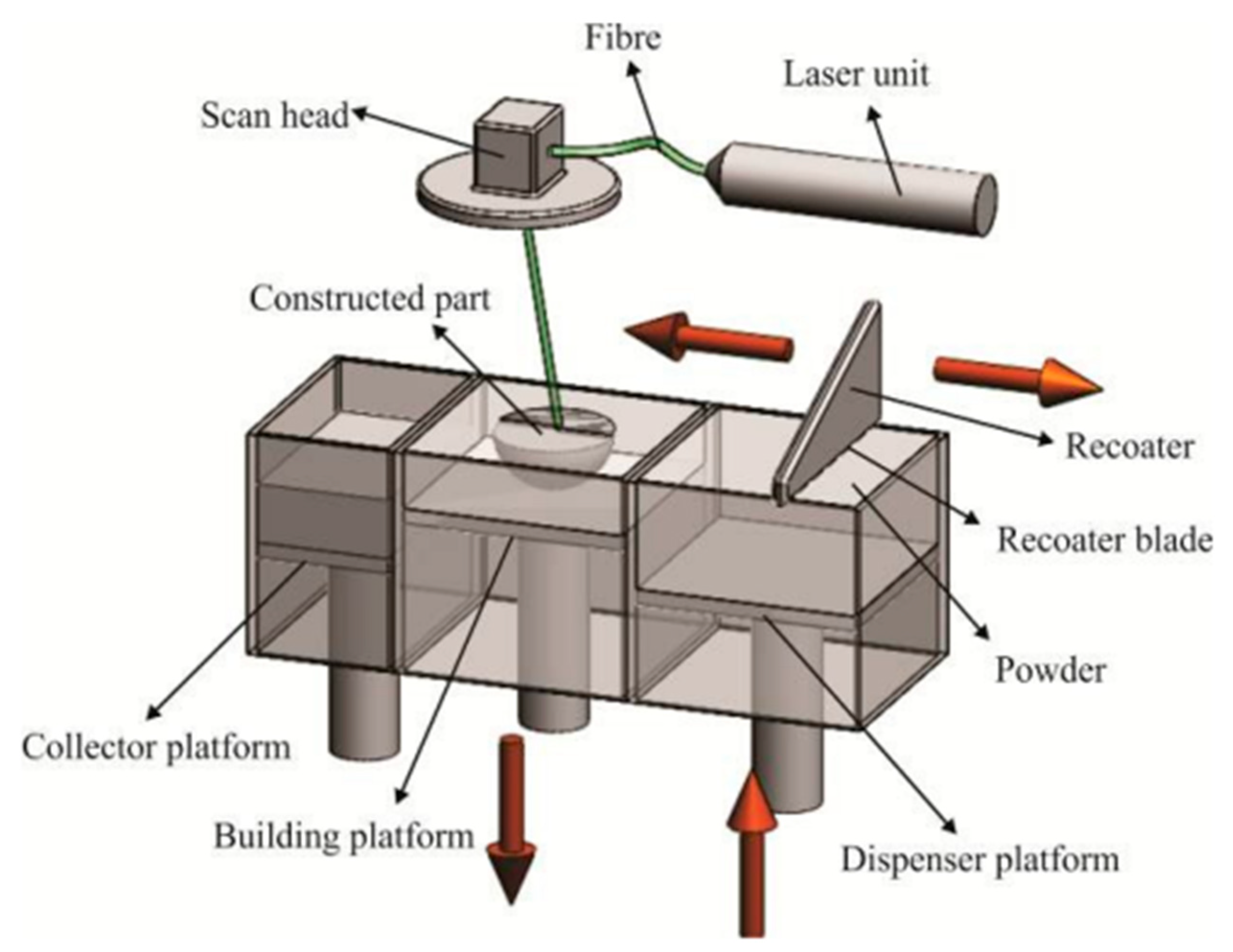
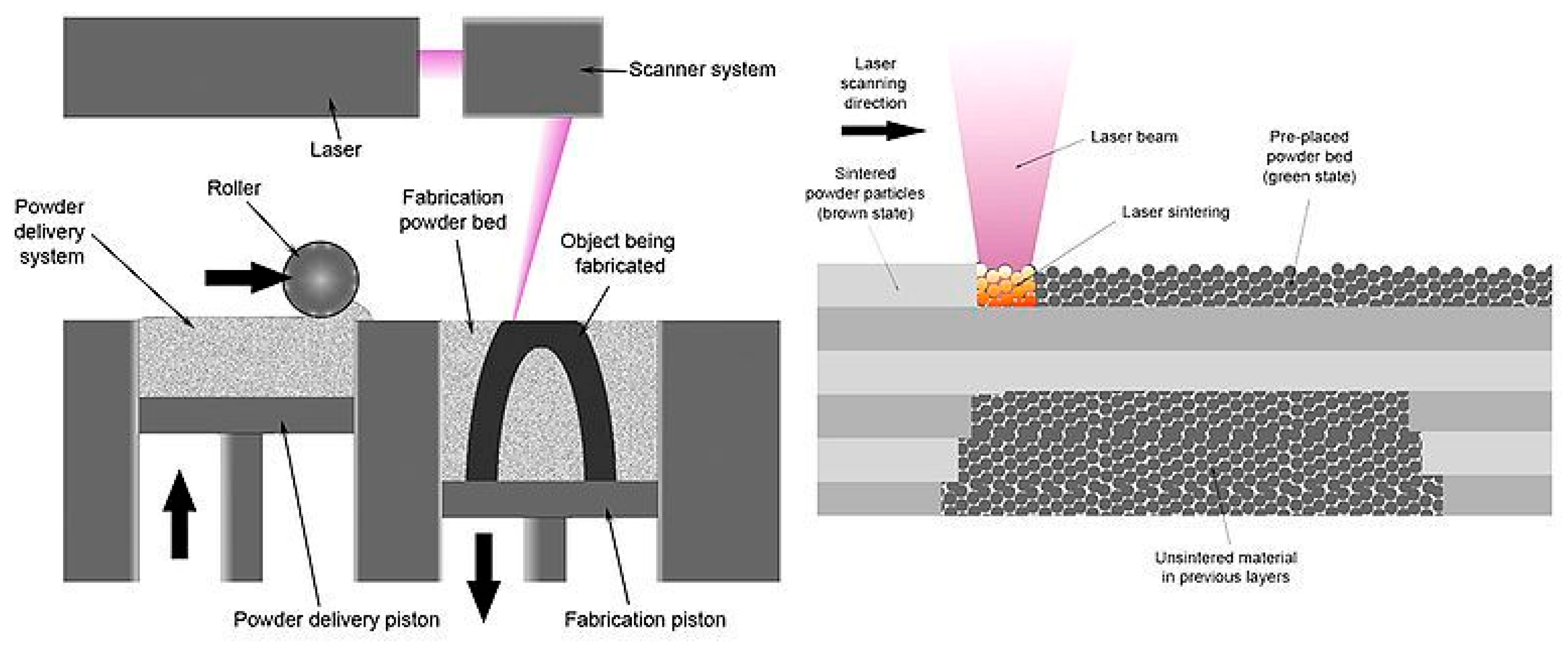
4.2. DMLS
4.3. SLA
4.4. SLS
4.5. Comparison of Selected Technologies
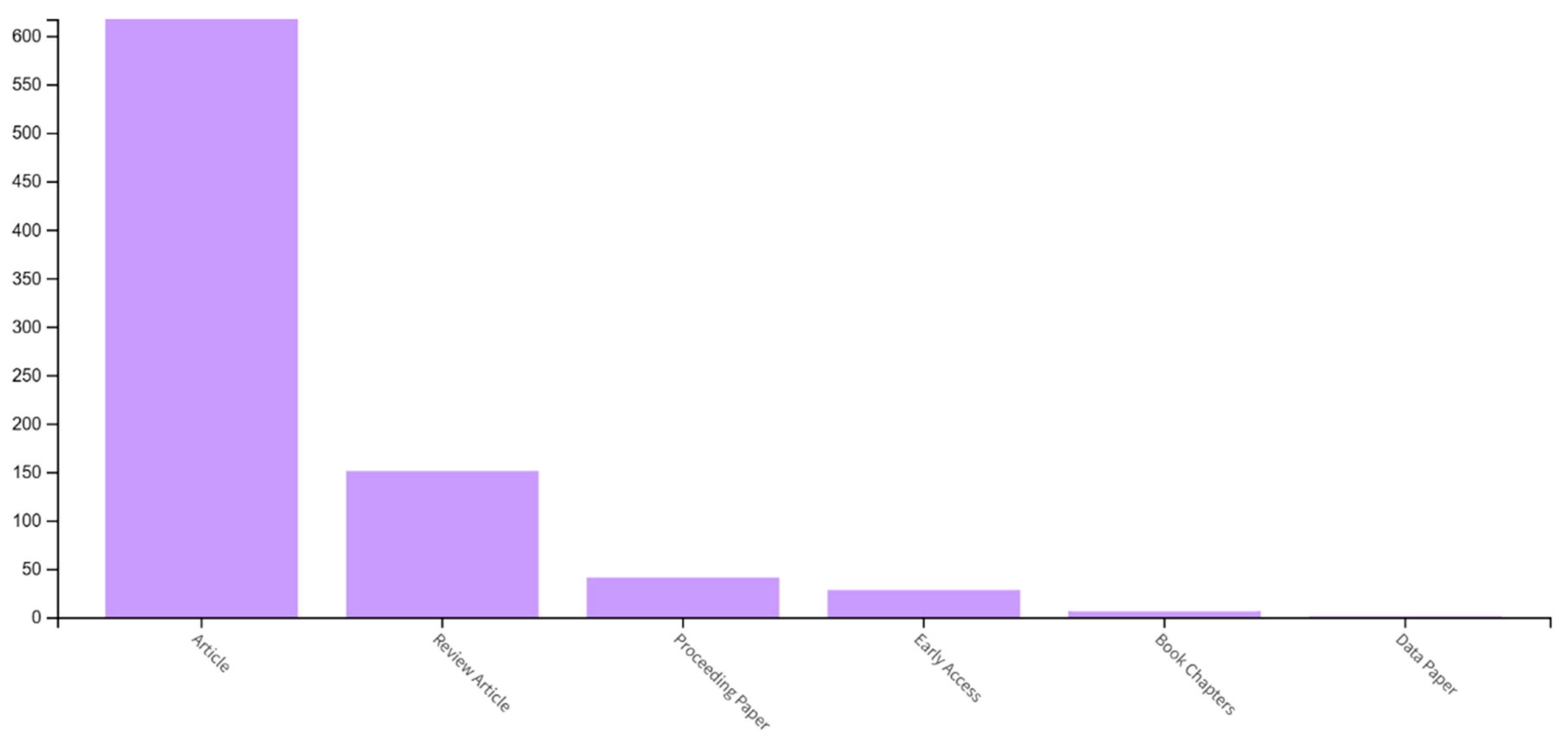
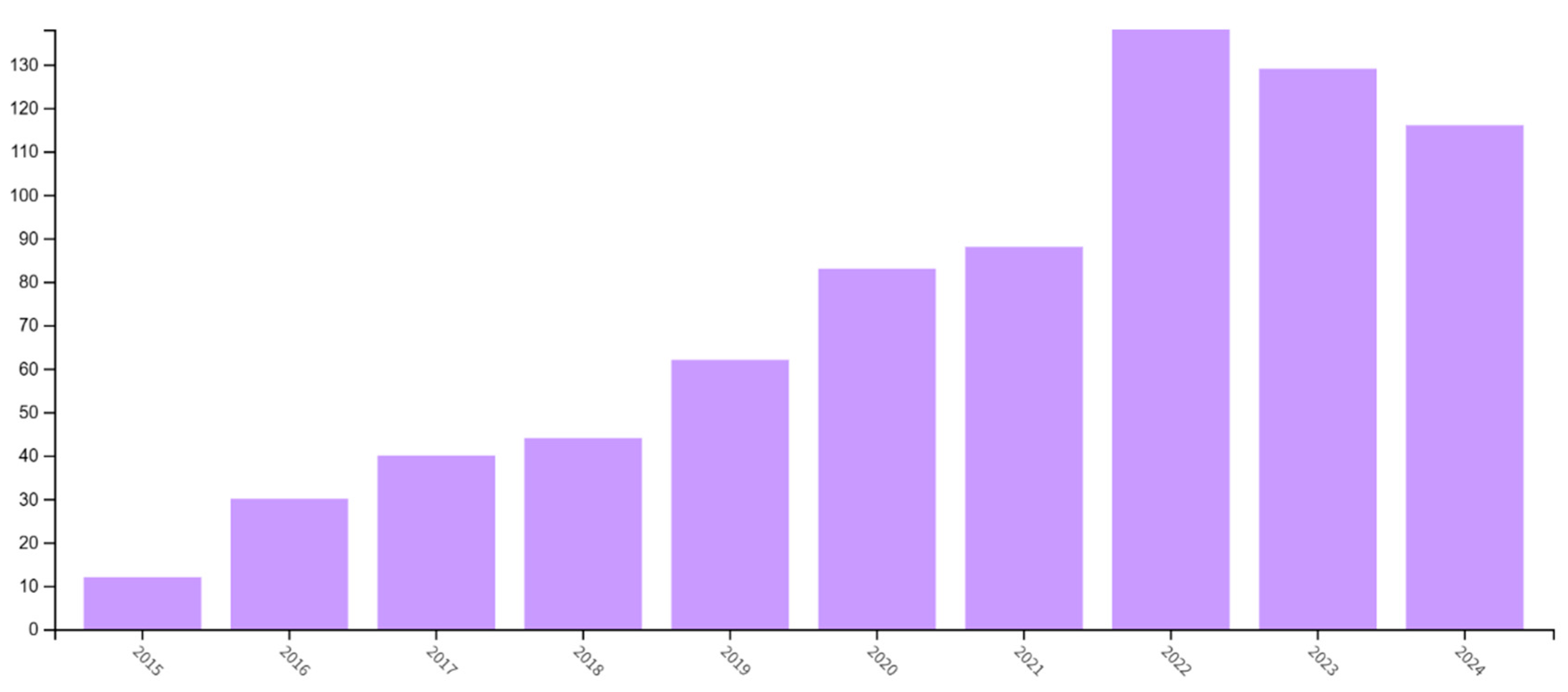
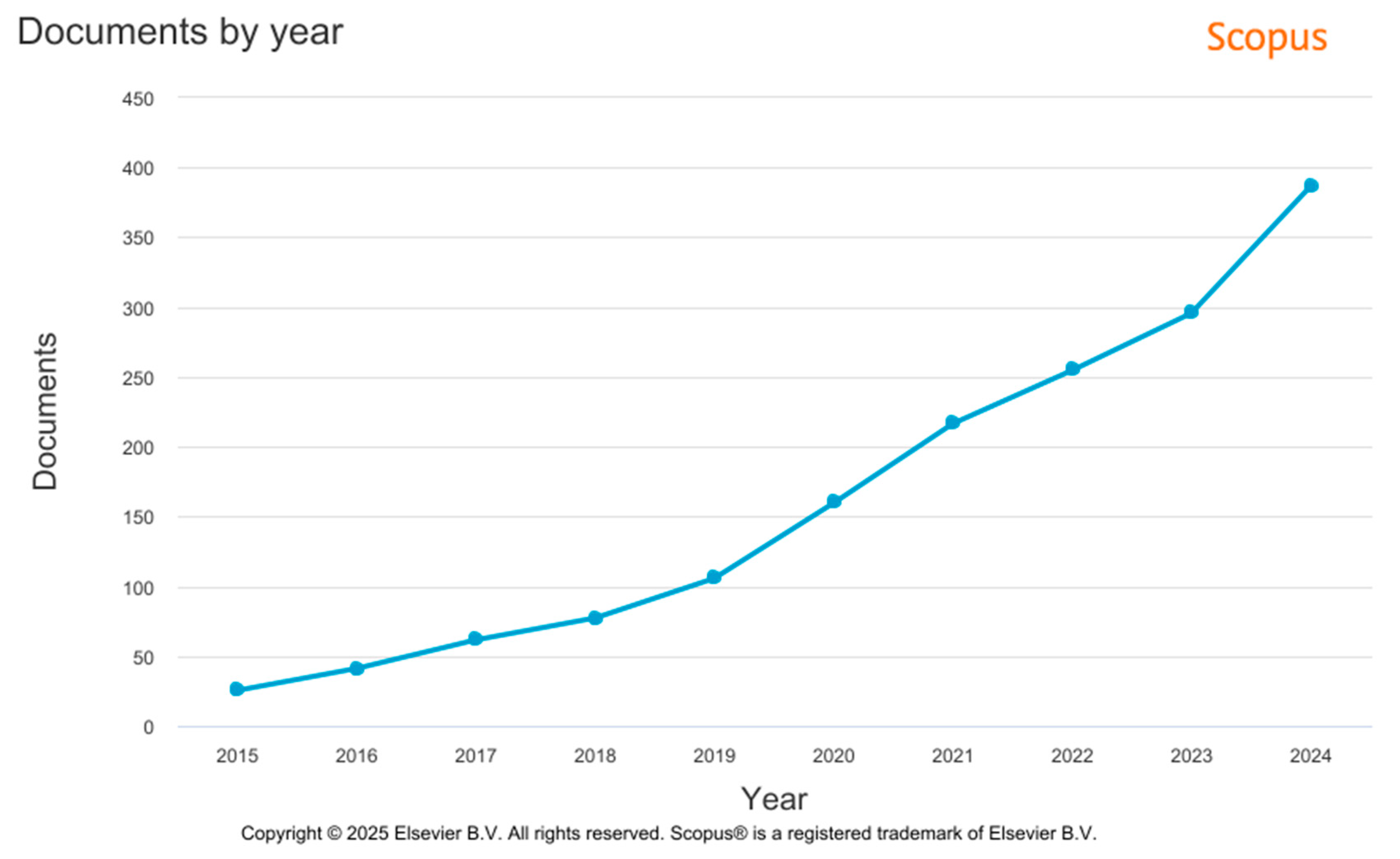
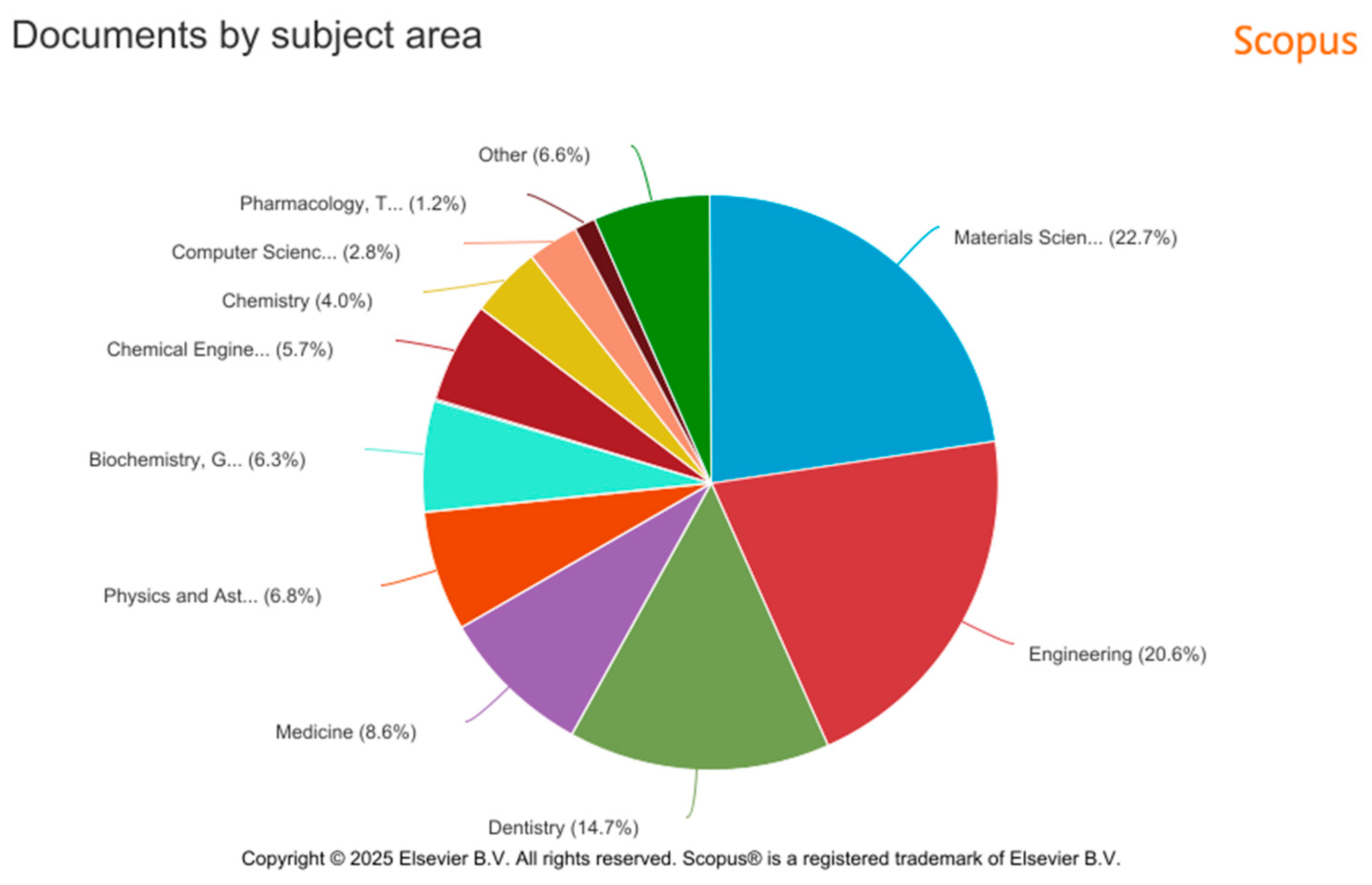
5. Additive Manufacturing in Dental Implant Production—Data Source Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Pan, Y.; Gao, Y.; Pang, H.R.; Sun, H.; Cheng, L.; Liu, J. Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings 2024, 14, 781. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Ahmed, M.M.; Chowdhury, M.A.; Mobarak, M.H.; Rahman, M.M.; Hossain, M.H. Advances and signifcances of titaniumin dental implant applications. Results Chem. 2024, 7, 101394. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Celik, H.K.; Koc, S.; Kustarci, A.; Caglayan, N.; Rennie, A.E. The state of additive manufacturing in dental research–A systematic scoping review of 2012–2022. Heliyon 2023, 9, e17462. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthcare Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Rezaie, F.; Farshbaf, M.; Dahri, M.; Masjedi, M.; Maleki, R.; Amini, F.; Wirth, J.; Moharamzadeh, K.; Weber, F.E.; Tayebi, L. 3D Printing of Dental Prostheses: Current and Emerging Applications. J. Compos. Sci. 2023, 7, 80. [Google Scholar] [CrossRef]
- Andreucci, C.A.; Fonseca, E.M.; Jorge, R.N. 3D Printing as an Effcient Way to Prototype and Develop Dental Implants. BioMedInformatics 2022, 2, 671–679. [Google Scholar] [CrossRef]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive manufacturing by digital light processing: A review. Prog. Addit. Manuf. 2022, 8, 331–351. [Google Scholar] [CrossRef]
- Kennedy, S.M.; Raghav, G.R.; Jeen Robert, R.B.; Manikandaraja, g.; Selvakumar, M. PEEK-based 3D printing: A paradigm shift in implant revolution for healthcare. Polym.-Plast. Technol. Mater. 2024, 63, 680–702. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Zhang, H.; Bai, J.; Jiang, B.; Jiang, C.; Ming, W.; Zhang, H.; Long, H.; Huang, X.; et al. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023, 17, 56. [Google Scholar] [CrossRef]
- Kechagias, J.D.; Zaoutsos, S.P. Optimising fused flament fabrication surface roughness for a dental implant. Mater. Manuf. Process. 2023, 38, 954–959. [Google Scholar] [CrossRef]
- Bakkal, B.; Topsakal, K.G.; Duran, G.S. New horizons in dentistry and orthodontics with 4D printing technologies 4D printing and orthodontics. Australas. Orthod. J. 2025. Available online: https://www.semanticscholar.org/paper/9135bb33b12d2d7d8a11d927835302be1783a7e3 (accessed on 14 February 2025).
- Aura Sium-Abel, L.; Keilig, I.; Dörsam, C. Bourauel Accuracy of template-based guided dental implant placement-An in vitro comparison of different manufacturing methods. Clin. Oral Implant. Res. 2024. Available online: https://www.semanticscholar.org/paper/204ec1b965411b7e494c57989320909d29efa67e (accessed on 23 March 2025).
- Li, B. The Investigation of Pore Characteristic and Mechanical Property of Porous Ti. Master’s Dissertation, Dalian Jiao Tong University, Dalian, China, 2005. [Google Scholar]
- Hu, H.B.; Liu, H.Q.; Wang, J.E.; Yi, D.Q.; Fu, S.; Sun, W.L. Research Progress of Biomedical Porous Titanium and Its Alloys. Mater. Rev. 2012, 26, 262–270. [Google Scholar]
- Li, Q.; Wang, H.; Zhang, L.X. The Surface Treatment of Artificial Joint Metal Materials. Chin. J. Med. Instrum. 2002, 26, 44–47. [Google Scholar]
- Agrawal, C.M. Reconstructing the Human-Body Using Biomaterials. JOM 1998, 50, 31–35. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, S.J.; Jia, M.T.; Hao, Y.L.; Yang, R.; Guo, Z.X. Porous Ti-24Nb-4Zr8Sn alloy for biomedical applications fabricated by space-holder method. Chin. J. Nonferrous Met. 2010, 20, 967–971. [Google Scholar]
- Zhao, J.; Lu, X.; Weng, J. Macro-porous Ti-Based Composite Scaffold Prepared by Polymer Impregnating Method with Calcium Phosphate Coatings. Mater. Lett. 2008, 62, 2921–2924. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Gu, D.; Yu, X. Preparation of Honeycomb-like Porous 316L Stainless Steel by Selective Laser Melting. Rare Met. Mater. Eng. 2011, 40, 161–164. [Google Scholar] [CrossRef]
- He, W.; Jia, W.; Liu, H. The Application of Rapid Prototyping Technology in Porous Metals Materials. Rare Met. Lett. 2008, 27, 1–6. [Google Scholar]
- Traini, T.; Mangano, C.; Sammons, R.I. Direct Laser metal sintering as a new approach to fabrication of isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef]
- Stamp, R.; Fox, P.; O’Neill, W. The development of a scanning strategy for the manufacture of porous biomaterials by selective laser melting. J. Mater. Sci. Mater. Med. 2009, 20, 1839–1848. [Google Scholar] [CrossRef]
- Heinl, P.; Muller, L.; Korner, C. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.M. Modeling of Porous Structure of Implants and Direct Manufacturing by Selective Laser Melting. Ph.D. Dissertation, South China University of Technology, Guangzhou, China, 2013. [Google Scholar]
- Li, X.; Wang, C.; Zhang, W. Fabrication of Porous Ti6Al4V Implant Using Electron Beam Melting and Mechanical Properties. J. Shanghai Jiao Tong Univ. 2009, 43, 1946–1949. [Google Scholar]
- Parthasarathy, J.; Starly, B. Mechanical Evaluation of Porous Titanium (Ti-6Al-4V) Structures with Electron Beam Melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tan-talum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Kaplan, R.B. Open Cell Tantalum Structures for Cancellousbone Implants and Cell and Tissue Structures. U.S. Patent No. 5282861, 1 February 1994. [Google Scholar]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Porous Tantalum Trabecular Metal Dental Implants. Clin. Implant Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef]
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechani-cal properties of a porous tantalum foam. J. Biomed. Mater. Res. 2001, 58, 180–187. [Google Scholar] [CrossRef]
- Cohen, R. A porous tantalum trabecular metal: Basic science. Am. J. Orthop. 2002, 31, 216–217. [Google Scholar]
- Heiner, A.D.; Brown, T.D.; Poggie, R.A. Structural efficacy of anovel porous tantalum implant for osteonecrosis grafting. Trans. Orthop. Res. Soc. 2001, 26, 480. [Google Scholar]
- Christie, M.J. Clinical applications of Trabecular Metal. Am. J. Orthop. 2002, 31, 219–220. [Google Scholar] [PubMed]
- Henry, P.J.; Laney, W.R.; Jemt, T.; Harris, D.; Krogh, P.H.; Polizzi, G.; Zarb, G.A.; Herrmann, I. Osseointegrated implantsfor single-tooth replacement: A prospective 5-year multi-center study. Int. J. Oral Maxillofac. Implant. 1996, 11, 450–455. [Google Scholar]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.; Lindhe, J.; Eriksson, B.; Sbordone, L. Marginal tissuereactions at osseointegrated titanium fixtures (I). A 3-yearlongitudinal prospective study. Int. J. Oral Maxillofac. Surg. 1986, 15, 39–52. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surfacetreatments of titanium dental implants for rapid osseointe-gration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydro-philicity through chemical modification of microstructuredtitanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Wilkes, J.; Hagedorn, Y.C.; Meiners, W.; Wissenbach, K. Additive manufacturing of ZrO2-Al2O3 ceramic components by selective laser melting. Papid Prototyp. J. 2013, 19, 51–57. [Google Scholar] [CrossRef]
- Khanlar, L.N.; Salazar Rios, A.; Tahmaseb, A.; Zandinejad, A. Additive manufacturing of zirconia ceramic and its application in clinical dentistry: A review. Dent. J. 2021, 9, 104. [Google Scholar] [CrossRef]
- Sandhaus, S. Technic and instrumentation of the implantCBS (Cristalline Bone Screw). Inf. Odontostomatol. 1967, 4, 19–24. [Google Scholar]
- Steflik, D.; Koth, D.; Robinson, F.; McKinney, R.; Davis, B.; Mor-ris, C.; Davis, Q. Prospective investigation of the single-crystal sapphire endosteal dental implant in humans: Ten-yearresults. J. Oral Implant. 1994, 21, 8–18. [Google Scholar]
- Nakamura, K.; Kanno, T.; Milleding, P.; Örtengren, U. Zirconiaas a dental implant abutment material: A systematicreview. Int. J. Prosthodont. 2010, 23, 299–309. [Google Scholar] [PubMed]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural cera-mic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Covacci, V.; Bruzzese, N.; Maccauro, G.; Andreassi, C.; Ricci, G.; Piconi, C.; Marmo, E.; Burger, W.; Cittadini, A. In vitroevaluation of the mutagenic and carcinogenic power of highpurity zirconia ceramic. Biomaterials 1999, 20, 371–376. [Google Scholar] [CrossRef]
- Sanon, C.; Chevalier, J.; Douillard, T.; Cattani-Lorente, M.; Scherrer, S.S.; Gremillard, L. A new testing protocol for zirconia dental implants. Dent. Mater. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Lughi, V.; Sergo, V. Low temperature degradation-aging-ofzirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Oliva, J.; Oliva, X.; Oliva, J.D. Five-year success rate of 831 con-secutively placed Zirconia dental implants in humans: Acomparison of three different rough surfaces. Int. J. OralMaxillofac. Implant. 2010, 25, 336–344. [Google Scholar]
- Payer, M.; Arnetzl, V.; Kirmeier, R.; Koller, M.; Arnetzl, G.; Jakse, N. Immediate provisional restoration of single-piece zirco-nia implants: A prospective case series–results after24 months of clinical function. Clin. Oral Implant. Res. 2013, 24, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zembić, A.; Johannesen, L.; Schou, S.; Malo, P.; Reichert, T.; Farella, M.; Hämmerle, C. Immediately restored one-piecesingle-tooth implants with reduced diameter: One-yearresults of a multi-center study. Clin. Oral Implant. Res. 2012, 23, 49–54. [Google Scholar] [CrossRef]
- Hermann, J.S.; Cochran, D.L.; Buser, D.; Schenk, R.K.; Schoolfield, J.D. Biologic Width around one-and two-piece titaniumimplants. Clin. Oral Implant. Res. 2001, 12, 559–571. [Google Scholar] [CrossRef]
- Hartman, G.A.; Cochran, D.L. Initial implant position deter-mines the magnitude of crestal bone remodeling. J. Peri-Odontol. 2004, 75, 572–577. [Google Scholar]
- Wilson, T.G., Jr. The positive relationship between excesscement and peri-implant disease: A prospective clinicalendoscopic study. J. Periodontol. 2009, 80, 1388–1392. [Google Scholar] [CrossRef]
- Linkevicius, T.; Vindasiute, E.; Puisys, A.; Linkeviciene, L.; Maslova, N.; Puriene, A. The influence of the cementationmargin position on the amount of undetected cement. Aprospective clinical study. Clin. Oral Implant. Res. 2013, 24, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Robra, B.-P.; Walther, W. Cement-associated signsof inflammation: Retrospective analysis of the effect ofexcess cement on peri-implant tissue. Int. J. Prosthodont. 2015, 28, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.-G.; Millen, C.; Brägger, U. Clinical Performanceof Screw-Versus Cement-Retained Fixed Implant-Sup-ported Reconstructions-A Systematic Review. Int. J. OralMaxillofac Implant. 2014, 29, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Payer, M.; Heschl, A.; Koller, M.; Arnetzl, G.; Lorenzoni, M.; Jakse, N. All-ceramic restoration of zirconia two-piece implants –a randomized controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 371–376. [Google Scholar] [CrossRef]
- Brüll, F.; van Winkelhoff, A.J.; Cune, M.S. Zirconia dentalimplants: A clinical, radiographic, and microbiologic evalu-ation up to 3 years. Int. J. Oral Maxillofac Implant. 2014, 29, 914–920. [Google Scholar]
- Becker, J.; John, G.; Becker, K.; Mainusch, S.; Diedrichs, G.; Schwarz, F. Clinical performance of two-piece zirconiaimplants in the posterior mandible and maxilla: A prospec-tive cohort study over 2 years. Clin. Oral Implants Res. 2015, 28, 29–35. [Google Scholar] [CrossRef]
- Cionca, N.; Müller, N.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: A prospectiveclinical study. Clin. Oral Implants Res. 2015, 26, 413–418. [Google Scholar] [CrossRef]
- Hodosh, M.; Povar, M.; Shklar, G. The dental polymer implant concept. J. Prosthet. Dent. 1969, 22, 371–380. [Google Scholar] [CrossRef]
- Hodosh, M.; Povar, M.; Shklar, G. Development of the self-supporting polymer tooth implant. J. Biomed. Mater. Res. 1973, 7, 205–216. [Google Scholar] [CrossRef]
- Jockusch, J.; Özcan, M. Additive manufacturing of dental polymers: An overview on processes. Mater. Appl. 2020. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Chang, P.C.; Liu, B.Y.; Liu, C.M.; Chou, H.H.; Ho, M.H.; Liu, H.C.; Wang, D.M.; Hou, L.T. Bone tissue engineering with novel rhBMP2-PLLA composite scaffolds. J. Biomed. Mater. Res. Part A 2007, 81, 771–780. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-based coatings: Promising strategies to improve the biocompatibility and functionality of materials used in biomedical engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Ivanov, D. Natural and Synthetic Polymers for Designing Composite Materials. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Lan, S.-F.; Kehinde, T.; Zhang, X.; Khajotia, S.; Schmidtke, D.W.; Starly, B. Controlled release of metronidazole from composite poly-e-caprolactone/alginate (PCL/alginate) rings for dental implants. Dent. Mater. 2013, 29, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Di Salle, A.; Riccitiello, F.; Petillo, O.; Peluso, G.; Calarco, A. Biodegradable polymers in dental tissue engineering and regeneration. AIMS Mater. Sci. 2018, 5, 1073–1101. [Google Scholar] [CrossRef]
- Hosseinalipour, M.; Javadpour, J.; Rezaie, H. Investigation of Mechanical Properties of Experimental Bis-GMA/TEGDMA Dental Composite Resins Containing Various Mass Fractions of Silica Nanoparticles. J. Prosthodont. 2010, 19, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Suwanprateeb, J.; Sanngam, R.; Suvannapruk, W.; Panyathanmaporn, T. Mechanical and in vitro performance of apatite–wollastonite glass ceramic reinforced hydroxyapatite composite fabricated by 3D-printing. J. Mater. Sci. Mater. Med. 2009, 20, 1281–1289. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Wang, Y.; Li, H. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dent. Mater. 2011, 27, 1187–1195. [Google Scholar] [CrossRef]
- Filipecki, J.; Chamerski, K.; Boyko, O.; Kotynia, K. Ageing phenomenon in acrylic polymer dental materials detected by means of positron annihilation lifetime spectroscopy. Polim. W Med. 2014, 44, 21–28. [Google Scholar] [CrossRef]
- Ganguly, S.; Tang, X.S. 3D Printing of High Strength Thermally Stable Sustainable Lightweight Corrosion-Resistant Nanocomposite by Solvent Exchange Postprocessing. ACS Sustain. Chem. Eng. 2024, 13, 423–435. [Google Scholar] [CrossRef]
- Huang, B.; Qian, J.; Wang, G.; Cai, M. Synthesis and Properties of Novel Copolymers of Poly (ether ketone diphenyl ketone ether ketone ketone) and Poly (ether amide ether amide ether ketone ketone). Polym. Eng. Sci. 2014, 54, 1757–1764. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; Klämpfl, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: Fabrication, biocompatibility analysis and photoelastic study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef] [PubMed]
- Carraro, F.; Bagno, A. Tantalum as trabecular metal for endosseous implantable applications. Biomimetics 2023, 8, 49. [Google Scholar] [CrossRef]
- Aldhuwayhi, S. Zirconia in Dental Implantology: A Review of the Literature with Recent Updates. Bioengineering 2025, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.J.; Van Overschelde, P.A.; Keulemans, F.; Kleverlaan, C.J.; Tribst, J.P.M. Mechanical properties of additive-manufactured composite-based resins for permanent indirect restorations: A scoping review. Materials 2024, 17, 3951. [Google Scholar] [CrossRef]
- Lu, B.; Li, D. Development of the Additive Manufacturing (3D printing) Technology. Mach. Build. Autom. 2013, 42, 1–4. [Google Scholar]
- Gong, S.; Suo, J.; Li, H. Development and Application of Metal Additive Manufacturing Technology. Aeronaut. Manuf. Technol. 2013, 13, 66–71. [Google Scholar]
- Zhou, L.; Yang, Q.; Zhang, G.; Zhao, F.; Shen, G.; Yu, B. Additive manufacturing technologies of porous metal implants. China Foundry 2014, 11, 322–331. [Google Scholar]
- Shi, Y.; Lu, Z.; Zhang, W. The Technology and Equipment of Selective Laser Melting. China Surf. Eng. 2006, 19, 150–153. [Google Scholar]
- Han, J.; Lin, F.; Qi, H. Effects of powder preheating in electron beam selective melting process. Trans. China Weld. Inst. 2008, 29, 77–81. [Google Scholar]
- Qi, H.; Yang, M.; Qi, F. Numerical Simulation of Effects of Scanning Path on Electron Beam Selective Melting Process of TC4. Trans. China Weld. Inst. 2009, 30, 5–8. [Google Scholar]
- Jia, W.; Tang, H.; He, W. The Development and Key Problems of Electric Beam Rapid Forming. Electromachining Mould. 2010, 2, 41–44. [Google Scholar]
- Yao, N.; Peng, X. The Preparation Method of Metal Powder for 3D Printing. Sichuan Nonferrous 2013, 12, 48–51. [Google Scholar]
- Bineli, A.R.R.; Peres, A.P.G.; Bernardes, L.F.; Jardini, A.L.; Maciel Filho, R. Design of microreactor by integration of reverse engineering and direct metal laser sintering process. In Proceedings of the 5th International Workshop on Hydrogen and Fuel Cells, Campinas, Brasil, 26–29 October 2010; EOS: New York City, NY, USA, 2009. [Google Scholar]
- Senthilkumaran, K.; Pandey, P.M.; Rao, P.V.M. Influence of building strategies on the accuracy of parts in selective laser sintering. Mater. Des. 2009, 30, 2946–2954. [Google Scholar] [CrossRef]
- Yu, N. Process Parameter Optimization for Direct Metal Laser Sintering (DMLS). Ph.D. Thesis, National University of Singapore, Singapore, 2005. [Google Scholar]
- Bineli, A.R.R.; Peres, A.P.G.; Jardini, A.L.; Maciel Filho, R. Direct metal laser sintering (DMLS): Technology for design and construction of microreactors. In Proceedings of the 6º Congress Obrasileiro De Engenharia De Fabrica Ção, Caxias do Sulm, Brazil, 11–15 April 2011; Volume 11. [Google Scholar]
- Kim, G.B.; Lee, S.; Kim, H.; Yang, D.H.; Kim, Y.H.; Kyung, Y.S.; Kim, C.S.; Choi, S.H.; Kim, B.J.; Ha, H.; et al. Three-dimensional printing: Basic principles and applications in medicine and radiology. Korean J. Radiol. 2016, 17, 182–197. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Liu, W.; He, R.; Zhou, M.; Wu, S.; Song, X.; Chen, Y. Effect of the particle size and the debinding process on the density of alumina ceramics fabricated by 3D printing based on stereolithography. Ceram. Int. 2016, 42, 17290–17294. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Günster, J. Additive manufacturing of ceramics: Issues, potentialities, and opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Lian, Q.; Sui, W.; Wu, X.; Yang, F.; Yang, S. Additive manufacturing of ZrO2 ceramic dental bridges by stereolithography. Rapid Prototyp. J. 2018, 24, 114–119. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, K.; Bourell, D.L.; Chen, F.; Sun, H.; Shi, Y.; Wang, J.; He, M.; Chen, J. Gelcasting of zirconia-based all-ceramic teeth combined with stereolithography. Ceram. Int. 2018, 44, 21556–21563. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Zhou, W.; Wang, L. Curing characteristics of ceramic stereolithography for an aqueous-based silica suspension. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2010, 224, 641–651. [Google Scholar] [CrossRef]
- Bertsch, A.; Jiguet, S.; Renaud, P. Microfabrication of ceramic components by microstereolithography. J. Micromech. Microeng. 2004, 14, 197–203. [Google Scholar] [CrossRef]
- Zhang, K.; He, R.; Xie, C.; Wang, G.; Ding, G.; Wang, M.; Song, W.; Fang, D. Photosensitive ZrO2 suspensions for stereolithography. Ceram. Int. 2019, 45, 12189–12195. [Google Scholar] [CrossRef]
- Gentry, S.P.; Halloran, J.W. Depth and width of cured lines in photopolymerizable ceramic suspensions. J. Eur. Ceram. Soc. 2013, 33, 1981–1988. [Google Scholar] [CrossRef]
- Badev, A.; Abouliatim, Y.; Chartier, T.; Lecamp, L.; Lebaudy, P.; Chaput, C.; Delage, C. Photopolymerization kinetics of a polyether acrylate in the presence of ceramic fillers used in stereolithography. J. Photochem. Photobiol. A Chem. 2011, 222, 117–122. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Shi, J. Additive manufacturing of zirconia ceramics: A state-of-the-art review. J. Mater. Res. Technol. 2020, 9, 9029–9048. [Google Scholar] [CrossRef]
- Wang, J.C.; Dommati, H. Fabrication of zirconia ceramic parts by using solvent-based slurry stereolithography and sintering. Int. J. Adv. Manuf. Technol. 2018, 98, 1537–1546. [Google Scholar] [CrossRef]
- Revilla-León, M.; Husain, N.A.; Ceballos, L.; Özcan, M. Flexural strength and Weibull characteristics of stereolithography additive manufactured versus milled zirconia. J. Prosthet. Dent. 2021, 125, 685–690. [Google Scholar] [CrossRef]
- Revilla-León, M.; Mostafavi, D.; Methani, M.M.; Zandinejad, A. Manufacturing accuracy and volumetric changes of stereolithography additively manufactured zirconia with different porosities. J. Prosthet. Dent. 2022, 128, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Inokoshi, M.; Nozaki, K.; Komatsu, K.; Kamijo, S.; Liu, H.; Shimizubata, M.; Minakuchi, S.; Van Meerbeek, B.; Vleugels, J.; et al. Additively manufactured zirconia for dental applications. Materials 2021, 14, 3694. [Google Scholar] [CrossRef] [PubMed]
- Deckard, C.R. Method and Apparatus for Producing Parts by Selective Sintering. U.S. Patent No. 4,863,538-A, 5 September 1986. [Google Scholar]
- Subramani, R.; Mustafa, M.A.; Ghadir, G.K.; Al-Tmimi, H.M.; Alani, Z.K.; Haridas, D.; Rusho, M.A.; Rajeswari, N.; Rajan, A.J.; Kumar, A.P. Advancements in 3D printing materials: A comparative analysis of performance and applications. Appl. Chem. Eng. 2024, 7, ACE-3867. [Google Scholar] [CrossRef]




| Material | Tensile Strength (MPa) | Fatigue Resistance | Elastic Modulus (GPa) |
|---|---|---|---|
| Porous titanium | 550–900 | High | 10–30 |
| Trabecular tantalum | 200–300 | Extremely high | 3–5 |
| Zirconia | 900–1200 | Medium | 200–210 |
| Polymers | 50–100 | Low | 1–5 |
| Composite | 100–300 | Medium to high | 5–20 |
| Technology | Energy Type | Applicable Materials | Displacement Accuracy | Surface Quality | Limitations |
|---|---|---|---|---|---|
| SLM | Laser (infrared) | Metals (titanium, aluminum, stainless steel, alloys) | High (20–50 µm) | Medium to high | Higher residual stress, need for support |
| EBM | Electron beam (vacuum) | Metals (especially titanium alloys) | Medium to high (50–100 µm) | Coarse, requires adjustment | Requires vacuum, rougher surface |
| DMLS | Laser (infrared) | Metals (stainless steel, cobalt–chromium, titanium) | High (20–50 µm) | Medium to high | Similar to SLM, there may be higher exposure density |
| SLA | UV–light | Polymers, ceramic suspensions | Extremely high (10–50 µm) | Very smooth | Sensitive to resin properties, limited materials |
| SLS | Laser (CO2) | Polymers, composites | Medium (80–150 µm) | Slightly medium | Low strength without sintering, limited detail |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duplák, J.; Dupláková, D.; Yeromina, M.; Mikuláško, S.; Török, J. The Role of Additive Manufacturing in Dental Implant Production—A Narrative Literature Review. Sci 2025, 7, 109. https://doi.org/10.3390/sci7030109
Duplák J, Dupláková D, Yeromina M, Mikuláško S, Török J. The Role of Additive Manufacturing in Dental Implant Production—A Narrative Literature Review. Sci. 2025; 7(3):109. https://doi.org/10.3390/sci7030109
Chicago/Turabian StyleDuplák, Ján, Darina Dupláková, Maryna Yeromina, Samuel Mikuláško, and Jozef Török. 2025. "The Role of Additive Manufacturing in Dental Implant Production—A Narrative Literature Review" Sci 7, no. 3: 109. https://doi.org/10.3390/sci7030109
APA StyleDuplák, J., Dupláková, D., Yeromina, M., Mikuláško, S., & Török, J. (2025). The Role of Additive Manufacturing in Dental Implant Production—A Narrative Literature Review. Sci, 7(3), 109. https://doi.org/10.3390/sci7030109








