Abstract
Antioxidants, such as stilbenes, anthocyanidins, coumarins, tannins and flavonoids, are often based on oxygen-containing redox systems and tend to feature several hydroxyl groups in their chemical structures. From a synthetic perspective, oxygen atoms are prone to bioisosteric replacement with sulfur and, notably, selenium. The main objective of this narrative literature review is to explore if and how bioisosteric substitution of oxygen with sulfur or selenium can enhance the biological activity of natural products. This replacement boosts the biological activity of the resulting molecules considerably as they now combine the redox and antioxidant properties of the original flavonoids and other natural products with the specific redox behavior of sulfur and selenium. Besides sequestering free radicals and peroxides, they may, for instance, also catalyze the removal of oxidative stressors, capture free metal ions and even provide scope for selenium supplementation. Since these molecules resemble their natural counterparts, they also exhibit considerable selectivity inside the body and a good pharmacokinetic profile. Still, the synthesis of such hybrid molecules integrating sulfur and selenium into flavonoids and other natural products is a challenge and requires innovative synthetic strategies and approaches.
1. Introduction
Antioxidants have a long tradition in the field of nutrition and health. Despite considerable—and often justified—criticism about their bioavailability and biological activities in vivo, including issues surrounding uptake and metabolism in more complex organisms, these natural products are still widely considered to be beneficial to human health. Indeed, their preventive, and in some instances even medicinal actions place them at the forefront of nutraceuticals, especially in the elderly [1,2,3,4,5,6,7].
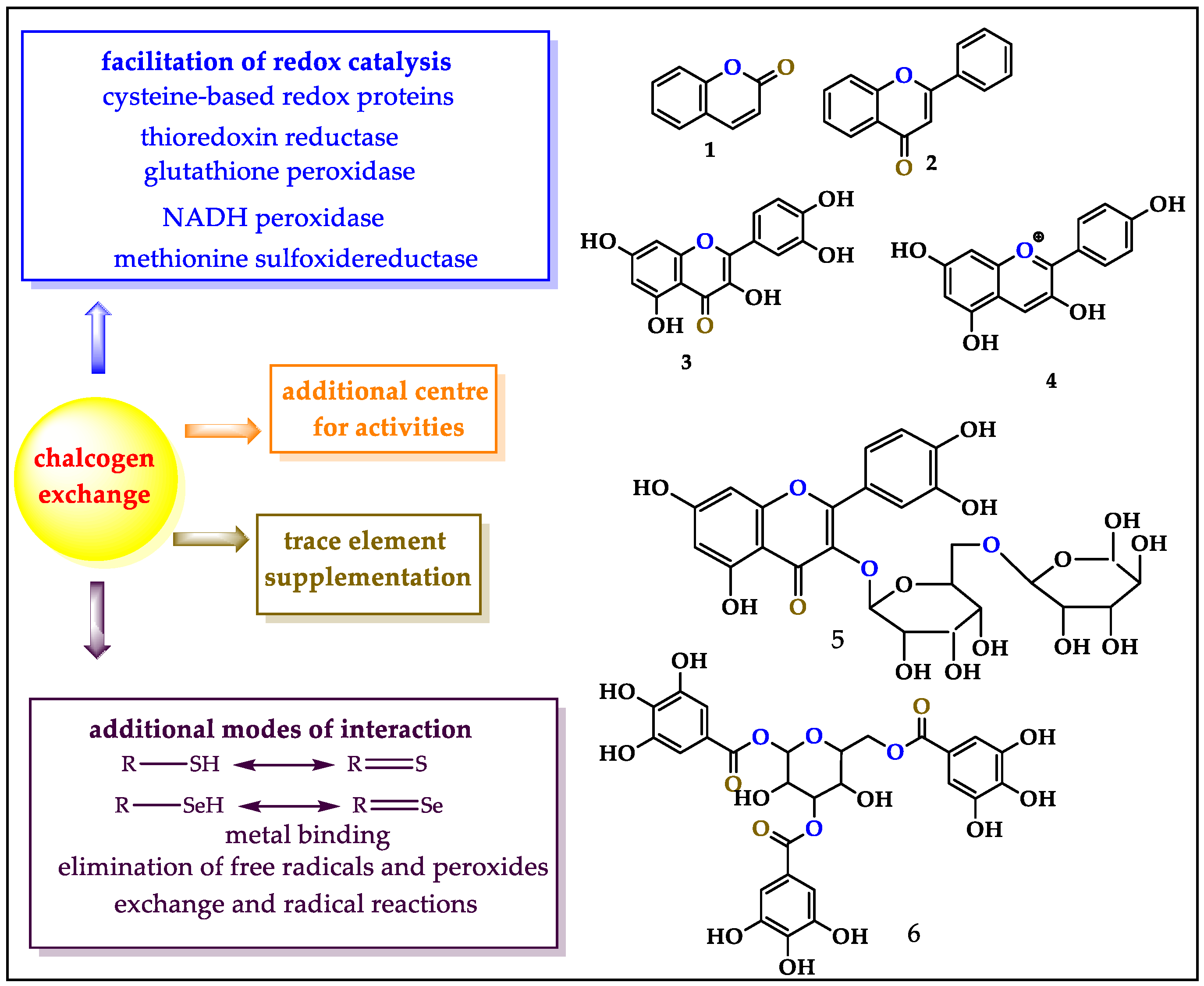
Interestingly, antioxidants are structurally diverse and range from fairly simple coumarins (1) and flavonoids (2,3) to complicated anthocyanidines (4), glycosides (5) and oligomeric tannins (6, Figure 1). Despite these structural differences, the ability of such antioxidants to counteract the typical ingredients of oxidative stress (OS) via their specific redox activity almost exclusively relies on oxygen-based redox moieties, such as (hydro-)quinones [8]. Indeed, many antioxidants are subsumed under the term “polyphenols”, an expression which has become almost synonymous to “antioxidants” [9].
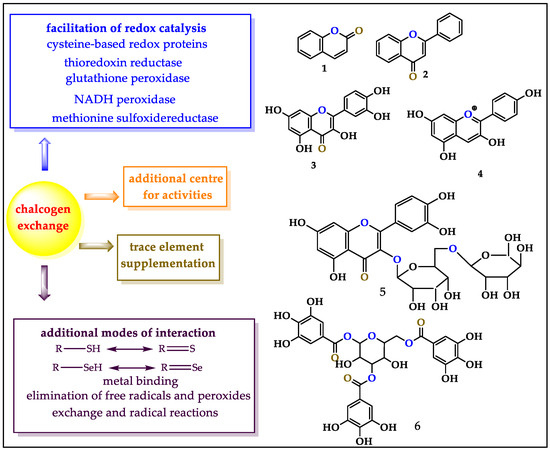
Figure 1.
The chalcogen exchange strategy provides several advantages, such as additional centers for biological activities, additional modes of interaction, facilitation of redox catalysis and supplementation of trace elements. Secondary metabolites such as 1–6 are amenable to bioisosteric replacements of oxygen for sulfur and selenium. The figure also highlights the “active”, “inactive/bystander” and “add-on” replacement options in green, blue and black, respectively.
Notably, the abundance—and importance—of oxygen atoms in such structures also provides a handle for a simple and powerful bioisosteric replacement strategy along the group of the chalcogen elements. In theory, it is possible to replace one or several oxygen atoms in such antioxidant structures with sulfur, and, more daringly, selenium, an essential trace element considered as an effective antioxidant on its own [10,11]. For strategic considerations, one may distinguish between the replacement of an oxygen atom actively involved in antioxidant, i.e., redox activity, or a ”bystander” oxygen with no direct antioxidant function on its own. In the first case, a change from blue to yellow or pink may increase an existing activity. In the second, it would incorporate an additional center of activity
Such a new center could, for instance, involve a thiol, thione, selenol or selenone able to remove free radicals and peroxides, facilitate redox catalysis and capture adventitious free metal ions such as Cu+ or Fe2+. Such sulfur and selenium sites may exhibit considerable synergy with the original (poly-)phenolic site(s), and, in the case of selenium, possibly serve as supplement for this trace element once the compound becomes metabolized. This Se supplementation is an added advantage since Se deficiency may lead to a plethora of complications, such as Keshan disease (KD), Kaschin–Beck disease (KBD), acquired immunodeficiency syndrome (AIDS), cardiovascular disease (CVD), cancer and inflammatory bowel disease (IBD) [12,13,14,15,16]. The World Health Organization (WHO) has confirmed an inadequate intake of Se not only in developing countries like Serbia, Slovenia and Turkey, but also in developed countries including the United Kingdom, France and Germany [16]. Natural products laced with Se, therefore, provide an interesting avenue to counter Se deficiency.
These various benefits of the “chalcogen exchange” strategy are highlighted in Figure 1, together with the basic and most common natural structures that are attractive for such an exchange. Besides exchanging an “active” or “inactive” oxygen atom in an existing natural product, one may also add chalcogen-containing functions to such molecules. Although we also consider selenium moieties linked to natural products, such “add-on” molecules without the relevant synergy are not the focus here, yet may be mentioned on occasion for comparison since they are discussed elsewhere [17,18,19,20]. The focus of this review is on the bioisosteric replacement of oxygen atoms in natural products, allowing us to showcase challenges in synthetic chalcogen chemistry, from simple selenides and selenols to selenoimidazoles, selenocarbonyls, selenoesters and selenosugars, each featuring specific reactivities and biological activities. It is important to mention that genetic engineering provides another alternative pathway for the substitution of oxygen with sulfur and selenium especially in proteins and enzymes. This review article focuses on chemical synthetic strategies for such substitution.
2. Inspired by Nature’s Symphony in Blue, Yellow and Pink
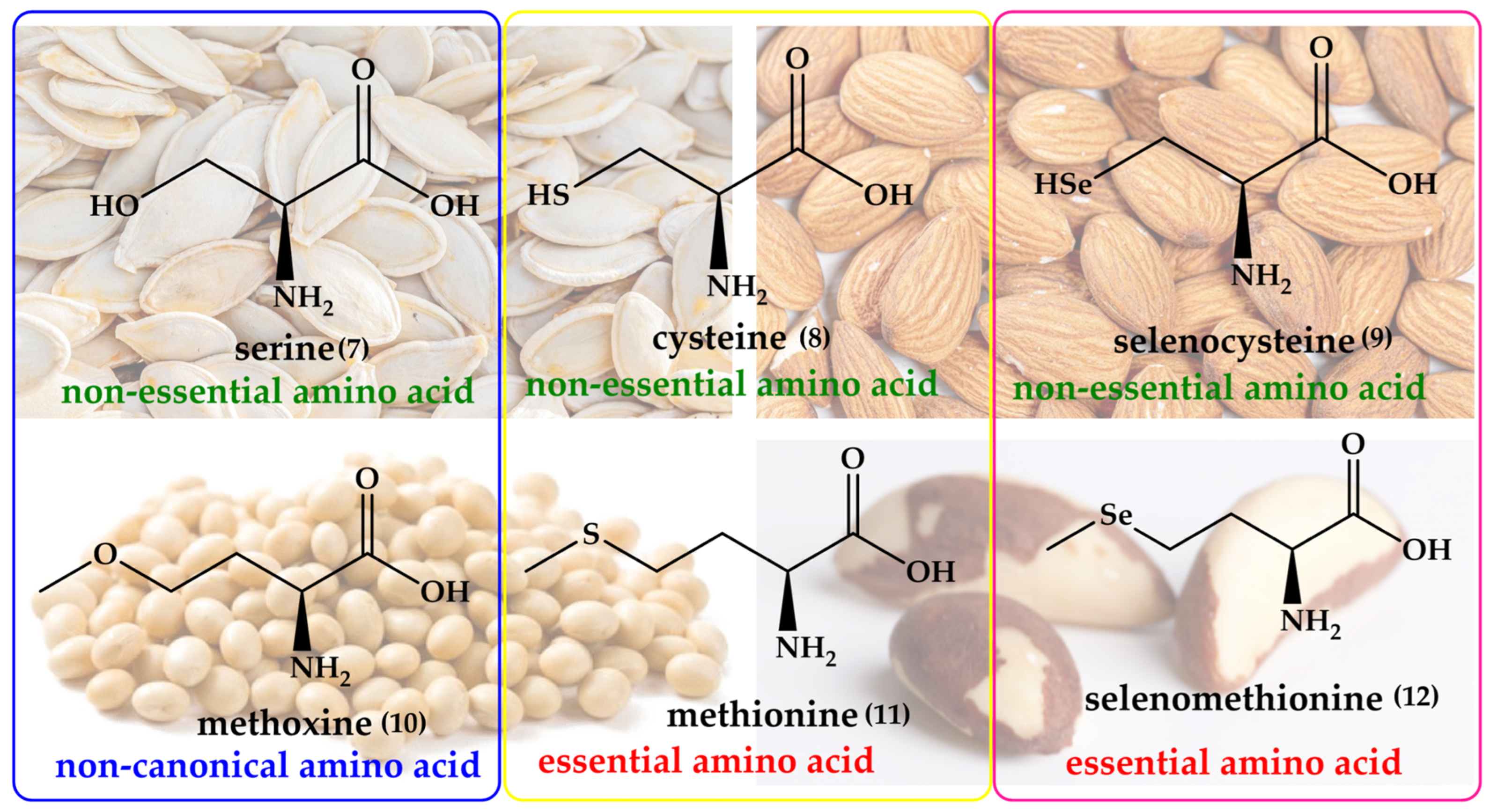
Interestingly, the idea of replacing oxygen for sulfur and then selenium in natural products is not as outlandish as one may initially expect. Nature itself provides such sets of oxygen, sulfur and selenium analogs already, albeit not systematically, and often not assigned to the same class of organisms. Replacing oxygen with sulfur and selenium may, therefore, rather be considered as a homage to nature. The most obvious example here is the trio of the amino acids serine (Ser), cysteine (Cys) and selenocysteine (SeCys), which differ only in their chalcogen atom. A similar shamrock is found in case of O-methyl-L-homoserine (methoxine, Mox), methionine (Met) and selenomethionine (SeMet). These amino acids are shown in Figure 2.
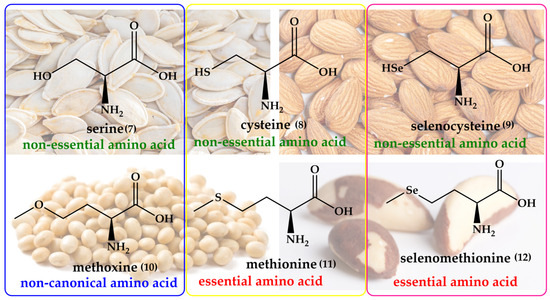
Figure 2.
Naturally occurring oxygen, sulfur, and selenium analogs of amino acids, such as Ser (7)/Cys (8)/SeCys (9) and Mox (10)/Met (11)/SeMet (12), which differ only in their respective chalcogen atom. Notably, SeCys and SeMet form the basis for the numerous selenoproteins and enzymes in biology.
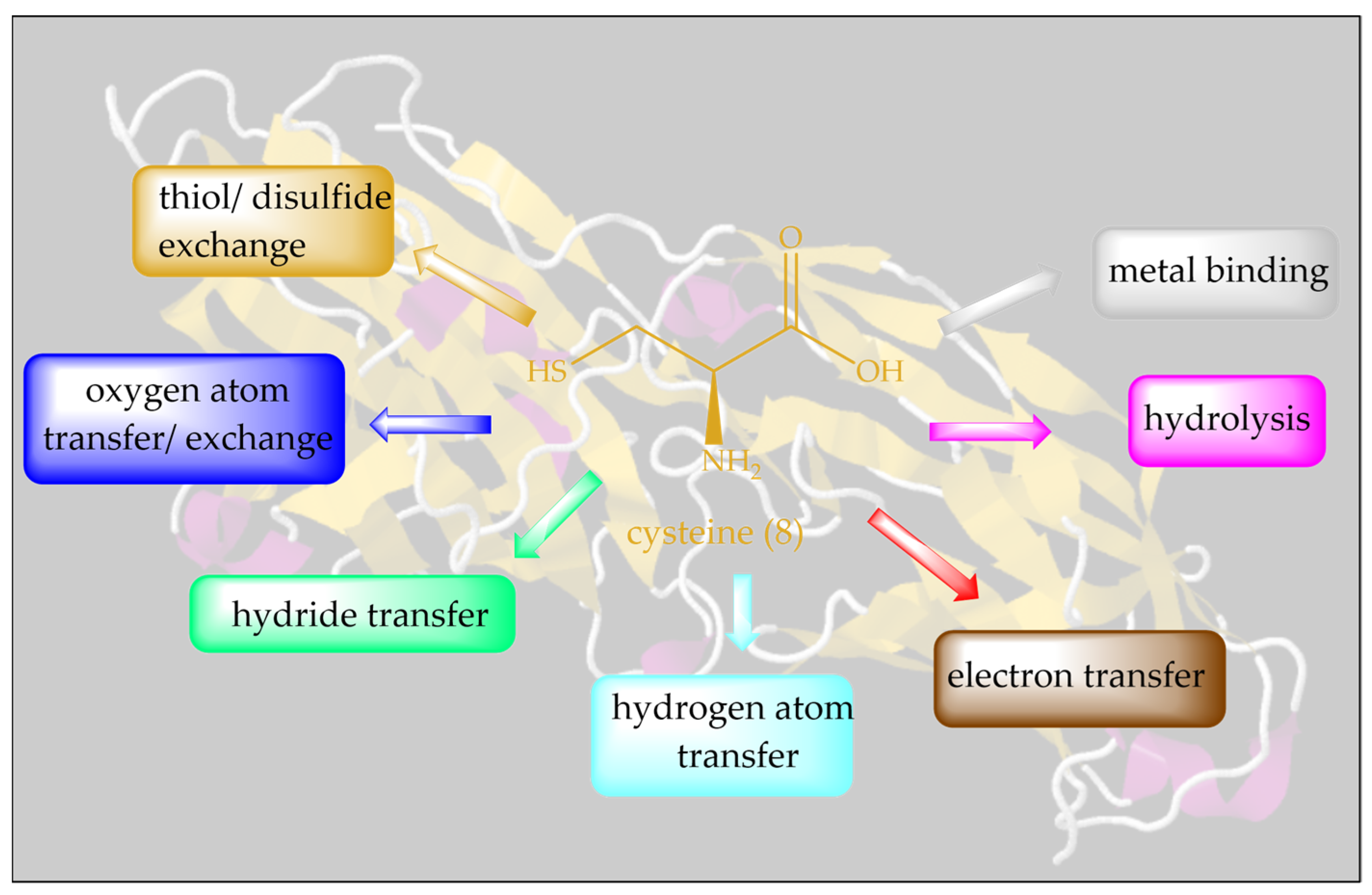
Isosteric replacement of oxygen with sulfur in serine results in one of the most important amino acids, Cys. Thanks to its ”redox chameleon” sulfur, Cys confers a broad range of different functions to proteins, such as disulfide formation, metal-binding, electron donation, hydrolysis and redox catalysis, as summarized in Figure 3.
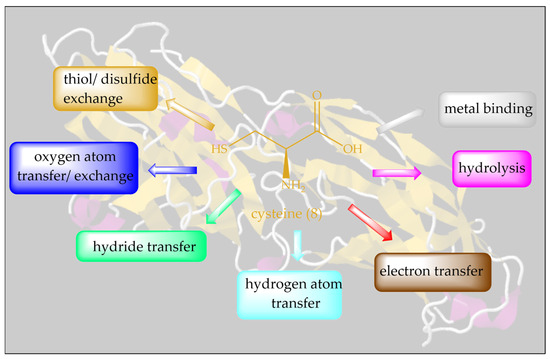
Figure 3.
A plethora of biological activities can be unlocked by a simple isosteric replacement of oxygen in Ser (7) with sulfur in Cys (8), and this is often achievable by simple genetic engineering.
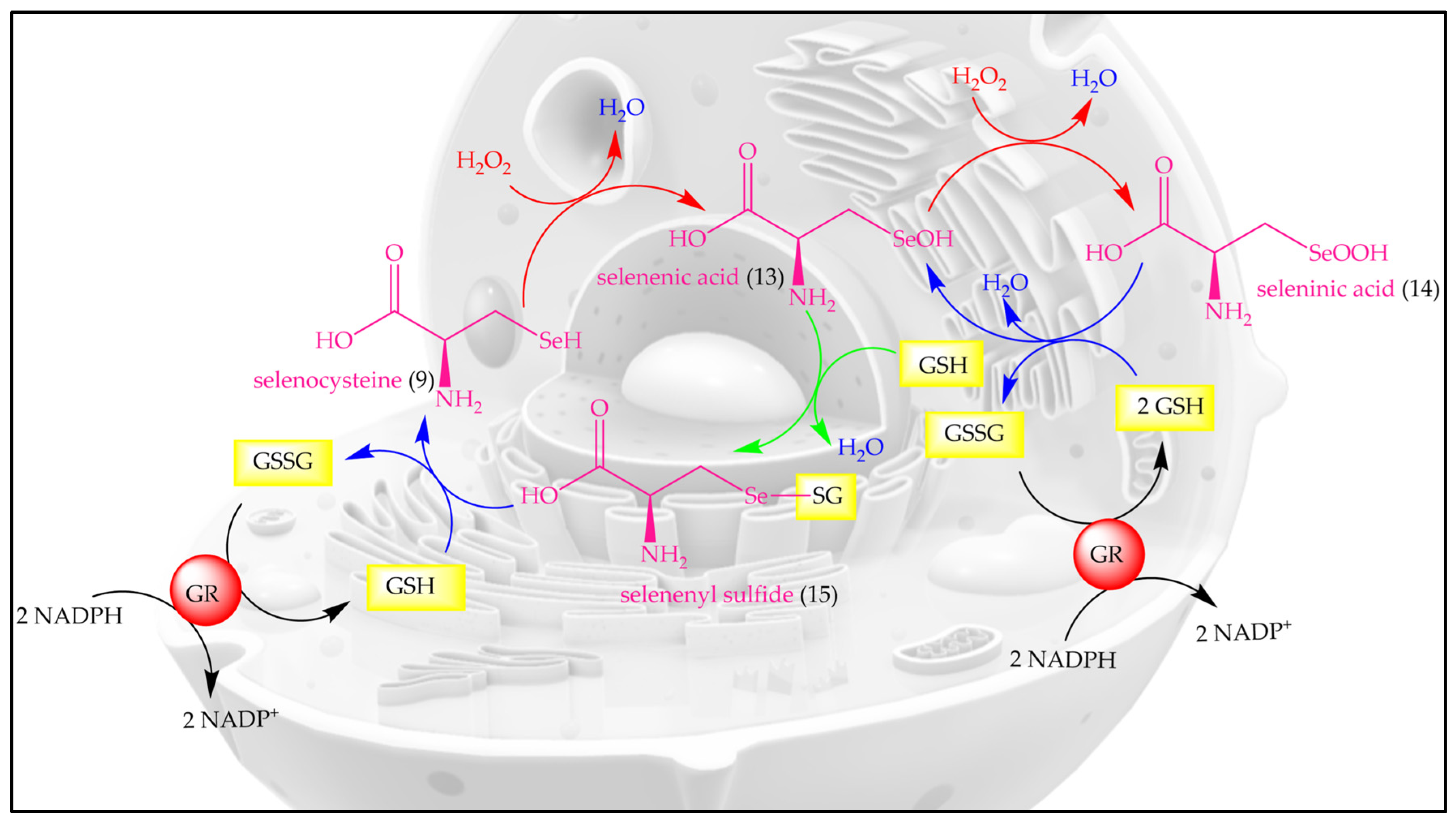
Let us, therefore, briefly consider “how nature does it”. Indeed, unlike the abundant oxygen and sulfur, selenium is an essential and beneficial trace element, occurring in just 20 mg in an average human adult, compared to around 140 g for sulfur [21,22]. Since its discovery as a trace element in humans by Flohé et al. and Rotruck et al., selenium has formed a center of interest in antioxidant research, as selenium is able to undergo various redox reactions quickly and efficiently, and with major consequences for biological systems [23,24,25,26]. In its redox behavior, the non-metal selenium acts alone, and therefore resembles metal ions, such as iron and copper, rather than more complex organic redox centers [27]. The redox catalytic cycle of SeCys is presented in Figure 4.
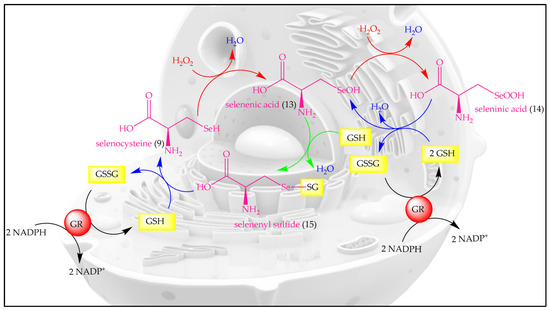
Figure 4.
The redox catalytic cycle of SeCys. H2O2, hydrogen peroxide; GSH, reduced glutathione; GSSG, glutathione disulfide; GR, glutathione reductase; NADPH, nicotinamide adenine dinucleotide phosphate.
The oxidation of selenol (-SeH) by H2O2 or other oxidants results in the formation of selenenic acid (-SeOH) which is reduced back to selenol (-SeH) by GSH via a two-step process involving selenenyl sulfide (-SeSG) as an intermediate. Intriguingly, high levels of OS or low concentrations of reduced glutathione (GSH) lead to the over-oxidation of selenenic acid (-SeOH) to seleninic acid (-SeO2H), which may also be reduced back to selenenic acid (-SeOH) by GSH.
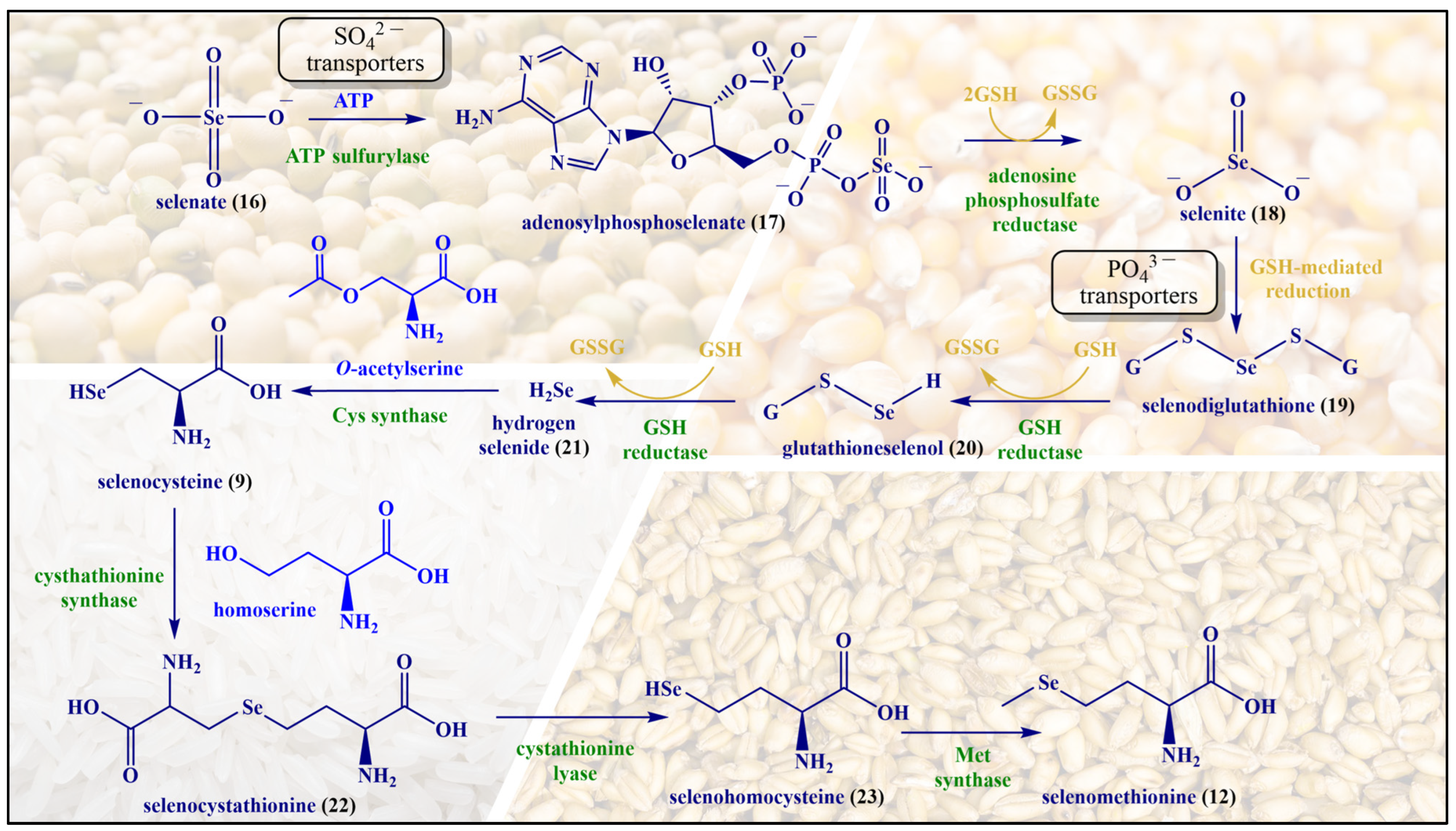
Although selenium is found in tens of proteins and enzymes, and there is a chemical analogy between Cys and Met on the one side and SeCys and SeMet on the other, the metabolism and natural metabolites of selenium tend to differ from the ones of sulfur. A few notable natural selenium metabolites, including glutathione selenotrisulfide (GSSeSG), methyl selenol ((CH3)SeH), dimethyl selenide ((CH3)2Se) and the trimethylselenonium ion ((CH3)3Se+), are formed as part of the selenium metabolism in animals and humans. These selenium compounds are not necessarily part of a natural chalcogen exchange series, as their sulfur and oxygen analogs are of less significance in animal metabolism. In contrast, the analogy between Met and SeMet is considerable, and enables animals and humans to incorporate SeMet more or less randomly in proteins and enzymes in positions designed for Met. In contrast to SeCys, humans and other animals are unable to synthesize SeMet (6) and obtain it from food sources [28]. SeMet (12) is naturally occurring in cereal grains, soybeans and grassland legumes providing, among others, a strong radioprotective effect [29,30]. The synthesis of SeMet (12) in plants begins with the uptake of selenate (SeO42−, 16) present in the soil via sulfate (SO42−) transporters of the plant root. The interaction of SeO42− with ATP results in the formation of adenosylphosphoselenate (17), which is subsequently reduced by adenosylphosphosulfate reductase to selenite (SeO32−, 18), followed by further reduction to selenide (Se2−, 21) by GSH via intermediates such as selenodiglutathione (19) and glutathioneselenol (20). Selenide (21) reacts with O-acetylserine to form SeCys (9), which later on interacts with homoserine in the presence of cystathionine synthase to produce selenocystathionine (22). The enzyme cystathionine lyase cleaves selenocystathionine (22) to selenohomocysteine (23), pyruvate and ammonia. Eventually,, methionine synthase produces SeMet (12) from selenohomocysteine (23) [28]. The biosynthesis of SeMet in plants is presented in Figure 5.
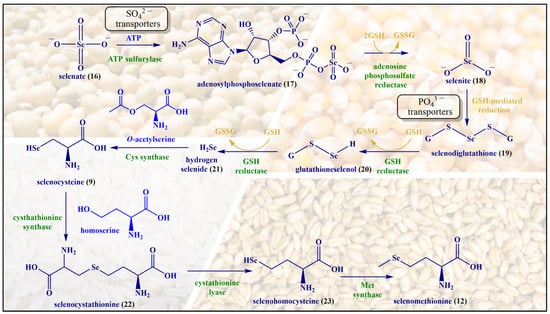
Figure 5.
The biosynthesis of SeMet (12) in plants.
The SeMet story in animals has an interesting twist to it, which is worth mentioning in passing. It is absorbed from the small intestine by a sodium-dependent transporter, binds with hemoglobin and accumulates in the liver and muscle, especially skeletal muscle, which accounts for approximately 28 to 46% of the total selenium pool [31]. Unlike SeCys, whose insertion into proteins is controlled extremely tightly, SeMet is incorporated into human proteins and enzymes non-specifically in concert with Met [32]. Once inserted into such proteins, SeMet provides considerable antioxidant protection [33]. In simple language, consumption of SeMet from natural sources may therefore be employed to “pimp” cellular proteins and enzymes with added antioxidant and redox catalytic features, a notion which has attracted and amazed nutritional scientists for decades.
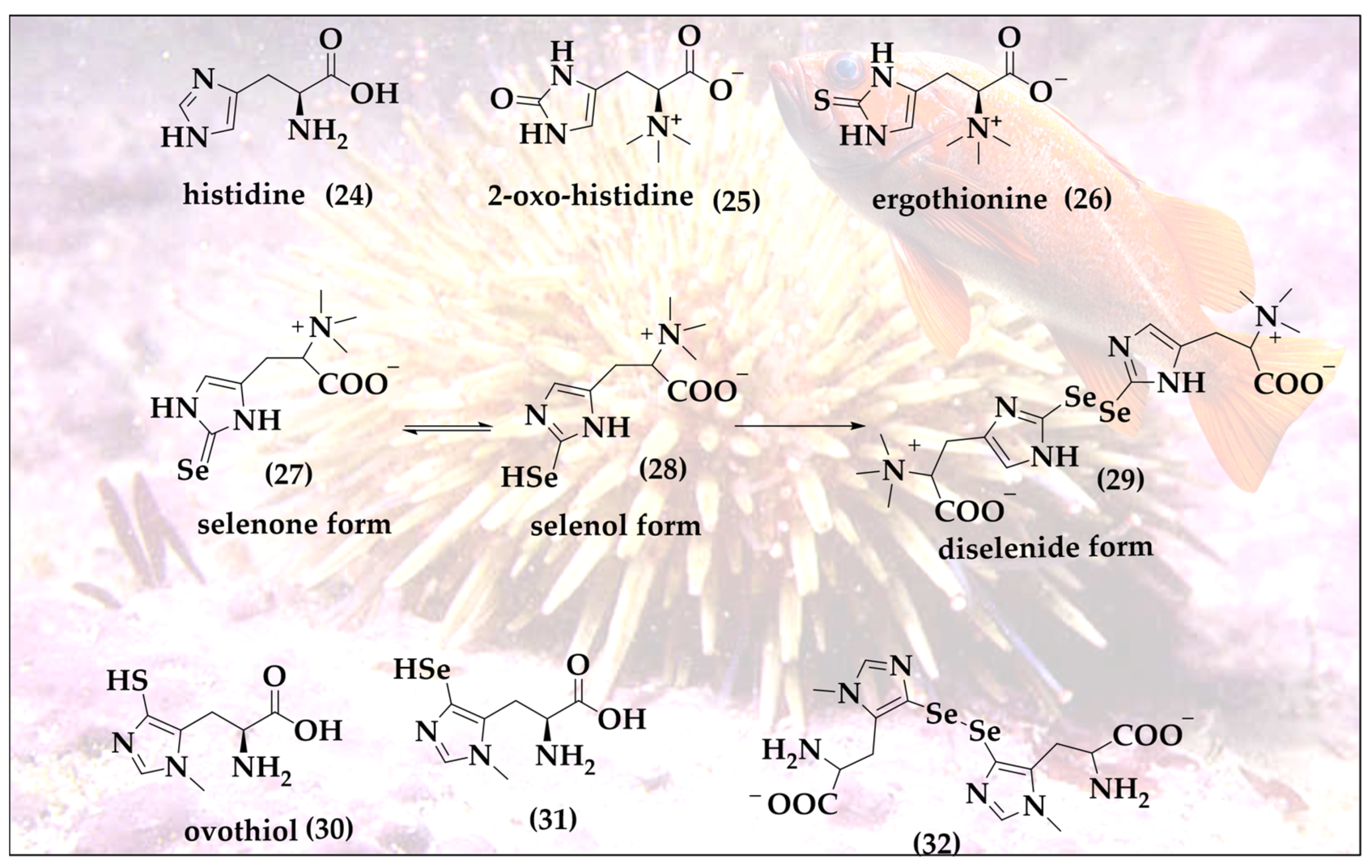
Selenoneine (SeN, 27) is the selenium analog of ergothioneine (ET, 26) and of 2-oxo-histidine (25); all three are based on the amino acid histidine (24) [34]. SeN is a powerful antioxidant and undergoes a specific selenol–selenone tautomerism with a distinct redox behavior associated with it (Figure 6) [35]. SeN and its oxidized diselenide form are at the center of catalytic activity. SeN exhibits a significantly higher antioxidant potential compared to ET. ET is very stable inside of mammals, dominated by the thione tautomer at physiological pH and a reasonable cellular antioxidant and its redox chemistry, thanks to the availability of the thiol tautomer allowing oxidative formation of disulfide bridges [36]. In a direct comparison, SeN by far outpaces ET, exhibiting a 50% radical scavenging concentration (RS50 value) in the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay at astonishing micromolar concentrations as compared to mM concentrations for ET [37].
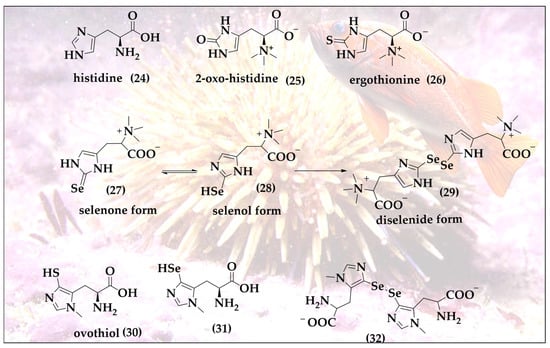
Figure 6.
SeN exhibits an unusual selenone (27)/selenol (28) tautomerism enabling efficient antioxidant action, binding to toxic metal ions, formation of diselenide bonds (29), interactions with thiols of the cellular thiolstat and also catalytic activity.
It should be emphasized that despite their similarities, chalcogen analogs, such as ET and SeN, are not synthesized or found in the same organism. ET is produced in ergot, a toxic fungus attacking rye, and also in edible mushrooms such as Agaricus bisporus, Lentinula edodes, Pleurotus ostreatus and Grifola frondosa [38,39]. In comparison, the sources of SeN are more limited and fishier. Initially isolated from the blood of tuna fish in 2010 by Yamashita et al., it is also found in various animal tissues, such as chicken heart, porcine kidney and tilapia blood [35]. Quite recently, it has also been found in the liver, kidneys, blood, brain and muscles of seabirds [40].
In tuna fish, SeN exhibits strong antioxidant activity by binding with haem proteins i.e., hemoglobin and myoglobin and thereby preventing the auto-oxidation of iron. This unique feature is central for tuna fish to endure in low-oxygen marine environments. The main source of SeN for humans is the daily diet. SeN is typically found in red blood cells, where it is considered as the major form of selenium and an important biomarker for selenium redox status in the red blood cells of fish-eating populations [41,42]. The occurrence of SeN in red blood cells is associated with the presence of carnitine/organic cation transporter (OCTN1), which are abundant in the cell membrane of the red blood cells [43].
Talking about mariners and marine organisms, it is also worth mentioning that quite a few other exotic sulfur compounds are also found in the sea, such as the ovothiols (30) present in sea urchins [44]. These ovothiols may represent one of the reactive sulfur species (RSS) serving as an incentive in the hunt for analogs, naturally occurring reactive selenium species (RSeS), with hypothetical structures (31 and 32), as shown in Figure 6. Indeed, one of the most exciting roles of oxygen- and sulfur-containing natural products is the opportunity to substitute these chalcogens for selenium and then either to hunt for the resulting hypothetical molecules in biology itself, or to simply prepare them in the laboratory.
3. Toolkit
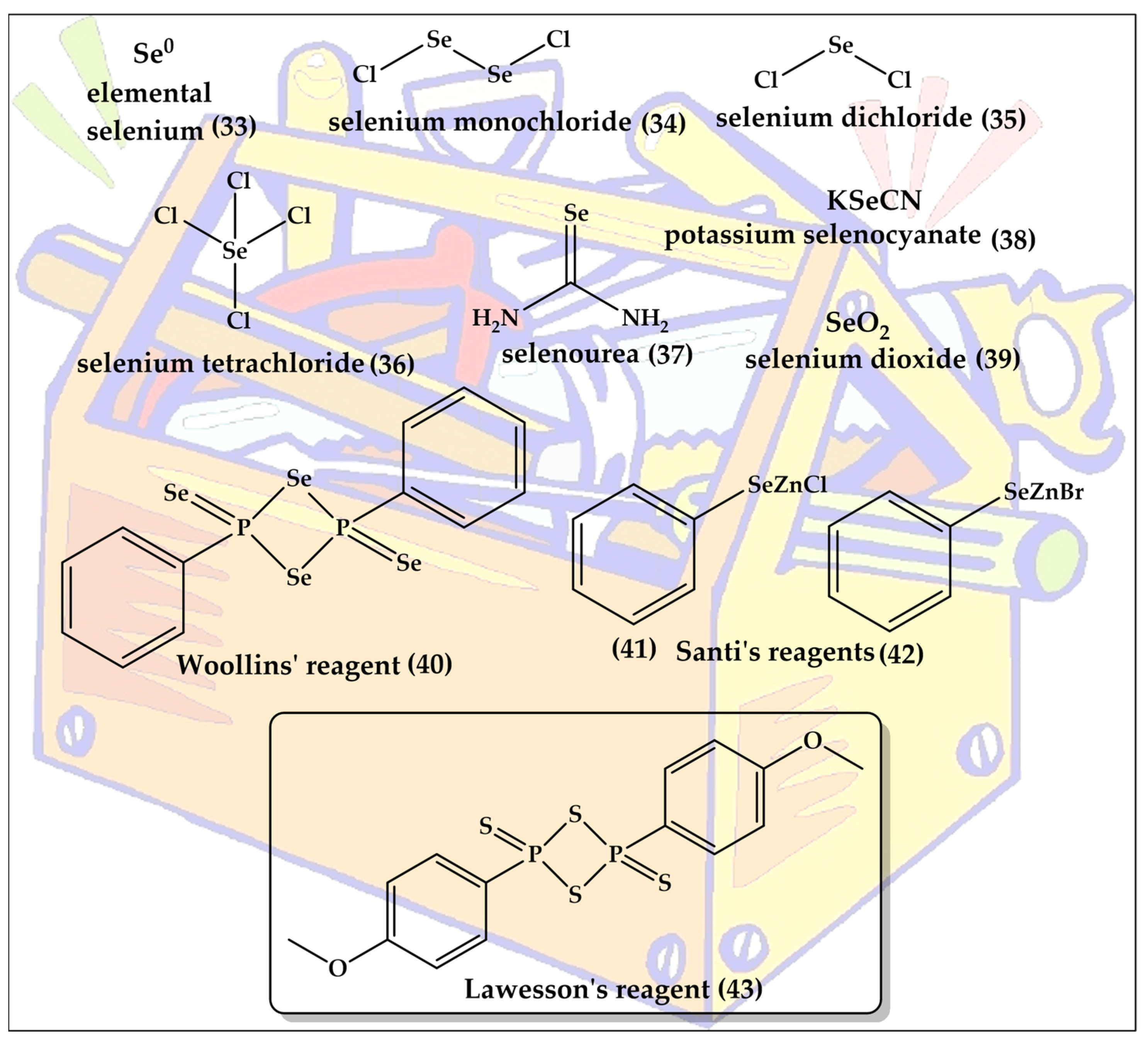
The expression “bioisosteric replacement” seems to imply that the chemistry behind the chalcogen exchange is exactly that, namely exchanging an oxygen or sulfur with a selenium atom in an already existing, and otherwise unaffected, molecule. Indeed, such direct exchange reactions are possible, for instance by methylating a thiol to a CH3S group ready to leave the molecule once a selenium nucleophile such as Se2− arrives on the scene [45]. There are specific strategies for such replacements in the literature, mostly involving extraction of the existing chalcogen atom as part of a newly formed leaving group and a nucleophilic sulfur or selenium taking its place [46,47]. In addition, specific reagents, such as Lawesson’s reagent and Woollin’s reagent, can be used for the direct replacement of oxygen with sulfur or of oxygen or sulfur with selenium, respectively [48,49]. These reagents are also shown in Figure 6 and are named after the Swedish chemist Sven-Olov Lawesson (1926–1985) and the British chemist John Derek Woollins.
Unfortunately, in many cases, such a direct replacement is difficult, and the synthesis fails because of the rather harsh reaction conditions required and undesired side-reactions. Indeed, the direct chalcogen exchange in rather sensitive and delicate molecules is a bit like extracting a blue tooth and replacing it with a pink denture in the absence of anesthesia.
It is therefore not surprising that most of the molecules presented in the following are not obtained by such direct replacements and chalcogen exchanges, but are synthesized from scratch with the sulfur or selenium inserted under the right conditions and regardless of whether there has been an oxygen atom before or not in that specific position. It should be noted that the synthesis of organo-selenium compounds has been discussed extensively in the literature and is beyond the scope of this review [50,51,52,53,54,55]. A brief list of commonly employed selenium reagents has been presented in Figure 7.
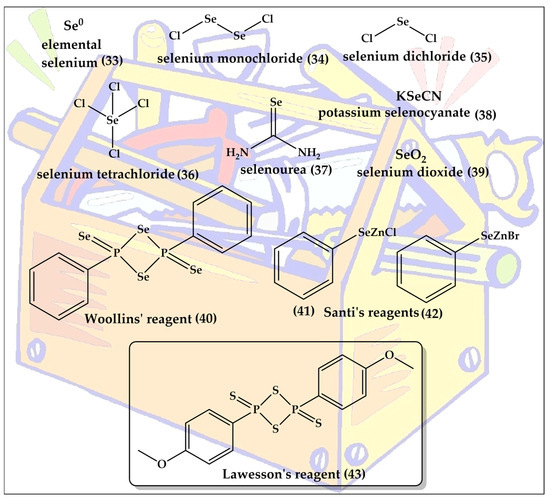
Figure 7.
The toolkit for the synthesis of organo-selenium compounds ranges in its composition from simple elemental selenium (33) to the rather complex Wollins’ reagent (40), which is the selenium analog of Lawesson’s reagent (43).
4. Blueprints and Blueberries
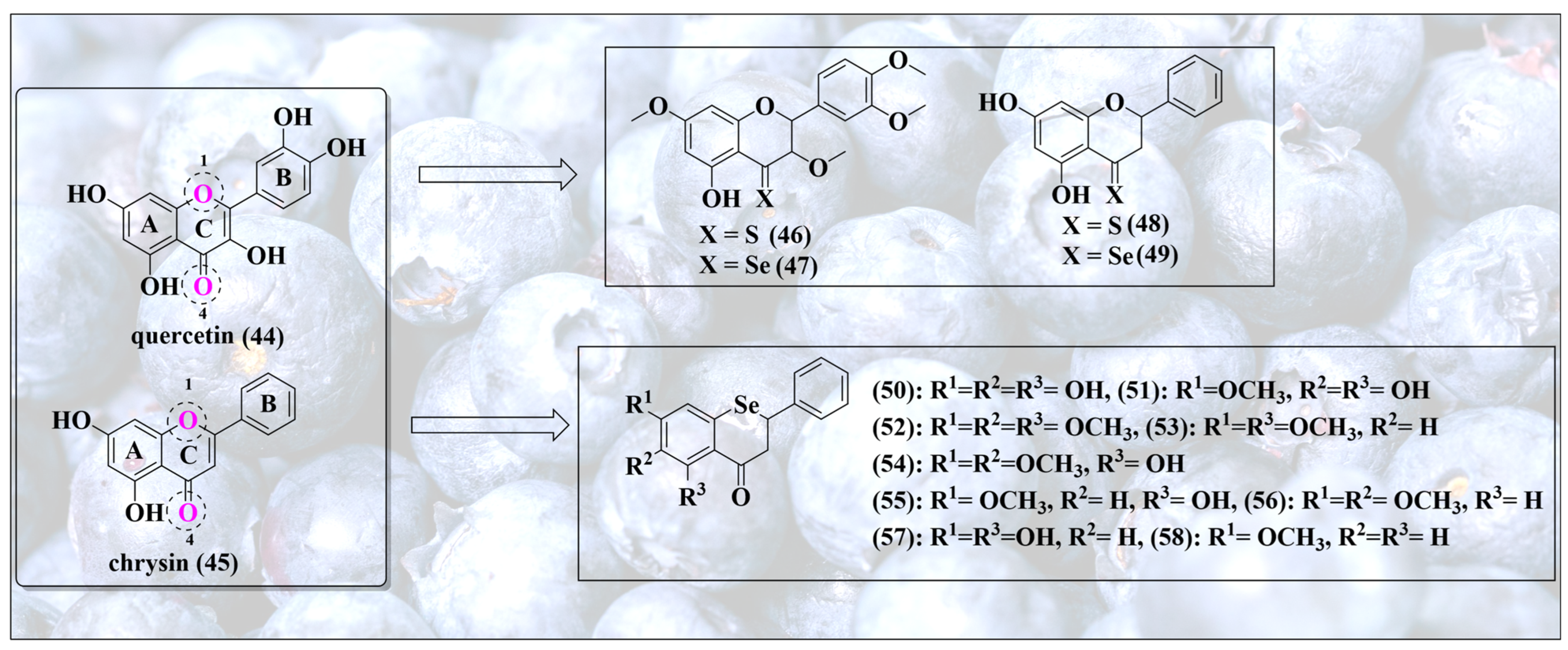
Healthy eating brings us to another group of powerful antioxidants, namely flavonoids, which naturally exclusively contain oxygen and encompass a range of structurally diverse and often highly active compounds, including flavones (e.g., apigenin), flavanones (e.g., hesperetin), isoflavones (e.g., genistin), flavonols (e.g., quercetin, myricetin), chalcones (e.g., phloretin), flavanols (e.g., catechins and epi-catechins) and anthocyanins (e.g., cyanidin) [56,57]. From a chemical perspective, these substances feature a similar scaffold in the form of oxygen-containing ring systems, yet differ in their respective substitutions and saturation patterns, as depicted in Figure 8. Together, these natural products provide ample opportunities for bioisosteric chalcogen exchanges. Some of the most popular sites for such replacements of oxygen for selenium are indicated in Figure 8 and include the oxygen atom inside the ring in position 1 and also the carbonyl function in position 4.
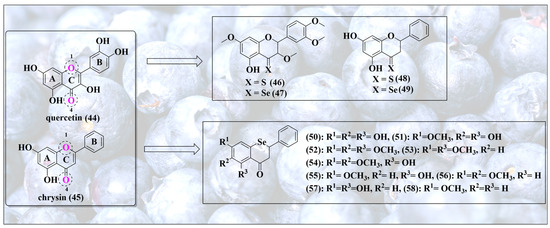
Figure 8.
Selenium-substituted flavonoids together with the natural products they are based upon. Please note that substitution of different oxygen atoms in the original secondary metabolites results in rather different types of selenium molecules, each with a specific redox activity.
Let us consider the carbonyl function at position 4 first, as it seems to be the more reactive, and thus interesting, one. The thione, as well as selenone, formed of natural flavonol quercetin (44) and flavone chrysin (45), for instance, have been prepared successfully (46–49) [58]. As predicted, switching from a carbonyl to a selenone group increases the antioxidant activity dramatically [58]. Notably, protection of hydroxyl groups of quercetin decreases this activity, indicating that the redox activity in these molecules is not—exclusively—linked to the presence of the selenium atom [58]. Indeed, the redox activity of the natural quinoid ring system B is dominant and controls biological activity; therefore, protection of one or more of the relevant OH groups abolishes most of this activity.
In stark contrast to the oxygen (44,45) and sulfur analogs (46,48), these selenium analogs (47,49) also exhibit excellent catalytic glutathione peroxidase (GPx)-like activity, reducing dithiothreitol (DTTred) in the presence of H2O2 at rates comparable to naphthyl selenourea and sugar-derived compounds [58,59]. This is not surprising as selenocarbonyls, i.e., selenones, differ considerably in their reactivity from carbonyls, and also from thiones. As one form of the selenol/selenone tautomerism, the selenol is also catalytic and may enter the respective catalytic cycle either as anti- or pro-oxidant, as already seen for SeN in Figure 6.
In biological cell-based assays, compound 49 inhibits thioredoxin reductase (TrxR) and diminishes the intracellular glutathione concentration in MCF-7 breast adenocarcinoma cells, thus de facto acting in these cells as an (indirect) pro-oxidant, interfering with the enzymatic antioxidant defense and the cellular thiolstat [58].Consequently, compound 49 may overcome cisplatin and multidrug resistance [58].
Besides the carbonyl oxygen in position 4, the pyran oxygen in position 1, i.e., inside the C ring, has also been replaced by selenium. Choi et al. have reported an impressive series of such selenium substituted flavonoids (50–58) [60]. Interestingly, this replacement of a more or less bystander oxygen generally does not increase the antioxidant capacity, as demonstrated in SHSY5Y cells treated with these oxygen and selenium compounds. Nonetheless, besides a similar antioxidant capacity, compound 51 has some advantages when compared to its oxygen analog, including a decreased polarity and increased lipophilicity [60,61]. Compounds 56 and 58 have been evaluated for biological activity in vivo in ischemia–reperfusion in mice. These compounds reduce the total infarction volumes in the ipsilateral hemisphere by 45% and 41%, respectively, thereby presenting much better neuroprotection compared to the original flavanones [60].
These observations are in line with a general trend observed for selenium in such ring systems, as such compounds often provide very disappointing redox and catalytic activities, yet still some good biological activity.
5. Selenium-Substituted Vitamin E
The structure and redox behavior of α-tocopherol, commonly referred to as vitamin E, is not dissimilar from the one of some of these flavonoids, with one oxygen inside at position 1 and one hydroxyl group outside a two-ring system in position 6. Vitamin E is an efficient radical scavenger able to donate a hydrogen atom H● or electron, subsequently stabilizing the tocopheryl radical in the quinoidal redox system, as depicted in Figure 5 [62,63].
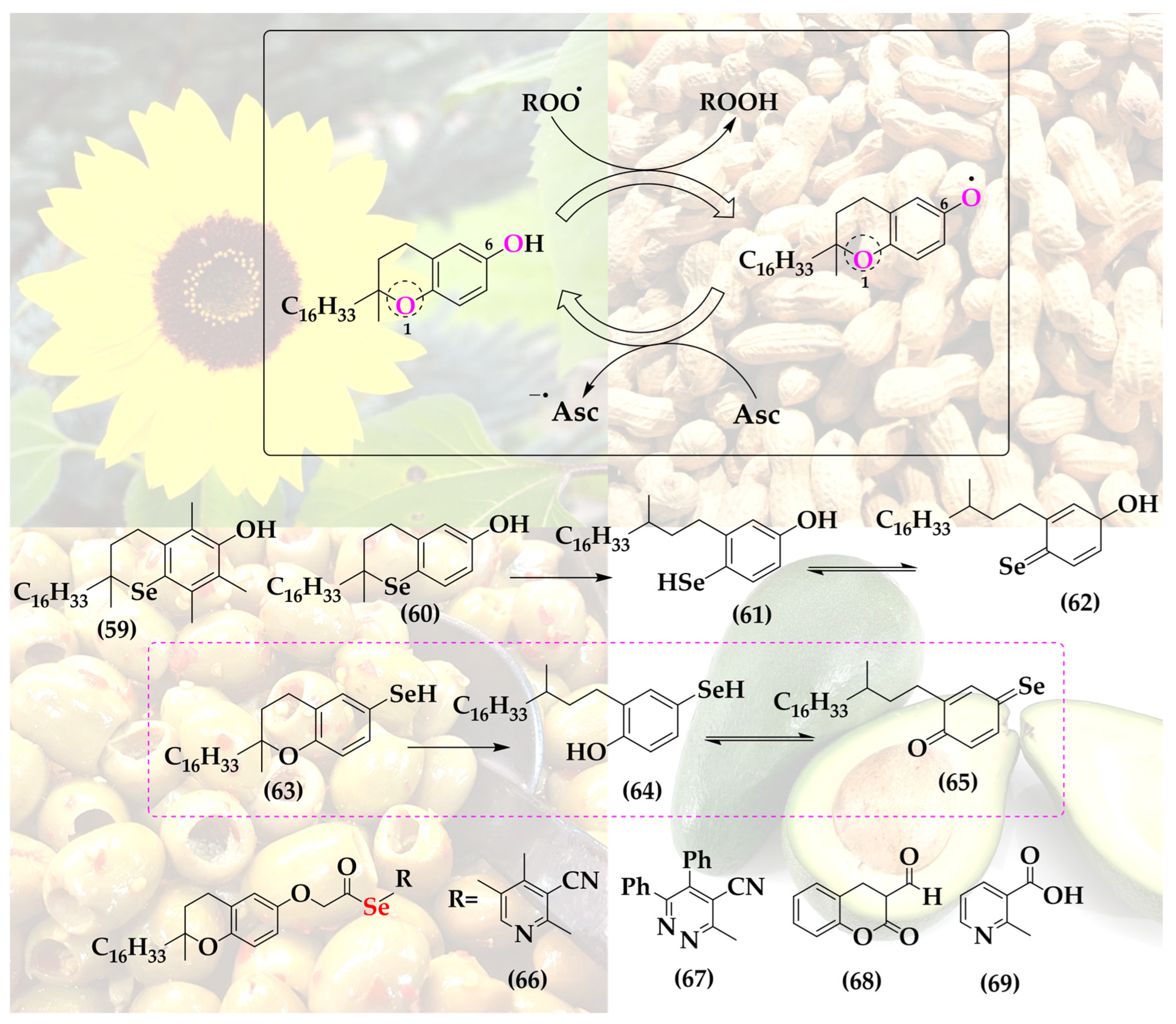
Since the more versatile selenium in the place of oxygen may provide additional stabilization or possibly also catalysis, a few selenium-substituted vitamin E analogs have been reported in the literature, including compounds 59 and 60, de facto manifestations of an interesting and otherwise unstable selenoquinone redox system (Figure 9).
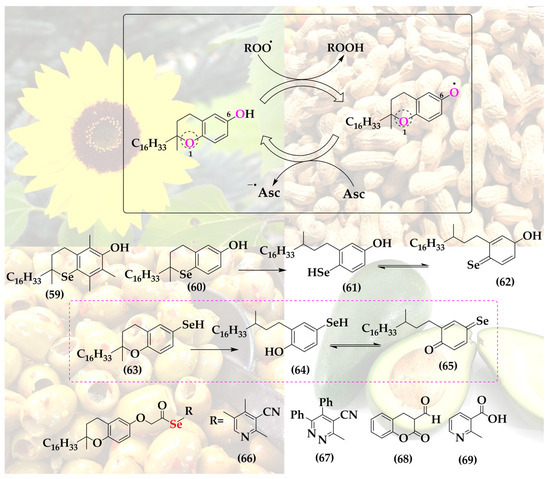
Figure 9.
The structure of Vitamin E provides two possible sites for the isosteric replacement of oxygen with selenium, as highlighted in the main structure.
These two selenium analogs, prepared by Al-Maharik et al., exhibit good antioxidant activity, albeit slightly lower compared to the original vitamin E and also not amenable to redox cycling, as may have been expected for selenium [64]. Oxidized α-selenotocopherol cannot be reduced by common reductants; hence, the hypothetical catalytic cycle stalls at the oxidized form, as has been noticed in a two-phase lipid peroxidation model [65].
A substitution of oxygen for selenium at the more reactive and thus interesting position 6 does not seem to have been reported so far (hypothetical structures of compounds 63–65). Then again, vitamin E has been coupled to selenium-containing groups via esterification at that specific hydroxyl group. Among these “add-on” selenium versions of vitamin E are, for instance, a range of compounds synthesized by Abdel-Hafez et al. [66]. This series includes vitamin E coupled to selenated pyridine (66), pyridazine (67), coumarin (68) and nicotine (69) [66]. These substances, in particular compound 69, exhibit significantly increased anticancer activity in a cell culture model of human MCF-7 breast cancer cells [66].
Notably, the complimentary antioxidant actions of the lipophilic radical scavenger vitamin E on the one side and the reducing, often catalytic selenium atom on the other have also attracted rather simpler solutions of combining both, for instance in form of vitamin E samples enriched with selenium. Such a cocktail is able to induce apoptosis in cultured human prostate cancer cells by changing the Bax/Bcl-2 ratio towards apoptosis [67]. This example of simply mixing two natural products—rather than combining their active redox moieties in a complicated, and clearly no longer natural, new agent—provides an argument against the synthetic approach discussed here. Eventually, in order to justify such hybrid molecules, one may therefore need to provide additional, possibly synergistic features.
6. Selenium-Containing Coumarins
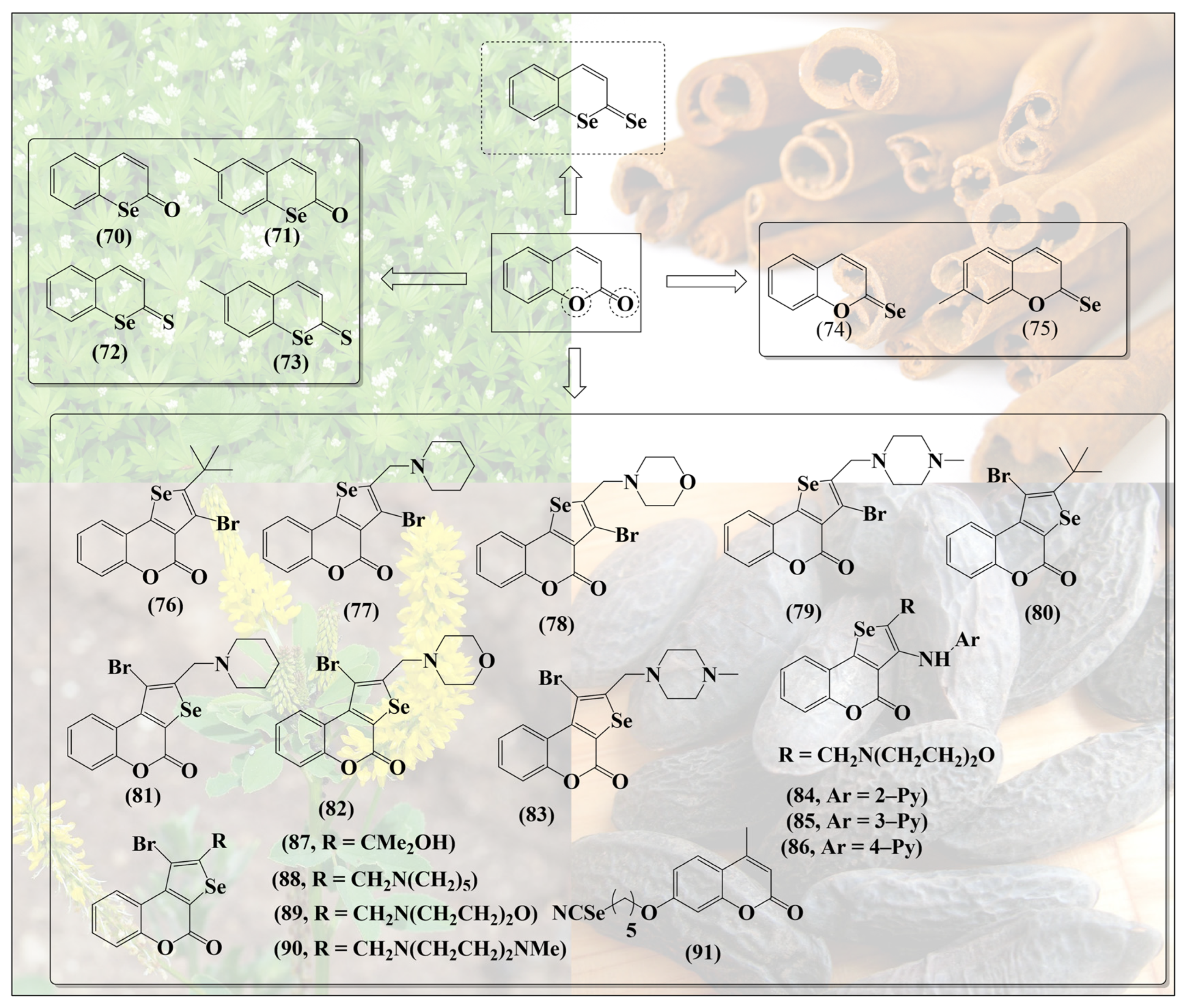
Such synergistic features may be expected in simple molecules such as selenium-based coumarins. In contrast to the ether-/pyrane-like structures discussed so far, coumarins are basically cyclic esters, implying that substitution of the relevant oxygen for sulfur and specially selenium may result in less stable and more reactive thioesters and selenoesters. Angeli et al. have synthesized a range of such selenium-containing coumarin derivatives (70–73, Figure 10) by substituting oxygen inside the ring for selenium [68]. Selenocoumarin 70 exhibits selective cytotoxicity against human prostate (PC3) and breast (MDA-MB-231) cancer cell lines [68]. Notably, compounds 72 and 73 feature a further substitution of the carbonyl oxygen for sulfur—albeit not for selenium. These coumarin-based selenium compounds have been reported to exhibit high selectivity and inhibitory action against carbonic anhydrase, an enzyme often associated with tumors [68]. Intriguingly, substitution of the carbonyl oxygen alone has also been reported in the literature (74,75. Figure 10) [69]. So far, compounds with substitution of both the oxygen atoms of coumarin with selenium, as shown at the top in Figure 10, do not seem to have been reported.
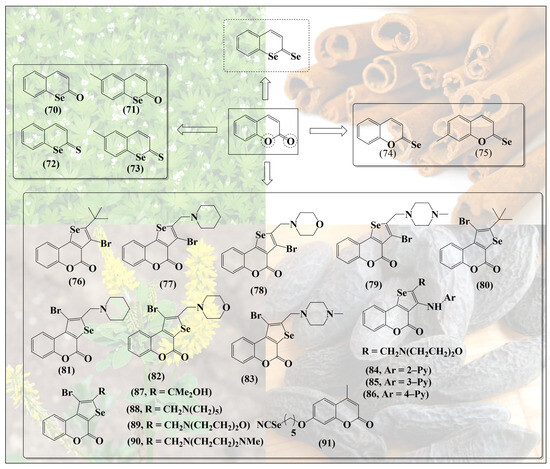
Figure 10.
Substitution of different oxygen atoms in the original secondary metabolites results in rather different types of selenium molecules, each with specific redox activity. Coumarins also lend themselves to the synthesis of add-on molecules.
Since substitution of oxygen atoms in coumarins for sulfur and especially for selenium is a challenging piece of synthesis, other teams have focused on the addition of selenium-containing substituents to the coumarin moiety, for instance in agents such as 76–83 (Figure 10). As expected, these “add-on” compounds are redox active and modulate the levels of reactive oxygen species (ROS) in cells, thereby influencing caspase activity and inducing apoptosis in certain cancer cell lines [70].
Similar selenium-substituted coumarins, such as 84–90, present antioxidant activity, selectively inhibit metalloproteinase MMP enzymes (MMP-1 and MMP-4) and prevent angiogenesis in several in vitro and in vivo models. Despite their antioxidant activities, they are also rather cytotoxic when compared to inorganic sodium selenite (Na2SeO3) in different cancer cell lines [71]. These, at first contradictory, activities result from the concurrent removal of ROS on the one side and depletion/consumption of GSH on the other, which obviously has a major impact on the cellular thiolstat of these cells.
It should also be mentioned that selenium and coumarins have been combined in and to more eloquent molecules, such as methyl-substituted umbelliferon selenocyanate (MUS) 91, a compound exhibiting adjuvant activity by counteracting the production of ROS and reactive nitrogen species (RNS) and restoring the glutathione redox pool. In cell culture, MUS has been reported to reduce the toxicity of carboplatin by decreasing chromosomal aberrations and micronuclei formation in order to protect DNA from damage in normal bone marrow cells and to induce apoptosis in damaged cells [72]. These multifunctional agents are based on individual “building blocks” and therefore reflect a different type of hybrid molecule chemistry, which often lacks the kind of synergy frequently provided by bioisosteric replacement.
7. Selenium-Substituted Sugars
Compared to the more or less stable natural scaffolds discussed so far, sugars such as glucose are, in fact, quite labile aldoses formed by the cyclization of an aldehyde with an alcohol to what is effectively a five-membered furanose or six-membered pyranose ring. From the perspective of bioisosteric replacement, these sugars represent a true challenge, as neither the seleno-aldose, nor the selenoaldehyde, or indeed the selenol per se, are particularly stable, especially under physiological conditions, i.e., in aqueous media and in the presence of dioxygen. It is, therefore, not surprising that there are no direct selenium analogs of glucose and related hexoses or pentoses, as may be naïvely anticipated, for instance in Figure 11.
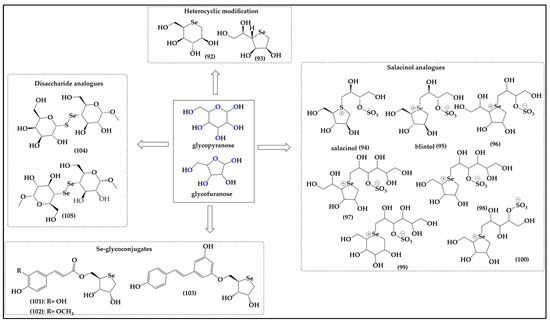
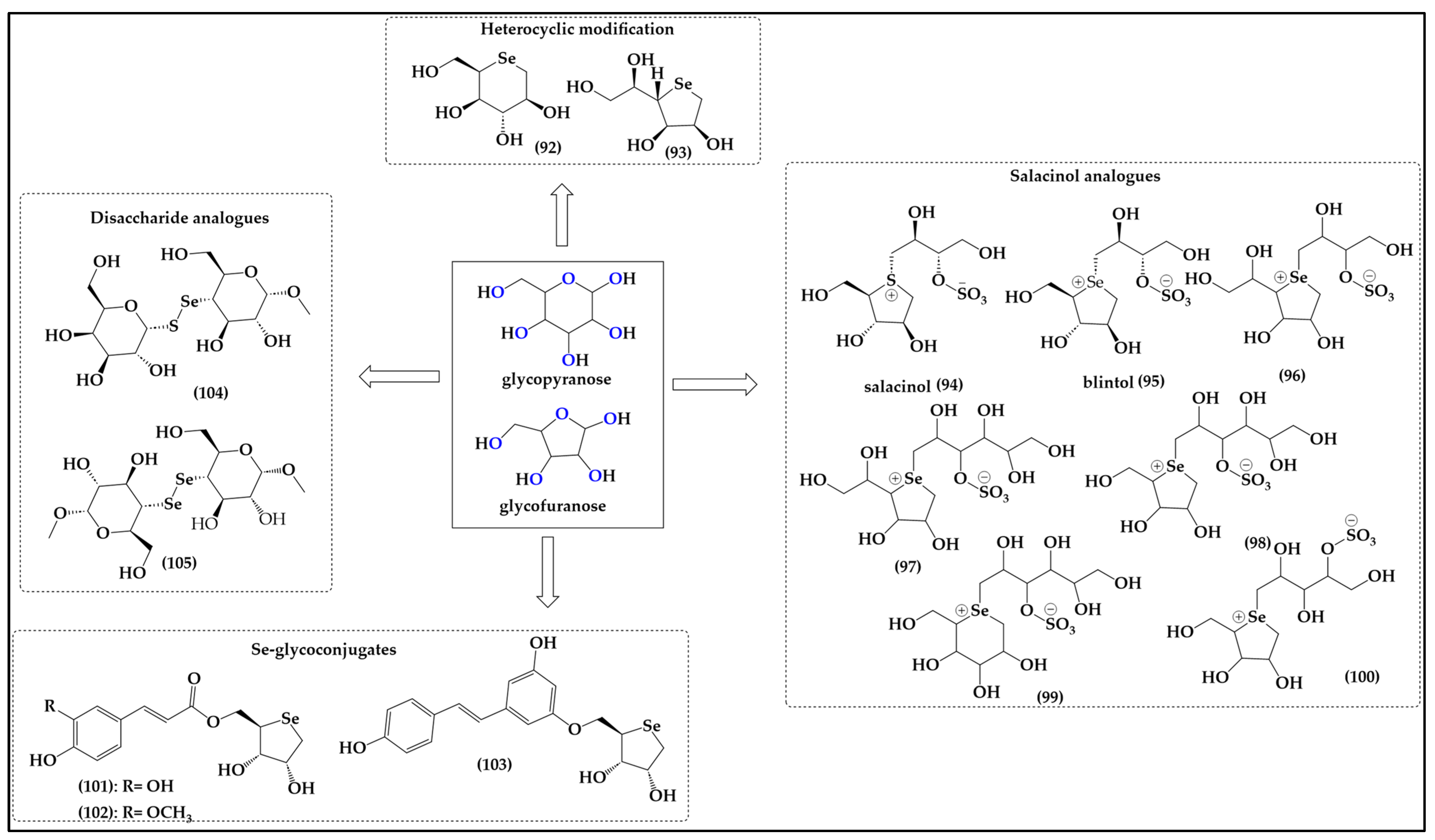
Figure 11.
Selenium-substituted sugar-like structures offer the possibilities of replacing the oxygen either inside the furan or pyran ring (92–103) or outside the ring in the form of diselenide or selenium–sulfide linkages in disaccharides (104,105).
Nonetheless, several teams of researchers have tried to circumvent this issue of instability by synthesizing more stable cyclic selenoethers resembling such sugars, albeit void of the labile functionalities, as also exemplified in Figure 11. Due to the sugar-like moiety in these molecules, such compounds are also rather soluble in aqueous media and permeable membranes, since they are non-ionic and frequently bioavailable [73].
Similarly to the coumarins, such sugars provide two major positions for bioisosteric replacement, namely the oxygen atom inside the furane/pyrane ring and one or several of the free hydroxyl groups of the sugars outside the ring, as depicted in Figure 11. As far as ring oxygen is concerned, moving from oxygen to sulfur requires adjustments for stability, as discussed already, and the sulfur and selenium analogs are, therefore, often dideoxy-seleno sugars, and no longer esters yet ethers, basically cyclic sulfides R2S and selenides R2Se.
These motifs are not that different from the ones found in Met and SeMet, respectively. The relevant compounds have been reported in the literature, for instance Storkey et al. describing the synthesis of 1,5-dideoxy-5-seleno-L-iditol (92) and 1,4-dideoxy-4-seleno-D-talitol (93) [74]. These seleno-hexose and seleno-furanose sugar-like structures are soluble in aqueous media and provide dose-dependent protection against HOCl-mediated oxidation of bovine serum albumin (BSA) and diluted human plasma samples [74]. They also prevent the formation of 3-chlorotyrosine (3-Cl-Tyr) in human plasma and mediate the detoxification of HOCl and N-chloramines in both isolated BSA and human plasma proteins, thus acting as effective antioxidants [74]. Seleno-furanose 93 is apparently fairly resistant to metabolic processing after oral administration, and exhibits an impressive range of such antioxidant properties in vitro and in vivo, including the protection of isolated primary human coronary artery endothelial cells (HCAEC) and smooth muscle cells (HCASMC), vaso-protection, antioxidant protection of isolated aortae of mice, repairing of damaged skin and enhanced healing of diabetic wounds [73,74,75].
Interestingly, the bioisosteric replacement of oxygen for selenium in such furanose-like structures adds an additional feature. In contrast to oxygen, which is mostly albeit not always divalent in organic molecules—anthocyanidines serving as one exception to this rule—sulfur, and especially selenium, are quite amenable to higher valencies and a positive charge in such organic molecules. Attempts to synthesize selenium analogs of the natural sulfur compound salacinol (94) isolated from Salacia reticulata, for instance, have yielded sugar-like charged selenium compounds 95–100 with interesting, literally sweet activities (Figure 7) [76,77,78]. Salacinol itself is employed in folk medicine to treat diabetes as it delays absorption of glucose and lowers blood glucose levels [79,80]. The selenium atom in the selenonium (R3Se+) moiety is de facto redox inactive, and it is therefore not surprising that despite the presence of selenium, there is no additional activity endowed on these molecules. Nonetheless, some of the original activity of salacinol in glucose metabolism has been maintained by the selenium analogs of salacinol such as blintol (95) and compound 98, confirming that the bioisosteric replacement of sulfonium for selenonium at least has no major negative impact on activity [81].
Notably, compound 100 also inhibits the recombinant human maltase glucoamylase, which is one of the most critical intestinal enzymes involved in the breakdown of glucose oligosaccharides in the small intestine, an activity which may be of interest in the field of glucose uptake, metabolism, fasting and possibly weight reduction [82]. Then again, compounds 96 and 97 are inactive against the recombinant human maltase glucoamylase (MGA), another critical intestinal glucosidase involved in the processing of oligosaccharides of glucose into glucose itself [82,83].
Intriguingly, Serpico et al. have reported the synthesis of Se glycoconjugates consisting of selenium-containing monosaccharides bound to a phenolic moiety (101,102) [84]. Compounds 101 and 102 provide promising radical scavenging, wound healing, and cell uptake properties. A preliminary cytotoxicity assessment on HaCaT and SH-SY5Y cell lines has shown the absence of basal toxicity below a 100 μM treatment dose, suggesting these compounds as promising candidates for further exploration in cosmeceutics [85]. Intriguingly, a dose dependent repair capacity of compounds 101 and 102 has been observed which is comparable to the corresponding hydroxycinnamic acids even at low doses. The design and the development of a new molecule for transdermal delivery of antioxidants has been successively patented by the same group for a single compound containing the resveratrol moiety (103) with the aim of optimizing the delivery of Se compounds directly to the wound site where their antioxidant properties can mitigate OS and support the healing process [86].
As discussed already, sulfur and selenium, once locked up and flanked by carbon atoms inside such ring systems, are surprisingly inactive as far as redox and catalytic properties are concerned. As a consequence, if one wants to bestow sugars with the classical selenium redox behavior and catalytic activity, substitution of one or more of the free hydroxyl groups for selenols may be the best option. To date, Chakka et al. have reported the synthesis of disaccharide analogs 104 and 105 comprising a diselenide and selenosulfide linkage, respectively, although they fell short of providing any antioxidant data or biological activity associated with them [87].
8. Selenium-Containing Esters of Polyphenolic Acids
Structurally, these furanose and hexose sugars are not that dissimilar from the more aromatic polyphenolic antioxidants, such as caffeic acid (106) and ferulic acid (107), and their respective esters, which are widely distributed in the plant kingdom [88,89]. These compounds have been associated with multiple functions in plants, mostly in plant mechanisms of stress tolerance. Caffeic acid is employed by plants in the synthesis of lignin, which ultimately thickens cell walls and protects plants from toxicity and heavy metal stress [90,91]. In addition, it shields mesophyll cells under drought stress against high-energy radiation via the production of ferulic acid [91,92,93]. In mammals, including humans, these plant secondary metabolites also exhibit a range of physiological activities, including antimicrobial and antioxidant activities [94,95,96]. Moreover, such secondary metabolites increase the production of collagen, prevent premature aging, decrease insulin resistance and exhibit certain chemo-preventive and antimutagenic activities, to name just a few effects [97,98,99,100,101,102,103,104].
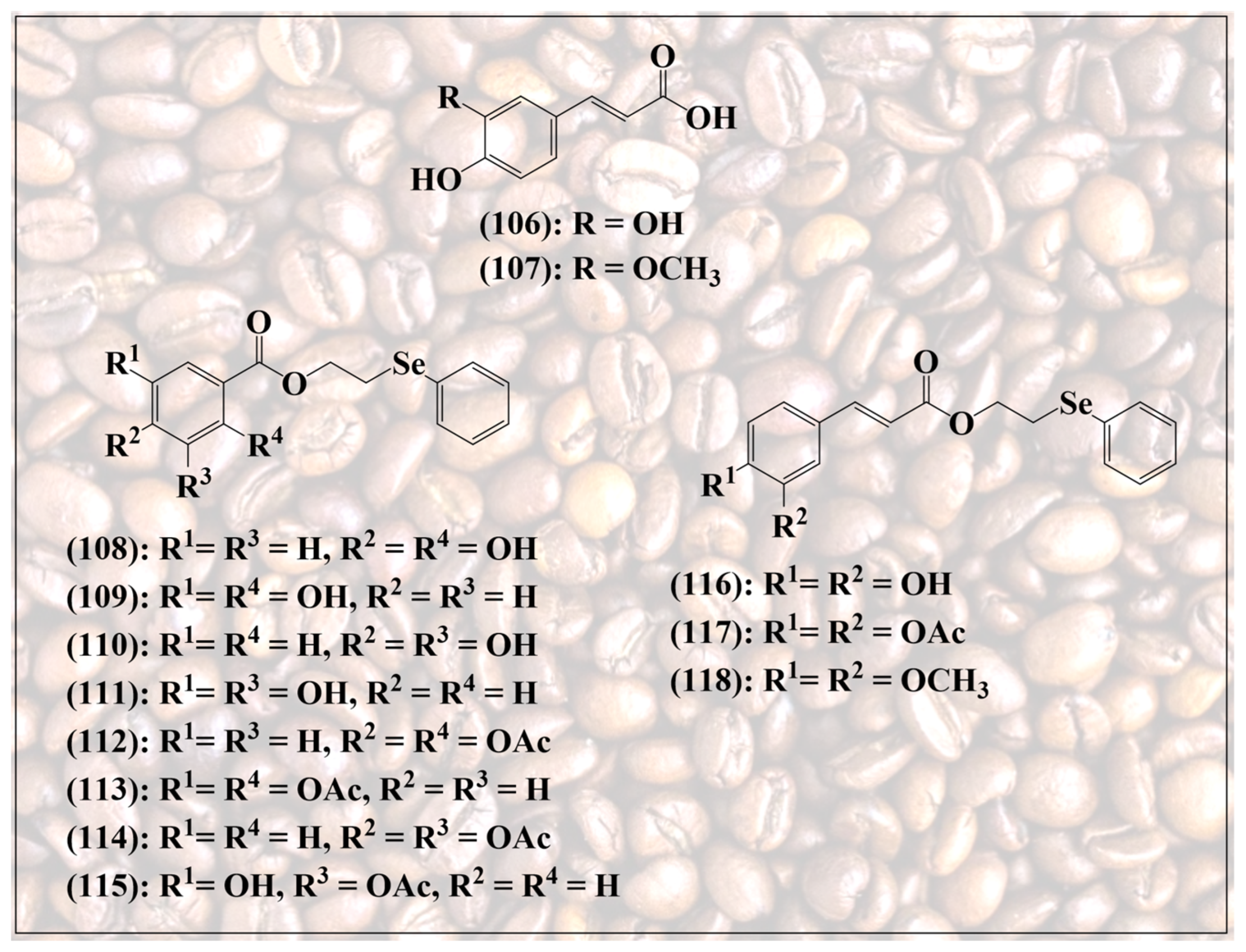
Similar activities have been observed for the esters of these polyphenolic compounds. Caffeic acid (3,4-dihydroxycinnamic acid) phenethyl ester (106, Figure 12), for instance, de facto a reduced form of an ortho-quinone, is one of the most active components of propolis from honeybee hives, exhibiting, for instance, anti-inflammatory, immunomodulatory, antiviral and anticancer activities in vitro, and preventing the proliferation of different types of transformed cells in cell culture, including the induction of apoptosis in non-tumorigenic human osteogenic sarcoma (N-HOS) cells [105,106,107,108,109,110].
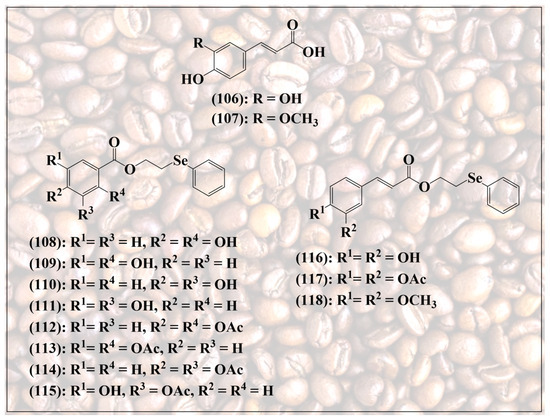
Figure 12.
Selenium-substituted esters of natural phenolic acids, including caffeic acid (106) and ferulic acid (107).
Since esterification provides a handle on synthetic modification of these acidic natural products, a wide range of synthetic analogs based on caffeic acid have been synthesized, some of them also containing “add-on” selenium, as depicted in Figure 12. These molecules often retain the original redox activity of their natural counterparts, and on occasion also present improved pharmacodynamic and pharmacokinetic properties, such as increased solubility in aqueous media and higher antioxidant activity [111,112].
Lin et al., for instance, have reported the synthesis of various selenium-containing esters of caffeic acid and ferulic acid, together with a range of comparable quinoidal structures (108–118) [113]. As expected, compounds with a free ortho-quinone redox system, such as 108–111,115 and 116, exhibit excellent antioxidant activity. In contrast, “freezing” this redox system by replacing the hydroxy groups with methoxy groups, as in compound 118, or also by adding an acetyl group as in compounds 112–114 and 117, significantly reduces this antioxidant activity measured in the DPPH assay. In different assays indicative of antioxidant activity, such as the 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH) assay measuring the inhibition of lipid peroxidation by this radical, the selenium analogs provide a slightly higher antioxidant protection compared to caffeic acid itself. Compounds 109 and 118, for instance, achieve an 87.7% and 80.2% inhibition of peroxidation, respectively, compared to 76.9% for caffeic acid [113].
To round this up by physically mixing rather than synthetically fusing selenium with polyphenols, a cocktail based on selenium compounds, such as SeMet and methylselenocysteine (MeSeCys), and polyphenols isolated from green tea, provides no additional activity compared to the selenium-free tea extract [114].
9. Conclusions
Our overview of selenium substituted natural products, albeit in many aspects incomplete, has demonstrated the enormous scope of such bioisosterically “enforced” compounds of the chalcogen exchange. Since most biologically active secondary metabolites contain oxygen in one place or another, they are prone to such a synthetic replacement involving sulfur and selenium, in order to add extra redox activity and—in the case of selenium—also redox catalysis. Indeed, different oxygen-based moieties present in such natural substances are amenable to replacement, from simple hydroxyl groups readily exchanged for thiols and selenols to ethers representing hypothetical placeholders for sulfides and selenides. In some instances, such as in the case of SeCys and SeMet, nature itself already provides us with the complete series of the chalcogen shamrock. In other instances, such a replacement may whet the appetite to look closer at nature’s treasure chest to see if the sulfur and selenium analogs may not be found there one day in one place or another, such as in the case of flavonoids and coumarins, where synthesis is achievable, albeit challenging. There are also many instances in which nature neither provides the respective chalcogen analogs, nor indicates that they may be found there anytime soon, yet gives us the inspiration and blueprints for their synthesis. In any case, sulfur and selenium are more colorful than oxygen. In some cases, this exchange also provides a few extras, such as sulfonium and selenonium ions. In addition, selenium compounds are not just “active”, but they may also be considered as nutritional supplements for this trace element selenium, as is already the case for SeMet [16,28,115,116]. Selenium deficiency can be quite detrimental to human health, as many studies have shown on a global scale. Indeed, selenium deficiency has been reported to deter the immune system from fighting viruses such as influenza, Ebola, the human immunodeficiency virus (HIV) and coronavirus [117,118,119,120,121]. Here, selenium strengthens the immune response by proliferation and differentiation of naive CD4-positive T lymphocytes toward T helper 1 cells, improves cardiac function in HIV infected patients suffering from cardiomyopathy and decreases progression and mortality caused by HIV [122,123,124]. Some reports have even suggested that Na2SeO3 protects against the Ebola virus as it oxidizes thiols and hence prevents the virus from entry and further intracellular actions [125].
Then again, if and how a chalcogen exchange is superior to simply physically mixing oxygen-containing natural products with sulfur agents and a pinch of selenium, for instance in form of Na2SeO3 or SeMet, needs to be shown. The chemical synthetic strategies have limitations when it comes to sensitive and complex compounds where one could employ genetic engineering for incorporating sulfur and selnium into proteins and enzymes. Such an approach may provide a more precise and efficient pathway for modifying structure and function tailored to the physiological context.
Regardless of this, forging our way through the jungle of plants and their active oxygen-rich secondary metabolites and other ingredients is a promising avenue to produce new and exciting sulfur and selenium compounds inspired by nature. Some of them may provide extra activity and synergism; others may be more for the record. So let’s put on our pink glasses and leave the blues behind.
Author Contributions
Conceptualization, C.J. and M.J.N. writing—original draft preparation, C.J. and M.J.N.; writing—review and editing, W.A., E.N.d.S.J., R.S.Z.S., C.G., J.H. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the support provided by the University of Saarland, Saarbruecken, Germany. E.N.d.S.J would like to thank CNPq, Capes and Fapemig for their support. The authors would also like to thank Sodomir Popojuk and Ken Rory for their advice, discussions, and proof-reading of the manuscript. Special thanks go to many other colleagues from the Academiacs International network (www.academiacs.eu) (accessed on 9 May 2024) and “Pharmasophy” for their helpful discussions and advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Číž, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes. Antioxidants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M. Dietary Antioxidants and Chronic Diseases. Antioxidants 2023, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Dueñas, M. Applications of Natural Products in Food. Foods 2021, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Katidi, A.; Pavlopoulou, A.; Vlassopoulos, A.; Kapsokefalou, M. The Nutritional Composition of Natural and Organic Branded Food Products: A Cross-Sectional Analysis of the Greek Foodscape. Nutrients 2022, 14, 808. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically Active Potent Dietary Supplements. BioMed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Moghadasian, M.H. Nutraceuticals and Nutrition Supplements: Challenges and Opportunities. Nutrients 2020, 12, 1593. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The Impact of Selenium Deficiency on Cardiovascular Function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Wang, F.; Sun, N.; Zeng, H.; Gao, Y.; Zhang, N.; Zhang, W. Selenium Deficiency Leads to Inflammation, Autophagy, Endoplasmic Reticulum Stress, Apoptosis and Contraction Abnormalities via Affecting Intestinal Flora in Intestinal Smooth Muscle of Mice. Front. Immunol. 2022, 13, 947655. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the Health-Promoting Effect of Adequate Selenium Status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.F.; Lima, D.B.; Alves, D.; Jacob, R.G.; Perin, G.; Lenardão, E.J.; Savegnago, L. Synthesis, Characterization and Antioxidant Activity of Organoselenium and Organotellurium Compound Derivatives of Chrysin. New J. Chem. 2015, 39, 3043–3050. [Google Scholar] [CrossRef]
- Kharma, A.; Jacob, C.; Bozzi, Í.A.O.; Jardim, G.A.M.; Braga, A.L.; Salomão, K.; Gatto, C.C.; Silva, M.F.S.; Pessoa, C.; Stangier, M.; et al. Electrochemical Selenation/Cyclization of Quinones: A Rapid, Green and Efficient Access to Functionalized Trypanocidal and Antitumor Compounds. Eur. J. Org. Chem. 2020, 2020, 4474–4486. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Capelo, J.L.; Lodeiro, C.; Santos, A.A.D. A Selective Emissive Chromogenic and Fluorogenic Seleno-Coumarin Probe for Cu2+ Detection in Aprotic Media. Photochem. Photobiol. Sci. 2017, 16, 1174–1181. [Google Scholar] [CrossRef]
- Mániková, D.; Medvecová Letavayová, L.; Vlasáková, D.; Košík, P.; Castellucci Estevam, E.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular Diagnostics: Hunting for the Mode of Action of Redox-Modulating Selenium Compounds in Selected Model Systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef]
- Ozbek, N.; Baysal, A. Determination of Sulfur in Human Hair Using High Resolution Continuum Source Graphite Furnace Molecular Absorption Spectrometry and Its Correlation with Total Protein and Albumin. Spectrochim. Acta Part B At. Spectrosc. 2017, 130, 17–20. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Huang, Y.; Li, J.; Cao, D.; Chen, Z.; Li, J.; Ran, B.; Yang, J.; Wang, R.; et al. Selenium Intake and Multiple Health-Related Outcomes: An Umbrella Review of Meta-Analyses. Front. Nutr. 2023, 10, 1263853. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Flohe, L.; Günzler, W.; Schock, H.H. Glutathione Peroxidase: A Selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Rotruck, J.; Swanson, A.; Pope, A.; Hoekstra, W.; Hafeman, D.; Ganther, H. Relationship of Selenium to GSH Peroxidase; Federation of American Societies for Experimental Biology: Bethesda, MD, USA, 1972; Volume 31, p. A691. [Google Scholar]
- Flohé, L.; Toppo, S.; Orian, L. The Glutathione Peroxidase Family: Discoveries and Mechanism. Free Rad. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Jacob, C.; Maret, W.; Vallee, B.L. Selenium Redox Biochemistry of Zinc–Sulfur Coordination Sites in Proteins and Enzymes. Proc. Nat. Acad. Sci. USA 1999, 96, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Nasim, M.J.; Zuraik, M.M.; Abdin, A.Y.; Ney, Y.; Jacob, C. Selenomethionine: A Pink Trojan Redox Horse with Implications in Aging and Various Age-Related Diseases. Antioxidants 2021, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Whanger, P.D. Selenocompounds in Plants and Animals and Their Biological Significance. J. Am. Coll. Nutr. 2002, 21, 223–232. [Google Scholar] [CrossRef]
- Weiss, J.F.; Srinivasan, V.; Kumar, K.S.; Landauer, M.R. Radioprotection by Metals: Selenium. Adv. Space Res. 1992, 12, 223–231. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Hussein, R.A.; Ahmed, M.; Heinemann, S.H. Selenomethionine Mis-Incorporation and Redox-Dependent Voltage-Gated Sodium Channel Gain of Function. J. Neurochem. 2023, 167, 262–276. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Tian, Y.; Huang, J.; Gao, P.; Zhao, Q.; Yang, Z. Antioxidant and Anti-Inflammatory Effects of Selenomethionine Promote Osteogenesis via Wnt/β-Catenin Pathway. Biochem. Biophys. Rep. 2023, 36, 101559. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamashita, Y.; Suzuki, T.; Kani, Y.; Mizusawa, N.; Imamura, S.; Takemoto, K.; Hara, T.; Hossain, M.A.; Yabu, T.; et al. Selenoneine, a Novel Selenium-Containing Compound, Mediates Detoxification Mechanisms against Methylmercury Accumulation and Toxicity in Zebrafish Embryo. Mar. Biotechnol. 2013, 15, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the Strong Antioxidant Selenoneine in Tuna and Selenium Redox Metabolism. World J. Biol. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef]
- Alhasan, R.; Nasim, M.J.; Jacob, C.; Gaucher, C. Selenoneine: A Unique Reactive Selenium Species From the Blood of Tuna with Implications for Human Diseases. Curr. Pharmacol. Rep. 2019, 5, 163–173. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M. Identification of a Novel Selenium-Containing Compound, Selenoneine, as the Predominant Chemical Form of Organic Selenium in the Blood of Bluefin Tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Kandasamy, R.; Ponnusamy, R.; Deruiter, J.; Dhanasekaran, M.; Thilagar, S. L-Ergothioneine: A Potential Bioactive Compound from Edible Mushrooms. In Medicinal Mushrooms: Recent Progress in Research and Development; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 391–407. ISBN 9789811363825. [Google Scholar] [CrossRef]
- Fahey, R.C. Novel Thiols of Prokaryotes. Annu. Rev. Microbiol. 2001, 55, 333–356. [Google Scholar] [CrossRef]
- El Hanafi, K.; Pedrero, Z.; Ouerdane, L.; Marchán Moreno, C.; Queipo-Abad, S.; Bueno, M.; Pannier, F.; Corns, W.T.; Cherel, Y.; Bustamante, P.; et al. First Time Identification of Selenoneine in Seabirds and Its Potential Role in Mercury Detoxification. Environ. Sci. Technol. 2022, 56, 3288–3298. [Google Scholar] [CrossRef]
- Kroepfl, N.; Francesconi, K.A.; Schwerdtle, T.; Kuehnelt, D. Selenoneine and Ergothioneine in Human Blood Cells Determined Simultaneously by HPLC/ICP-QQQ-MS. J. Anal. At. Spectrom. 2019, 34, 127–134. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamashita, Y.; Ando, T.; Wakamiya, J.; Akiba, S. Identification and Determination of Selenoneine, 2-Selenyl-Nα,Nα,Nα -Trimethyl-l-Histidine, as the Major Organic Selenium in Blood Cells in a Fish-Eating Population on Remote Japanese Islands. Biol. Trace Elem. Res. 2013, 156, 36–44. [Google Scholar] [CrossRef]
- Little, M.; Achouba, A.; Dumas, P.; Ouellet, N.; Ayotte, P.; Lemire, M. Determinants of Selenoneine Concentration in Red Blood Cells of Inuit from Nunavik (Northern Québec, Canada). Environ. Int. 2019, 127, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Milito, A.; Cocurullo, M.; Columbro, A.; Nonnis, S.; Tedeschi, G.; Castellano, I.; Arnone, M.I.; Palumbo, A. Ovothiol Ensures the Correct Developmental Programme of the Sea Urchin Paracentrotus Lividus Embryo. Open Biol. 2022, 12, 210262. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, R.; Martins, G.M.; de Castro, P.P.; Saleem, R.S.Z.; Zaiter, A.; Fries-Raeth, I.; Kleinclauss, A.; Perrin-Sarrado, C.; Chaimbault, P.; da Silva Júnior, E.N.; et al. Selenoneine-Inspired Selenohydantoins with Glutathione Peroxidase-like Activity. Bioorganic Med. Chem. 2023, 94, 117479. [Google Scholar] [CrossRef]
- Abdulnabi, Z.A.; Al-doghachi, F.A.J.; Abdulsahib, H.T. Synthesis, Characterization and Thermogravimetric Study of Some Metal Complexes of Selenazone Ligand Nanoparticles Analogue of Dithizone. Indones. J. Chem. 2021, 21, 1231–1243. [Google Scholar] [CrossRef]
- Kaya, B.; Gholam Azad, M.; Suleymanoglu, M.; Harmer, J.R.; Wijesinghe, T.P.; Richardson, V.; Zhao, X.; Bernhardt, P.V.; Dharmasivam, M.; Richardson, D.R. Isosteric Replacement of Sulfur to Selenium in a Thiosemicarbazone: Promotion of Zn(II) Complex Dissociation and Transmetalation to Augment Anticancer Efficacy. J. Med. Chem. 2024, 67, 12155–12183. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Abdulmalek, E. A Focused Review of Synthetic Applications of Lawesson’s Reagent in Organic Synthesis. Molecules 2021, 26, 6937. [Google Scholar] [CrossRef]
- Ascherl, L.; Nordheider, A.; Arachchige, K.S.A.; Cordes, D.B.; Karaghiosoff, K.; Bühl, M.; Slawin, A.M.Z.; Woollins, J.D. The Activation of Woollins’ Reagent. Isolation of Pyridine Stabilised PhPSe2. Chem. Commun. 2014, 50, 6214–6216. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and Tellurium in the Development of Novel Small Molecules and Nanoparticles as Cancer Multidrug Resistance Reversal Agents. Drug Resist. Updat. 2022, 63, 100844. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs-A Promising Approach to Fight Cancer Drug Resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef]
- Sonego, J.M.; de Diego, S.I.; Szajnman, S.H.; Gallo-Rodriguez, C.; Rodriguez, J.B. Organoselenium Compounds: Chemistry and Applications in Organic Synthesis. Chem. Eur. J. 2023, 29, e202300030. [Google Scholar] [CrossRef]
- Hou, W.; Dong, H.; Zhang, X.; Wang, Y.; Su, L.; Xu, H. Selenium as an Emerging Versatile Player in Heterocycles and Natural Products Modification. Drug Discov. Today 2022, 27, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Santoro, S.; Battistelli, B.; Testaferri, L.; Tiecco, M. Preparation of the First Bench-Stable Phenyl Selenolate: An Interesting “On Water” Nucleophilic Reagent. Eur. J. Org. Chem. 2008, 2008, 5387–5390. [Google Scholar] [CrossRef]
- Lai, S.; Liang, X.; Zeng, Q. Recent Progress in Synthesis and Application of Chiral Organoselenium Compounds. Chem. Eur. J. 2024, 30, e202304067. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.L.; Charneira, C.; Gandin, V.; Ferreira da Silva, J.L.; Justino, G.C.; Telo, J.P.; Vieira, A.J.S.C.; Marzano, C.; Antunes, A.M.M. Selenium-Containing Chrysin and Quercetin Derivatives: Attractive Scaffolds for Cancer Therapy. J. Med. Chem. 2015, 58, 4250–4265. [Google Scholar] [CrossRef]
- Merino-Montiel, P.; Maza, S.; Martos, S.; López, Ó.; Maya, I.; Fernández-Bolaños, J.G. Synthesis and Antioxidant Activity of O-Alkyl Selenocarbamates, Selenoureas and Selenohydantoins. Eur. J. Pharm. Sci. 2013, 48, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Kim, D.-M.; Kim, Y.-J.; Yang, S.; Lee, K.-T.; Ryu, J.H.; Jeong, J.-H. Synthesis and Evaluation of Neuroprotective Selenoflavanones. Int. J. Mol. Sci. 2015, 16, 29574–29582. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.J.; Lee, J.Y.; Lee, J.; Jeong, J.H. Synthesis and Evaluation of Selenoflavones That Have Potential Neuroprotective Effects. Heterocycles 2014, 89, 2794–2805. [Google Scholar] [CrossRef]
- Niki, E. Evidence for Beneficial Effects of Vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar] [CrossRef]
- Gamna, F.; Spriano, S. Vitamin E: A Review of Its Application and Methods of Detection When Combined with Implant Biomaterials. Materials 2021, 14, 3691. [Google Scholar] [CrossRef] [PubMed]
- Al-Maharik, N.; Engman, L.; Malmström, J.; Schiesser, C.H. Intramolecular Homolytic Substitution at Selenium: Synthesis of Novel Selenium-Containing Vitamin E Analogues. J. Org. Chem. 2001, 66, 6286–6290. [Google Scholar] [CrossRef] [PubMed]
- Shanks, D.; Amorati, R.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L.; Engman, L. Synthesis and Antioxidant Profile of All-Rac-α-Selenotocopherol. J. Org. Chem. 2006, 71, 1033–1038. [Google Scholar] [CrossRef]
- Design, Synthesis and Cytotoxic Activity of Vitamin E Bearing Selenium Compounds against Human Breast Cancer Cell Line (MCF-7). Phosphorus Sulfur. Silicon Relat. Elem. 2017, 192, 1114–1118. [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahsan, H.; Mukhtar, H.; Ahmad, N. Combination of Vitamin E and Selenium Causes an Induction of Apoptosis of Human Prostate Cancer Cells by Enhancing Bax/Bcl-2 Ratio. Prostate 2008, 68, 1624–1634. [Google Scholar] [CrossRef]
- Angeli, A.; Trallori, E.; Carta, F.; Di Cesare Mannelli, L.; Ghelardini, C.; Supuran, C.T. Heterocoumarins Are Selective Carbonic Anhydrase IX and XII Inhibitors with Cytotoxic Effects against Cancer Cells Lines. ACS Med. Chem. Lett. 2018, 9, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Yoshida, A.; Mizutani, T.; Kubuki, H.; Yamaguchi, K.; Maruyama, T.; Shibahara, F. The First Selenium Isologues of 2-Pyrones and Coumarins: Synthesis, Structures, and Reactions. Chem. Lett. 2017, 46, 1017–1019. [Google Scholar] [CrossRef]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno Quinolinones and Coumarins Promote Cancer Cell Apoptosis by ROS Depletion and Caspase-7 Activation. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef]
- Arsenyan, P.; Vasiljeva, J.; Shestakova, I.; Domracheva, I.; Jaschenko, E.; Romanchikova, N.; Leonchiks, A.; Rudevica, Z.; Belyakov, S. Selenopheno[3,2-c]- and [2,3-c]Coumarins: Synthesis, Cytotoxicity, Angiogenesis Inhibition, and Antioxidant Properties. Comptes Rendus Chim. 2015, 18, 399–409. [Google Scholar] [CrossRef]
- Patra, A.R.; Roy, S.S.; Basu, A.; Bhuniya, A.; Bhattacharjee, A.; Hajra, S.; Hossain Sk, U.; Baral, R.; Bhattacharya, S. Design and synthesis of coumarin-based organoselenium as a new hit for myeloprotection and synergistic therapeutic efficacy in adjuvant therapy. Sci. Rep. 2018, 8, 2194. [Google Scholar] [CrossRef]
- Davies, M.J.; Schiesser, C.H. 1,4-Anhydro-4-Seleno-D-Talitol (SeTal): A Remarkable Selenium-Containing Therapeutic Molecule. New J. Chem. 2019, 43, 9759–9765. [Google Scholar] [CrossRef]
- Storkey, C.; Pattison, D.I.; White, J.M.; Schiesser, C.H.; Davies, M.J. Preventing Protein Oxidation with Sugars: Scavenging of Hypohalous Acids by 5-Selenopyranose and 4-Selenofuranose Derivatives. Chem. Res. Toxicol. 2012, 25, 2589–2599. [Google Scholar] [CrossRef]
- Zacharias, T.; Flouda, K.; Jepps, T.A.; Gammelgaard, B.; Schiesser, C.H.; Davies, M.J. Effects of a Novel Selenium Substituted-Sugar (1,4-Anhydro-4-Seleno-d-Talitol, SeTal) on Human Coronary Artery Cell Lines and Mouse Aortic Rings. Biochem. Pharmacol. 2020, 173, 113631. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pinto, B.M. Efficient Synthesis of the Glucosidase Inhibitor Blintol, the Selenium Analogue of the Naturally Occurring Glycosidase Inhibitor Salacinol. J. Org. Chem. 2005, 70, 753–755. [Google Scholar] [CrossRef]
- Liu, H.; Pinto, B.M. Design and Synthesis of Selenonium and Sulfonium Ions Related to the Naturally Occurring Glucosidase Inhibitor Salacinol. Can. J. Chem. 2006, 84, 1351–1362. [Google Scholar] [CrossRef]
- Liu, H.; Pinto, B.M. Synthesis of Zwitterionic Selenonium and Sulfonium Sulfates from D-Mannose as Potential Glycosidase Inhibitors. Can. J. Chem. 2006, 84, 497–505. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Tanabe, G.; Matsuda, H.; Yoshikawa, M.; Muraoka, O. A Review of Antidiabetic Active Thiosugar Sulfoniums, Salacinol and Neokotalanol, from Plants of the Genus Salacia. J. Nat. Med. 2021, 75, 449–466. [Google Scholar] [CrossRef]
- Morikawa, T.; Akaki, J.; Ninomiya, K.; Kinouchi, E.; Tanabe, G.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O. Salacinol and Related Analogs: New Leads for Type 2 Diabetes Therapeutic Candidates from the Thai Traditional Natural Medicine Salacia Chinensis. Nutrients 2015, 7, 1480–1493. [Google Scholar] [CrossRef]
- Nasi, R.; Sim, L.; Rose, D.R.; Pinto, B.M. New Chain-Extended Analogues of Salacinol and Blintol and Their Glycosidase Inhibitory Activities. Mapping the Active-Site Requirements of Human Maltase Glucoamylase. J. Org. Chem. 2007, 72, 180–186. [Google Scholar] [CrossRef]
- Liu, H.; Nasi, R.; Jayakanthan, K.; Sim, L.; Heipel, H.; Rose, D.R.; Pinto, B.M. New Synthetic Routes to Chain-Extended Selenium, Sulfur, and Nitrogen Analogues of the Naturally Occurring Glucosidase Inhibitor Salacinol and Their Inhibitory Activities against Recombinant Human Maltase Glucoamylase. J. Org. Chem. 2007, 72, 6562–6572. [Google Scholar] [CrossRef]
- Nasi, R.; Sim, L.; Rose, D.R.; Pinto, B.M. Synthesis and Glycosidase Inhibitory Activities of Chain-Modified Analogues of the Glycosidase Inhibitors Salacinol and Blintol. Carb. Res. 2007, 342, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Serpico, L.; De Nisco, M.; Cermola, F.; Manfra, M.; Pedatella, S. Stereoselective Synthesis of Selenium-Containing Glycoconjugates via the Mitsunobu Reaction. Molecules 2021, 26, 2541. [Google Scholar] [CrossRef]
- Cimmino, G.; De Nisco, M.; Alonso, C.; Gravina, C.; Piscopo, V.; Lemos, R.; Coderch, L.; Piccolella, S.; Pacifico, S.; Pedatella, S. Novel Synthesized Seleno-Glycoconjugates as Cosmeceutical Ingredients: Antioxidant Activity and in Vitro Skin Permeation. Eur. J. Med. Chem. Rep. 2024, 12, 100240. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; De Stefano, L.; De Martino, S.; Battisti, M.; Dardano, P.; Pedatella, S.; De Nisco, M. PH-Sensitive Release of Antioxidant Se-Glycoconjugates through a Flexible Polymeric Patch. Eur. Polym. J. 2022, 178, 111486. [Google Scholar] [CrossRef]
- Chakka, N.; Johnston, B.D.; Pinto, B.M. Synthesis and Conformational Analysis of Disaccharide Analogues Containing Disulfide and Selenosulfide Functionalities in the Interglycosidic Linkages. Can. J. Chem. 2005, 83, 929–936. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and Mechanisms of Dietary Polyphenols Affected by Non-Thermal Processing Technology in Fruits and Vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Wang, K.; Lu, C.; Luo, M.; Shan, T.; Zhang, Z. A Wheat Caffeic Acid 3-O-Methyltransferase TaCOMT-3D Positively Contributes to Both Resistance to Sharp Eyespot Disease and Stem Mechanical Strength. Sci. Rep. 2018, 8, 6543. [Google Scholar] [CrossRef]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S.; et al. Exploring the Role of Caffeic Acid in Mitigating Abiotic Stresses in Plants: A Review. Plant Stress. 2024, 12, 100487. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Sangwan, N.; Jadaun, J.S.; Bansal, S.; Sangwan, R.S. Defining the Role of a Caffeic Acid 3-O-Methyltransferase from Azadirachta Indica Fruits in the Biosynthesis of Ferulic Acid through Heterologous over-Expression in Ocimum Species and Withania Somnifera. Planta 2021, 253, 20. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Akbar, M.N.; Iftikhar, Y.; Abbas, M.; Zahid, A.; Fahad, S.; Datta, R.; Ali, M.; Elgorban, A.M.; Ansari, M.J.; et al. Rhizobacteria Inoculation and Caffeic Acid Alleviated Drought Stress in Lentil Plants. Sustainability 2021, 13, 9603. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic Acid: A Review of Its Potential Use in Medications and Cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic Acid Inhibits Neuro-Inflammation in Mice Exposed to Chronic Unpredictable Mild Stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, F.; Han, C.; Yang, H.; Han, L.; Li, K.; Li, J.; Xu, Q.; Li, Z.; Yuan, B.; et al. Ferulic Acid Ameliorated Placental Inflammation and Apoptosis in Rat with Preeclampsia. Clin. Exp. Hypertens. 2019, 41, 524–530. [Google Scholar] [CrossRef]
- Huang, D.-W.; Shen, S.-C.; Wu, J.S.-B. Effects of Caffeic Acid and Cinnamic Acid on Glucose Uptake in Insulin-Resistant Mouse Hepatocytes. J. Agric. Food Chem. 2009, 57, 7687–7692. [Google Scholar] [CrossRef]
- Azhar, M.K.; Anwar, S.; Hasan, G.M.; Shamsi, A.; Islam, A.; Parvez, S.; Hassan, M.I. Comprehensive Insights into Biological Roles of Rosmarinic Acid: Implications in Diabetes, Cancer and Neurodegenerative Diseases. Nutrients 2023, 15, 4297. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential Therapeutic Implications of Caffeic Acid in Cancer Signaling: Past, Present, and Future. Front. Pharmacol. 2022, 13, 845871. [Google Scholar] [CrossRef]
- Chrrng, J.-M.; Shieh, D.-E.; Chiang, W.; Chang, M.-Y.; Chiang, L.-C. Chemopreventive Effects of Minor Dietary Constituents in Common Foods on Human Cancer Cells. Biosci. Biotech. Biochem. 2007, 71, 1500–1504. [Google Scholar] [CrossRef]
- Jaganathan, S.K. Growth Inhibition by Caffeic Acid, One of the Phenolic Constituents of Honey, in HCT 15 Colon Cancer Cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Chiou, S.-Y.; Chan, K.-C.; Sung, J.-M.; Lin, S.-D. Caffeic Acid Derivatives, Total Phenols, Antioxidant and Antimutagenic Activities of Echinacea purpurea Flower Extracts. LWT-Food Sci. Technol. 2012, 46, 169–176. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Jin, L. Caffeic Acid Alleviates Inflammatory Response in Rheumatoid Arthritis Fibroblast-like Synoviocytes by Inhibiting Phosphorylation of IκB Kinase α/β and IκBα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shifa ul Haq, H.M.; Ashfaq, R.; Mehmood, A.; Shahid, W.; Azam, H.G.; Azam, M.; Tasneem, S.; Akram, S.J.; Malik, K.; Riazuddin, S. Priming with Caffeic Acid Enhances the Potential and Survival Ability of Human Adipose-Derived Stem Cells to Counteract Hypoxia. Regen. Ther. 2023, 22, 115–127. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, J.; Wang, J.; Liu, J.; Sun, P.; Li, J.; Li, G.; Sun, Y.; Ying, J.; Li, K.; et al. Caffeic Acid Phenethyl Ester: An Effective Antiviral Agent against Porcine Reproductive and Respiratory Syndrome Virus. Antivir. Res. 2024, 225, 105868. [Google Scholar] [CrossRef]
- Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; de Lima Menezes, G.; da Silva, R.A.; Nebo, L.; da Silva, G.C.D.; de Aguiar Milhim, B.H.G.; da Silva Teixeira, I.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses 2023, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic Acid Derivatives (CAFDs) as Inhibitors of SARS-CoV-2: CAFDs-Based Functional Foods as a Potential Alternative Approach to Combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Hsu, L.-Y.; Lin, C.-F.; Hsu, W.-C.; Hsu, W.-L.; Chang, T.-C. Evaluation of Polyphenolic Acid Esters as Potential Antioxidants. Biol. Pharm. Bull. 2005, 28, 1211–1215. [Google Scholar] [CrossRef]
- Kougan, G.B.; Tabopda, T.; Kuete, V.; Verpoorte, R. 6—Simple Phenols, Phenolic Acids, and Related Esters from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 225–249. ISBN 978-0-12-405927-6. [Google Scholar] [CrossRef]
- Lin, C.-F.; Chang, T.-C.; Chiang, C.-C.; Tsai, H.-J.; Hsu, L.-Y. Synthesis of Selenium-Containing Polyphenolic Acid Esters and Evaluation of Their Effects on Antioxidation and 5-Lipoxygenase Inhibition. Chem. Pharm. Bull. 2005, 53, 1402–1407. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Investigation of Antioxidant Activity of Selenium Compounds and Their Mixtures with Tea Polyphenols. Mol. Biol. Rep. 2019, 46, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Olsen, A.-K. Selenium—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10320. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sagada, G.; Wang, R.; Li, P.; Xu, B.; Zhang, C.; Qiao, J.; Yan, Y. Different Forms of Selenium Supplementation in Fish Feed: The Bioavailability, Nutritional Functions, and Potential Toxicity. Aquaculture 2022, 549, 737819. [Google Scholar] [CrossRef]
- Ivory, K.; Prieto, E.; Spinks, C.; Armah, C.N.; Goldson, A.J.; Dainty, J.R.; Nicoletti, C. Selenium Supplementation Has Beneficial and Detrimental Effects on Immunity to Influenza Vaccine in Older Adults. Clin. Nutr. 2017, 36, 407–415. [Google Scholar] [CrossRef]
- Harthill, M. Review: Micronutrient Selenium Deficiency Influences Evolution of Some Viral Infectious Diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Tamargo, J.; Campa, A.; Baum, M.K. Role of Selenium in Viral Infections with a Major Focus on SARS-CoV-2. Int. J. Mol. Sci. 2021, 23, 280. [Google Scholar] [CrossRef]
- Larvie, D.Y.; Perrin, M.T.; Donati, G.L.; Armah, S.M. COVID-19 Severity Is Associated with Selenium Intake among Young Adults with Low Selenium and Zinc Intake in North Carolina. Curr. Dev. Nutr. 2023, 7, 100044. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency in COVID-19—A Possible Long-Lasting Toxic Relationship. Nutrients 2022, 14, 283. [Google Scholar] [CrossRef]
- Stone, C.A.; Kawai, K.; Kupka, R.; Fawzi, W.W. Role of Selenium in Hiv Infection. Nutr. Rev. 2010, 68, 671–681. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Al-Quraishy, S.; Dkhil, M.A.; Wunderlich, F.; Sies, H. Dietary Selenium in Adjuvant Therapy of Viral and Bacterial Infections12. Adv. Nutr. 2015, 6, 73–82. [Google Scholar] [CrossRef]
- Di Bella, S.; Grilli, E.; Cataldo, M.A.; Petrosillo, N. Selenium Deficiency and HIV Infection. Infect. Dis. Rep. 2010, 2, e18. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Can Selenite Be an Ultimate Inhibitor of Ebola and Other Viral Infections? J. Adv. Med. Med. Res. 2015, 6, 319–324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).