Dapagliflozin and Silymarin Ameliorate Cisplatin-Induced Nephrotoxicity via Nrf2/HO-1 Upregulation: A Preclinical Mechanistic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals
2.3. Justification for Cisplatin, Silymarin, and Dapagliflozin Dosage Selection
2.4. Preparation and Administration of Drugs
2.5. Experimental Design
2.6. Collection of Blood and Serum Preparation
2.7. Kidney Collection and Gross Examination
2.8. Preparation of Kidney Homogenate
2.9. Biochemical Estimations in Serum and Kidney Homogenate
2.10. Histopathological Examination of the Kidney
2.11. Statistical Analysis
3. Results
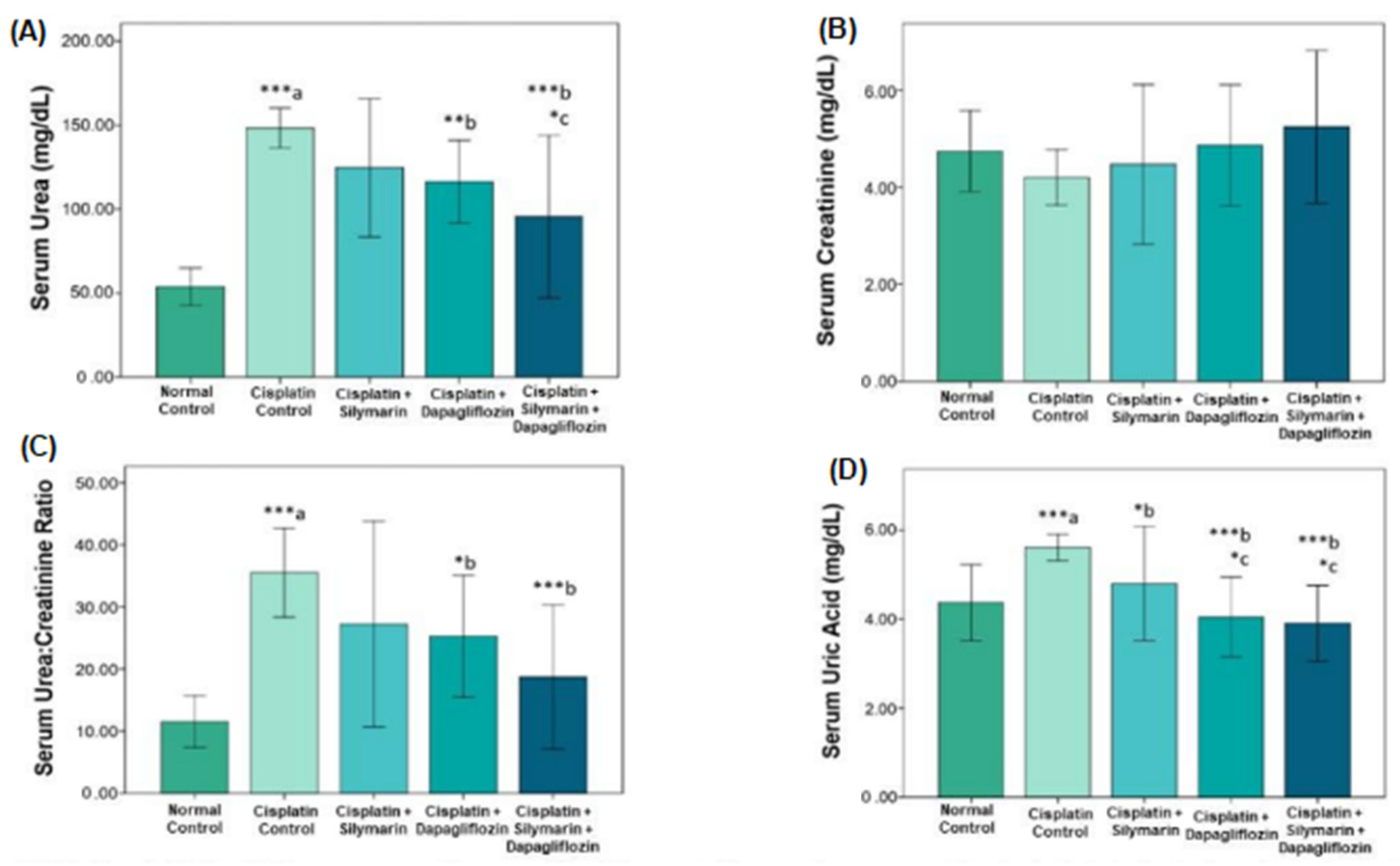
3.1. Impact on Tests of Renal Function
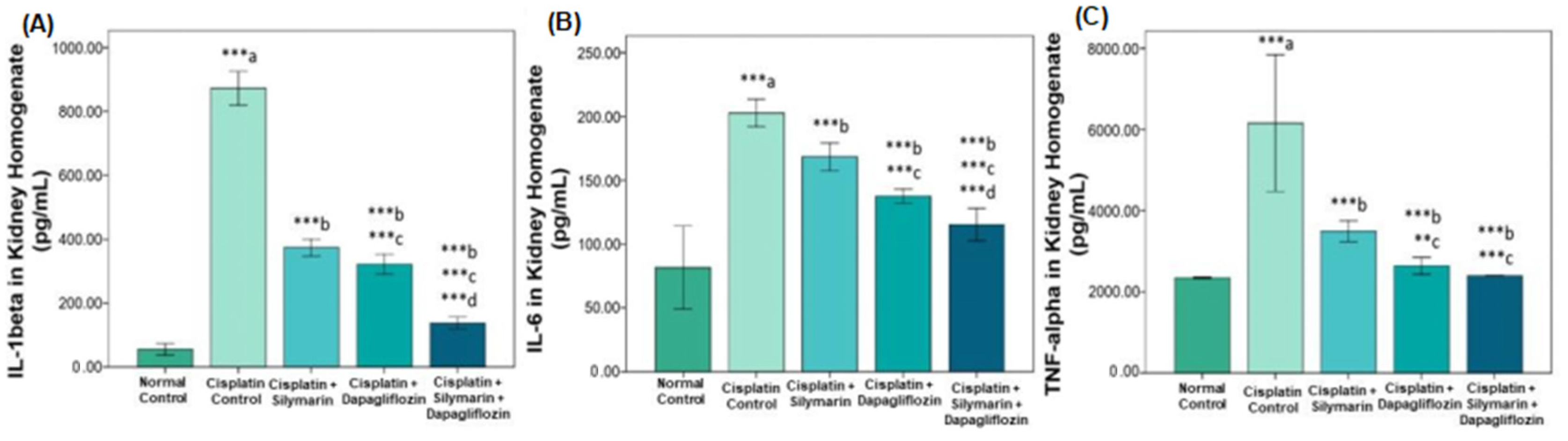
3.2. Impact on Inflammatory Cytokines
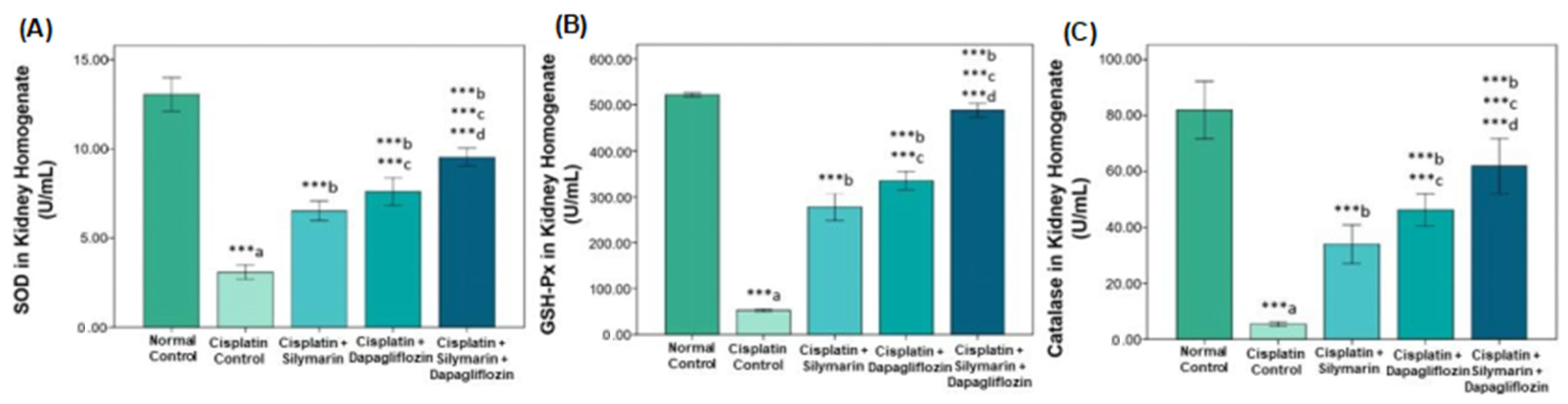
3.3. Effect on Oxidative Stress Biomarkers
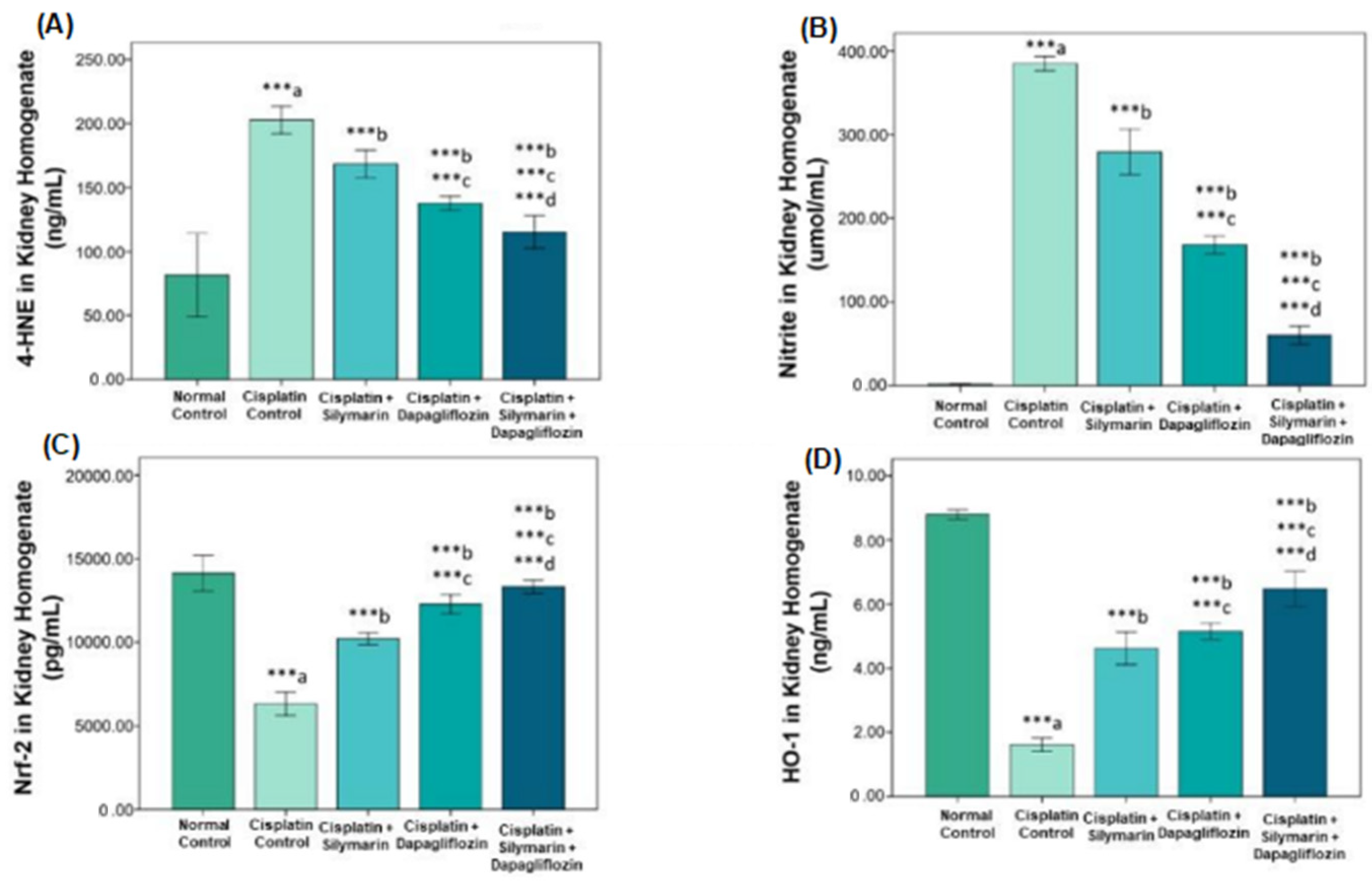
3.4. Nrf2/HO-1 Signaling Pathway Modification
3.5. Impact on Fasting Blood Glucose Levels and Body Weight
3.6. Effect on Kidney Histology
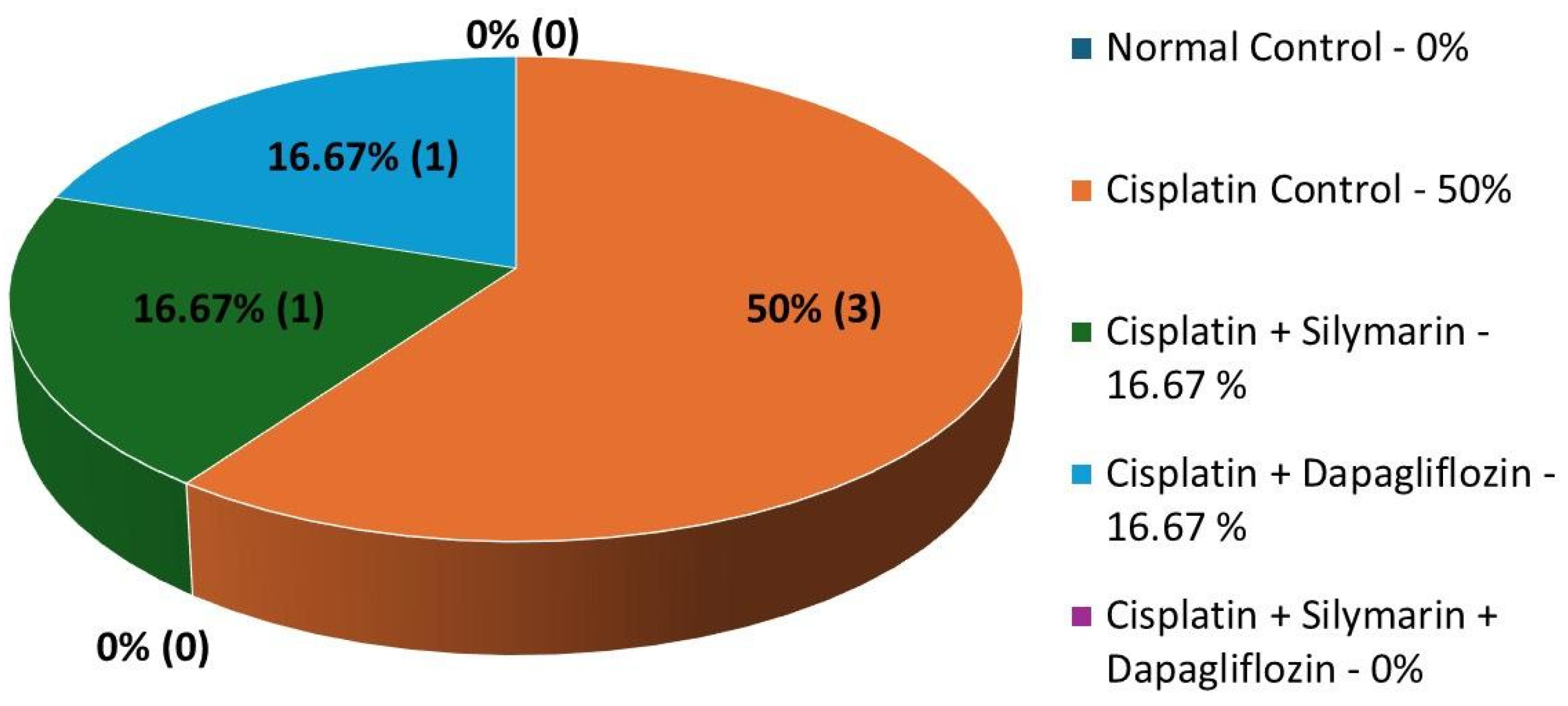
3.7. Impact on Mortality Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Pérez, J.M. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Launay-Vacher, V.; Rey, J.-B.; Isnard-Bagnis, C.; Deray, G.; Daouphars, M. Prevention of cisplatin nephrotoxicity: State of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother. Pharmacol. 2008, 61, 903–909. [Google Scholar] [CrossRef]
- Wang, J.; Pabla, N.; Wang, C.-Y.; Wang, W.; Schoenlein, P.V.; Dong, Z. Caspase-mediated cleavage of ATM during cisplatin-induced tubular cell apoptosis: Inactivation of its kinase activity toward p53. Am. J. Physiol.-Ren. Physiol. 2006, 291, F1300–F1307. [Google Scholar] [CrossRef]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- Sikking, C.; Niggebrugge-Mentink, K.L.; van der Sman, A.S.E.; Smit, R.H.P.; Bouman-Wammes, E.W.; Beex-Oosterhuis, M.M.; van Kesteren, C. Hydration methods for cisplatin containing chemotherapy: A systematic review. Oncologist 2024, 29, e173–e186. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhu, S.; Li, X.; Wu, H.; Li, Y.; Hua, F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis based on randomized controlled trials. PLoS ONE 2014, 9, e95968. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of amifostine in radiation protection: Administration and delivery. Mol. Pharm. 2023, 20, 5383–5395. [Google Scholar] [CrossRef]
- Ibrahim, M.E.-T.; El Bana, E.; El-Kerdasy, H.I. Role of bone marrow derived mesenchymal stem cells and the protective effect of silymarin in cisplatin-induced acute renal failure in rats. Am. J. Med. Sci. 2018, 355, 76–83. [Google Scholar] [CrossRef]
- Iskander, A.; Yan, L.-J. Cisplatin-induced kidney toxicity: Potential roles of major NAD+-dependent enzymes and plant-derived natural products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011, 14, 308. [Google Scholar]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Kordkheyli, V.; Abbaszadeh-Goudarzi, K.; Nejati-Laskokalayeh, M.; Zarpou, S.; Khonakdar-Tarsi, A. The protective effects of silymarin on ischemia-reperfusion injuries: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 968. [Google Scholar]
- Karimi, G.; Ramezani, M.; Tahoonian, Z. Cisplatin nephrotoxicity and protection by milk thistle extract in rats. Evid. -Based Complement. Altern. Med. 2005, 2, 383–386. [Google Scholar] [CrossRef]
- Mansour, H.H.; Hafez, H.F.; Fahmy, N.M. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. BMB Rep. 2006, 39, 656–661. [Google Scholar] [CrossRef]
- Nicholson, M.K.; Asswad, R.G.; Wilding, J.P. Dapagliflozin for the treatment of type 2 diabetes mellitus—An update. Expert Opin. Pharmacother. 2021, 22, 2303–2310. [Google Scholar] [CrossRef]
- Shahbazi, F.; Dashti-Khavidaki, S.; Khalili, H.; Lessan-Pezeshki, M. Potential renoprotective effects of silymarin against nephrotoxic drugs: A review of literature. J. Pharm. Pharm. Sci. 2012, 15, 112–123. [Google Scholar] [CrossRef]
- Sonnenbichler, J.; Scalera, F.; Sonnenbichler, I.; Weyhenmeyer, R. Stimulatory effects of silibinin and silicristin from the milk thistle Silybum marianum on kidney cells. J. Pharmacol. Exp. Ther. 1999, 290, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, E.-G.; Kim, S.; Kim, S.G.; Hahn, S.; Kim, N.H. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2019, 9, 13009. [Google Scholar] [CrossRef] [PubMed]
- Chino, Y.; Samukawa, Y.; Sakai, S.; Nakai, Y.; Yamaguchi, J.; Nakanishi, T.; Tamai, I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm. Drug Dispos. 2014, 35, 391–404. [Google Scholar] [CrossRef]
- van Bommel, E.J.; Muskiet, M.H.; van Baar, M.J.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212. [Google Scholar] [CrossRef]
- Tanna, M.S.; Goldberg, L.R. The pleiotropic cardiovascular effects of sodium-glucose cotransporter-2 inhibitors. Curr. Opin. Cardiol. 2021, 36, 764–768. [Google Scholar] [CrossRef]
- Oraby, M.A.; El-Yamany, M.F.; Safar, M.M.; Assaf, N.; Ghoneim, H.A. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed. Pharmacother. 2019, 109, 910–920. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Tian, M.; Sjöström, C.D.; Johansson, U.; Peng, X.-R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef]
- Garvey, W.T.; Van Gaal, L.; Leiter, L.A.; Vijapurkar, U.; List, J.; Cuddihy, R.; Ren, J.; Davies, M.J. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism 2018, 85, 32–37. [Google Scholar] [CrossRef]
- Zaibi, N.; Li, P.; Xu, S.-Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Večerić-Haler, Ž. Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. BioMed Res. Int. 2018, 2018, 1462802. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Ern, O.T.; Yen, Y.G.; Kanasin, A.; Muthaiah, T.; Ratnam, U.S.; Yadav, K. Influence of combination of docosahexaenoic acid supplement and a polyherbal formulation (Liv. 52) on carbon tetrachloride-induced hepatic injury: A preclinical study. J. Datta Meghe Inst. Med. Sci. Univ. 2020, 15, 114–117. [Google Scholar] [CrossRef]
- Paget, G.; Barnes, J. Toxicity tests. In Evaluation of Drug Activities; Elsevier: Amsterdam, The Netherlands, 1964; pp. 135–166. [Google Scholar]
- Satyam, S.M.; Bairy, L.K.; Shetty, P.; Sainath, P.; Bharati, S.; Ahmed, A.Z.; Singh, V.K.; Ashwal, A.J. Metformin and dapagliflozin attenuate doxorubicin-induced acute cardiotoxicity in Wistar rats: An electrocardiographic, biochemical, and histopathological approach. Cardiovasc. Toxicol. 2023, 23, 107–119. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.-M. Acidic solvent improves cisplatin action in in-vitro. Biochem. Biophys. Res. Commun. 2024, 712, 149936. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Rehman, A.; Attia, M.; Ahmed, L.; Emad, K.; Jaafer, Y.; Bahaaeldin, A. Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology 2024, 13, 473. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Rehman, A.; Farook, M.; Khan, S.; Nair, A.A.; Binu, N.N.; Yehya, M.; Khan, M.M. Dapagliflozin: A Promising Strategy to Combat Cisplatin-Induced Hepatotoxicity in Wistar Rats. Biology 2024, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Anees, L.M.; Abdel-Hamid, G.R.; Elkady, A.A. A nano based approach to alleviate cisplatin induced nephrotoxicity. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211066441. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Lou, D.-Y.; Zhou, L.-Q.; Wang, J.-C.; Yang, B.; He, Q.-J.; Wang, J.-J.; Weng, Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, X.; Ruan, L.; Ye, T.; Wen, Y.; Song, Z.; Hu, S.; Chen, Y.; Peng, B.; Li, S. Protective effect of mannitol on cisplatin-induced nephrotoxicity: A systematic review and meta-analysis. Front. Oncol. 2021, 11, 804685. [Google Scholar] [CrossRef]
- Mercantepe, F.; Mercantepe, T.; Topcu, A.; Yılmaz, A.; Tumkaya, L. Protective effects of amifostine, curcumin, and melatonin against cisplatin-induced acute kidney injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 915–931. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Bouma, H.R.; Lobb, I.; Sener, A. Hydrogen sulfide: A novel nephroprotectant against cisplatin-induced renal toxicity. Nitric Oxide 2016, 57, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Suchal, K.; Gamad, N.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Telmisartan ameliorates cisplatin-induced nephrotoxicity by inhibiting MAPK mediated inflammation and apoptosis. Eur. J. Pharmacol. 2015, 748, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-S.; Kim, H.-J.; Shen, A.; Lee, S.-B.; Yang, S.-H.; Shim, H.; Cho, E.-Y.; Kwon, K.-B.; Kwak, T.H.; So, H.-S. New therapeutic concept of NAD redox balance for cisplatin nephrotoxicity. BioMed Res. Int. 2016, 2016, 4048390. [Google Scholar] [CrossRef]
- Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 2015, 66, 255–270. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Chi, P.-J.; Lee, C.-J.; Hsieh, Y.-J.; Lu, C.-W.; Hsu, B.-G. Dapagliflozin ameliorates lipopolysaccharide related acute kidney injury in mice with streptozotocin-induced diabetes mellitus. Int. J. Med. Sci. 2022, 19, 729–739. [Google Scholar] [CrossRef]
- Afsar, B.; Afsar, R.E. Sodium–glucose cotransporter inhibitors and kidney fibrosis: Review of the current evidence and related mechanisms. Pharmacol. Rep. 2023, 75, 44–68. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; Del Prato, S.; Chilton, R.; DeFronzo, R.A. SGLT2 inhibitors and cardiovascular risk: Lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016, 39, 717–725. [Google Scholar] [CrossRef]
- Cai, A.; Shen, J.; Yang, X.; Shao, X.; Gu, L.; Mou, S.; Che, X. Dapagliflozin alleviates renal inflammation and protects against diabetic kidney diseases, both dependent and independent of blood glucose levels. Front. Immunol. 2023, 14, 1205834. [Google Scholar] [CrossRef]
- Tai, S.; Zhou, Y.; Fu, L.; Ding, H.; Zhou, Y.; Yin, Z.; Yang, R.; Liu, Z.; Zhou, S. Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes. Heliyon 2023, 9, e19152. [Google Scholar] [CrossRef]
- Lee, O.Y.A.; Wong, A.N.N.; Ho, C.Y.; Tse, K.W.; Chan, A.Z.; Leung, G.P.-H.; Kwan, Y.W.; Yeung, M.H.Y. Potentials of natural antioxidants in reducing inflammation and oxidative stress in chronic kidney disease. Antioxidants 2024, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Ishihara, T.; Oku, A.; Nawano, M.; Ueta, K.; Kitamura, K.; Matsumoto, M.; Saito, A. Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na+-glucose cotransporter inhibitor T-1095. Br. J. Pharmacol. 2001, 132, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013, 45, 817–826. [Google Scholar] [CrossRef]
- Jung, K.-A.; Kwak, M.-K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide dismutase administration: A review of proposed human uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Dinić, S.; Grdović, N.; Uskoković, A.; ĐORĐEVIĆ, M.; Mihailović, M.; Jovanović, J.A.; Poznanović, G.; Vidaković, M. CXCL12 protects pancreatic β-cells from oxidative stress by a Nrf2-induced increase in catalase expression and activity. Proc. Jpn. Acad. Ser. B 2016, 92, 436–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, H.; Zhou, L.; Guo, Z. Nrf2-dependent induction of NQO1 in mouse aortic endothelial cells overexpressing catalase. Free Radic. Biol. Med. 2011, 51, 97–106. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Luo, J.-F.; Shen, X.Y.; Lio, C.K.; Dai, Y.; Cheng, C.S.; Liu, J.X.; Yao, Y.D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.; Li, J.; Xia, M.; Li, Y.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Kan, D.; et al. Oxidative stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and treatment of aging-related diseases. J. Immunol. Res. 2022, 2022, 2233906. [Google Scholar] [CrossRef]
- Ahmed, A.Z.; Shetty, P.; Satyam, S.M.; D’souza, M.R.; Herle, A.M.; Singh, V.K. Methyl gallate mitigates doxorubicin-induced peripheral cytopenias: A preclinical experimental study. Res. J. Pharm. Technol. 2021, 14, 4529–4534. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K. Neuronutraceuticals combating Neuroinflammaging: Molecular insights and translational challenges—A systematic review. Nutrients 2022, 14, 3029. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.M.; Bairy, L.K.; Pirasanthan, R. Influence of grape seed extract and zinc containing multivitamin-mineral nutritional food supplement on lipid profile in normal and diet-induced hypercholesterolemic rats. J. Clin. Diagn. Res. JCDR 2014, 8, HC12. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Pirasanthan, R.; Vaihnav, R.L. Grape seed extract and zinc containing nutritional food supplement decreases the oxidative stress induced by carbon tetrachloride in rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 626–631. [Google Scholar]
- Satyam, S.M.; Kurady, L.B.; Prakash, J.; Syed, M.; Kumar, N.; Patil, J. Influence of ageless liquid with or without piperine on gentamicin-induced nephrotoxicity in wistar rats. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1963–1976. [Google Scholar]
- Alanazi, W.A.; Alharbi, T.; Bin Anzan, K.M.; Alyahiya, M.K.; El-Nagar, D.M.; Almutairi, M.M.; Alhamami, H.N.; Albogami, A.M.; Mohany, M. The Role of Dapagliflozin in the Modulation of Hypothermia and Renal Injury Caused by Septic Shock in Euglycemic and Hyperglycemic Rat Models. Curr. Mol. Pharmacol. 2024, 17, e18761429329635. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin inhibits IL-1β-mediated inflammatory response in human proximal tubular cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef]
- Song, J.; Li, X.; Ni, J. A Role for Sodium-Glucose Cotransporter 2 Inhibitors in the Treatment of Chronic Kidney Disease: A Mini Review. Kidney Blood Press. Res. 2023, 48, 599–610. [Google Scholar] [CrossRef]
- Speedtsberg, E.S.; Tepel, M. Narrative review investigating the nephroprotective mechanisms of sodium glucose cotransporter type 2 inhibitors in diabetic and nondiabetic patients with chronic kidney disease. Front. Endocrinol. 2023, 14, 1281107. [Google Scholar] [CrossRef]
- Chalmoukou, K.; Polyzos, D.; Manta, E.; Tatakis, F.; Konstantinidis, D.; Thomopoulos, C.; Costas, T. Renal outcomes associated with glucose-lowering agents: Systematic review and meta-analysis of randomized outcome trials. Eur. J. Intern. Med. 2022, 97, 78–85. [Google Scholar] [CrossRef]
- Ferrannini, E.; Ramos, S.J.; Salsali, A.; Tang, W.; List, J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010, 33, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Vico, M.; Wei, L.; Salsali, A.; List, J.F. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA1c, body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012, 35, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef]
- Devenny, J.J.; Godonis, H.E.; Harvey, S.J.; Rooney, S.; Cullen, M.J.; Pelleymounter, M.A. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity 2012, 20, 1645–1652. [Google Scholar] [CrossRef]
- Pereira, M.J.; Lundkvist, P.; Kamble, P.G.; Lau, J.; Martins, J.G.; Sjöström, C.D.; Schnecke, V.; Walentinsson, A.; Johnsson, E.; Eriksson, J.W. A randomized controlled trial of dapagliflozin plus once-weekly exenatide versus placebo in individuals with obesity and without diabetes: Metabolic effects and markers associated with bodyweight loss. Diabetes Ther. 2018, 9, 1511–1532. [Google Scholar] [CrossRef]
- Perše, M. Cisplatin mouse models: Treatment, toxicity and translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satyam, S.M.; Bairy, L.K.; Rehman, A.; Nair, A.A.; Farook, M.; Binu, N.N.; Khan, S.; Yehya, M.; Khan, M.M. Dapagliflozin and Silymarin Ameliorate Cisplatin-Induced Nephrotoxicity via Nrf2/HO-1 Upregulation: A Preclinical Mechanistic Study. Sci 2025, 7, 59. https://doi.org/10.3390/sci7020059
Satyam SM, Bairy LK, Rehman A, Nair AA, Farook M, Binu NN, Khan S, Yehya M, Khan MM. Dapagliflozin and Silymarin Ameliorate Cisplatin-Induced Nephrotoxicity via Nrf2/HO-1 Upregulation: A Preclinical Mechanistic Study. Sci. 2025; 7(2):59. https://doi.org/10.3390/sci7020059
Chicago/Turabian StyleSatyam, Shakta Mani, Laxminarayana Kurady Bairy, Abdul Rehman, Anuradha Asokan Nair, Mohamed Farook, Nirmal Nachiketh Binu, Sofiya Khan, Mohamed Yehya, and Mohammed Moin Khan. 2025. "Dapagliflozin and Silymarin Ameliorate Cisplatin-Induced Nephrotoxicity via Nrf2/HO-1 Upregulation: A Preclinical Mechanistic Study" Sci 7, no. 2: 59. https://doi.org/10.3390/sci7020059
APA StyleSatyam, S. M., Bairy, L. K., Rehman, A., Nair, A. A., Farook, M., Binu, N. N., Khan, S., Yehya, M., & Khan, M. M. (2025). Dapagliflozin and Silymarin Ameliorate Cisplatin-Induced Nephrotoxicity via Nrf2/HO-1 Upregulation: A Preclinical Mechanistic Study. Sci, 7(2), 59. https://doi.org/10.3390/sci7020059







