Abstract
Background: Previous research has examined the acute effects of high-intensity exercise on muscle contractility, revealing potential interference in reaching peak contraction. This study aims to evaluate the impact of a standardized kettlebell swing protocol on low back musculature contractility, measured by tensiomyography (TMG), and pain sensitivity, measured by pressure algometry. Methods: Forty participants were randomly assigned to one of three groups: control, kettlebell swing, and kettlebell isometric hold. Pre-intervention TMG and pressure pain threshold (PPT) measurements were taken, followed by the intervention and post-intervention measurements. Results: Participants averaged 23.85 years (SD ± 2.73), 162.39 lbs (SD ± 28.69), and 174.29 cm (SD ± 12.45). Baseline ANOVAs showed no significant differences between groups for pre-intervention DM or PPT measurements, nor for demographics (p > 0.05). Although no significant within-group differences in TMG measurements were observed, the kettlebell swing group showed small mean differences in muscle displacement and contraction time for the gluteus maximus with effect sizes ranging from 0.09 to 0.49. Conclusions: The study suggests posterior chain muscles, such as the gluteus maximus, are involved in kettlebell swings. Also, despite the lack of significant TMG differences within groups, the kettlebell swing group exhibited small changes in muscle characteristics, enhancing the understanding of exercise-induced hypoalgesia and posterior chain involvement in resistance exercises.
1. Introduction
Previous literature has evaluated the acute changes in muscle contractility due to high-intensity exercise [1,2]. It has become evident that these effects from acute high-intensity exercise seemingly increase the difficulty for a muscle to reach its peak contraction [1]. Furthermore, these acute changes in contractility from high-intensity exercises may be due to peripheral fatigue, where muscles accumulate metabolites such as inorganic phosphate (Pi), hydrogen, and adenosine diphosphate (ADP) within the muscle fibers as byproducts of muscle metabolism [3]. The accumulation of these metabolites ultimately can decrease the ability for the fatigued muscle groups to contract [3]. In similar studies, high-intensity exercise can also lead to changes in pain sensitivity through the exercise-induced hypoalgesia (EIH) effect [4]. This effect stems from the descending pain modulatory system, leading to increases in pain threshold [4]. Despite the existing literature on high-intensity exercise, there are still a limited number of studies showing that acute high-intensity exercise influences both contractility and sensitivity [2,5]. Additionally, these changes may be accomplished through utilizing various exercise equipment during high-intensity exercise.
The equipment used to influence muscle contractility and sensitivity varies in both shape and complexity. For instance, studies have examined the effects of acute exercise on muscle contractility using traditional equipment such as dumbbells and exercise machines [6,7]. Similarly, Piqueras-Sanchez investigated acute changes in muscle contractility following a high-intensity squat program utilizing a Smith machine [1]. Additionally, more advanced equipment like the isokinetic machine has demonstrated its ability to induce changes in contractility, as evidenced by alterations in maximal muscle displacement measured via TMG [8]. Dumbbells have also been utilized in high-intensity exercise to modify pain sensitivity through the EIH effect [6]. The kettlebell, known for its affordability and efficacy in various training programs, has the potential to alter contractility and sensitivity through its use in high-intensity exercises [9]. The kettlebell’s unique shape enables individuals to perform cyclic ballistic movements such as the kettlebell swing, presenting a distinct mode of exercise [9]. However, there are limited studies utilizing this equipment in research protocols. While a variety of equipment can induce changes in musculature, the type of high-intensity exercise protocol employed also plays a crucial role in influencing contractility and sensitivity.
Various high-intensity exercises and protocols have been utilized to induce acute changes in muscle contractility and sensitivity. Among these, high-intensity interval training (HIIT) and aerobic exercises have demonstrated the ability to reduce pain sensitivity [10,11]. For instance, Vaegter’s systematic review highlighted how HIIT can lead to EIH immediately following such interventions [10,11]. One particular form of HIIT is the Tabata-style workout, which typically involves intense interval-style training consisting of 20 s of high-intensity exercise followed by 10 s of rest for a set number of intervals [12]. This approach is noteworthy for its minimal rest intervals, potentially contributing to increased musculature fatigue when executed effectively. Additionally, there Consequently, there is potential for this approach to induce changes in muscle contractility and sensitivity. Nevertheless, the current literature lacks substantial evidence to ascertain whether this protocol can indeed bring about such acute changes, especially when combining a HIIT-like protocol with an acute bout of exercise.
The kettlebell swing represents a novel training approach integrating ballistic movements with an eccentric loading phase of hip hinging, succeeded by an explosive concentric phase to drive the weight forward [5]. While existing studies demonstrate the kettlebell swing’s capacity to enhance muscular properties over extended durations, evidenced by its efficacy in improving explosive power in athletes compared to traditional jump squat training [13], there is a shortage of evidence regarding its acute impact on the contractility of specific muscle groups, particularly those in the lumbosacral and pelvic-hip regions. Exploring changes in muscle contractile parameters, such as muscle displacement (Dm), which measures a muscle group’s displacement from its original position following an electrical stimulus [14], remains uncharted territory within the context of a high-intensity kettlebell swing protocol. Despite indications that the kettlebell swing activates muscles in the low back and gluteus maximus regions [15,16], along with other hip extensor musculature like the biceps femoris, as evidenced by EMG studies [17], the potential for the kettlebell swing to influence these contractile properties post-fatiguing acute exercise warrants investigation. Moreover, while one study has examined muscle sensitivity in the lumbosacral region [5], revealing an increase in pressure pain threshold following an acute bout of a high-intensity kettlebell swing protocol, further research is essential to fully comprehend how this exercise modality impacts muscular contractility and sensitivity in the lumbosacral region.
Despite evidence suggesting that kettlebell swings may alter motor control, power, and endurance of the low back and hip musculature [17], to date, no studies have assessed contractility after the kettlebell swing while following a modified Tabata protocol, with only one study investigating the relationship between kettlebell swings and lumbopelvic pressure pain threshold [5]. Therefore, the purpose of this study is to evaluate how a standardized kettlebell swing protocol influences the contractility of low back musculature, measured by TMG, and pain sensitivity, measured by pressure algometry. The results of this study have the potential to lay the groundwork for future research examining kettlebell swings with modified Tabata protocols, particularly among patients with mechanical low back pain.
2. Materials and Methods
2.1. Subjects
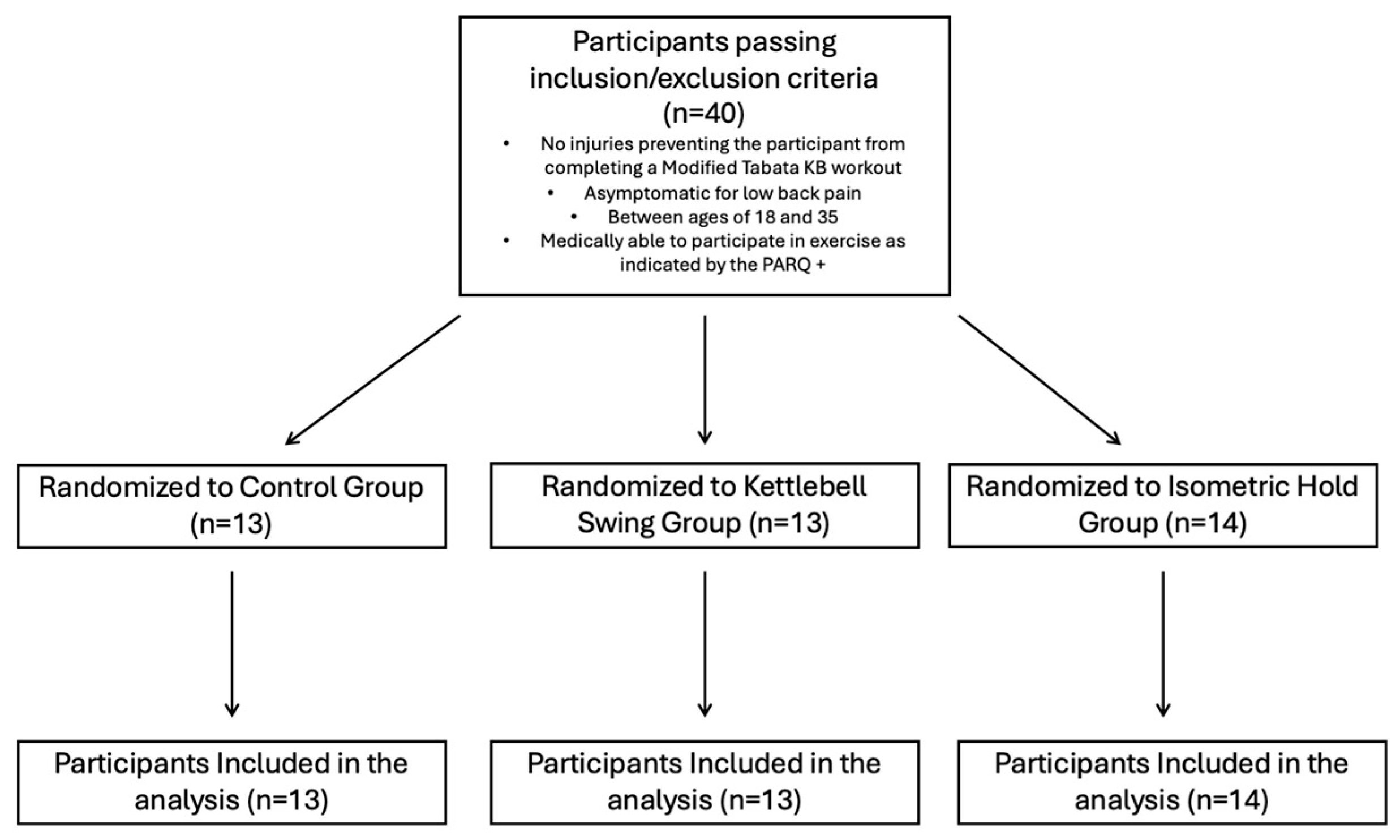
This study was registered on clinicaltrials.gov (NCT05607212) and approved by the Institutional Review Board at the University of Central Florida prior to beginning data collection. Participants were recruited from the student body at the University of Central Florida (STUDY00004622). Information about the study was distributed through social media, fliers, and word of mouth across the campus. A survey was created and distributed to interested participants to assess whether they met the inclusion criteria and contained a small demographics portion that asked about training experience, age, and sex at birth. Participants were also asked about how long they have been involved in regular physical activity (which includes sports, weight training, or aerobic exercise), with options being “Yes, less than 1 year”, “Yes, more than 1 year”, or “I do not exercise regularly”. Participants were eligible to participate in the study if they were (a) between 18 and 35, (b) asymptomatic for low back pain, and (c) did not have any injury that would prevent them from performing a Tabata-style kettlebell swing workout. Eligible participants were asked to participate in a single, 2 h visit to the Spine and Mobility Lab at the University of Central Florida. They were told the study involves potentially participating in a modified Tabata-style kettlebell swing workout, measurements of muscle contractility through the TMG, and measurements of pressure pain threshold through handheld pressure algometry. Prior to starting the research trial, participants were screened with the 2020 version of the PARQ+ to indicate that they were medically ready to participate in the study. All participants completed a written informed consent form prior to the start of the trial. After obtaining consent, all participants were randomly assigned to one of three groups: (1) control group, (2) kettlebell swing group, and (3) kettlebell isometric hold group, by selecting from note cards containing a number corresponding to their assigned group. Height and weight were collected prior to beginning the research trial. All the methods for each group are listed below. After group randomization, participants completed the research trial in the following order: Pre-Intervention TMG and PPT measurements were taken first; participants were then assigned an intervention. After the intervention, TMG and PPT measurements were taken (Figure 1). The power analysis was conducted utilizing G-power v.3.1.9.7 analysis for a repeated-measures ANOVA with a within–between interaction, indicating 40 participants provided 80% power with alpha level = 0.05 to determine time x intervention interaction effects with a small effect size (partial eta2 = 0.03).
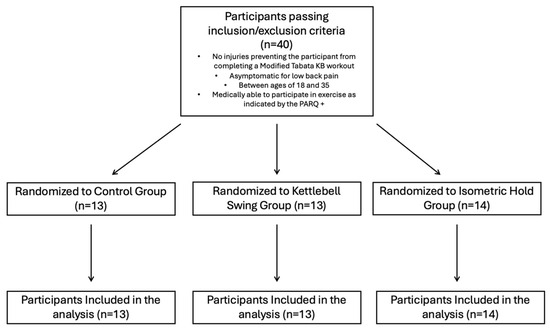
Figure 1.
Inclusion criteria and randomization of participants to groups.
A total of forty individuals qualified and completed the research trial. There were no dropouts out of all the participants who completed the study. To standardize measurement procedures and to ensure the stimulus administered by the TMG would not potentially affect pressure algometry measurements, all TMG measurements were taken on the left side of the participant’s body while all pressure algometry measurements were taken on the right side. Tasks were divided so that 1 researcher conducted all TMG and PPT measurements, and 1 other researcher instructed all participants on their assigned intervention. Methods for the use of the TMG, pressure algometry, and interventions are described in the sections below. Evaluation of the erector spinae, gluteus maximus, and biceps femoris is essential during kettlebell swing performance, as these muscles play key roles in hip extension, trunk stabilization, and force generation. Assessing their function provides insight into neuromuscular coordination, power output, and potential compensatory patterns that may influence performance efficiency and injury risk. Additionally, all assessment procedures were conducted by the same investigator to improve consistency.
2.2. Procedures
2.2.1. Tensiomyography Procedures
TMG was used to measure differences in muscle contractile properties between the three groups before and after performing the intervention. TMG evaluates contractile properties of muscles by administering an electrical stimulus to a target muscle through electrodes placed distal and proximal from the targeted muscle group [14]. The stimulus causes an isometric contraction of the target muscle, from which a sensor that is placed in between the electrodes and at the center of the targeted muscle belly gathers contractile data about the muscle group [14]. Relative reliability for completing TMG ranges from good to excellent for several different muscle groups [18].
The procedures for conducting TMG are based off Lohr et al. [19]. Each participant laid prone on a massage table for all the testing procedures. To collect TMG measurements, the muscle bellies of ES, GM, and BF were first palpated by the testing researcher, and markings were made using a non-permanent marker to indicate electrode and sensor placement. This was performed so the testing researcher could quickly and accurately place the electrodes and sensor tip in the center of the muscle belly after the intervention period when post-intervention measurements needed to be collected. The sensor tip was placed in the marked area (perpendicular to the muscle belly), followed by an electrode placed 1.5 cm distally and proximally on either side of the sensor [19]. An electrical stimulus was then distributed to each of the muscle groups, causing the muscle to contract and moving the sensor tip from its initial position [19]. The TMG software (v3.6) (recorded values for each of the TMG variables based on this sensor movement from its initial position. The initial amplitude of the electrical current sent to each was 30 mA and was increased by 10 mA for every measurement until the muscle displacement (Dm) value plateaued or decreased from the previous value [19]. At any point during the assessment process, procedures were discontinued if the participant reported discomfort or pain. Participant responses were monitored and evaluated at each stage to ensure safety and tolerability. Due to the increasing amplitude with every measurement, the researcher asked the participant for consent to proceed with the trial after taking each TMG measurement. Participants were also informed prior to attaining written informed consent that at any point in the study they are allowed to stop participating in the study and all data collected would be deleted. Increments of at least 10 s were taken between TMG measurements to ensure there was no muscle fatigue prior to the next measurement. This process is repeated for all tested muscle groups before and after the administered intervention, and all collected data were then stored in TMG software for data analysis. The TMG variables collected for data analysis include muscle displacement (Dm), time to contraction (Tc), time to relaxation (Tr), sustained contraction time (Ts), and delay time (Td).
Procedures for palpating the center of the muscle belly for accurate placement of the sensor tip are drawn from other TMG protocols for consistent placement of the sensor tip on each of the participants. Participants laid prone on a massage table during palpation of muscle groups. Measurements were conducted in a private room, and draping was used to minimize exposure of muscle groups.
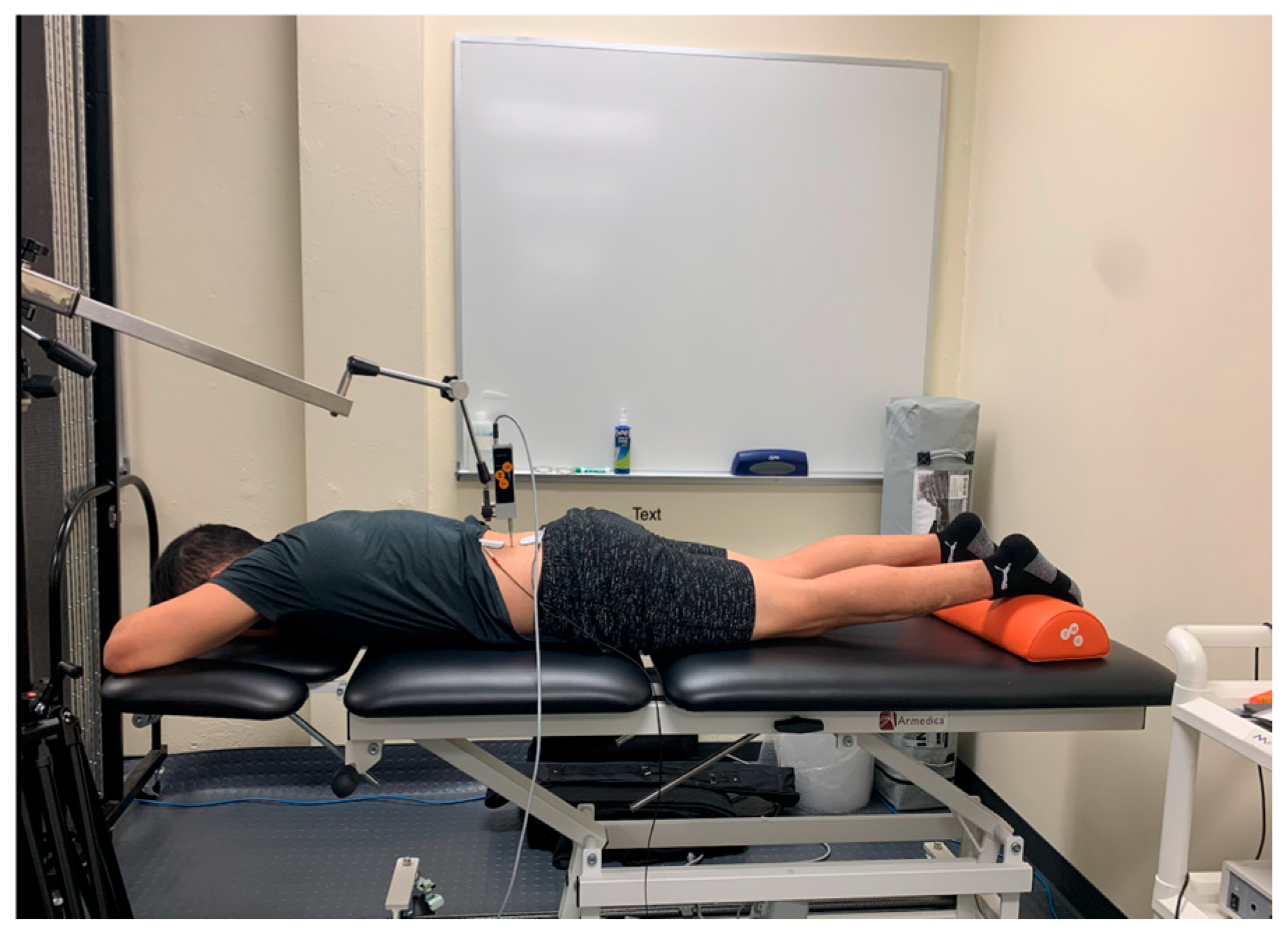
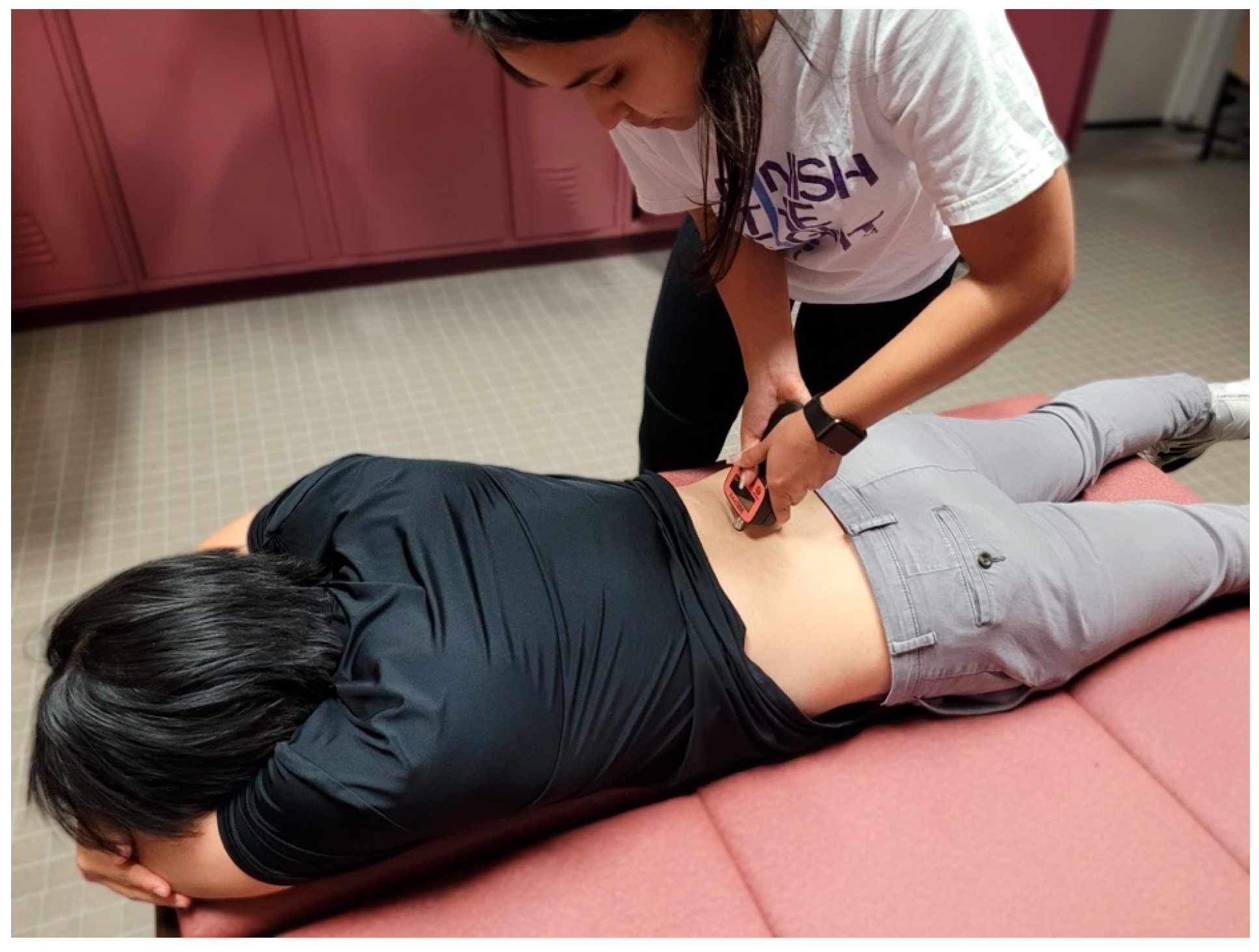
To palpate the sensor placement for the erector spinae, the researcher followed the protocol as demonstrated by Lohr et al. [19]. To find the center of the muscle belly, the researcher palpated 3 cm lateral to the L2 spinous process, which is found by locating the iliac crest, following it medially and posteriorly to L4, and then palpating proximally until the spinous process of L2 is located (Figure 2) [19].
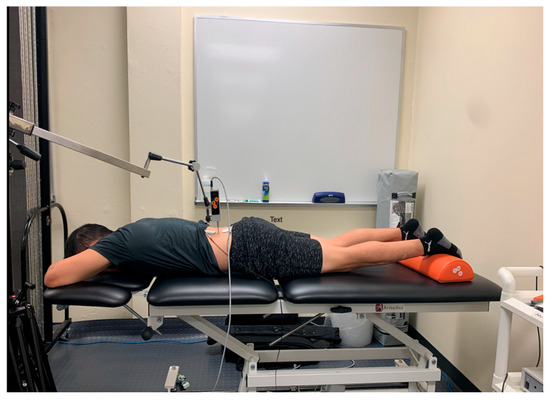
Figure 2.
Tensiomyography testing position for the erector spinae.
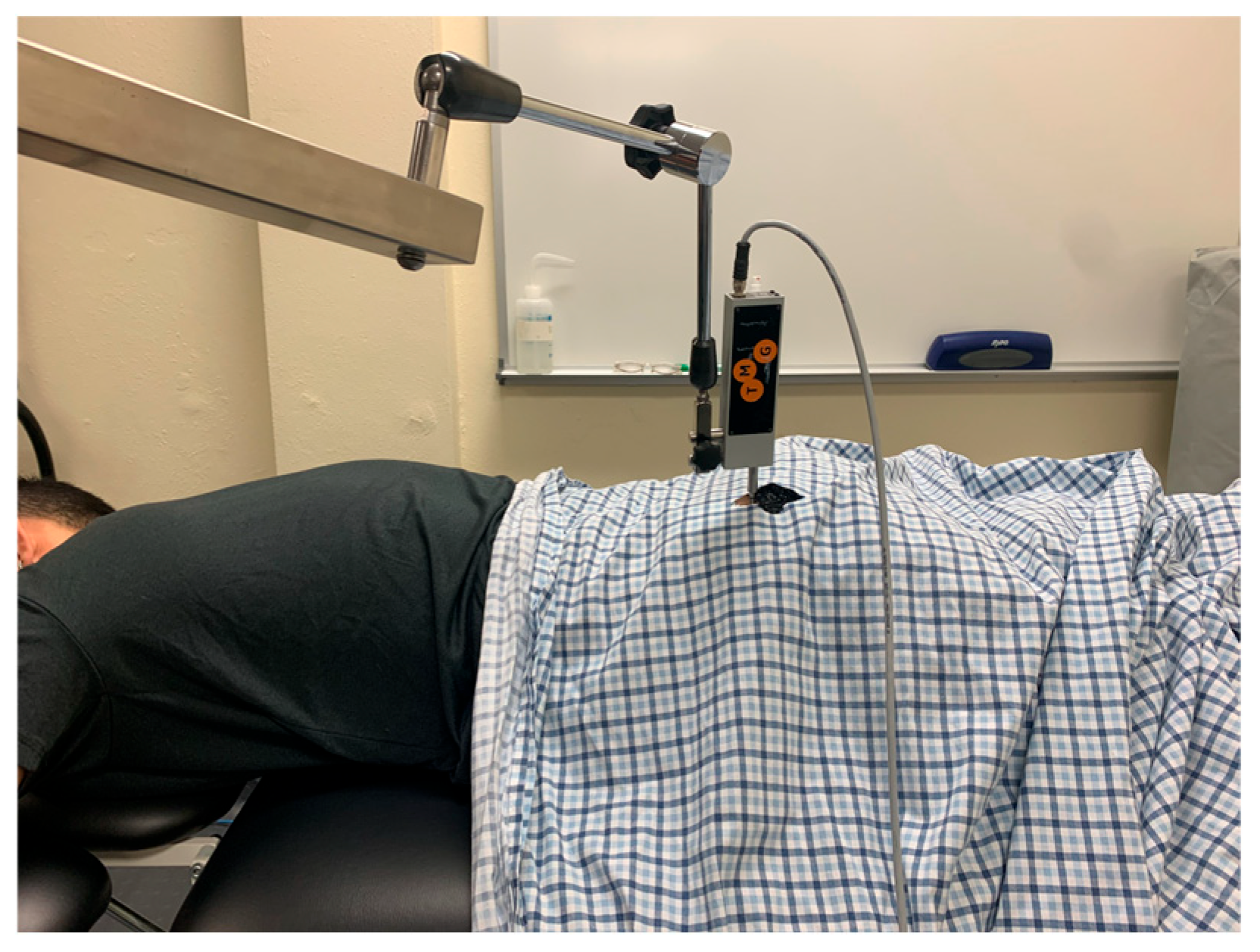
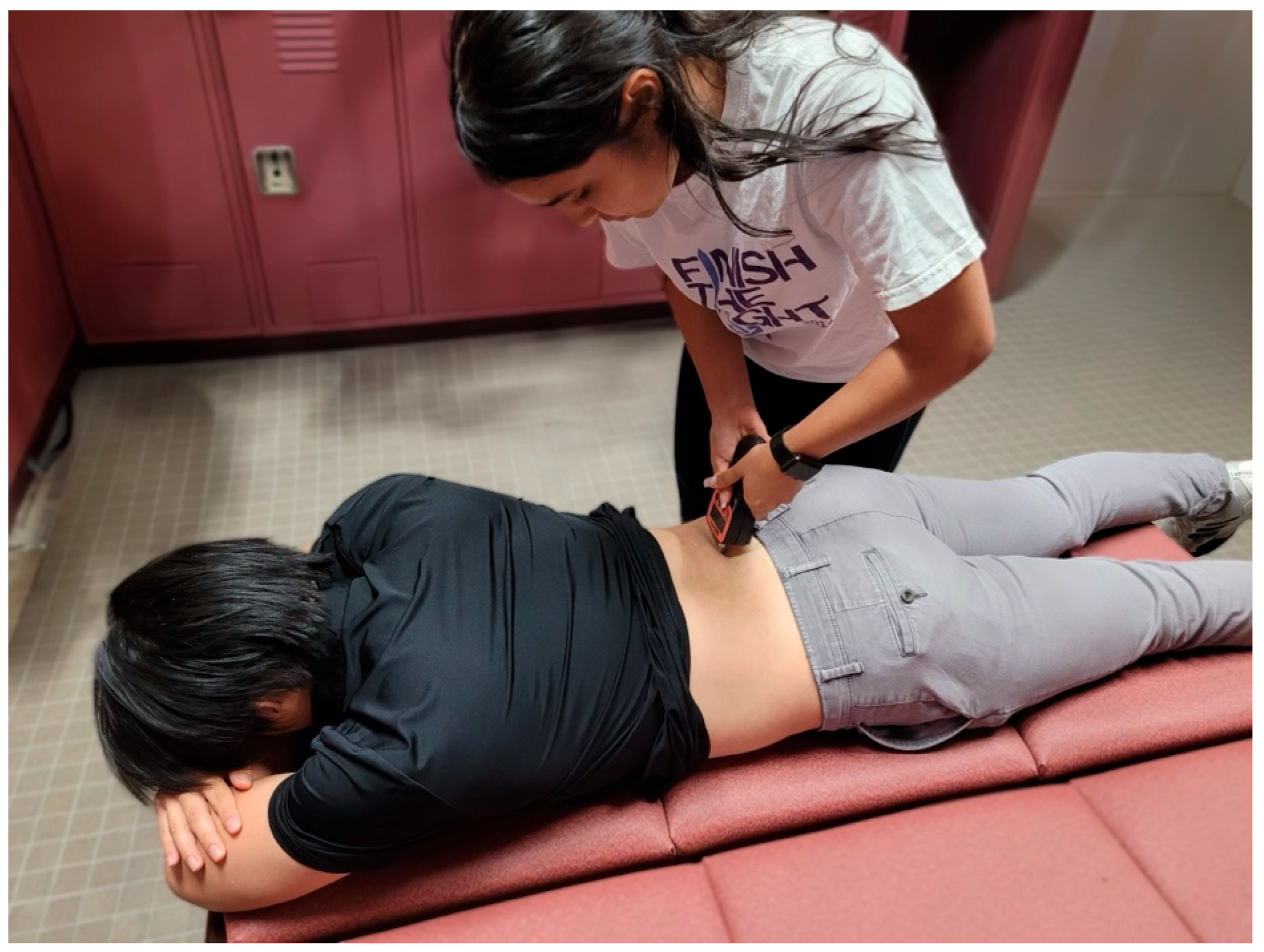
The participant was kept prone for the gluteus maximus measurements. The sensor placement for gluteus maximus was marked using the procedures as described by Burger et al. [20]. First, the PSIS and coccyx were palpated by the examiner. One mark was made ¼ the distance from the PSIS to the coccyx on this line. The greater trochanter was then palpated, and a line was made from this landmark to the first mark made earlier. On this new line, a second mark was made ⅓ of the distance from the first mark made to the greater trochanter. This second mark was used for the sensor placement of the TMG (Figure 3).
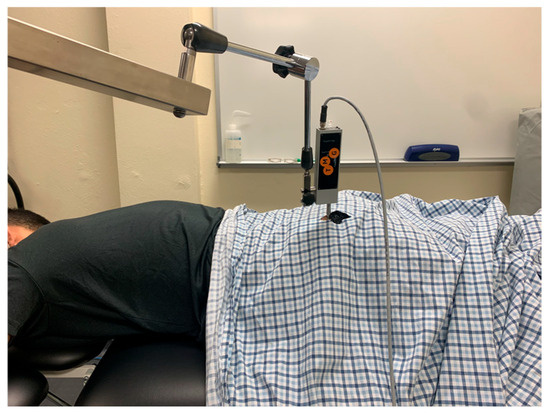
Figure 3.
Tensiomyography testing position for the gluteus maximus.
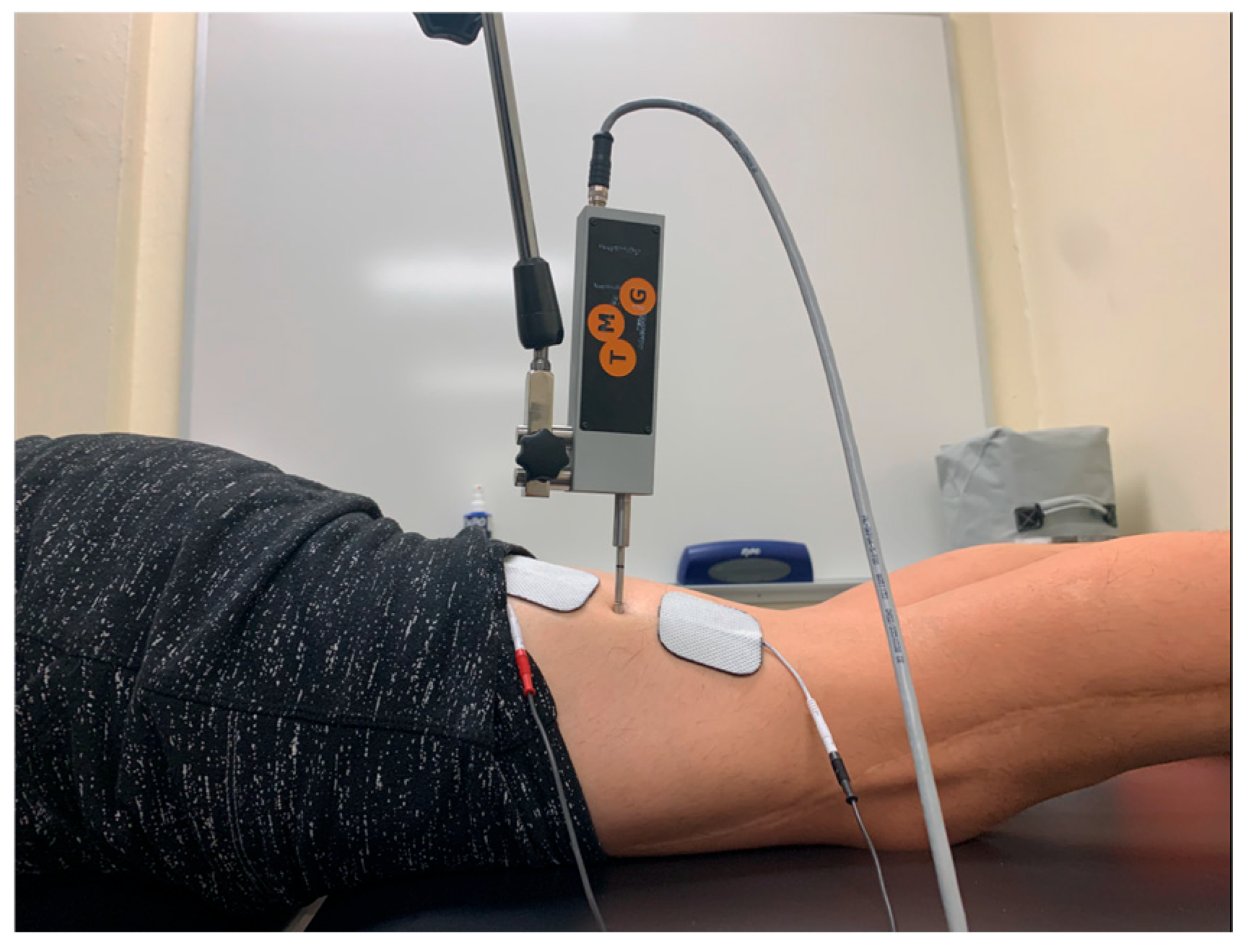
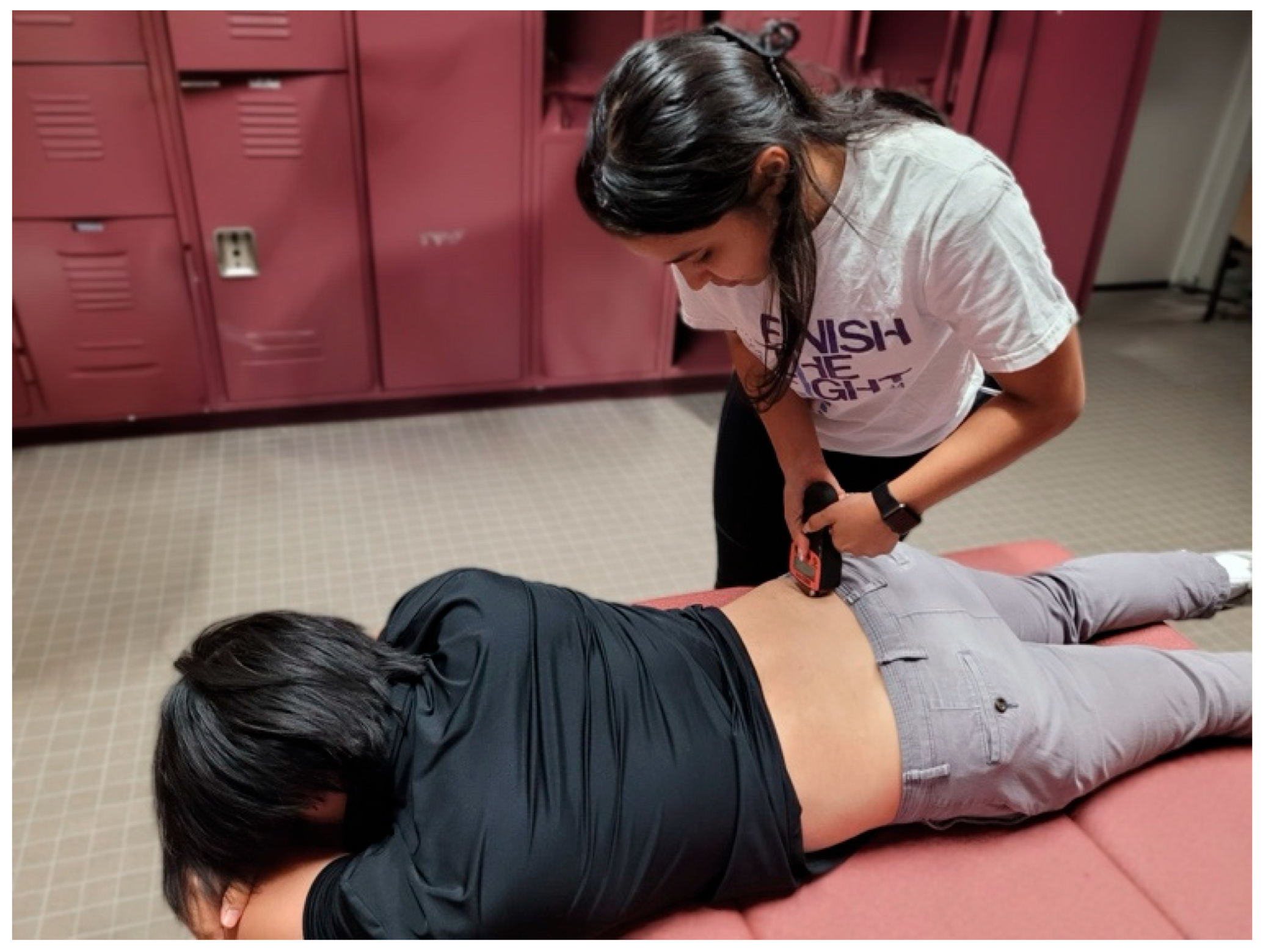
For sensor placement of the biceps femoris, the participant was first placed in 15 degrees of knee flexion while lying prone as described by Dordevic et al. [21]. The midway point between the participant’s ischial tuberosity and fibula was palpated and marked (Figure 4).
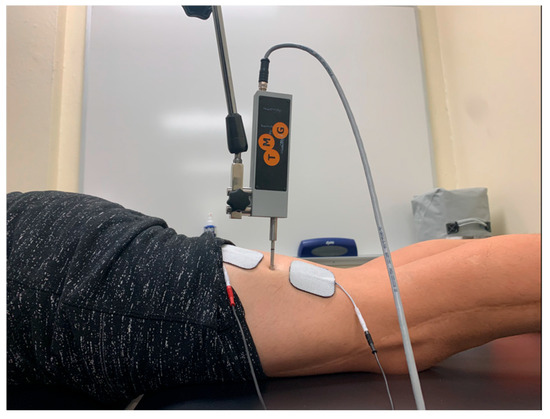
Figure 4.
Tensiomyography testing position for the biceps femoris.
2.2.2. Pressure Algometry
Pressure algometry measurements were taken immediately after all TMG measurements were completed during the pre-intervention and post-intervention periods. The Wagner FX-25 pressure was used to conduct all pressure algometry measurements (Figure 5) [5]. This device contains a rubberized end measuring around 1 cm2 that was applied to each muscle site. All measurements were collected in Newtons for the trial. This method of collecting PPT has been shown to have excellent intra-rater reliability in healthy participants [22]. All pressure algometry measures were taken on the participant’s right side. The pressure algometry palpation and application procedures were based on a normalized protocol used by Keilman et al. [5]. This protocol provided procedures for palpating the erector spinae, quadratus lumborum, and piriformis muscles. Each muscle was palpated using these procedures while the subject laid on the table prone, and each spot for the targeted muscle group was marked with a non-permanent marker. The specific palpations for each muscle group are listed below.

Figure 5.
Pressure algometer used for pressure pain threshold measurements.
The erector spinae were marked by palpating the last rib, located at the level of T12, and moving one vertebra inferior from that level (Figure 6) [5]. Quadratus lumborum was marked by palpating the iliac crest (L5), moving up 2 vertebrae, and moving 5 cm lateral (Figure 7). Piriformis was marked by palpating the posterior superior iliac spine and the tip of the sacrum and marking a spot halfway between and 2.5 cm lateral (Figure 8) [5].
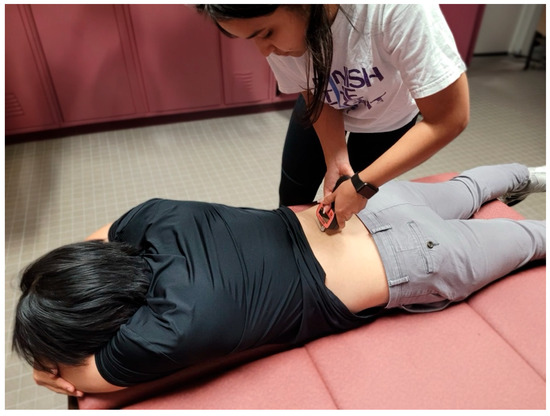
Figure 6.
Testing position for pressure pain threshold measurements of the erector spinae.
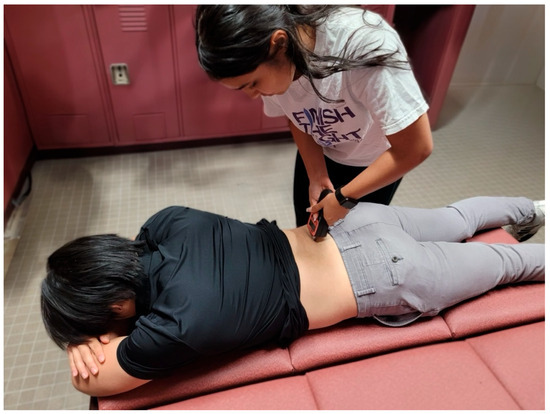
Figure 7.
Testing position for pressure pain threshold measurements of the quadratus lumborum.
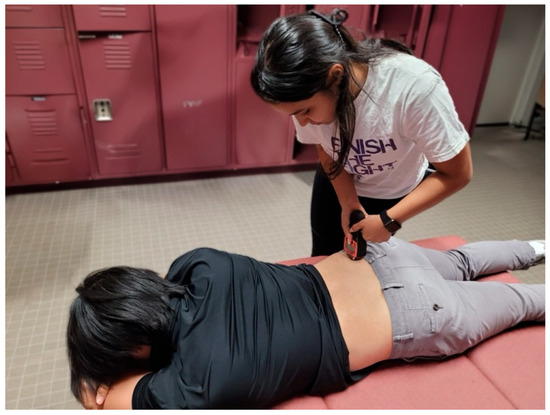
Figure 8.
Testing position for pressure pain threshold measurements of the piriformis.
The application of pressure algometry also was applied as described by Keilman et al. [5] where the units were converted from N • cm−2 to kilopascals (kPa), where 1 N • cm−2 is equal to 10 kPa. The researcher pushed down with pressure using the pressure algometer at a rate of 5 Newtons per second for each of the three muscles. The participant was asked by the researcher to disclose when “pressure changes to slight pain”. At this point, the researcher stopped application of the instrument, and the value was then recorded. The researcher waited at least 20 s after each pressure algometry measurement before collecting another measurement. A total of three measurements of PPT per muscle group were collected, and the average of those three was used during data analysis.
2.3. Intervention
After collection of pre-intervention TMG and PPT measurements, subjects assigned to an intervention group performed their respective protocol as described below. Following completion of the assigned intervention, participants underwent a standardized 5 min rest period prior to the initiation of post-intervention assessments. It is important to note that the setup procedures for tensiomyography (TMG) and pressure pain threshold (PPT) measurements commenced only after this 5 min rest period had elapsed. While the setup required additional time, it was not included within the designated rest interval, ensuring a consistent recovery duration prior to data collection. Subjects assigned to the control group rested for 15 min after collection of baseline PPT measurements before post-intervention measurements were collected. If the participant was placed in the kettlebell swing or the isometric hold group, they performed their specified exercise intervention in an interval training format as specified by Jay et al. [23].
2.3.1. Warm-Up
All participants placed in the KBS or isometric group were required to perform a standardized warmup. Prior to the warmup, participants were taught the proper technique of the kettlebell swing or isometric hold with a 5 kg kettlebell by our researcher using a standardized script that was read to each patient. The researcher made corrections to the participants’ forms under their discretion to ensure accurate completion of the intervention. Then, a general warm-up was performed consisting of 10 non-weighted squats, 10 non-weighted Romanian deadlifts, and 10 dowel rod shoulder flexion repetitions.
All male participants performed the intervention with a 16 kg kettlebell, and all female participants performed the intervention with a 12 kg kettlebell to standardize the weight consistent with the study by Jay et al. [23]. Depending on the group the participant was randomized into, participants performed two-handed kettlebell swings or isometric holds using the interval training protocol outlined in the study carried out by Jay et al. [23]. The protocol calls for 30 s of work, followed by 30 s of the control group. While the original Tabata protocol called for 20 s of work and 10 s of rest, we elected to utilize the modified version as reported by Jay et al. [23] to maximize the potential fatigue effect. The protocol calls for 30 s of work, followed by 30 s of the control group and was utilized to maximize the potential influence of fatigue. All participants randomized into the control group were asked to sit quietly in a room for 15 min prior to repeating post-intervention measurements without reading material or use of electronic devices.
2.3.2. Kettlebell Swing Group
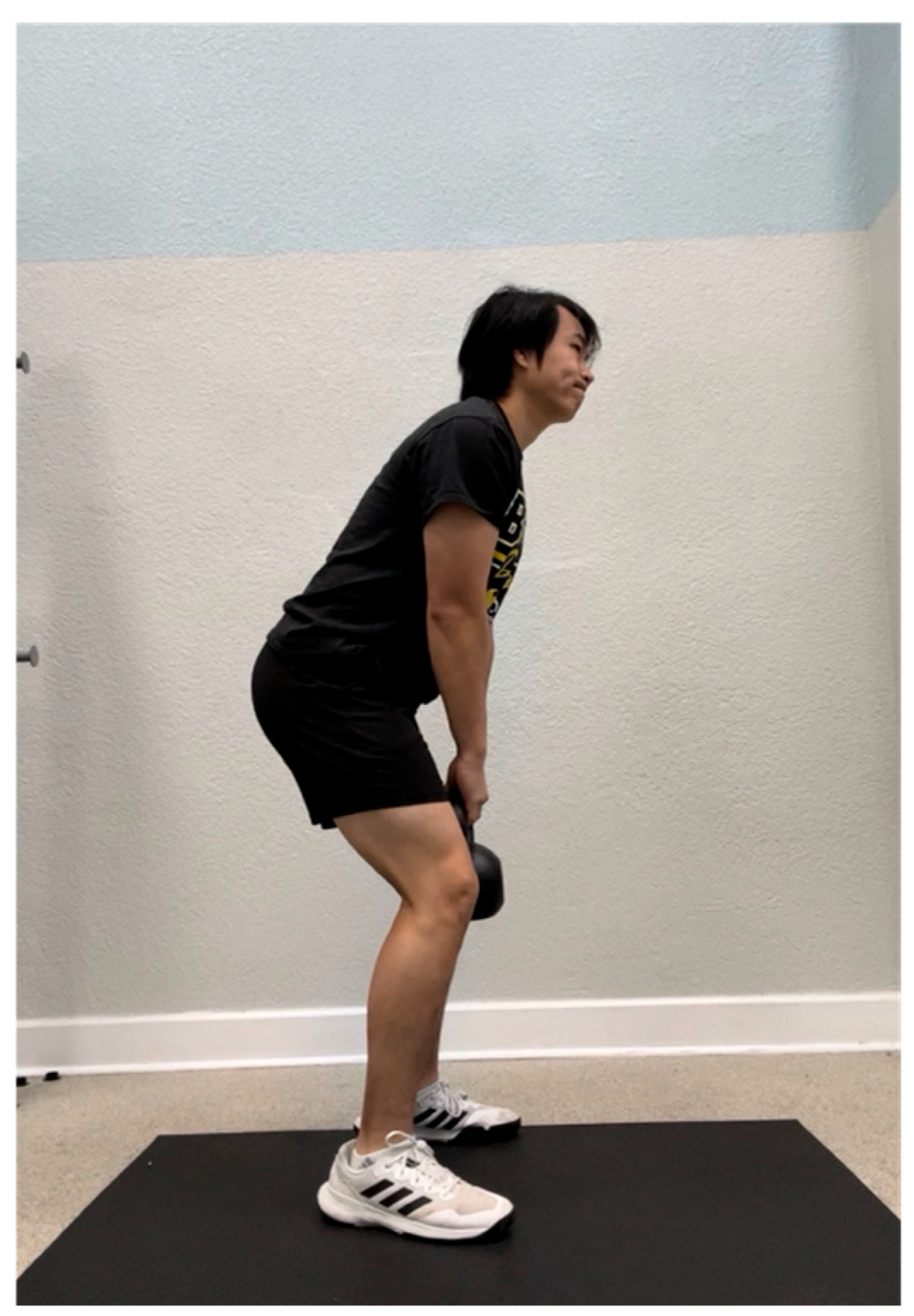
The mechanics of the kettlebell swing followed those outlined in the study carried out by McGill et al. [16]. As described above, participants were taught how to perform a kettlebell swing by a single researcher prior to the warm-up to ensure proper technique during the trial (Figure 9 and Figure 10). During the trial, the researcher provided cues for technique correction if any of the following errors were made: (1) the spine was not kept in a neutral position (e.g., the back was arched), (2) the kettlebell was not in between shoulder level and head height at the end of the swing, (3) the hips and knees were not slightly flexed at the beginning of the swing, and (4) the hips and knees were not in full extension at the end of the swing. The participant was instructed to perform the protocol to the best of their ability, exerting maximal effort as tolerated. Intensity was self-regulated and subjective, reflecting each individual’s perceived level of maximal exertion.

Figure 9.
Initial position of the kettlebell swing.

Figure 10.
Ending position of the kettlebell swing.
2.3.3. Isometric Hold
Participants in the isometric hold group performed an isometric hold of the kettlebell for the intervals as previously mentioned (Figure 11). To perform the isometric hold, participants mimicked the initial kettlebell swing position by standing shoulder-width apart with their back in a neutral position and hips and knees slightly flexed while holding the kettlebell in between their legs. Participants were told to “squeeze their glutes” and look straight ahead while they held the weight for each 30 s interval. Verbal cues were provided for form correction during the trial when any of the following errors were made: (1) the back was not in a neutral position, (2) the hips and knees were not flexed, and (3) the shoulders were not retracted and depressed.
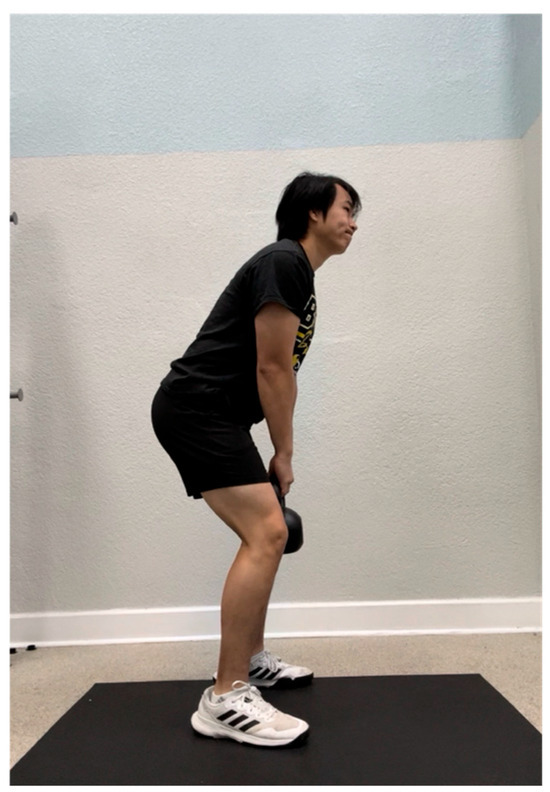
Figure 11.
Position of the isometric hold.
3. Results
Forty participants met the inclusion criteria and participated in the study. All data were analyzed using the 2022 version of JASP (version 0.16.4). All data are based on a 95% confidence interval, with a p < 0.05 being used as a marker for significant differences. The average age of the participants = 23.85 (standard deviation (SD) = ± 2.726). The average weight of the participants is 73.69 kg (SD = ± 13.62). The average height of the participants is 174.287 cm (SD ± 12.448). There were no dropouts, and no reports of adverse events occurred for any participant. Baseline ANOVAs were used to indicate whether there were any baseline differences between groups based on the pre-intervention muscle displacement (Dm) for each muscle group tested by TMG (ES: p = 0.645, GM: p = 0.733, BF: p = 0.586) and the average pre-intervention PPT for each tested muscle group (ES: p = 0.232, QL: p = 0.425, PF: p = 0.386). No significant differences were found between each of the groups based on these results for each of these tested muscle groups. Also using baseline ANOVAs, no significant differences were also found between groups based on demographics (Table 1). T-tests were used to test for within-group differences before and after the intervention period. Repeated measures ANOVA was used to test for group-time interactions for both TMG and PPT measurements.

Table 1.
Participant baseline characteristics.
3.1. Tensiomyography Results
For the erector spinae, no significant within-group differences were found for each of the experimental groups for any of the TMG variables: control, kettlebell swing, and isometric hold groups (Table 2). No significant group-time interactions were seen for any of the TMG variables (Table 2).

Table 2.
Pre- to post-intervention changes in erector spinae parameters (Tc, Tr, Td, Ts).
For the gluteus maximus, no significant within-group differences were found for each of the experimental groups for any of the TMG variables: control, kettlebell, and isometric hold groups (Table 3). No significant group-time interactions were seen for any of the TMG variables (Table 3).

Table 3.
Pre- to post-intervention changes in gluteus maximus parameters (Tc, Tr, Td, Ts).
For the biceps femoris, no significant within-group differences were found for each of the experimental groups for any of the TMG variables: control, kettlebell swing, and isometric hold groups (Table 4 and Table 5). No significant group-time interactions were seen for any of the TMG variables (Table 4 and Table 5).

Table 4.
Pre- to post-intervention changes in biceps femoris parameters (Tc, Tr, Td, Ts).

Table 5.
Pre- to post-intervention changes in TMG displacement (Dm) for erector spinae, quadratus lumborum, and piriformis.
3.2. Pressure Pain Threshold Results
There were no significant within-group differences in PPT for paravertebral muscles for any of the three groups: control, swing, and isometric (Table 6). There are also no significant group-time interactions for this muscle group (Table 6).

Table 6.
Pre- to post-intervention changes in pressure pain threshold for erector spinae, quadratus lumborum, and piriformis.
No significant within-group differences in PPT were found for QL for any of the three groups: control, swing, and isometric (Table 6). There are also no significant group-time interactions for this muscle group (Table 6).
No significant within-group differences in PPT were found for the control group (Table 6). Significant within-group differences were found for both the swing group (p = 0.017) and the isometric group (p = 0.015) (Table 6). However, there are no significant group-time interactions for any of the three groups (Table 6).
4. Discussion
The goal of this study was to assess changes in the muscular contractility and sensitivity of the ES, GM, and BF and assess changes in pressure pain threshold of the ES, QL, and PF after completing a two-handed kettlebell swing following a modified Tabata protocol. No significant differences in TMG measurements were detected among the participants. Mean increases in pressure pain threshold were found in lumbosacral musculature following the KBS protocol and isometric hold protocol. These results are consistent with Keilman et al. [5], who also demonstrated increases in lumbopelvic muscle sensitivity. Within-group differences for changes in PPT for the piriformis muscle reached statistical significance for both the KBS group and the isometric hold group.
The insignificant differences for TMG measurements in the lumbosacral muscle groups may be attributed to differences in contributions of muscle groups during the kettlebell swing and isometric hold groups. There are EMG studies suggesting that anterior muscle groups such as the rectus femoris [16] and vastus lateralis [24] may play a bigger role in the KBS. The kettlebell swing initially begins with hip flexion, knee flexion, and dorsiflexion, followed by triple extension to propel the weight to just above shoulder height. The rectus femoris and vastus lateralis may contribute to the extension moment at the knee. Despite contributions from anterior muscles, research shows that the ES primarily contributes to the kettlebell swing at the initiation of the movement, followed by the GM further into the extension portion of the swing. Although the quadriceps may function synergistically with the gluteus maximus and erector spinae during a kettlebell swing, it is not the primary contributor to the joint moments involved in the movement and may not fully reflect the associated fatigue characteristics. This suggests that the posterior chain muscles might play a larger role in the swing than anterior muscles. The KB swing utilizes these posterior chain muscles to complete the movement, such as the GM, potentially influencing its myofascial characteristics.
Despite no significant within-group differences being seen for TMG measurements, the KBS does demonstrate small mean differences in Dm and Tc for the glute max. Although our study shows no significant results regarding within-group differences for TMG measurements, studies do show greater contractility of lumbosacral muscles during EMG studies [16,24]. Therefore, TMG should be able to detect acute changes in contractile parameters such as muscle displacement. In comparison, the isometric hold should also demonstrate changes in contractility detectable by the TMG due to the hip hinge position of the kettlebell swing.
Participants in this study were instructed to perform an isometric hold of the kettlebell in the hip hinge position of the kettlebell swing. Holding the kettlebell in this hip hinge position potentially elicits glute muscle activation while the participant completes the protocol. Studies demonstrate the GM plays a significant role in other exercises containing hip hinges, such as the squat and deadlift [25]. In Liu’s study, the GM has the highest torque with isometric contractions around 45 degrees of hip flexion [26], while in a different study, peak glute activation of the kettlebell swing occurs around 70% of the swing [16]. With the GM’s role as a powerful hip extensor, the contractility of the GM may be acutely affected because of this exercise. Due to the discrepancy between glute muscle activation at different angles of hip flexion, future research should examine varying levels of hip flexion for the kettlebell isometric hold to see if this will affect the contractility of GM. The isometric hold not only potentially affects contractility of lumbosacral muscles but can also acutely influence the PPT in these muscle groups.
While significant within-group differences were not seen with TMG measurements, the KBS and isometric groups both had significant within-group differences with pressure pain threshold measurements for the piriformis. Exercise-induced hypoalgesia has been shown to occur after aerobic exercise [27] and dynamic resistance exercise [27]. Isometric holds also have been shown to cause acute analgesic responses, even at lower intensities [28]. The increases in PPT for the piriformis are in line with Keilman et al. [5] who completed the only other study that has examined this muscle in the context of a Tabata-style protocol. They also found significant within-group differences in PPT in this muscle group after having participants follow a similar high-intensity kettlebell swing training protocol like this study [5]. However, our study expands upon this study by incorporating another form of exercise, the isometric hold, which also showed significant changes in PPT after completing the exercise in an interval-style format. The piriformis muscle is often used in the differential diagnosis of lower back pain due to pathologies relating to this muscle, such as piriformis syndrome and active myofascial trigger points [29]. There is also evidence for its role in lumbopelvic dysfunction for those with patellofemoral pain syndrome [30]. Due to the piriformis’s role in pathologies, future studies should explore the effects of both the isometric hold and KBS on clinically relevant lumbosacral pathologies to decrease symptoms localized to this muscle group. The isometric hold specifically may be of interest in other clinically relevant populations, such as those with cardiovascular pathology.
Those with a high risk of cardiovascular events may benefit more from the isometric hold by allowing them to attain analgesic effects in this muscle group without having to perform exercises at a high intensity. The traditional Tabata-style protocol involves exercising at >80% of the person’s maximum heart rate [12]. This may present a problem for individuals with a cardiovascular condition since high-intensity exercise temporarily increases the risk of adverse effects such as cardiac arrest [31]. Athletic populations, specifically those with cardiovascular disease, have also been shown to have an increased risk of cardiovascular events during exercise [32]. Isometric exercise may be less risky to perform in these populations since this kind of exercise has been shown to not push an individual’s systolic blood pressure above the upper limits of ACSM guidelines for exercise termination [33]. In this study, the participants placed in the isometric hold group were able to continuously complete the isometric hold of the kettlebell for all intervals (14/14). However, not all the participants placed in the kettlebell swing group could continuously swing the kettlebell for all the required intervals (8/13). In these cases, the participants completed the 30 s bout but required a brief pause. This may be due to the higher intensity level of the kettlebell swing in contrast to the isometric hold. The isometric hold may be a useful tool for clinicians looking to harness the analgesic effects of exercise in this muscle group without the patient needing to perform high-intensity exercise. This may be especially effective for those who are at risk of adverse effects due to underlying cardiovascular pathologies.
Limitations
Some limitations warrant notes in the study. First is the difficulty with blinding during post-intervention TMG and PPT measurements. While the researcher collecting measurements is blinded to participant group, there are physiological changes participants experienced in the KBS and isometric hold groups that may make it difficult for the researcher to stay blinded. Performing a modified Tabata-style protocol may lead to patient responses to exercise such as sweat, redness, and an increase in the rate of breathing, which may introduce a level of bias during the assessment procedure of 30 mA. Second, there is a time lag between the time the participants perform the exercise and when the data are collected. Participants performed the kettlebell swings prior to having post-intervention measurements collected. Also, it was discussed earlier that TMG results may be limited due to varying levels of contribution to the kettlebell swing differing individually. Some untested muscle groups, such as the rectus femoris and vastus lateralis, may demonstrate changes in contractility not measured in our study. Future studies should incorporate more muscle groups to see how contractility differs between anteriorly and posteriorly located muscles. The process of collecting the TMG measurements was also time-consuming and may allow the participant increased time for muscle recovery from exercise if they were placed in that group. This means that measurements collected later in the post-intervention measurement period, such as the biceps femoris, may demonstrate differences in contractility parameters due to having more time to recover from muscle fatigue. Another limitation is the sample of participants. Recruitment for this study was limited in the variability of individuals due to participants being mostly composed of students from the university, as well as a greater number of males in the study compared to females. The number of participants, n=40, is also another limiting factor in this study, as a greater number of participants could demonstrate different results.
5. Conclusions
TMG on the erector spinae, gluteus maximus, and biceps femoris did not reproduce significant within-group differences in the control, isometric, and experimental groups. Pressure algometry showed no within-group differences for the paravertebral muscles and the quadratus lumborum. There were significant within-group differences for the isometric and swing groups for PPT on the piriformis.
These findings indicate that modified Tabata-style kettlebell swings and an isometric hold protocol can produce exercise-induced hypoalgesia (EIH) in the piriformis muscle, suggesting a potential non-pharmacological intervention for managing pain sensitivity in this region. The piriformis is often implicated in individuals experiencing low back pain, radiating symptoms (e.g., sciatica-like discomfort), and broader lumbopelvic dysfunction due to its anatomical proximity to the sciatic nerve and its role in hip stabilization and external rotation.
The observed increase in pressure pain threshold post-intervention supports the idea that targeted high-intensity and isometric training can desensitize hyperirritable myofascial tissue, potentially reducing discomfort and improving functional outcomes. These results may inform rehabilitation strategies by integrating structured, time-efficient kettlebell-based protocols to address myofascial sensitivity in the piriformis, which is frequently overlooked in conventional spine-focused rehabilitation plans. Furthermore, such interventions could be particularly beneficial in clinical populations where traditional movement-based treatments are limited by pain or fear-avoidance behaviors.
Further research could be conducted to examine the implications of exercise-induced hypoalgesia in individuals with disorders related to the piriformis and low back pain. The generalizability of this study is low, as the participant demographics are narrow and look at a relatively healthy population. This study presents the need for further research in individuals with chronic and acute low back pain and the implications of exercise-induced hypoalgesia after a bout of high-intensity kettlebell protocol.
Author Contributions
Conceptualization, W.J.H., A.W.A., M.J.K., C.L., J.C.-H. and J.L.; methodology, W.J.H., A.W.A. and M.J.K.; formal analysis, W.J.H. and A.W.A.; writing—original draft preparation, W.J.H., C.L., J.C.-H. and J.L.; writing—review and editing, W.J.H., A.W.A., M.J.K., C.L., J.C.-H. and J.L.; project administration, W.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Central Florida (protocol code 00004622) 24 October 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piqueras-Sanchiz, F.; Cornejo-Daza, P.J.; Sánchez-Valdepeñas, J.; Bachero-Mena, B.; Sánchez-Moreno, M.; Martín-Rodríguez, S.; García-García, Ó.; Pareja-Blanco, F. Acute Mechanical, Neuromuscular, and Metabolic Responses to Different Set Configurations in Resistance Training. J. Strength Cond. Res. 2022, 36, 2983–2991. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Cargnel, A.; Raffini, A.; Mazzari, L.; Martini, M.; Ajčević, M.; Accardo, A.; Deodato, M.; Murena, L. Shoulder Tensiomyography and Isometric Strength in Swimmers Before and After a Fatiguing Protocol. J. Athl. Train. 2024, 59, 738–744. [Google Scholar] [CrossRef]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Carolyna Gianlorenço, A.; Machado, R.; Queiroga, M.; Zeng, H.; Shaikh, E.; Yang, Y.; Nogueira, B.; Castelo-Branco, L.; Fregni, F. Exercise-induced pain threshold modulation in healthy subjects: A systematic review and meta-analysis. Princ. Pract. Clin. Res. 2020, 6, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Keilman, B.M.; Hanney, W.J.; Kolber, M.J.; Pabian, P.S.; Salamh, P.A.; Rothschild, C.E.; Liu, X. The Short-Term Effect of Kettlebell Swings on Lumbopelvic Pressure Pain Thresholds: A Randomized Controlled Trial. J. Strength Cond. Res. 2017, 31, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Maruyama, T.; Naito, H.; Sinclair, P.J. Acute effects of high-intensity dumbbell exercise after isokinetic eccentric damage: Interaction between altered pain perception and fatigue on static and dynamic muscle performance. J. Strength Cond. Res. 2010, 24, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.G.; Dawes, H.; Howells, K.; Scott, O.M.; Cramp, M.; Izadi, H. Alterations in peripheral muscle contractile characteristics following high and low intensity bouts of exercise. Eur. J. Appl. Physiol. 2012, 112, 337–343. [Google Scholar] [CrossRef]
- Muñoz-López, A.; De Hoyo, M.; Nuñez, F.J.; Sañudo, B. Using Tensiomyography to Assess Changes in Knee Muscle Contraction Properties After Concentric and Eccentric Fatiguing Muscle Actions. J. Strength Cond. Res. 2022, 36, 935–940. [Google Scholar] [CrossRef]
- Jaiswal, P.R.; Ramteke, S.U.; Shedge, S. Enhancing Athletic Performance: A Comprehensive Review on Kettlebell Training. Cureus 2024, 16, e53497. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Hoeger Bement, M.; Madsen, A.B.; Fridriksson, J.; Dasa, M.; Graven-Nielsen, T. Exercise increases pressure pain tolerance but not pressure and heat pain thresholds in healthy young men. Eur. J. Pain 2017, 21, 73–81. [Google Scholar] [CrossRef]
- Wewege, M.A.; Jones, M.D. Exercise-Induced Hypoalgesia in Healthy Individuals and People With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J. Pain 2021, 22, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tabata, I. Tabata training: One of the most energetically effective high-intensity intermittent training methods. J. Physiol. Sci. 2019, 69, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.P.; Lauder, M.A. Kettlebell swing training improves maximal and explosive strength. J. Strength Cond. Res. 2012, 26, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Čular, D.; Babić, M.; Zubac, D.; Kezić, A.; Macan, I.; Peyré-Tartaruga, L.A.; Ceccarini, F.; Padulo, J. Tensiomyography: From muscle assessment to talent identification tool. Front. Physiol. 2023, 14, 1163078. [Google Scholar] [CrossRef]
- Edinborough, L.; Fisher, J.P.; Steele, J. A Comparison of the Effect of Kettlebell Swings and Isolated Lumbar Extension Training on Acute Torque Production of the Lumbar Extensors. J. Strength Cond. Res. 2016, 30, 1189–1195. [Google Scholar] [CrossRef]
- McGill, S.M.; Marshall, L.W. Kettlebell swing, snatch, and bottoms-up carry: Back and hip muscle activation, motion, and low back loads. J. Strength Cond. Res. 2012, 26, 16–27. [Google Scholar] [CrossRef]
- Van Gelder, L.H.; Hoogenboom, B.J.; Alonzo, B.; Briggs, D.; Hatzel, B. EMG Analysis and Sagittal Plane Kinematics of the Two-Handed and Single-Handed Kettlebell Swing: A Descriptive Study. Int. J. Sports Phys. Ther. 2015, 10, 811–826. [Google Scholar]
- Martín-Rodríguez, S.; Loturco, I.; Hunter, A.M.; Rodríguez-Ruiz, D.; Munguia-Izquierdo, D. Reliability and Measurement Error of Tensiomyography to Assess Mechanical Muscle Function: A Systematic Review. J. Strength Cond. Res. 2017, 31, 3524–3536. [Google Scholar] [CrossRef]
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur. J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef]
- Burger, H.; Valencic, V.; Marincek, C.; Kogovsek, N. Properties of musculus gluteus maximus in above-knee amputees. Clin. Biomech. 1996, 11, 35–38. [Google Scholar] [CrossRef]
- Đorđević, S.; Rozman, S.; Zupet, P.; Dopsaj, M.; Maffulli, N. Tensiomyography Allows to Discriminate between Injured and Non-Injured Biceps Femoris Muscle. Biology 2022, 11, 746. [Google Scholar] [CrossRef]
- Walton, D.M.; Macdermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Jay, K.; Frisch, D.; Hansen, K.; Zebis, M.K.; Andersen, C.H.; Mortensen, O.S.; Andersen, L.L. Kettlebell training for musculoskeletal and cardiovascular health: A randomized controlled trial. Scand. J. Work. Environ. Health 2011, 37, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.C.; Mayo, J.J.; Tucker, W.S.; Wax, B.; Hendrix, R.C. Electromyographical Comparison of Muscle Activation Patterns Across Three Commonly Performed Kettlebell Exercises. J. Strength Cond. Res. 2017, 31, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.K.; Soares, E.G.; Vieira, T.L.; Aguiar, R.; Chola, T.A.; Sampaio, V.L.; Gama, E.F. Gluteus Maximus Activation during Common Strength and Hypertrophy Exercises: A Systematic Review. J. Sports Sci. Med. 2020, 19, 195–203. [Google Scholar]
- Liu, J.; Teng, H.L.; Selkowitz, D.M.; Asavasopon, S.; Powers, C.M. Influence of hip and knee positions on gluteus maximus and hamstrings contributions to hip extension torque production. Physiother. Theory Pract. 2022, 38, 2650–2657. [Google Scholar] [CrossRef]
- Niwa, Y.; Shimo, K.; Ohga, S.; Tokiwa, Y.; Hattori, T.; Matsubara, T. Effects of Exercise-Induced Hypoalgesia at Different Aerobic Exercise Intensities in Healthy Young Adults. J. Pain Res. 2022, 15, 3615–3624. [Google Scholar] [CrossRef]
- Hoeger Bement, M.K.; Dicapo, J.; Rasiarmos, R.; Hunter, S.K. Dose response of isometric contractions on pain perception in healthy adults. Med. Sci. Sports Exerc. 2008, 40, 1880–1889. [Google Scholar] [CrossRef]
- Probst, D.; Stout, A.; Hunt, D. Piriformis Syndrome: A Narrative Review of the Anatomy, Diagnosis, and Treatment. PM R. 2019, 11 (Suppl. 1), S54–S63. [Google Scholar] [CrossRef]
- Samani, M.; Ghaffarinejad, F.; Abolahrari-Shirazi, S.; Khodadadi, T.; Roshan, F. Prevalence and sensitivity of trigger points in lumbo-pelvic-hip muscles in patients with patellofemoral pain syndrome. J. Bodyw. Mov. Ther. 2020, 24, 126–130. [Google Scholar] [CrossRef]
- Thompson, P.D.; Franklin, B.A.; Balady, G.J.; Blair, S.N.; Corrado, D.; Estes, N.A., 3rd; Fulton, J.E.; Gordon, N.F.; Haskell, W.L.; Link, M.S.; et al. Exercise and acute cardiovascular events placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007, 115, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- van Teeffelen, W.M.; de Beus, M.F.; Mosterd, A.; Bots, M.L.; Mosterd, W.L.; Pool, J.; Doevendans, P.A.; Grobbee, D.E. Risk factors for exercise-related acute cardiac events. A case-control study. Br. J. Sports Med. 2009, 43, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.D.; Taylor, K.; Coleman, D.; Sharma, R.; O’Driscoll, J.M. The safety of isometric exercise: Rethinking the exercise prescription paradigm for those with stage 1 hypertension. Medicine 2018, 97, e0105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).