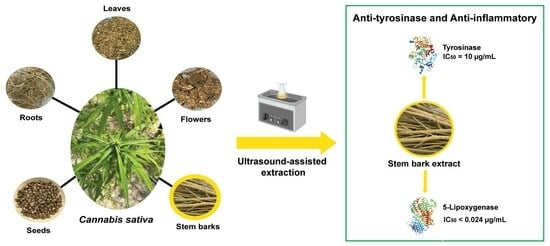
Exploring Anti-Inflammatory and Anti-Tyrosinase Potentials and Phytochemical Profiling of Cannabis sativa Stems Byproducts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Sample Preparation
2.3. Ultrasonic-Assisted Extraction of Cannabis sativa L.
2.4. Determination of Total Phenolic Content (TPC) and Total Flavonoids Content (TFC)
2.5. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and Nitric Oxide (NO) Radical Scavenging Activities
2.6. 5-Lipoxygenase (5-LOX) Inhibition of the C. sativa L. Extracts
2.7. In Vitro Anti-Tyrosinase Activity of the C. sativa L. Extracts
2.8. Cytotoxicity of the C. sativa L. Extracts on Human Keratinocyte (HaCaT) Cells
2.9. Gas Chromatography-Mass Spectrometry (GC-MS/MS) Analysis
2.10. Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS/MS) Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Extraction of the C. sativa L. Extracts
3.2. Biological Assessment of the C. sativa L. Extracts
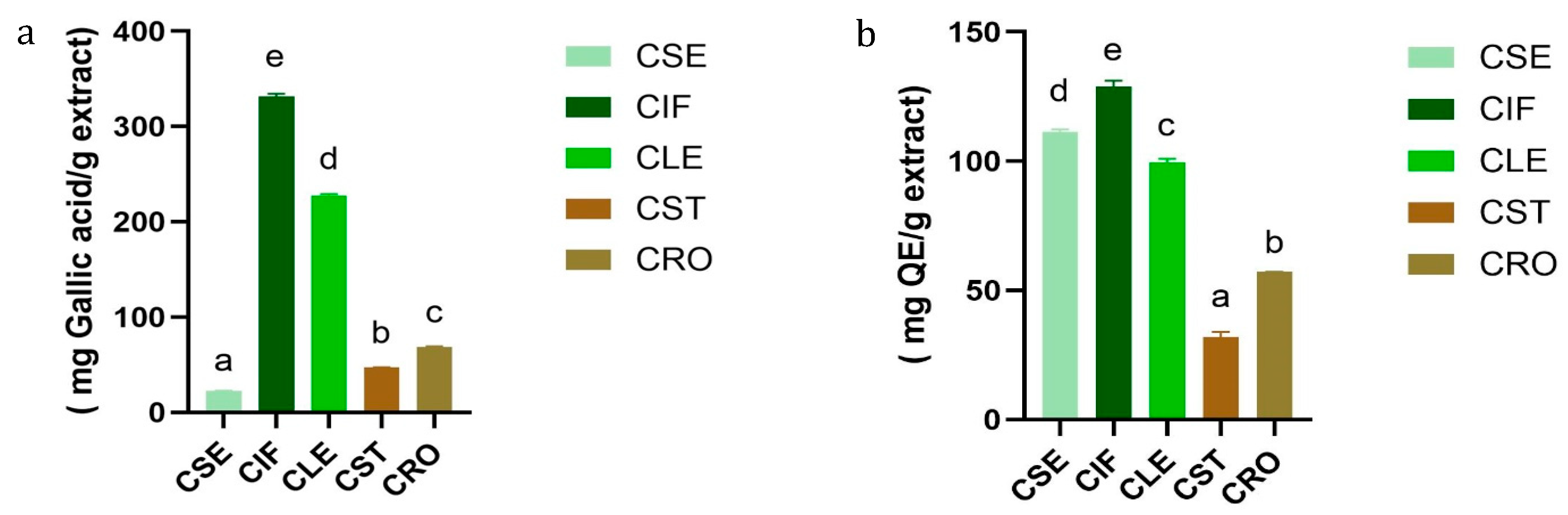
3.2.1. Determination of Total Phenolic and Total Flavonoids Contents of the C. sativa L. Extracts
3.2.2. DPPH and NO Radical Scavenging Activities of the C. sativa L. Extracts
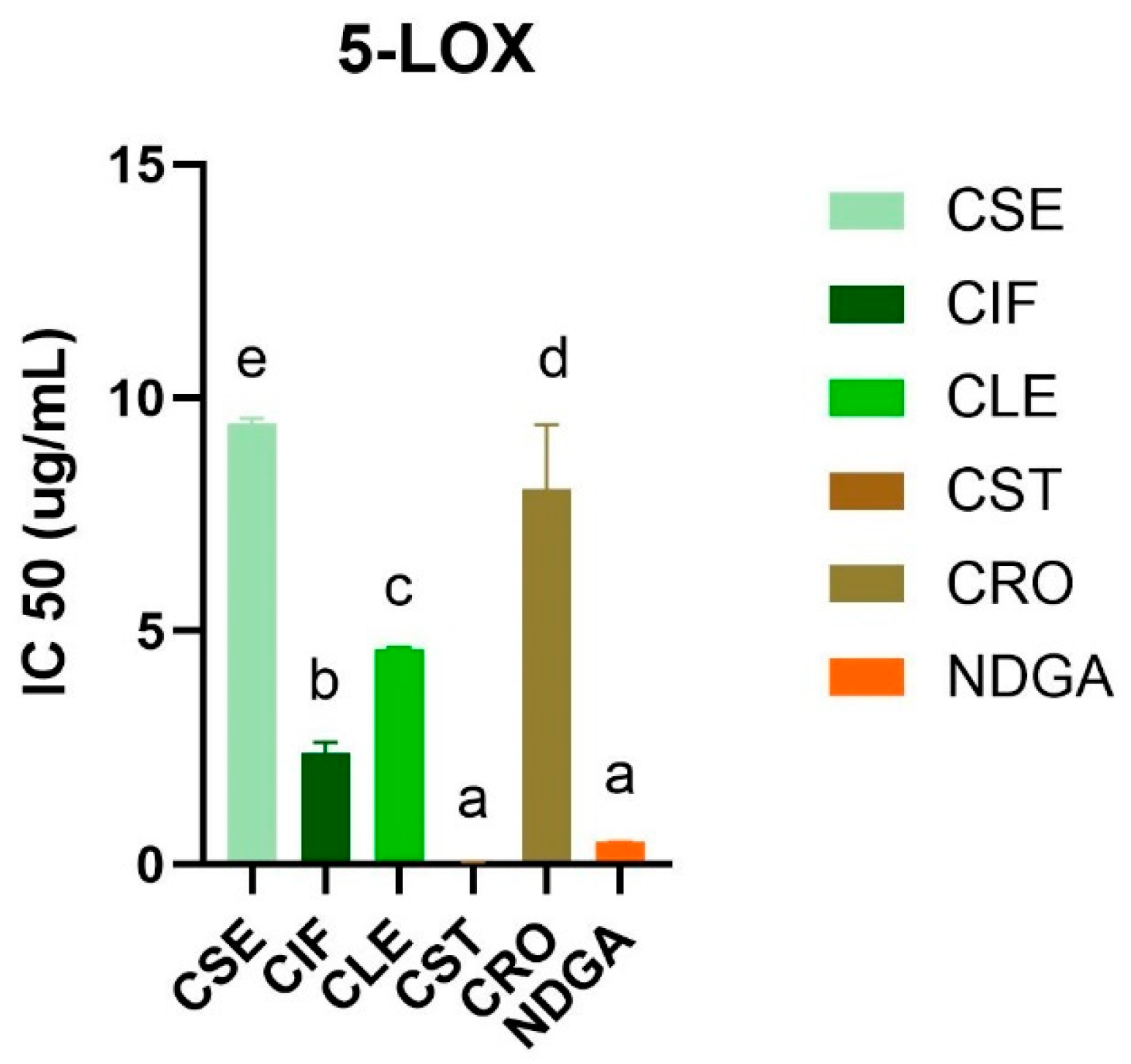
3.2.3. 5-Lipoxygenase Inhibitory Activity of the C. sativa L. Extracts
3.2.4. Tyrosinase Inhibitory Activity of the C. sativa L. Extracts
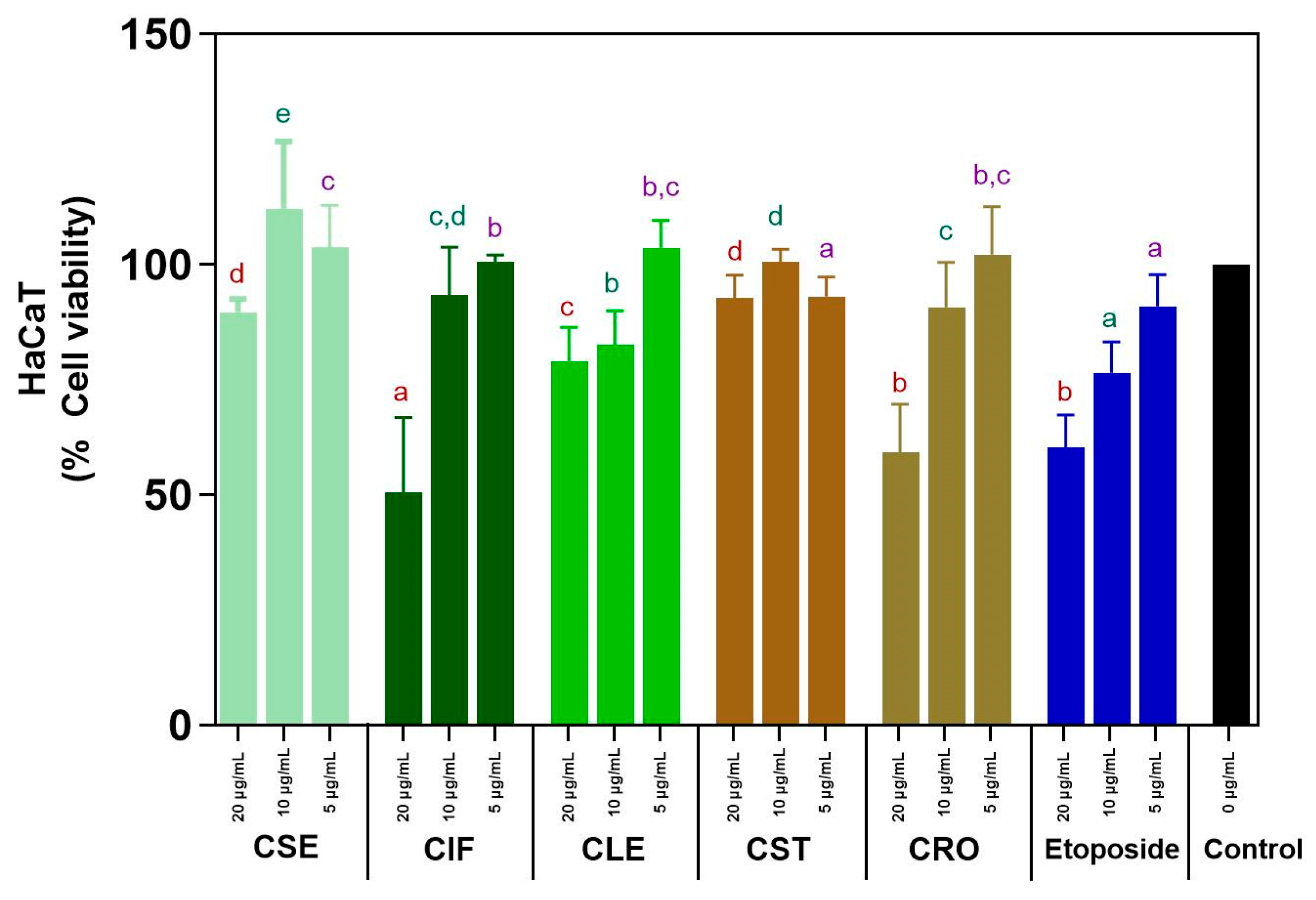
3.2.5. Cytotoxicity on HaCaT Cells of the C. sativa L. Extracts
3.3. Chemical Analysis of Bioactive Compounds in the C. sativa L. Extracts by LC-QTOF-MS/MS and GC-MS/MS Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
References
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Andra, C.; Suwalska, A.; Dumitrescu, A.M.; Kerob, D.; Delva, C.; Hasse-Cieślińska, M.; Solymosi, A.; Arenbergerova, M. A Corrective Cosmetic Improves the Quality of Life and Skin Quality of Subjects with Facial Blemishes Caused by Skin Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 253–257. [Google Scholar] [CrossRef]
- Skala, T.; Kahánková, Z.; Tauchen, J.; Janatová, A.; Klouˇcek, P.; Hubka, V.; Fraˇnková, A. Medical cannabis dimethyl ether, ethanol and butane extracts inhibit the in vitro growth of bacteria and dermatophytes causing common skin diseases. Front. Microbiol. 2022, 13, 953092. [Google Scholar] [CrossRef]
- Di Costanzo, L.F. Structural characterization of tyrosinases and an update on human enzymes. Enzymes 2024, 56, 55–83. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Muddathir, A.M.; Yamauchi, K.; Batubara, I.; Mohieldin, E.A.M.; Mitsunaga, T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S. Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Ma, W.; Wlaschek, M.; Tantcheva-Poór, I.; Schneider, L.A.; Naderi, L.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermatol. 2001, 26, 592–599. [Google Scholar] [CrossRef]
- Khojah, H.; Ahmed, S.R.; Alharbi, S.Y.; AlSabeelah, K.K.; Alrayyes, H.Y.; Almusayyab, K.B.; Alrawiliy, S.R.; Alshammari, R.M.; Qasim, S. Skin anti-aging potential of Launaea procumbens extract: Antioxidant and enzyme inhibition activities supported by ADMET and molecular docking studies. Saudi Pharm. J. 2024, 32, 102107. [Google Scholar] [CrossRef]
- Podder, D.; Sasmal, S.; Haldar, D. Sonication Responsive Gelator: Synthesis and Applications. Curr. Org. Synth. 2015, 12, 440–456. [Google Scholar] [CrossRef]
- French, J.A.; Koepp, M.; Naegelin, Y.; Vigevano, F.; Auvin, S.; Rho, J.M.; Rosenberg, E.; Devinsky, O.; Olofsson, P.S.; Dichter, M.A. Clinical studies and anti-inflammatory mechanisms of treatments. Epilepsia 2017, 58 (Suppl. S3), 69–82. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xu, J.; Zhou, H.; Seeram, N.P.; Ma, H.; Gu, Q. Chemical constituents of industrial hemp roots and their anti-inflammatory activities. J. Cannabis Res. 2023, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Rea Martinez, J.; Montserrat-de la Paz, S.; De la Puerta, R.; García-Giménez, M.D.; Fernández-Arche, M.Á. Characterization of bioactive compounds in defatted hempseed (Cannabis sativa L.) by UHPLC-HRMS/MS and anti-inflammatory activity in primary human monocytes. Food Funct. 2020, 11, 4057–4066. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Rožanc, J.; Kotnik, P.; Milojević, M.; Gradišnik, L.; Knez Hrnčič, M.; Knez, Ž.; Maver, U. Different Cannabis sativa Extraction Methods Result in Different Biological Activities against a Colon Cancer Cell Line and Healthy Colon Cells. Plants 2021, 10, 566. [Google Scholar] [CrossRef]
- Liana, D.; Eurtivong, C.; Phanumartwiwath, A. Boesenbergia rotunda and Its Pinostrobin for Atopic Dermatitis: Dual 5-Lipoxygenase and Cyclooxygenase-2 Inhibitor and Its Mechanistic Study through Steady-State Kinetics and Molecular Modeling. Antioxidants 2024, 13, 74. [Google Scholar] [CrossRef]
- Manosroi, A.; Boonpisuttinant, K.; Winitchai, S.; Manosroi, W.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori). Pharm. Biol. 2010, 48, 855–860. [Google Scholar] [CrossRef]
- Wan, L.; Song, Z.; Wang, Z.; Dong, J.; Chen, Y.; Hu, J. Repair effect of Centella asiatica (L.) extract on damaged HaCaT cells studied by atomic force microscopy. J. Microsc. 2023, 292, 148–157. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.H.; Khan, I.A.; Chandra, S.; Lata, H.; et al. Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Povedano, M.M.; Sánchez-Carnerero Callado, C.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted characterization of extracts from Cannabis sativa L. cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode. Talanta 2020, 208, 120384. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of Vitis vinifera, Foeniculum vulgare, Cannabis sativa and Punica granatum By-Product Seeds as Dermo-Cosmetic Agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, N.; Shanker, K.; Kumar, B.; Kumar, N. Monoecious Cannabis sativa L. discloses the organ-specific variation in glandular trichomes, cannabinoids content and antioxidant potential. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100476. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Bakari, G.; Misinzo, G.; Nho, C.W.; Kim, H.Y. Variation in Phenolic Compounds and Antioxidant Activity of Various Organs of African Cabbage (Cleome gynandra L.) Accessions at Different Growth Stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef]
- Kim, Y.N.; Sim, K.S.; Park, S.; Sohn, H.Y.; Kim, T.; Kim, J.H. In Vitro and In Vivo Anti-Inflammatory Effects of Cannabis sativa Stem Extract. J. Med. Food 2022, 25, 408–417. [Google Scholar] [CrossRef]
- Radi, M.H.; El-Shiekh, R.A.; El-Halawany, A.M.; Abdel-Sattar, E. Friedelin and 3β-Friedelinol: Pharmacological Activities. Rev. Bras. Farmacog. 2023, 33, 886–900. [Google Scholar] [CrossRef]
- Rashed, K. Beta-sitosterol medicinal properties: A review article. J. Sci. Innov. Technol. 2020, 9, 208–212. [Google Scholar]
- Da Marinho, A.M.N.; da Silva Neto, R.W.G. Anti-inflammatory effects of cannabinoids. BrJP 2023, 6, 31–37. [Google Scholar] [CrossRef]
- Jin, S.; Lee, M.-Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Kumari, R.; Meyyappan, A.; Selvamani, P.; Mukherjee, J.; Jaisankar, P. Lipoxygenase inhibitory activity of crude bark extracts and isolated compounds from Commiphora berryi. J. Ethnopharmacol. 2011, 138, 256–259. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Kim, J.K.; Heo, H.-Y.; Park, S.; Kim, H.; Oh, J.J.; Sohn, E.-H.; Jung, S.-H.; Lee, K. Characterization of Phenethyl Cinnamamide Compounds from Hemp Seed and Determination of Their Melanogenesis Inhibitory Activity. ACS Omega 2021, 6, 31945–31954. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ(9)-tetrahydrocannabinol: Δ(9)-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 20335. [Google Scholar] [CrossRef]
- Ross, S.A.; Mehmedic, Z.; Murphy, T.P.; Elsohly, M.A. GC-MS analysis of the total Δ9-THC content of both drug- and fiber-type cannabis seeds. J. Anal. Toxicol. 2000, 24, 715–717. [Google Scholar] [CrossRef]
- Kitamura, M.; Kiba, Y.; Suzuki, R.; Tomida, N.; Uwaya, A.; Isami, F.; Deng, S. Cannabidiol Content and In Vitro Biological Activities of Commercial Cannabidiol Oils and Hemp Seed Oils. Medicines 2020, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Kubiliene, A.; Mickute, K.; Baranauskaite, J.; Marksa, M.; Liekis, A.; Sadauskiene, I. The Effects of Cannabis sativa L. Extract on Oxidative Stress Markers In Vivo. Life 2021, 11, 647. [Google Scholar] [CrossRef]
- Nakkliang, K.; Areesantichai, C.; Rungsihirunrat, K. Assessment of pharmacognostic specification of Cannabis sativa leaves in Thailand. J. Adv. Pharm. Technol. Res. 2022, 13, 226–231. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wu, H.; Wang, X. Beneficial health effects of lupenone triterpene: A review. Biomed. Pharmacother. 2018, 103, 198–203. [Google Scholar] [CrossRef]
- Chen, X.; Zong, C.; Gao, Y.; Cai, R.; Fang, L.; Lu, J.; Liu, F.; Qi, Y. Curcumol exhibits anti-inflammatory properties by interfering with the JNK-mediated AP-1 pathway in lipopolysaccharide-activated RAW264.7 cells. Eur. J. Pharmacol. 2014, 723, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kornpointner, C.; Sainz Martinez, A.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crop. Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Javaid, A.; Khan, I.H.; Ferdosi, M.F. Bioactive constituents of wild Cannabis sativa roots from Pakistan. Pak. J. Weed Sci. Res. 2021, 27, 359. [Google Scholar] [CrossRef]

| Extract | Percent Yield (%) |
|---|---|
| C. sativa L. seed (CSE) | 20.01 |
| C. sativa L. inflorescence (CIF) | 6.62 |
| C. sativa L. leaf (CLE) | 3.46 |
| C. sativa L. stem (CST) | 0.28 |
| C. sativa L. root (CRO) | 0.23 |
| Antioxidant Activities | ||
|---|---|---|
| Extract | DPPH, IC50 (µg/mL) | NO, IC50 (µg/mL) |
| C. sativa L. seed (CSE) | - | - |
| C. sativa L. inflorescence (CIF) | 3849.01 ± 5.25 b | - |
| C. sativa L. leaf (CLE) | 4709.98 ± 1.48 c | - |
| C. sativa L. stem (CST) | - | - |
| C. sativa L. root (CRO) | - | - |
| Ascorbic acid | 40.41 ± 0.79 a | NA |
| Quercetin | NA | 0.06 ± 0.00 |
| Extract | IC50 (mg/mL) |
|---|---|
| C. sativa L. seed (CSE) | >1000 c |
| C. sativa L. inflorescence (CIF) | >1000 c |
| C. sativa L. leaf (CLE) | 5.28 ± 1.98 a |
| C. sativa L. stem (CST) | 0.01 ± 0.00 a |
| C. sativa L. root (CRO) | 59.51 ± 8.95 b |
| Kojic acid | 0.004 ± 0.00 a |
| Major Classes | Bioactive Compound | Molecular Formula | Detected Ion | Exact Mass | m/z | Fragments | Retention Time (min) | Part of the Plant |
|---|---|---|---|---|---|---|---|---|
| Cannabinoids | Cannabigerol | C21H32O2 | [M+H]+ | 316.2405 | 317.2472 | 123.0434, 193.1207 | 10.2772 | Inflorescence (CIF), Leaf (CLE) |
| Cannabidiolic acid | C22H30O4 | [M+H]+ | 358.2136 | 359.2201 | 219.081, 261.1467 | 11.4921 | Inflorescence (CIF), Leaf (CLE) | |
| Deta-11-tetrahydrocannabinol | C21H32O2 | [M+H]+ | 315.2298 | 315.2291 | 193.1188, 259.1662 | 10.9532 | Inflorescence (CIF), Leaf (CLE) | |
| Cannabinol | C21H26O2 | [M+H]+ | 310.1936 | 311.2004 | 240.1141 | 8.9473 | Leaf (CLE) | |
| Phenolic compounds | Plantamajoside | C29H36O15 | [M+H]+ | 662.3959 | 663.4027 | 529.2924 | 14.9209 | Inflorescence (CIF) |
| 5,7-Dihydroxy-4-methylcoumarin | C10H8O4 | [M+H]+ | 192.1156 | 193.1224 | 123.0437 | 11.7230 | Inflorescence (CIF) | |
| 3-Methoxybenzenepropanoic acid | C10H12O3 | [M+H]+ | 180.1158 | 181.1225 | 179.1423 | 6.5724 | Inflorescence (CIF) | |
| Pinoresinol | C20H22O6 | [M+H]+ | 340.2594 | 341.2662 | 147.1165, 193.1310 | 10.3300 | Leaf (CLE) | |
| 6-hydroxy-4-methylcoumarin | C10H8O3 | [M+H]+ | 176.1571 | 177.1639 | - | 5.4458 | Leaf (CLE) | |
| 2,6-Di-tert-butyl-4-hydroxymethylphenol | C15H24O2 | [M+H]+ | 218.1677 | 219.1744 | 84.9591, 136.9312 | 5.9681 | Stem (CST) | |
| Curculigoside | C23H32O11 | [M+H]+ | 450.3564 | 489.3194 | - | 10.3229 | Seed (CSE) | |
| Valeroyl salicylate | C12H14O3 | [M+H]+ | 204.0792 | 205.0856 | 65.0385, 121.091, 149.0238 | 9.7979 | Seed (CSE), Stem (CST) | |
| Flavonoids | N-Isorhamnetin | C16H12O7 | [M+H]+ | 316.1725 | 317.1792 | - | 10.7044 | Inflorescence (CIF), Leaf (CLE), Seed (CSE) |
| 2′,5′-Dimethoxy-6-fluoroflavone | C17H13FO4 | [M+H]+ | 300.1012 | 301.1394 | - | 9.7999 | Leaf (CLE), Root (CRO) | |
| Alkaloids | Vincamine | C21H26N2O3 | [M+H]+ | 354.1834 | 355.1900 | 235.1116, 253.1232 | 11.2074 | Inflorescence (CIF), Leaf (CLE) |
| Thebaine | C19H21NO3 | [M+H]+ | 311.1899 | 312.1966 | 146.0971, 282.1491 | 11.4521 | Inflorescence (CIF), Leaf (CLE) | |
| Saponins | Astrasieversianin XV | C41H68O14 | [M+H]+ | 884.5466 | 923.5093 | 645.2117 | 31.2773 | Inflorescence (CIF), Root (CRO), Stem (CST) |
| Astragaloside II | C41H68O14 | [M+H]+ | 848.6213 | 849.6283 | - | 13.5875 | Root (CRO), Stem (CST) | |
| Astragaloside III | C57H92O26 | [M+H]+ | 806.5739 | 807.5812 | - | 11.2879 | Inflorescence (CIF), Leaf (CLE), Root (CRO), Seed (CSE), Stem (CST) | |
| Saikosaponin D | C42H68O13 | [M+H]+ | 762.6693 | 763.6756 | 149.1326, 189.1648 | 24.7425 | Inflorescence (CIF), Leaf (CLE), Root (CRO), Seed (CSE), Stem (CST) | |
| Soyasaponin II | C48H78O18 | [M+H]+ | 934.7231 | 935.7305 | 680.4992, 805.5938 | 38.0745 | Seed (CSE) | |
| Terpenoids | Lupeol | C30H50O | [M+H]+ | 408.3398 | 409.3833 | 95.0854, 135.1168, 161.1324 | 18.9885 | Inflorescence (CIF), Leaf (CLE) |
| alpha.-Cyperone | C15H22O | [M+H]+ | 218.1680 | 219.1748 | 91.0541 | 8.1689 | Inflorescence (CIF) | |
| Ç Curcumol | C15H26O2 | [M+H]+ | 236.1779 | 237.1847 | 151.1117, 179.1425 | 8.1093 | Inflorescence (CIF), Leaf (CLE), Stem (CST) | |
| Lupenone | C30H48O | [M+H]+ | 424.3715 | 425.3785 | 135.1167 | 13.3515 | Inflorescence (CIF), Leaf (CLE), Root (CRO), Stem (CST) | |
| Farnesal | C15H24O | [M+H]+ | 220.1836 | 221.1902 | 81.0697, 105.0698 | 9.7006 | Inflorescence (CIF), Leaf (CLE) | |
| Steviolbioside | C38H60O18 | [M+H]+ | 664.4172 | 665.4251 | 149.0250, 271.1844 | 10.2717 | Inflorescence (CIF) | |
| Friedelin | C30H50O | [M+H]+ | 426.3480 | 427.3572 | 173.0966, 325.3103 | 17.0872 | Inflorescence (CIF), Leaf (CLE), Root (CRO), Stem (CST) | |
| Kahweol palmitate | C40H58O4 | [M+H]+ | 534.2623 | 535.2700 | - | 12.2929 | Leaf (CLE), Root (CRO) | |
| Aristolone | C15H22O | [M+H]+ | 218.1680 | 219.1748 | 84.9600, 136.9313, 161.1330 | 8.1759 | Root (CRO), Seed (CSE) | |
| Cucurbitacin E-2-O-glucoside | C38H58O11 | [M+H]+ | 740.3889 | 741.3955 | 313.0734, 413.1930 | 11.5132 | Leaf (CLE) | |
| Kahweol | C20H26O3 | [M+H]+ | 314.2242 | 315.2320 | - | 12.0785 | Root (CRO) | |
| Uvaol | C30H50O2 | [M+H]+ | 410.3555 | 443.3885 | 95.0857, 177.9638 | 13.5105 | Root (CRO) | |
| Cycloartenol acetate | C32H52O2 | [M+H]+ | 408.3768 | 409.3832 | 137.1325, 271.2415 | 20.7737 | Root (CRO) | |
| Betulin | C30H50O2 | [M+H]+ | 442.3820 | 443.3886 | 177.1636, 229.1945 | 13.5390 | Stem (CST) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongtananeti, P.; Liana, D.; Myo, H.; Phanumartwiwath, A.; Areesantichai, C. Exploring Anti-Inflammatory and Anti-Tyrosinase Potentials and Phytochemical Profiling of Cannabis sativa Stems Byproducts. Sci 2025, 7, 77. https://doi.org/10.3390/sci7020077
Kongtananeti P, Liana D, Myo H, Phanumartwiwath A, Areesantichai C. Exploring Anti-Inflammatory and Anti-Tyrosinase Potentials and Phytochemical Profiling of Cannabis sativa Stems Byproducts. Sci. 2025; 7(2):77. https://doi.org/10.3390/sci7020077
Chicago/Turabian StyleKongtananeti, Pannita, Desy Liana, Hla Myo, Anuchit Phanumartwiwath, and Chitlada Areesantichai. 2025. "Exploring Anti-Inflammatory and Anti-Tyrosinase Potentials and Phytochemical Profiling of Cannabis sativa Stems Byproducts" Sci 7, no. 2: 77. https://doi.org/10.3390/sci7020077
APA StyleKongtananeti, P., Liana, D., Myo, H., Phanumartwiwath, A., & Areesantichai, C. (2025). Exploring Anti-Inflammatory and Anti-Tyrosinase Potentials and Phytochemical Profiling of Cannabis sativa Stems Byproducts. Sci, 7(2), 77. https://doi.org/10.3390/sci7020077