Advancements of Biohydrogen Production Based on Anaerobic Digestion: Technologies, Substrates, and Future Prospects
Abstract
1. Introduction
2. Materials and Methods
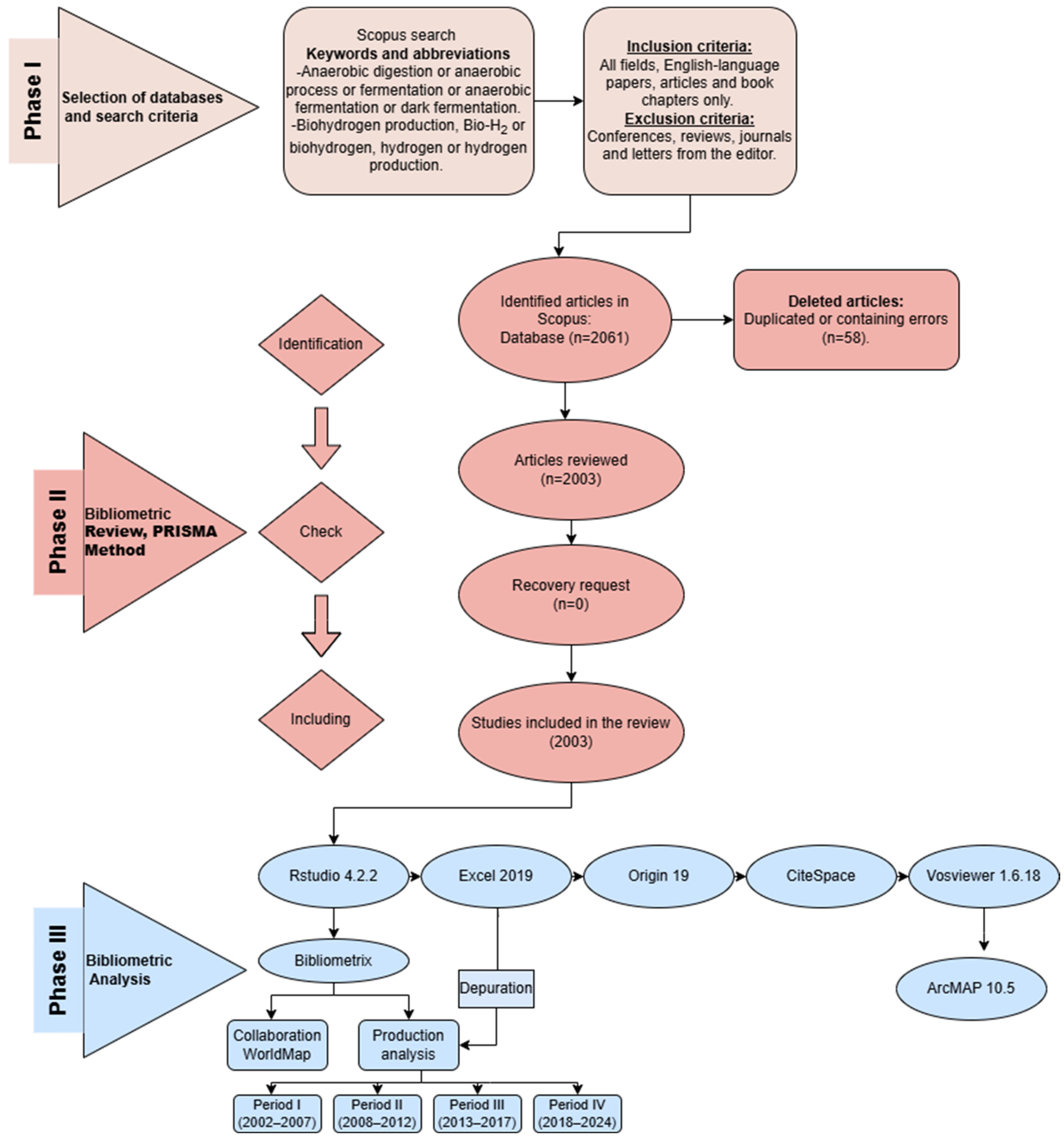
2.1. Selection of Databases and Search Criteria
2.2. Phase II—Screening and Eligibility
- Identification: retrieval of 2061 documents from Scopus.
- Screening: elimination of 58 documents with metadata inconsistencies or duplications.
- Eligibility: Inclusion of 2003 full-length, peer-reviewed documents in English. No manual recovery of excluded records was performed.
2.3. Phase III—Bibliometric and Content Analysis
2.3.1. Performance Analysis
2.3.2. Bibliometric Mapping
2.3.3. Prospective Analysis
2.4. Exploring Research Frontiers in Biohydrogen Production by the Use of CiteSpace Network Analysis
3. Results and Discussion
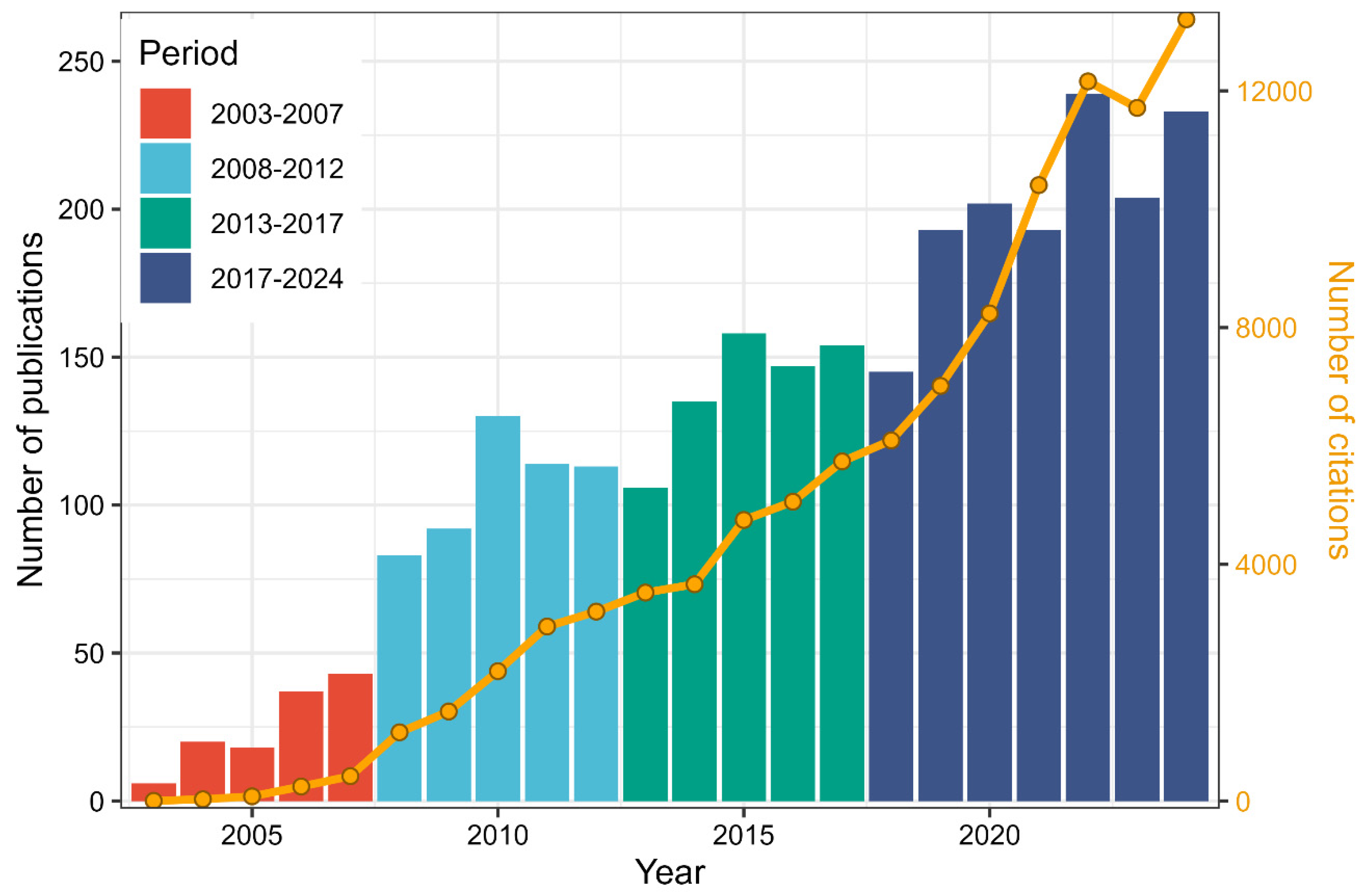
3.1. Annual Scientific Production by Period
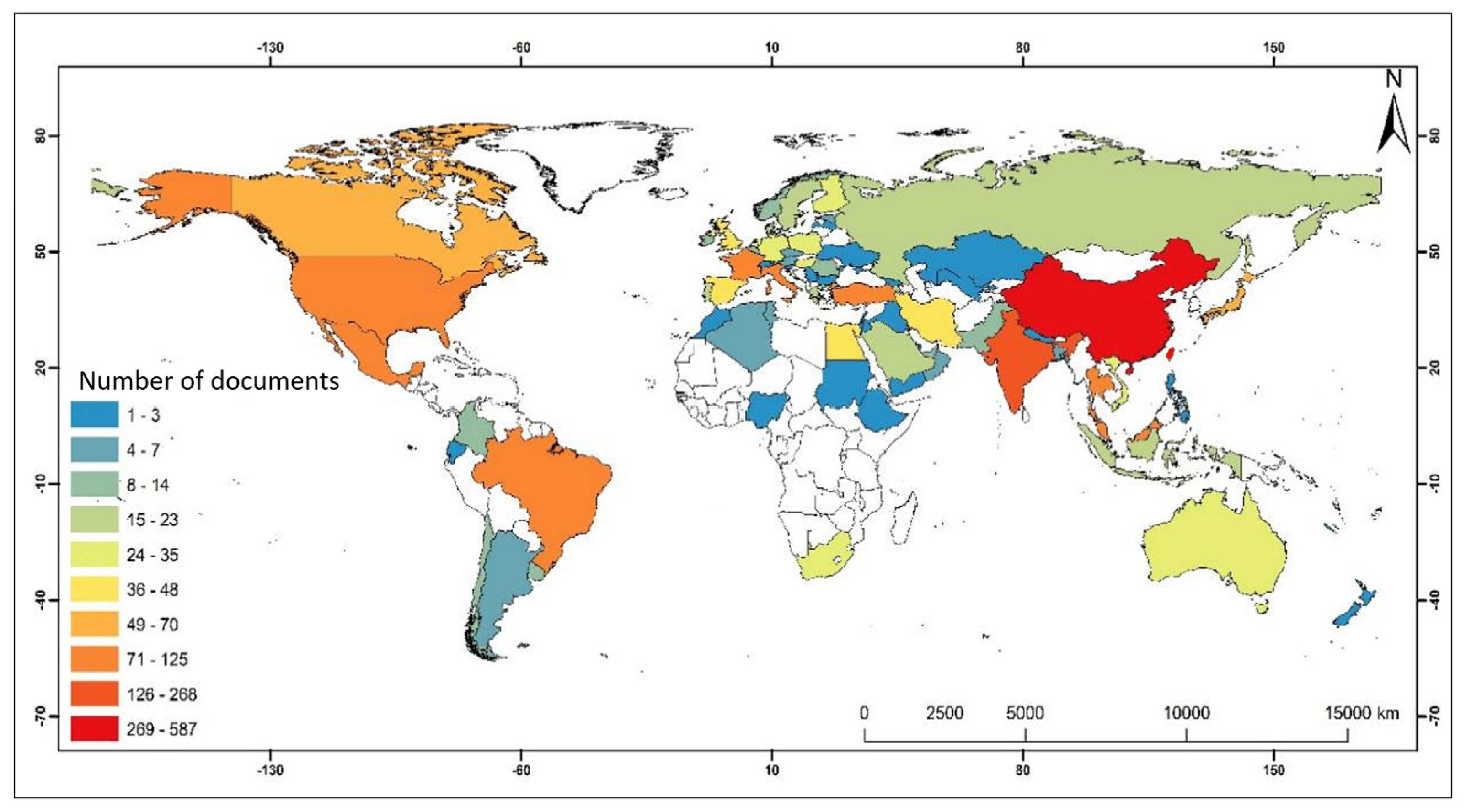
3.2. Analysis of Cooperation Between Countries and Regions
3.3. Contributions by Country and Collaborations
3.4. Journals with the Highest Number of Articles
3.5. Top-Cited Articles
| N° | Article | Authors | Journals | Citations | Open Access Article |
|---|---|---|---|---|---|
| 1 | Biohydrogen production: Prospects and limitations to practical application | Levin and Love [71] | International Journal of Hydrogen Energy | 1275 | No |
| 2 | Hydrogen production from agricultural waste by dark fermentation: A review | Guo et al. [76] | International Journal of Hydrogen Energy | 599 | No |
| 3 | Advances in biological hydrogen production processes | Das and Veziroglu [68] | International Journal of Hydrogen Energy | 561 | No |
| 4 | Use of algae as biofuel sources | Demirbas [77] | Energy Conversion and Management | 543 | No |
| 5 | Sustainable and efficient biohydrogen production via electrohydrogenesis | Cheng and Logan [67] | Proceedings of the National Academy of Sciences of the United States of America | 530 | Yes |
| 6 | Biohydrogen production by anaerobic fermentation of food waste | Han and Shin [74] | International Journal of Hydrogen Energy | 439 | No |
| 7 | Comparison of biohydrogen production processes | Manish and Banerjee [69] | International Journal of Hydrogen Energy | 385 | No |
| 8 | Enhanced biohydrogen production from sewage sludge with alkaline pretreatment | Cai et al. [78] | Environmental Science and Technology | 379 | Yes |
| 9 | Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge | Kim et al. [75] | International Journal of Hydrogen Energy | 367 | No |
| 10 | Biofuels generation from sweet sorghum: Fermentative hydrogen production and anaerobic digestion of the remaining biomass | Antonopoulou et al. [79] | Bioresource Technology | 362 | No |
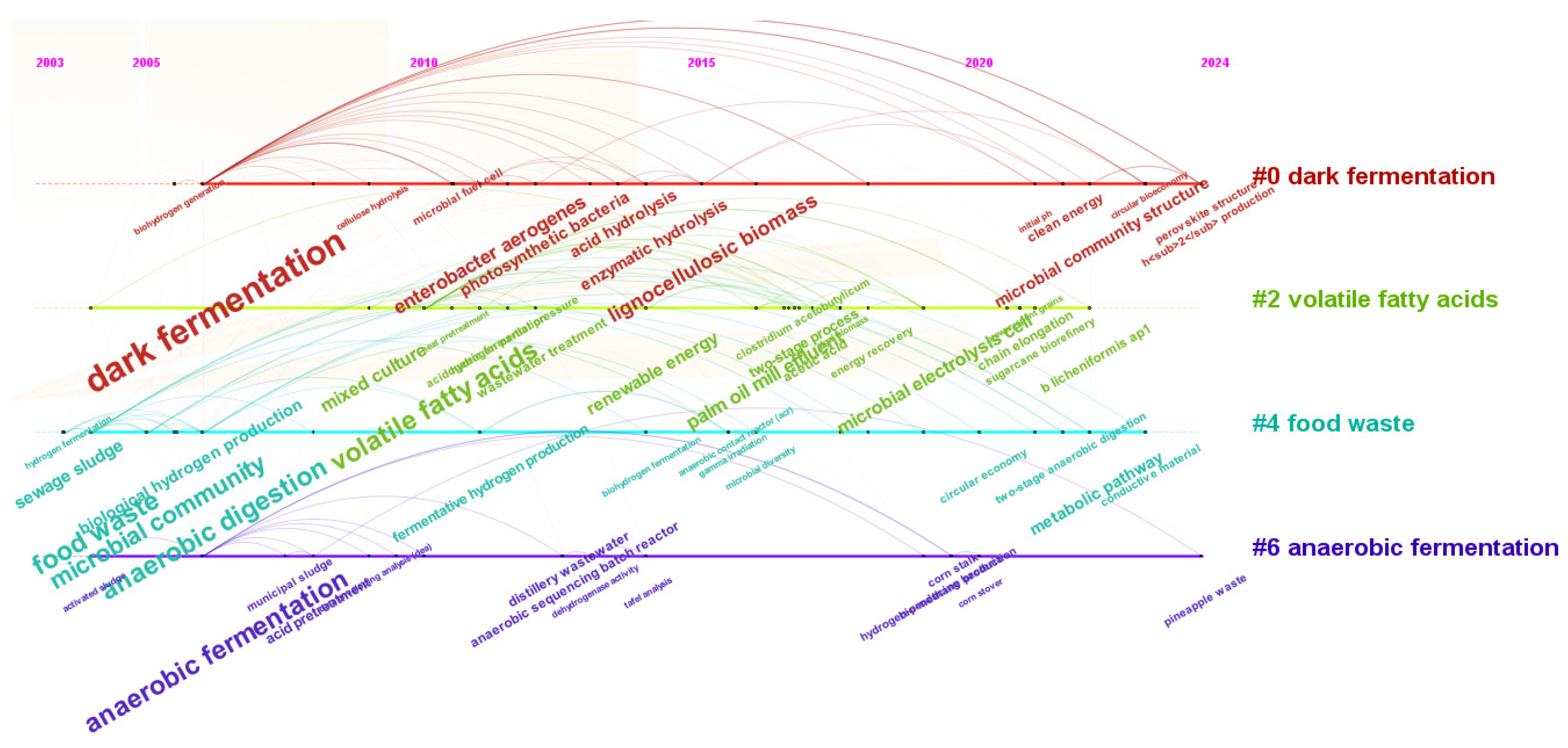
3.6. Keyword Analysis
3.7. Network Analysis of Biohydrogen Production
3.8. Main Research Areas
3.9. Scientific Progress in Biohydrogen: Innovations in Microbial Pathways, Feedstock Additives, and Environmental Strategies
3.10. Advancing Sustainability with Biohydrogen and Anaerobic Digestion
4. Analysis of the Current Practices for Biohydrogen Production
4.1. Applications of AD in Biohydrogen Production
4.2. The Main Bioenergy Outputs in AD for Biohydrogen Production
4.3. Key Biomass Degradation and Fermentation Indicators for Biohydrogen Production by AD
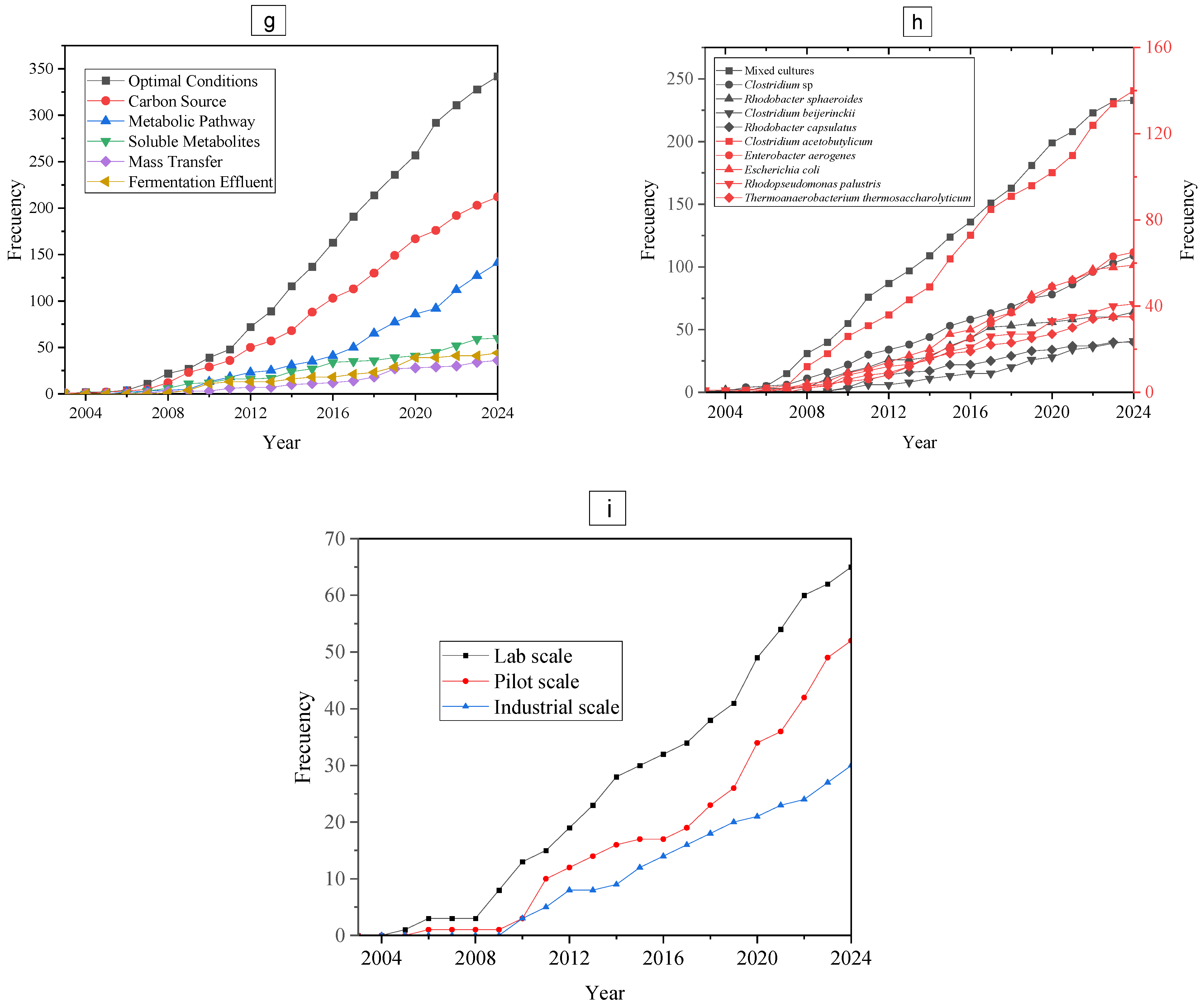
4.4. Key Factors Affecting Biohydrogen Production Kinetics in AD Systems
4.5. Diversity of Biomass Feedstocks for Biohydrogen Production via AD
4.6. Biohydrogen Production Pathways and Process Conditions in AD
4.7. AD Reactor Configurations for Biohydrogen Production
4.8. The Main Factors Influencing Biohydrogen Production in AD Systems
4.9. Microbial Strains for Biohydrogen Production in AD Studies
4.10. Occurrences of Lab, Industry, and Pilot Scale in Publications
5. Future Perspectives and Final Remarks
5.1. Integration of Advanced Tools Based on Artificial Intelligence into Biohydrogen Production
5.2. Life Cycle Assessment and Environmental Impact Modeling
5.3. Substrate Selection and Mixture Optimization
5.4. Real-Time Monitoring and Anomaly Detection
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zohuri, B.; McDaniel, P. Energy Insight: An Energy Essential Guide. Introd. Energy Essent. 2021, 321–370. [Google Scholar] [CrossRef]
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Tezel, E.; Figen, H.E.; Baykara, S.Z. Hydrogen Production by Methane Decomposition Using Bimetallic Ni–Fe Catalysts. Int. J. Hydrogen Energy 2019, 44, 9930–9940. [Google Scholar] [CrossRef]
- Arimi, M.M.; Knodel, J.; Kiprop, A.; Namango, S.S.; Zhang, Y.; Geißen, S.-U. Strategies for Improvement of Biohydrogen Production from Organic-Rich Wastewater: A Review. Biomass Bioenergy 2015, 75, 101–118. [Google Scholar] [CrossRef]
- Lubbe, F.; Rongé, J.; Bosserez, T.; Martens, J.A. Golden Hydrogen. Curr. Opin. Green. Sustain. Chem. 2023, 39, 100732. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The Future of Hydrogen: Challenges on Production, Storage and Applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Beasy, K.; Lodewyckx, S.; Mattila, P. Industry Perceptions and Community Perspectives on Advancing a Hydrogen Economy in Australia. Int. J. Hydrogen Energy 2023, 48, 8386–8397. [Google Scholar] [CrossRef]
- Van Renssen, S. The Hydrogen Solution? Nat. Clim. Change 2020, 10, 799–801. [Google Scholar] [CrossRef]
- Romero, A.; Jobbagy, M.; Laborde, M.; Baronetti, G.; Amadeo, N. Ni (II)–Mg (II)–Al (III) Catalysts for Hydrogen Production from Ethanol Steam Reforming: Influence of the Mg Content. Appl. Catal. A Gen. 2014, 470, 398–404. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Zuo, Z.; Han, Z.; Yao, X.; Qin, Q. Hydrogen Production via Sorption-Enhanced Catalytic Steam Reforming of Bio-Oil. Int. J. Hydrogen Energy 2016, 41, 2345–2353. [Google Scholar] [CrossRef]
- Dokhani, S.; Assadi, M.; Pollet, B.G. Techno-Economic Assessment of Hydrogen Production from Seawater. Int. J. Hydrogen Energy 2023, 48, 9592–9608. [Google Scholar] [CrossRef]
- Catumba, B.D.; Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Lopes, A.A.S.; de Sousa Rios, M.A.; Desai, A.S.; Bilal, M.; dos Santos, J.C.S. Sustainability and Challenges in Hydrogen Production: An Advanced Bibliometric Analysis. Int. J. Hydrogen Energy 2023, 48, 7975–7992. [Google Scholar] [CrossRef]
- Machhirake, N.P.; Vanapalli, K.R.; Kumar, S.; Mohanty, B. Biohydrogen from Waste Feedstocks: An Energy Opportunity for Decarbonization in Developing Countries. Environ. Res. 2024, 252, 119028. [Google Scholar] [CrossRef] [PubMed]
- European Biogas Association Biohydrogen: Affordable, Green and Yet Overlooked. Available online: https://www.europeanbiogas.eu/biohydrogen-affordable-green-and-yet-overlooked/ (accessed on 30 November 2024).
- Barghash, H.; Okedu, K.E.; Al Balushi, A. Bio-Hydrogen Production Using Landfill Leachate Considering Different Photo-Fermentation Processes. Front. Bioeng. Biotechnol. 2021, 9, 644065. [Google Scholar] [CrossRef]
- Sa, K.; Ravi, Y.K.; Ma, G.; Chattopadhyay, I.; Palani, S.; Kumar, V.; Kumar, G.; Jeyakumar, R.B. Development of an Integrated Biorefinery System for Bioconversion of Lignocellulosic Biomass to Polyhydroxyalkanoates and Biohydrogen. ACS Sustain. Chem. Eng. 2023, 11, 4606–4622. [Google Scholar] [CrossRef]
- Trancone, G.; Policastro, G.; Spasiano, D.; Race, M.; Parrino, F.; Fratino, U.; Fabbricino, M.; Pirozzi, F. Treatment of Concrete Waste from Construction and Demolition Activities: Application of Organic Acids from Continuous Dark Fermentation in Moving Bed Biofilm Reactors. Chem. Eng. J. 2025, 505, 159536. [Google Scholar] [CrossRef]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A Combined System for Asbestos-Cement Waste Degradation by Dark Fermentation and Resulting Supernatant Valorization in Anaerobic Digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Gençer, E. Hydrogen—MIT Climate Portal. Available online: https://climate.mit.edu/explainers/hydrogen (accessed on 11 December 2024).
- Sohale, A.P.; Janardanan, S.; Yadav, D.; Dash, B.; Yadav, M.D. Dark Fermentative Biohydrogen Production: Recent Advances and Challenges. Ind. Eng. Chem. Res. 2023, 62, 14755–14771. [Google Scholar] [CrossRef]
- Muddasar, M.; Liaquat, R.; Aslam, A.; Ur Rahman, M.Z.; Abdullah, A.; Khoja, A.H.; Latif, K.; Bahadar, A. Performance Efficiency Comparison of Microbial Electrolysis Cells for Sustainable Production of Biohydrogen—A Comprehensive Review. Int. J. Energy Res. 2022, 46, 5625–5645. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Luft, L.; Ugalde, G.A.; Tovar, L.P.; Mayer, F.D.; Mazutti, M.A. Estimation of Bioethanol, Biohydrogen, and Chemicals Production from Biomass Wastes in Brazil. Clean 2022, 50, 2200155. [Google Scholar] [CrossRef]
- Thulasisingh, A.; Ananthakrishnan, K.; Nandakumar, J.; Kannaiyan, S. Fermentative Biohydrogen Fuel Production Utilizing Wastewater: A Review. Clean 2023, 51, 2300112. [Google Scholar] [CrossRef]
- Pomdaeng, P.; Kongthong, O.; Tseng, C.H.; Dokmaingam, P.; Chu, C.Y. An Immobilized Mixed Microflora Approach to Enhancing Hydrogen and Methane Productions from High-Strength Organic Loading Food Waste Hydrolysate in Series Batch Reactors. Int. J. Hydrogen Energy 2024, 52, 160–169. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as Potential Feedstock for the Production of Biofuels and Value-Added Products: Opportunities and Challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.; Orr, M.; Cordray, K.; Greenbaum, E. Enzymatic Production of Biohydrogen. Nature 2000, 405, 1014–1015. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Modern Technologies of Hydrogen Production. Processes 2022, 11, 56. [Google Scholar] [CrossRef]
- Castiblanco, O.; Cárdenas, D.J. Producción de Hidrógeno y Su Perspectiva En Colombia: Una Revisión. Gest. Ambiente 2020, 23, 299–311. [Google Scholar] [CrossRef]
- Yuan, T.; Shi, X.; Sun, R.; Ko, J.H.; Xu, Q. Simultaneous Addition of Biochar and Zero-Valent Iron to Improve Food Waste Anaerobic Digestion. J. Clean. Prod. 2021, 278, 123627. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Li, L.; Dai, X.; Dai, L. A Review on Application of Single and Composite Conductive Additives for Anaerobic Digestion: Advances, Challenges and Prospects. Resour. Conserv. Recycl. 2021, 174, 105844. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, Y.; Ma, X.; Guan, W.; Gao, M.; Li, Y.-Y.; Wang, Q.; Wu, C. Effect of Zero-Valent Iron Addition on the Biogas Fermentation of Food Waste after Anaerobic Preservation. J. Environ. Chem. Eng. 2021, 9, 106013. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Ventaja, L.; Tomé, L.C.; Marrucho, I.M. Towards Biohydrogen Separation Using Poly(Ionic Liquid)/Ionic Liquid Composite Membranes. Membranes 2018, 8, 124. [Google Scholar] [CrossRef]
- Mete, M.; Pattyn, P.; Robidart, A.; Beringuier, G.; Thomas, H.; Grandjean, C.; Irague, R.; Andres, Y. Isolation and Characterisation of an Environmental Clostridium Beijerinckii Strain for Biohydrogen Production from Dairy Wastes. Int. J. Hydrogen Energy 2024, 49, 371–383. [Google Scholar] [CrossRef]
- Bai, R.; Chu, W.; Qiao, Z.; Lu, P.; Jiang, K.; Xu, Y.; Yang, J.; Gao, T.; Xu, F.; Zhao, H. Metabolic Regulation of NADH Supply and Hydrogen Production in Enterobacter Aerogenes by Multi-Gene Engineering. Int. J. Hydrogen Energy 2023, 48, 909–920. [Google Scholar] [CrossRef]
- Ramprakash, B.; Incharoensakdi, A. Dark Fermentative Hydrogen Production from Pretreated Garden Wastes by Escherichia Coli. Fuel 2022, 310, 122217. [Google Scholar] [CrossRef]
- Umunnawuike, C.; Mahat, S.Q.A.; Nwaichi, P.I.; Money, B.; Agi, A. Biohydrogen Production for Sustainable Energy Transition: A Bibliometric and Systematic Review of the Reaction Mechanisms, Challenges, Knowledge Gaps and Emerging Trends. Biomass Bioenergy 2024, 188, 107345. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Ghosh, S. State-of-the-Art of Biochar Amended Dark Fermentative Hydrogen Production: A Sustainable Coupling of Decarbonization Pathways towards Low Carbon Future. J. Clean. Prod. 2024, 443, 141208. [Google Scholar] [CrossRef]
- Nazari, M.T.; Mazutti, J.; Basso, L.G.; Colla, L.M.; Brandli, L. Biofuels and Their Connections with the Sustainable Development Goals: A Bibliometric and Systematic Review. Environ. Dev. Sustain. 2021, 23, 11139–11156. [Google Scholar] [CrossRef]
- Meena, P.K.; Kumar, D.; Sharma, S.; Didwania, M.; Singh, L. Advancing Sustainable Energy: Biohydrogen, Biogas, and Biohythane Production from Waste Materials through Life Cycle Analysis. Biofuels 2025, 1–17. [Google Scholar] [CrossRef]
- Madurai Elavarasan, R.; Nadarajah, M.; Pugazhendhi, R.; Sinha, A.; Gangatharan, S.; Chiaramonti, D.; Abou Houran, M. The Untold Subtlety of Energy Consumption and Its Influence on Policy Drive towards Sustainable Development Goal 7. Appl. Energy 2023, 334, 120698. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 1–19. [Google Scholar]
- Wang, C.; Deng, L.; Zhang, Y.; Zhao, M.; Liang, M.; Lee, L.C.; Cristhian, C.O.; Yang, L.; He, T. Farmland Phytoremediation in Bibliometric Analysis. J. Environ. Manag. 2024, 351, 119971. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Deng, L.; Zhao, M.; Liang, M.; Lee, L.C.; Chicaiza-Ortiz, C.; Yang, L.; He, T. Visualization Network Analysis of Studies on Agricultural Drainage Water Treatment. Processes 2023, 11, 2952. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nahrin, M.; Chowdhury, S.N.; Nuzhat, S.; Alherek, M.; Rafa, N.; Ong, H.C.; Nghiem, L.D.; Mahlia, T.M.I. Biohydrogen Production from Wastewater-Based Microalgae: Progresses and Challenges. Int. J. Hydrogen Energy 2022, 47, 37321–37342. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Wall, D.; Murphy, J.D. Improvement in Biohydrogen and Volatile Fatty Acid Production from Seaweed through Addition of Conductive Carbon Materials Depends on the Properties of the Conductive Materials. Energy 2022, 239, 122188. [Google Scholar] [CrossRef]
- Feng, H.; Sun, C.; Zhang, C.; Chang, H.; Zhong, N.; Wu, W.; Wu, H.; Tan, X.; Zhang, M.; Ho, S.-H. Bioconversion of Mature Landfill Leachate into Biohydrogen and Volatile Fatty Acids via Microalgal Photosynthesis Together with Dark Fermentation. Energy Convers. Manag. 2022, 252, 115035. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen Production from Microalgae for Environmental Sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef]
- Kundu, P.; Vineetha, S.V.; Mohan, A.; Ravikumar, A. Bio-Hydrogen Production from Various Waste Resources through Circular Economy: Current Technologies and Future Perspective. J. Mater. Cycles Waste Manag. 2025, 1–20. [Google Scholar] [CrossRef]
- Lagioia, G.; Spinelli, M.P.; Amicarelli, V. Blue and Green Hydrogen Energy to Meet European Union Decarbonisation Objectives. an Overview of Perspectives and the Current State of Affairs. Int. J. Hydrogen Energy 2023, 48, 1304–1322. [Google Scholar] [CrossRef]
- Bistline, J.; Blanford, G.; Brown, M.; Burtraw, D.; Domeshek, M.; Farbes, J.; Fawcett, A.; Hamilton, A.; Jenkins, J.; Jones, R.; et al. Emissions and Energy Impacts of the Inflation Reduction Act. Science (1979) 2023, 380, 1324–1327. [Google Scholar] [CrossRef]
- Loyte, A.; Suryawanshi, J.; Bellala, S.S.K.; Marode, R.V.; Devarajan, Y. Current Status and Obstacles in the Sustainable Synthesis of Biohydrogen from Microalgal Species. Results Eng. 2024, 24, 103455. [Google Scholar] [CrossRef]
- Ingle, A.P.; Saxena, S.; Moharil, M.P.; Rivaldi, J.D.; Ramos, L.; Chandel, A.K. Production of Biomaterials and Biochemicals from Lignocellulosic Biomass through Sustainable Approaches: Current Scenario and Future Perspectives. Biotechnol. Sustain. Mater. 2025, 2, 1–30. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Dinesh Aravind, V.; Ranjith, G.; Kishore, V.; Ewe, L.S.; Yew, W.K.; Baskaran, R. Sustainability Considerations in Bio-Hydrogen from Bio-Algae with the Aid of Bio-Algae Cultivation and Harvesting: Critical Review. MRS Energy Sustain. 2024, 11, 317–342. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Li, Y.; Zhang, H. Modeling and Optimization of Photo-Fermentation Biohydrogen Production from Co-Substrates Basing on Response Surface Methodology and Artificial Neural Network Integrated Genetic Algorithm. Bioresour. Technol. 2023, 374, 128789. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Y.; Zhang, Z.; Ai, F.; Zhang, H.; Li, Y.; Wang, Y.; Zhang, Q. Ascorbic Acid-Mediated Zero-Valent Iron Enhanced Hydrogen Production Potential of Bean Dregs and Corn Stover by Photo Fermentation. Bioresour. Technol. 2023, 374, 128761. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Berrocal-Bravo, M.J.; Valdez-Vazquez, I. Biohydrogen Production from Food Waste and Waste Activated Sludge in Codigestion: Influence of Organic Loading Rate and Changes in Microbial Community. J. Chem. Technol. Biotechnol. 2023, 98, 230–237. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; García-Depraect, O.; Muñoz, R. Elucidating the Role of PH and Total Solids Content in the Co-Production of Biohydrogen and Carboxylic Acids from Food Waste via Lactate-Driven Dark Fermentation. Fuel 2023, 338, 127238. [Google Scholar] [CrossRef]
- Shanmugam, S.; Mathimani, T.; Rajendran, K.; Sekar, M.; Rene, E.R.; Chi, N.T.L.; Ngo, H.H.; Pugazhendhi, A. Perspective on the Strategies and Challenges in Hydrogen Production from Food and Food Processing Wastes. Fuel 2023, 338, 127376. [Google Scholar] [CrossRef]
- Lee, D.H. Efficiency and Economic Benefit of Dark-Fermentative Biohydrogen Production in Asian Circular Economies: Evaluation Using Soft-Link Methodology with Data Envelopment Analysis (DEA) and Computable General Equilibrium Model (CGE). Int. J. Hydrogen Energy 2020, 45, 3688–3698. [Google Scholar] [CrossRef]
- National Archives. Hydrogen Market Research in Malaysia and Thailand Final Report; National Archives: Richmond, VA, USA, 2023. [Google Scholar]
- Arellano-Yasaca, D.V.; Chu, C.Y. Insights into Nutrients Recovery from Food Waste Liquid Digestate: A Critical Review and Systematic Analysis. Waste Manag. 2025, 200, 114743. [Google Scholar] [CrossRef]
- Cudjoe, D.; Zhu, B.; Wang, H. Towards the Realization of Sustainable Development Goals: Benefits of Hydrogen from Biogas Using Food Waste in China. J. Clean. Prod. 2022, 360, 132161. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen Production from Molasses by Anaerobic Fermentation with a Pilot-Scale Bioreactor System. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, F.; Li, Y.; Zhu, S.; Wu, Q.; Duan, Z.; Liu, H.; Qian, L.; Zhang, Q.; Zhang, Y. Co-Production Process Optimization and Carbon Footprint Analysis of Biohydrogen and Biofertilizer from Corncob by Photo-Fermentation. Bioresour. Technol. 2023, 375, 128814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Sustainable and Efficient Biohydrogen Production via Electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Manish, S.; Banerjee, R. Comparison of Biohydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 279–286. [Google Scholar] [CrossRef]
- Guo, W.-Q.; Ren, N.-Q.; Chen, Z.-B.; Liu, B.-F.; Wang, X.-J.; Xiang, W.-S.; Ding, J. Simultaneous Biohydrogen Production and Starch Wastewater Treatment in an Acidogenic Expanded Granular Sludge Bed Reactor by Mixed Culture for Long-Term Operation. Int. J. Hydrogen Energy 2008, 33, 7397–7404. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen Production: Prospects and Limitations to Practical Application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- SJR: Scientific Journal Rankings. Available online: https://www.scimagojr.com/journalrank.php#google_vignette (accessed on 22 March 2025).
- Kurian, N.; Gandhi, N.; Daniel, A.Y.; Varghese, K.G.; Dhawan, K.; Mathew, J.E.; Kaur, P. A 10-Year (2010 to 2019) Scientometric Analysis of Prosthodontic Journals Based on SCImago Journal and Country Rank Indicators. J. Prosthet. Dent. 2023, 129, 913–919. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Biohydrogen Production by Anaerobic Fermentation of Food Waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Shin, H.-S. Feasibility of Biohydrogen Production by Anaerobic Co-Digestion of Food Waste and Sewage Sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Demirbas, A. Use of Algae as Biofuel Sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wei, Y. Enhanced Biohydrogen Production from Sewage Sludge with Alkaline Pretreatment. Environ. Sci. Technol. 2004, 38, 3195–3202. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Angelopoulos, K.; Lyberatos, G. Biofuels Generation from Sweet Sorghum: Fermentative Hydrogen Production and Anaerobic Digestion of the Remaining Biomass. Bioresour. Technol. 2008, 99, 110–119. [Google Scholar] [CrossRef]
- Abdelqader, A.A.; Abdelsalam, E.M.; Attia, Y.A.; Moselhy, M.; Ali, A.S.; Arisha, A.H.; Samer, M. Application of Helium-Neon Red Laser for Increasing Biohydrogen Production from Anaerobic Digestion of Biowastes. Egypt. J. Chem. 2022, 65, 11–17. [Google Scholar] [CrossRef]
- Delavar, M.A.; Wang, J. Numerical Investigation of PH Control on Dark Fermentation and Hydrogen Production in a Microbioreactor. Fuel 2021, 292, 120355. [Google Scholar] [CrossRef]
- Angeriz-Campoy, R.; Fdez-Güelfo, L.A.; Álvarez-Gallego, C.J.; Romero-García, L.I. Pre-Composting of Municipal Solid Wastes as Enhancer of Bio-Hydrogen Production through Dark Fermentation Process. Fuel 2023, 333, 126575. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kalia, V.C.; Lee, J.-K. Integration of Biogas Derived from Dark Fermentation and Anaerobic Digestion of Biowaste to Enhance Methanol Production by Methanotrophs. Bioresour. Technol. 2023, 369, 128427. [Google Scholar] [CrossRef]
- Zainal, B.S.; Zaini, S.; Zinatizadeh, A.A.; Mohd, N.S.; Ibrahim, S.; Ker, P.J.; Mohamed, H. Preliminary Investigation of Different Types of Inoculums and Substrate Preparation for Biohydrogen Production. Fermentation 2023, 9, 127. [Google Scholar] [CrossRef]
- Özgür, E.; Mars, A.E.; Peksel, B.; Louwerse, A.; Yücel, M.; Gündüz, U.; Claassen, P.A.M.; Eroğlu, İ. Biohydrogen Production from Beet Molasses by Sequential Dark and Photofermentation. Int. J. Hydrogen Energy 2010, 35, 511–517. [Google Scholar] [CrossRef]
- Wang, W.; Xie, L.; Chen, J.; Luo, G.; Zhou, Q. Biohydrogen and Methane Production by Co-Digestion of Cassava Stillage and Excess Sludge under Thermophilic Condition. Bioresour. Technol. 2011, 102, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; Elshobary, M.; Abdullah, E.; Abdel-Basset, R.; Metwally, M. Application of a Novel Biological-Nanoparticle Pretreatment to Oscillatoria Acuminata Biomass and Coculture Dark Fermentation for Improving Hydrogen Production. Microb. Cell Fact. 2023, 22, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mohanakrishna, G.; Modestra, J.A. Value Addition through Biohydrogen Production and Integrated Processes from Hydrothermal Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2023, 369, 128386. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.N.B.; Lay, C.-H.; Nguyen, D.D.; Chang, S.W.; Banu, J.R.; Hong, Y.; Park, J.-H. Improving the Biohydrogen Production Potential of Macroalgal Biomass through Mild Acid Dispersion Pretreatment. Fuel 2023, 332, 125895. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.P.; Ruiz-Filippi, G.; Trably, E. Microbial Ecology of Fermentative Hydrogen Producing Bioprocesses: Useful Insights for Driving the Ecosystem Function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Xuan, J.; He, L.; Wen, W.; Feng, Y. Hydrogenase and Nitrogenase: Key Catalysts in Biohydrogen Production. Molecules 2023, 28, 1392. [Google Scholar] [CrossRef]
- Boeckaert, C.; Vlaeminck, B.; Fievez, V.; Maignien, L.; Dijkstra, J.; Boon, N. Accumulation of Trans C18:1 Fatty Acids in the Rumen after Dietary Algal Supplementation Is Associated with Changes in the Butyrivibrio Community. Appl. Environ. Microbiol. 2008, 74, 6923–6930. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium Species for Fermentative Hydrogen Production: An Overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and Applications of Clostridium Co-Culture Systems in Biotechnology. Front. Microbiol. 2020, 11, 560223. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, B.; Zhang, H.; Qian, S.; Wei, T.; Zhang, Z.; Song, L.; Yang, X. Fermentative Hydrogen Production from Lignocellulose by Mesophilic Clostridium Populeti FZ10 Newly Isolated from Microcrystalline Cellulose-Acclimated Compost. Appl. Sci. 2022, 12, 9562. [Google Scholar] [CrossRef]
- Verdezoto-Prado, J.; Chicaiza-Ortiz, C.; Belén Mejía-Pérez, A.; Freire-Torres, C.; Viteri-Yánez, M.; Deng, L.; Barba-Ostria, C.; Guamán, L.P. Advances in Environmental Biotechnology with CRISPR/Cas9: Bibliometric Review and Cutting-Edge Applications. Discov. Appl. Sci. 2025, 7, 1–33. [Google Scholar] [CrossRef]
- Terrell, J.L.; Tschirhart, T.; Jahnke, J.P.; Stephens, K.; Liu, Y.; Dong, H.; Hurley, M.M.; Pozo, M.; McKay, R.; Tsao, C.Y.; et al. Bioelectronic Control of a Microbial Community Using Surface-Assembled Electrogenetic Cells to Route Signals. Nat. Nanotechnol. 2021, 16, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Alavi-Borazjani, S.A.; da Cruz Tarelho, L.A.; Capela, M.I. Biohythane Production via Anaerobic Digestion Process: Fundamentals, Scale-up Challenges, and Techno-Economic and Environmental Aspects. Environ. Sci. Pollut. Res. 2024, 31, 49935–49984. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Liao, Z.; Qu, C.; Fu, H.; Wang, J. Metabolic Engineering of Clostridium Tyrobutyricum for Enhanced Butyric Acid Production from Undetoxified Corncob Acid Hydrolysate. Bioresour. Technol. 2019, 271, 266–273. [Google Scholar] [CrossRef]
- Chicaiza-Ortiz, C.; Peñafiel-Arcos, P.; Herrera-Feijoo, R.J.; Ma, W.; Logroño, W.; Tian, H.; Yuan, W. Waste-to-Energy Technologies for Municipal Solid Waste Management: Bibliometric Review, Life Cycle Assessment, and Energy Potential Case Study. J. Clean. Prod. 2024, 480, 143993. [Google Scholar] [CrossRef]
- Hassan, A.N.; Nelson, B.K. Invited Review: Anaerobic Fermentation of Dairy Food Wastewater. J. Dairy. Sci. 2012, 95, 6188–6203. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, L.; Lin, R.; Yue, L.; Liu, J.; Zhou, J.; Cen, K. Fermentative Biohydrogen and Biomethane Co-Production from Mixture of Food Waste and Sewage Sludge: Effects of Physiochemical Properties and Mix Ratios on Fermentation Performance. Appl. Energy 2016, 184, 1–8. [Google Scholar] [CrossRef]
- Cichy, P.; Tomczak-Wandzel, R.; Szatkowska, B.; Kalka, J.; Yadav, R.S. Closing the Loop: Can Anaerobic Digestates from Food Waste Be Universal Source of Nutrients for Plant Growth? Sustainability 2024, 16, 6171. [Google Scholar] [CrossRef]
- Shiyan, G.; Wenyi, Z.; Huige, X.; Ruji, W.; Jiyang, S.; Min, Z.; Yi, L. The Effect of Anaerobic Co-Fermentation on Acidification Performance of Food Waste and Cardboard Waste. Water Sci. Technol. 2022, 85, 839–850. [Google Scholar] [CrossRef]
- da Costa Freire, C.C.; Marin, D.F.C.; da Silva Mazareli, R.C.; de Freitas, C.; Brienzo, M.; Maintinguer, S.I. Unravelling the Biohydrogen Production Potential from a Co-Digestion Process of Banana Processing Wastewater and Synthetic Sewage by Anaerobic Fermentation: Performance Evaluation and Microbial Community Analysis. Waste Biomass Valorization 2024, 15, 1587–1601. [Google Scholar] [CrossRef]
- Sillero, L.; Sganzerla, W.G.; Forster-Carneiro, T.; Solera, R.; Perez, M. A Bibliometric Analysis of the Hydrogen Production from Dark Fermentation. Int. J. Hydrogen Energy 2022, 47, 27397–27420. [Google Scholar] [CrossRef]
- Sheng, T.; Zhao, L.; Gao, L.; Liu, W.; Wu, G.; Wu, J.; Wang, A. Enhanced Biohydrogen Production from Nutrient-Free Anaerobic Fermentation Medium with Edible Fungal Pretreated Rice Straw. RSC Adv. 2018, 8, 22924–22930. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chang, C.W.; Chu, C.P.; Lee, D.J.; Chang, B.V.; Liao, C.S.; Tay, J.H. Using Filtrate of Waste Biosolids to Effectively Produce Bio-Hydrogen by Anaerobic Fermentation. Water Res. 2003, 37, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Fang, H.H.P. Biohydrogen Production from Starch in Wastewater under Thermophilic Condition. J Environ. Manag. 2003, 69, 149–156. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K. Light Fermentation of Dark Fermentation Effluent for Bio-Hydrogen Production by Different Rhodobacter Species at Different Initial Volatile Fatty Acid (VFA) Concentrations. Int. J. Hydrogen Energy 2008, 33, 7405–7412. [Google Scholar] [CrossRef]
- Radjaram, B.; Saravanane, R.; Sundararajan, T. Feasibility Study of Biohydrogen Production from Pressmud by UASB Process and Assessment of Operating Parameters. Glob. Nest J. 2013, 15, 560–567. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic Materials Into Biohydrogen and Biomethane: Impact of Structural Features and Pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Di Bonito, R.; Marone, A.; Massini, G.; Patriarca, C.; Rosa, S.; Signorini, A.; Varrone, C.; Viola, C.; Izzo, G. Characterization by Length Heterogeneity (LH)-PCR of a Hydrogen-Producing Community Obtained in Dark Fermentation Using Coastal Lake Sediment as an Inoculum. Energy Sustain. Soc. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Nobre, B.P.; Villalobos, F.; Barragán, B.E.; Oliveira, A.C.; Batista, A.P.; Marques, P.A.S.S.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A Biorefinery from Nannochloropsis sp. Microalga—Extraction of Oils and Pigments. Production of Biohydrogen from the Leftover Biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef]
- Sheehan, N.P.; Plante, L.; Martinez, E.; Murray, K.; Bier, P.; Ouellette, C. Sustainable Bioenergy from Biofuel Residues and Waste. Water Environ. Res. 2018, 90, 1073–1090. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-Energy Nexus for Circular Economy and Environmental Protection: Recent Trends in Hydrogen Energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, D.; Liu, Y.; Wang, Q.; Ni, B.J.; Chen, F.; Yang, Q.; Li, X.; Zeng, G.; et al. Free Nitrous Acid Promotes Hydrogen Production from Dark Fermentation of Waste Activated Sludge. Water Res. 2018, 145, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Akram, J.; Song, C.; El Mashad, H.M.; Chen, C.; Zhang, R.; Liu, G. Advances in Microbial Community, Mechanisms and Stimulation Effects of Direct Interspecies Electron Transfer in Anaerobic Digestion. Biotechnol. Adv. 2024, 76, 108398. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Chang, J.; Liang, J.; Zhang, P.; Zhang, G. Improvement of Direct Interspecies Electron Transfer via Adding Conductive Materials in Anaerobic Digestion: Mechanisms, Performances, and Challenges. Front. Microbiol. 2022, 13, 860749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Cristhian, C.O.; Zhang, J. Additive Strategies for Enhanced Anaerobic Digestion for Bioenergy and Biochemicals. In Biomass, Biofuels, Biochemicals: Microbial Fermentation of Biowastes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 131–158. [Google Scholar] [CrossRef]
- Liu, J.; Jin, F.; Fan, M.; Zhu, L.; Tang, C.; Chang, R.; Jia, Q.; Li, Q. Production of High-Pure Hydrogen by an Integrated Catalytic Process: Comparison of Different Lignocellulosic Biomasses and Three Major Components. Fuel 2018, 226, 322–330. [Google Scholar] [CrossRef]
- Youcai, Z.; Tao, Z. Pretreatment and Aged Refuse Dosage on Biohydrogen Production from Food Waste. In Biohydrogen Production and Hybrid Process Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–238. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of Hydrogen and Methane from Lignocellulose Waste by Fermentation. A Review of Chemical Pretreatment for Enhancing the Efficiency of the Digestion Process. J. Clean. Prod. 2020, 267, 121721. [Google Scholar] [CrossRef]
- Barrena, R.; Moral-Vico, J.; Font, X.; Sánchez, A. Enhancement of Anaerobic Digestion with Nanomaterials: A Mini Review. Energies 2022, 15, 5087. [Google Scholar] [CrossRef]
- Dell’Orto, A.; Trois, C. Double-Stage Anaerobic Digestion for Biohydrogen Production: A Strategy for Organic Waste Diversion and Emission Reduction in a South African Municipality. Sustainability 2024, 16, 7200. [Google Scholar] [CrossRef]
- Mei, C.; Zhang, M.; Chen, Y.C.; Dong, K.; Sun, R.Z.; Zhang, X.H.; Li, H.X. Exploring the Applications and Carbon Reduction of Multi-Technology-Coupled Membrane Biofilm Reactors for Sustainable Wastewater Treatment: A Review. Environ. Sci. 2025, 11, 793–808. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, S.; Gu, W.; Zhuang, W.; Gao, M.; Chan, C.C.; Zhang, X. Coordinated Planning Model for Multi-Regional Ammonia Industries Leveraging Hydrogen Supply Chain and Power Grid Integration: A Case Study of Shandong. Appl. Energy 2025, 377, 124456. [Google Scholar] [CrossRef]
- Zhu, X.; Yellezuome, D.; Zhang, H.; Lv, W.; Liu, R. Effects of Reduced Hydraulic Retention Time and Self-Induced Mixing on Two-Stage Anaerobic Digestion Utilizing Acidogenic off-Gas. Chem. Eng. J. 2025, 504, 158886. [Google Scholar] [CrossRef]
- Nawaz, A.; Aamir, F.; Huang, R.; Haq, I.U.; Wu, F.; Munir, M.; Chaudhary, R.; Rafique, A.; Jiang, K. Co-Production of Biohydrogen and Biomethane Utilizing Halophytic Biomass Atriplexcrassifolia by Two-Stage Anaerobic Fermentation Process. Front. Chem. 2023, 11, 1233494. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. The Promotion of Anaerobic Digestion Technology Upgrades in Waste Stream Treatment Plants for Circular Economy in the Context of “Dual Carbon”: Global Status, Development Trend, and Future Challenges. Water 2024, 16, 3718. [Google Scholar] [CrossRef]
- Chicaiza-Ortiz, C.; Zhang, P.; Zhang, J.; Zhang, T.; Yang, Q.; He, Y. CO₂-Enhanced Methane Production by Integration of Bamboo Biochar during Anaerobic Co-Digestion. J. Environ. Manag. 2025, 373, 123603. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies 2022, 15, 4876. [Google Scholar] [CrossRef]
- Kumar, G.; Mathimani, T.; Sivaramakrishnan, R.; Shanmugam, S.; Bhatia, S.K.; Pugazhendhi, A. Application of Molecular Techniques in Biohydrogen Production as a Clean Fuel. Sci. Total Environ. 2020, 722, 137795. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Biohydrogen Production from Waste Activated Sludge Pretreated by Combining Sodium Citrate with Ultrasonic: Energy Conversion and Microbial Community. Energy Convers. Manag. 2020, 225, 113436. [Google Scholar] [CrossRef]
- Kamyab, S.; Ataei, S.A.; Masoumeh, T.; Mirhosseinei, S.A. Optimizing Parameters for Bio-Hydrogen Production from Mixed Culture and Food Wastewater. Iran. J. Chem. Chem. Eng. 2022, 41, 3204–3213. [Google Scholar] [CrossRef]
- Mamimin, C.; Prasertsan, P.; Kongjan, P.; O-Thong, S. Effects of Volatile Fatty Acids in Biohydrogen Effluent on Biohythane Production from Palm Oil Mill Effluent under Thermophilic Condition. Electron. J. Biotechnol. 2017, 29, 78–85. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. The Effects of PH on the Production of Volatile Fatty Acids and Microbial Dynamics in Long-Term Reactor Operation. J. Environ. Manag. 2022, 319, 115700. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Z.H.; Wu, J.T.; Ren, H.Y.; Yang, S.S.; Nan, J.; Cao, G.L.; Sheng, Y.C.; Wang, A.J.; Ren, N.Q. Co-Fermentation of a Mixture of Glucose and Xylose to Hydrogen by Thermoanaerobacter Thermosaccharolyticum W16: Characteristics and Kinetics. Int. J. Hydrogen Energy 2019, 44, 9248–9255. [Google Scholar] [CrossRef]
- Hung, C.H.; Chang, Y.T.; Chang, Y.J. Roles of Microorganisms Other than Clostridium and Enterobacter in Anaerobic Fermentative Biohydrogen Production Systems—A Review. Bioresour. Technol. 2011, 102, 8437–8444. [Google Scholar] [CrossRef]
- Alavi-Borazjani, S.A.; Capela, I.; Tarelho, L.A.C. Dark Fermentative Hydrogen Production from Food Waste: Effect of Biomass Ash Supplementation. Int. J. Hydrogen Energy 2019, 44, 26213–26225. [Google Scholar] [CrossRef]
- Kriswantoro, J.A.; Chu, C.Y. Biohydrogen Production Kinetics from Cacao Pod Husk Hydrolysate in Dark Fermentations: Effect of Pretreatment, Substrate Concentration, and Inoculum. J. Clean. Prod. 2024, 434, 140407. [Google Scholar] [CrossRef]
- Sitthikitpanya, N.; Khamtib, S.; Sittijunda, S.; Imai, T.; Reungsang, A. Valorization of Sugarcane Leaves and Co-Digestion with Microalgal Biomass to Produce Biofuels and Value-Added Products under the Circular Economy and Zero-Waste Concepts. Energy Convers. Manag. 2024, 299, 117854. [Google Scholar] [CrossRef]
- Arellano-Yasaca, D.V.; Chu, C.Y.; Petracchini, F. Harnessing Sustainable Opportunities for Nutrient Removal and Recovery from Liquid Digestate in a Modern Municipal Food Waste Biogas Power Plant. J. Environ. Chem. Eng. 2024, 12, 112362. [Google Scholar] [CrossRef]
- Qi, N.; Zhao, X.; Hu, X.; Wang, J.; Yang, C. Enhancing Biohydrogen Production by Peanut Shell Carrier Assisted Fermentation at Different Hydraulic Retention Time. Renew. Energy 2023, 219, 119492. [Google Scholar] [CrossRef]
- Kumari, M.; Chandel, M.K. Anaerobic Co-Digestion of Sewage Sludge and Organic Fraction of Municipal Solid Waste: Focus on Mix Ratio Optimization and Synergistic Effects. J. Environ. Manag. 2023, 345, 118821. [Google Scholar] [CrossRef]
- Barak, H.; Fuchs, N.; Liddor-Naim, M.; Nir, I.; Sivan, A.; Kushmaro, A. Microbial Dark Matter Sequences Verification in Amplicon Sequencing and Environmental Metagenomics Data. Front. Microbiol. 2023, 14, 1247119. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public. Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Weinrich, S.; Nelles, M. DBFZ Report No. 40: Basics of Anaerobic Digestion—Biochemical Conversion and Process Modelling.
- Shaterzadeh, M.J.; Ataei, S.A. The Effects of Temperature, Initial PH, and Glucose Concentration on Biohydrogen Production from Clostridium Acetobutylicum. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1118–1123. [Google Scholar] [CrossRef]
- Reyna-Gómez, L.M.; Molina-Guerrero, C.E.; Alfaro, J.M.; Vázquez, S.I.S.; Robledo-Olivo, A.; Cruz-López, A. Effect of Carbon/Nitrogen Ratio, Temperature, and Inoculum Source on Hydrogen Production from Dark Codigestion of Fruit Peels and Sewage Sludge. Sustainability 2019, 11, 2139. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Khashayar, P.; Amereh, M.; Tasnim, N.; Hoorfar, M.; Akbari, M. Microfluidic-Based Oxygen (O2) Sensors for On-Chip Monitoring of Cell, Tissue and Organ Metabolism. Biosensors 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Boni, M.R.; Polettini, A.; Pomi, R.; Rossi, A.; De Gioannis, G.; Muntoni, A.; Spiga, D. Fermentative H2 Production from Food Waste: Parametric Analysis of Factor Effects. Bioresour. Technol. 2019, 276, 349–360. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen Production from Dark Fermentation of Cheese Whey: Influence of PH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Murugan, R.S.; Dinesh, G.H.; Swetha, T.R.A.; Boobalan, T.; Jothibasu, M.; Manimaran, P.S.; Selvakumar, G.; Arun, A. Acinetobacter Junii AH4-A Potential Strain for Bio-Hydrogen Production from Dairy Industry Anaerobic Sludge. J. Pure Appl. Microbiol. 2018, 12, 1761–1769. [Google Scholar] [CrossRef]
- Kumar Awasthi, M.; Paul, A.; Kumar, V.; Sar, T.; Kumar, D.; Sarsaiya, S.; Liu, H.; Zhang, Z.; Binod, P.; Sindhu, R.; et al. Recent Trends and Developments on Integrated Biochemical Conversion Process for Valorization of Dairy Waste to Value Added Bioproducts: A Review. Bioresour. Technol. 2022, 344, 126193. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Li, Y.; Tahir, N.; Liu, H.; Zhang, Q. Analysis of Shaking Effect on Photo-Fermentative Hydrogen Production under Different Concentrations of Corn Stover Powder. Int. J. Hydrogen Energy 2018, 43, 20465–20473. [Google Scholar] [CrossRef]
- Jin, M.; Wei, X.; Mu, X.; Ren, W.; Zhang, S.; Tang, C.; Cao, W. Life-Cycle Analysis of Biohydrogen Production via Dark-Photo Fermentation from Wheat Straw. Bioresour. Technol. 2024, 396, 130429. [Google Scholar] [CrossRef]
- Rani, P.; Kumar Yadav, D.; Yadav, A.; Ram Bishnoi, N.; Kumar, V.; Ram, C.; Pugazhendhi, A.; Kumar, S.S. Frontier in Dark Fermentative Biohydrogen Production from Lignocellulosic Biomass: Challenges and Future Prospects. Fuel 2024, 366, 131187. [Google Scholar] [CrossRef]
- Ji, J.; Cai, Z.; Shen, L. Comparison Analysis on Biohydrogen Production of Mesophilic and Thermophilic Dark Fermentation Using Nickel Doped Lanthanum-Iron Oxide Nanoparticles as Supplementation. Int. J. Hydrogen Energy 2024, 49, 90–106. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Rohtagi, B.; Darjee, S.; Khandelwal, A.; Shrivastava, M.; Kothari, R.; Mohan, H.; Raina, S.; Kaur, J.; et al. Production of Biofuels Options by Contribution of Effective and Suitable Enzymes: Technological Developments and Challenges. Mater. Sci. Energy Technol. 2022, 5, 294–310. [Google Scholar] [CrossRef]
- Dhanya, B.S.; Mishra, A.; Chandel, A.K.; Verma, M.L. Development of Sustainable Approaches for Converting the Organic Waste to Bioenergy. Sci. Total Environ. 2020, 723, 138109. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the Potential of Bio-Hydrogen Production by Fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Jing, Y.; Tahir, N.; Zhang, Y.; Zhang, Q. Comparative Study on Bio-Hydrogen Production from Corn Stover: Photo-Fermentation, Dark-Fermentation and Dark-Photo Co-Fermentation. Int. J. Hydrogen Energy 2020, 45, 3807–3814. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Altaee, A.; Li, D. Machine Learning Modeling and Analysis of Biohydrogen Production from Wastewater by Dark Fermentation Process. Bioresour. Technol. 2022, 343, 126111. [Google Scholar] [CrossRef]
- Bar-Even, A. Does Acetogenesis Really Require Espec. Low Reduct. Potential? Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 395–400. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Z.; Xu, J.; Liu, H.; Dai, X.; Gu, S.; Ruan, W. Dosing Effect of Nano Zero Valent Iron (NZVI) on the Dark Hydrogen Fermentation Performance via Lake Algae and Food Waste Co-Digestion. Energy Rep. 2020, 6, 3192–3199. [Google Scholar] [CrossRef]
- Show, K.Y.; Yan, Y.; Lee, D.J. Chapter 16-Bioreactor and Bioprocess Design for Biohydrogen Production. In Biohydrogen-Biomass Biofuels Biochem; Springer: Berlin/Heidelberg, Germany, 2019; pp. 391–411. [Google Scholar]
- Niño-Navarro, C.; Chairez, I.; Torres-Bustillos, L.; Ramírez-Muñoz, J.; Salgado-Manjarrez, E.; Garcia-Peña, E.I. Effects of Fluid Dynamics on Enhanced Biohydrogen Production in a Pilot Stirred Tank Reactor: CFD Simulation and Experimental Studies. Int. J. Hydrogen Energy 2016, 41, 14630–14640. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.Y. Using Sucrose as a Substrate in an Anaerobic Hydrogen-Producing Reactor. Adv. Environ. Res. 2003, 7, 695–699. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Hernández, A.; Mar-Alvarez, I.; Moreno-Andrade, I. Start-up and Operation of Continuous Stirred-Tank Reactor for Biohydrogen Production from Restaurant Organic Solid Waste. Int. J. Hydrogen Energy 2015, 40, 17239–17245. [Google Scholar] [CrossRef]
- Morales Hernández, S.A.; Rizo-Acosta, P.; Hernández-Rojas, M.E.; Dávila-Gómez, J.A. Producción de Biohidrógeno En Un Reactor Continuo UASB. Rev. Cuba. De Química 2015, 27, 65–78. [Google Scholar]
- Jamali, N.S.; Md Jahim, J.; O-Thong, S.; Jehlee, A. Hydrodynamic Characteristics and Model of Fluidized Bed Reactor with Immobilised Cells on Activated Carbon for Biohydrogen Production. Int. J. Hydrogen Energy 2019, 44, 9256–9271. [Google Scholar] [CrossRef]
- Show, K.Y.; Zhang, Z.P.; Tay, J.H.; Liang, D.T.; Lee, D.J.; Ren, N.; Wang, A. Critical Assessment of Anaerobic Processes for Continuous Biohydrogen Production from Organic Wastewater. Int. J. Hydrogen Energy 2010, 35, 13350–13355. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ma, S.; Huang, H.; Ma, Y.; Li, Z. Enhancing Hydrogen Productivity of Photosynthetic Bacteria from the Formulated Carbon Source by Mixing Xylose with Glucose. Appl. Biochem. Biotechnol. 2021, 193, 3996–4017. [Google Scholar] [CrossRef]
- Tepari, E.A.; Nakhla, G.; Haroun, B.M.; Hafez, H. Co-Fermentation of Carbohydrates and Proteins for Biohydrogen Production: Statistical Optimization Using Response Surface Methodology. Int. J. Hydrogen Energy 2020, 45, 2640–2654. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Chang, J.S. Recent Insights into Consolidated Bioprocessing for Lignocellulosic Biohydrogen Production. Int. J. Hydrogen Energy 2019, 44, 14362–14379. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Rego, G.C.; Ramos, L.R.; Soares, L.A.; Sakamoto, I.K.; de Oliveira, L.L.; Varesche, M.B.A.; Silva, E.L. Selection of Metabolic Pathways for Continuous Hydrogen Production under Thermophilic and Mesophilic Temperature Conditions in Anaerobic Fluidized Bed Reactors. Int. J. Hydrogen Energy 2018, 43, 18908–18917. [Google Scholar] [CrossRef]
- Rogeri, R.C.; Fuess, L.T.; Eng, F.; do Borges, A.V.; de Araujo, M.N.; Damianovic, M.H.R.Z.; Silva, A.J. da Strategies to Control PH in the Dark Fermentation of Sugarcane Vinasse: Impacts on Sulfate Reduction, Biohydrogen Production and Metabolite Distribution. J Environ. Manag. 2023, 325, 116495. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ren, N.; Shi, Y.; Lee, D.J. Bioaugmented Hydrogen Production from Microcrystalline Cellulose Using Co-Culture—Clostridium Acetobutylicum X9 and Ethanoigenens Harbinense B49. Int. J. Hydrogen Energy 2008, 33, 912–917. [Google Scholar] [CrossRef]
- Hakobyan, L.; Gabrielyan, L.; Blbulyan, S.; Trchounian, A. The Prospects of Brewery Waste Application in Biohydrogen Production by Photofermentation of Rhodobacter Sphaeroides. Int. J. Hydrogen Energy 2021, 46, 289–296. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Role of Chemicals Addition in Affecting Biohydrogen Production through Photofermentation. Energy Convers. Manag. 2018, 165, 509–527. [Google Scholar] [CrossRef]
- Singh, S.; Sarma, P.M.; Lal, B. Biohydrogen Production by Thermoanaerobacterium Thermosaccharolyticum TERI S7 from Oil Reservoir Flow Pipeline. Int. J. Hydrogen Energy 2014, 39, 4206–4214. [Google Scholar] [CrossRef]
- Arellano-Yasaca, D.V.; Chu, C.Y.; Ríos-García, I.; Wan Jusoh, W.N.L. Process Optimization for Ammonium Removal from Municipal Food Waste Treatment Plant Liquid Digestate Using Natural Rocks and Volcanic Ash. Biomass Bioenergy 2025, 193, 107581. [Google Scholar] [CrossRef]
- Lenzuni, M.; Converti, A.; Casazza, A.A. From Laboratory- to Industrial-Scale Plants: Future of Anaerobic Digestion of Olive Mill Solid Wastes. Bioresour. Technol. 2024, 394, 130317. [Google Scholar] [CrossRef]
- Roque, B.A.C.; Cavalcanti, M.H.C.; Brasileiro, P.P.F.; Gama, P.H.R.P.; dos Santos, V.A.; Converti, A.; Benachour, M.; Sarubbo, L.A. Hydrogen-Powered Future: Catalyzing Energy Transition, Industry Decarbonization and Sustainable Economic Development: A Review. Gondwana Res. 2025, 140, 159–180. [Google Scholar] [CrossRef]
- Romeiko, X.X.; Zhang, X.; Pang, Y.; Gao, F.; Xu, M.; Lin, S.; Babbitt, C. A Review of Machine Learning Applications in Life Cycle Assessment Studies. Sci. Total Environ. 2024, 912, 168969. [Google Scholar] [CrossRef]
- De Jesus, J.O.; Oliveira-Esquerre, K.; Medeiros, D.L. Integration of Artificial Intelligence and Life Cycle Assessment Methods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1196, 012028. [Google Scholar] [CrossRef]
- Jamali, M.; Hajialigol, N.; Fattahi, A. An Insight into the Application and Progress of Artificial Intelligence in the Hydrogen Production Industry: A Review. Mater. Today Sustain. 2025, 30, 101098. [Google Scholar] [CrossRef]
- Kheirrouz, M.; Melino, F.; Ancona, M.A. Fault Detection and Diagnosis Methods for Green Hydrogen Production: A Review. Int. J. Hydrogen Energy 2022, 47, 27747–27774. [Google Scholar] [CrossRef]
- Zhou, Q.; Qu, S.; Wang, Q.; She, Y.; Yu, Y.; Bi, J. Sliding Window-Based Machine Learning for Environmental Inspection Resource Allocation. Environ. Sci. Technol. 2023, 57, 16743–16754. [Google Scholar] [CrossRef]
- Mira, K.; Bugiotti, F.; Morosuk, T. Artificial Intelligence and Machine Learning in Energy Conversion and Management. Energies 2023, 16, 7773. [Google Scholar] [CrossRef]
- Sanghvi, A.H.; Manjoo, A.; Rajput, P.; Mahajan, N.; Rajamohan, N.; Abrar, I. Advancements in Biohydrogen Production—A Comprehensive Review of Technologies, Lifecycle Analysis, and Future Scope. RSC Adv. 2024, 14, 36868–36885. [Google Scholar] [CrossRef]
- Long, F.; Liu, H. An Integration of Machine Learning Models and Life Cycle Assessment for Lignocellulosic Bioethanol Platforms. Energy Convers. Manag. 2023, 292, 117379. [Google Scholar] [CrossRef]
- Khandelwal, K.; Nanda, S.; Dalai, A.K. Machine Learning Modeling of Supercritical Water Gasification for Predictive Hydrogen Production from Waste Biomass. Biomass Bioenergy 2025, 197, 107816. [Google Scholar] [CrossRef]
- Alagumalai, A.; Devarajan, B.; Song, H.; Wongwises, S.; Ledesma-Amaro, R.; Mahian, O.; Sheremet, M.; Lichtfouse, E. Machine Learning in Biohydrogen Production: A Review. Biofuel Res. J. 2023, 10, 1844–1858. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Smoliński, A.; Sanchez, A. A Meta-Analysis of Research Trends on Hydrogen Production via Dark Fermentation. Int. J. Hydrogen Energy 2022, 47, 13300–13339. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdul-Wahab, M.F.; Hashim, M.; Omar, A.H.; Md Reba, M.N.; Muhamad Said, M.F.; Soeed, K.; Alias, S.A.; Smykla, J.; Abba, M.; et al. Biohydrogen Production by Antarctic Psychrotolerant Klebsiella Sp. ABZ11. Pol. J. Microbiol. 2018, 67, 283–290. [Google Scholar] [CrossRef]
- Faizollahzadeh Ardabili, S.; Mosavi, A.; Várkonyi-Kóczy, R.A. Systematic Review of Deep Learning and Machine Learning Models in Biofuels Research; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Hino, M.; Benami, E.; Brooks, N. Machine Learning for Environmental Monitoring. Nat. Sustain. 2018, 1, 583–588. [Google Scholar] [CrossRef]
- Rezk, H.; Olabi, A.G.; Abdelkareem, M.A.; Alami, A.H.; Sayed, E.T. Optimal Parameter Determination of Membrane Bioreactor to Boost Biohydrogen Production-Based Integration of ANFIS Modeling and Honey Badger Algorithm. Sustainability 2023, 15, 1589. [Google Scholar] [CrossRef]
- Wang, L.; Long, F.; Liao, W.; Liu, H. Prediction of Anaerobic Digestion Performance and Identification of Critical Operational Parameters Using Machine Learning Algorithms. Bioresour. Technol. 2020, 298, 122495. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Lee, C.; Yoon, J. Machine Learning Approach for Predicting Anaerobic Digestion Performance and Stability in Direct Interspecies Electron Transfer-Stimulated Environments. Biochem. Eng. J. 2023, 193, 108840. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, T.; Zhang, J.; Liu, H.; Chicaiza-Ortiz, C.; Lee, J.T.E.; He, Y.; Dai, Y.; Tong, Y.W. A Machine Learning Assisted Prediction of Potential Biochar and Its Applications in Anaerobic Digestion for Valuable Chemicals and Energy Recovery from Organic Waste. Carbon. Neutrality 2024, 3, 1–21. [Google Scholar] [CrossRef]
- Gueguim Kana, E.B.; Schmidt, S.; Azanfack Kenfack, R.H. A Web-Enabled Software for Real-Time Biogas Fermentation Monitoring—Assessment of Dark Fermentations for Correlations between Medium Conductivity and Biohydrogen Evolution. Int. J. Hydrogen Energy 2013, 38, 10235–10244. [Google Scholar] [CrossRef]
- Pratap, V.; Kumar, S.; Yadav, B.R. Optimization of Biogas Production from Thermal-Alkali Pre-Treated Sludge Using Response Surface Methodology and Random Forest Regressor Model. J. Taiwan Inst. Chem. Eng. 2024, 105571. [Google Scholar] [CrossRef]
- Iqbal, S.; Aftab, K.; tul Jannat, F.; Ali Baig, M.; Kalsoom, U. A Bibliographic Analysis of Optimization of Hydrogen Production via Electrochemical Method Using Machine Learning. Fuel 2024, 372, 132126. [Google Scholar] [CrossRef]
- Duong-Trung, N.; Born, S.; Kim, J.W.; Schermeyer, M.T.; Paulick, K.; Borisyak, M.; Cruz-Bournazou, M.N.; Werner, T.; Scholz, R.; Schmidt-Thieme, L.; et al. When Bioprocess Engineering Meets Machine Learning: A Survey from the Perspective of Automated Bioprocess Development. Biochem. Eng. J. 2023, 190, 108764. [Google Scholar] [CrossRef]
- Bhat, M.A.; Bhat, M.A.; Jan, S.; Shah, A.A.; Jan, A.T. Lignocellulosic Biomass in Circular Economy: A Techno-Transition in Carbon Neutrality towards Sustainable Energy Production. Biomass Bioenergy 2024, 189, 107349. [Google Scholar] [CrossRef]
- Abdoos, B.; Pourfayaz, F.; Ahmadi, M.H.; Gholami, A. Energetic/Exergetic Parametric Study of a Combined Cooling, Heating, and Power (CCHP) System Coupled with a Local Cellulosic Biomass Combustion Chamber. Energy Rep. 2024, 12, 3770–3777. [Google Scholar] [CrossRef]
- Jawaid, S.A.; Ahmed, S. The Transformative Economic Impact of Artificial Intelligence. Preprints 2023. [Google Scholar] [CrossRef]
- Abhulimen, A.O.; Ejike, O.G. Ethical Considerations in AI Use for SMEs and Supply Chains: Current Challenges and Future Directions. Int. J. Appl. Res. Soc. Sci. 2024, 6, 1653–1679. [Google Scholar] [CrossRef]
- Bourdin, S.; Nadou, F. The Role of a Local Authority as a Stakeholder Encouraging the Development of Biogas: A Study on Territorial Intermediation. J Environ. Manag. 2020, 258, 110009. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Van Nguyen, P.; Vrontis, D.; Vo, N.T.T. Enhancing Organizational Sustainable Performance through Green Innovation: The Roles of Knowledge Application, Government Policy, and Green Market Orientation. J. Knowl. Manag. 2024, 29, 870–890. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, C. A “Circular” World: Reconciling Profitability with Sustainability. In The Circular Economy; Academic Press: Cambridge, MA, USA, 2019; pp. 207–279. [Google Scholar] [CrossRef]
- Saeed, M.; Shahzad, U.; Fazle Rabbee, M.; Manzar, R.; Al-Humaidi, J.Y.; Siddique, A.; Sheikh, T.A.; Althomali, R.H.; Qamar, T.; Rahman, M.M. Potential Development of Porous Carbon Composites Generated from the Biomass for Energy Storage Applications. Chem. Asian J. 2024, 19, e202400394. [Google Scholar] [CrossRef]
- Chicaiza-Ortiz, C.D.; Vintimilla, W.L.; Ortiz, Á.C.; Chávez, W.N.; Cañar, M.E.O. Environmental Management Strategies in Kichwa Communities of the Amazon of Ecuador. Cienc. Unemi 2022, 15, 27–34. [Google Scholar] [CrossRef]
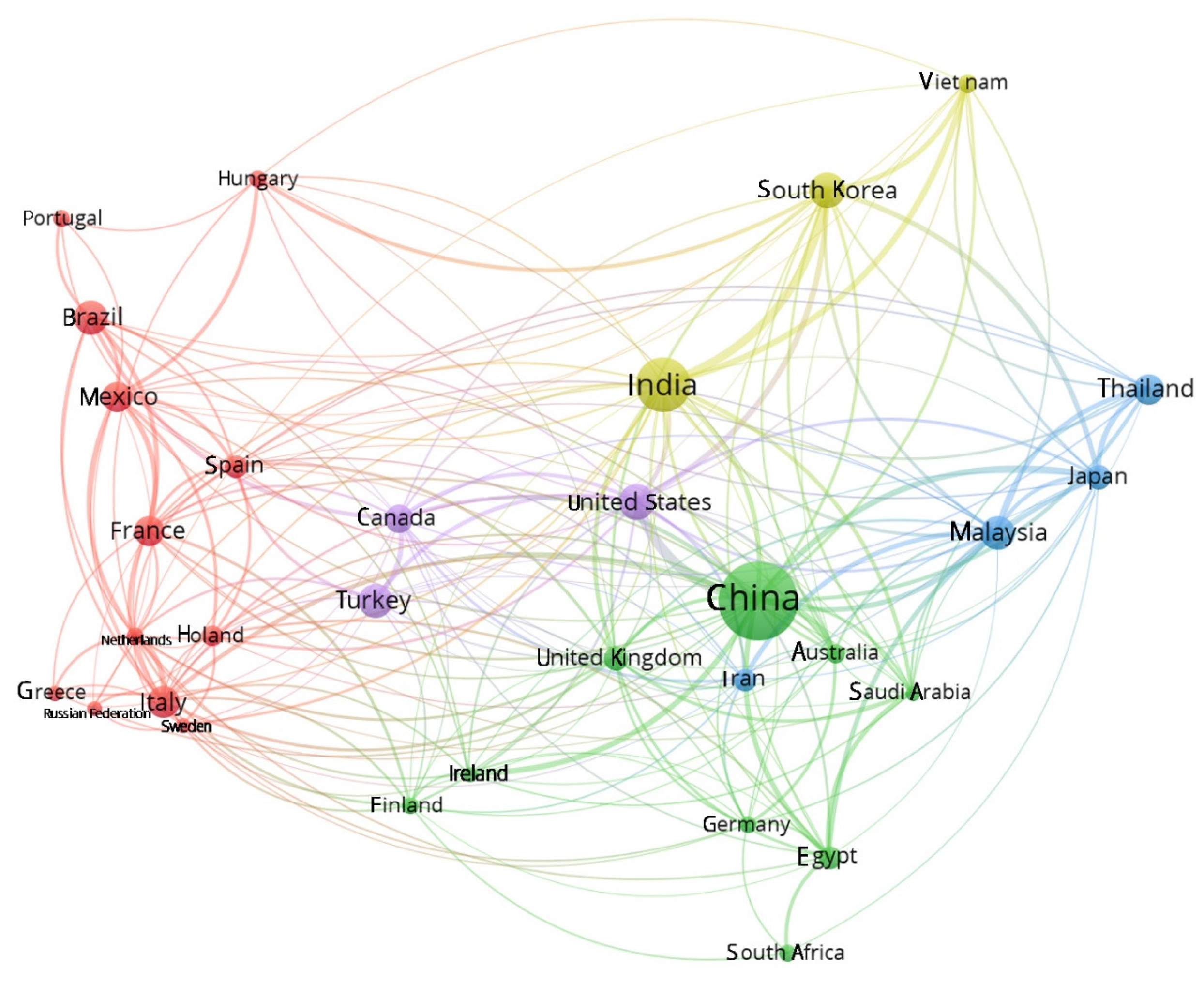
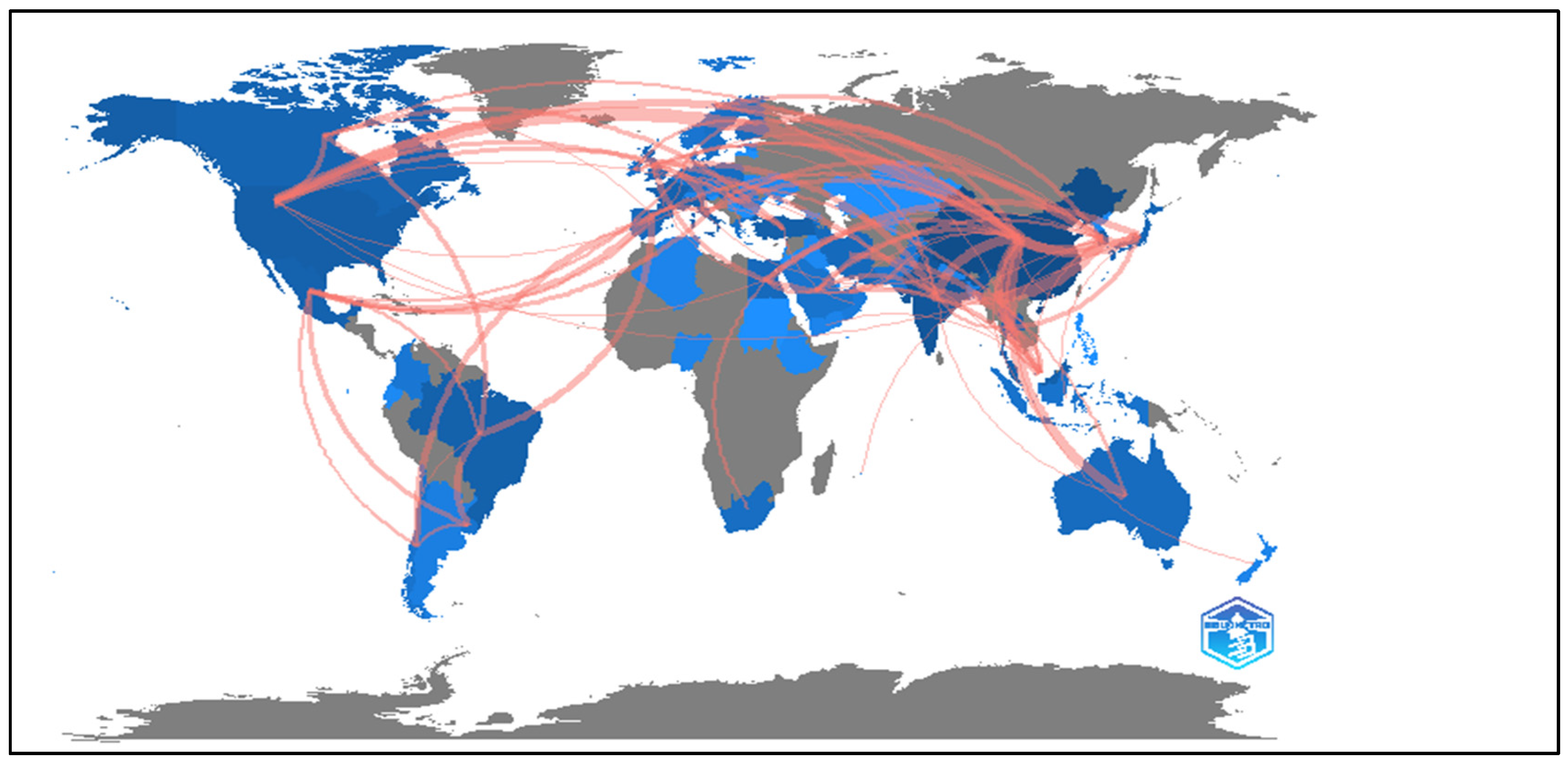


| Country/Regions | Bond | Total Bond Strength | Documents | Contribution Rate (%) | Citations by Country | Population (Estimated in 2024 by UN) * | Documents Per Million Inhabitants |
|---|---|---|---|---|---|---|---|
| China | 39 | 5428 | 552 | 27.56 | 16,435 | 1.42e + 09 | 0.39 |
| India | 39 | 3457 | 259 | 12.93 | 9611 | 1.45e + 09 | 0.18 |
| United States | 39 | 1959 | 122 | 6.09 | 6668 | 3.45e + 08 | 0.35 |
| South Korea | 39 | 1861 | 116 | 5.79 | 4570 | 5.17e + 07 | 2.24 |
| Turkey | 39 | 1500 | 106 | 5.29 | 4633 | 8.75e + 07 | 1.21 |
| France | 39 | 1489 | 84 | 4.19 | 3718 | 6.65e + 07 | 1.26 |
| Malaysia | 39 | 1319 | 95 | 4.74 | 2854 | 3.40e + 07 | 2.79 |
| Brazil | 39 | 1319 | 95 | 4.74 | 2854 | 2.12e + 08 | 0.45 |
| Canada | 38 | 1170 | 67 | 3.34 | 3634 | 3.93e + 07 | 1.71 |
| Thailand | 38 | 1099 | 83 | 4.14 | 2355 | 7.17e + 06 | 11.58 |
| Ranking | Journal | Publisher | Country | Number of Articles | SJR 2022 | Quartile | Open Access Documents |
|---|---|---|---|---|---|---|---|
| 1 | International Journal of Hydrogen Energy | Elsevier | United Kingdom | 604 | 1.32 | Q1 | All Open Access/Green Open Access/Gold Open Access/Bronze Open Access/Hybrid gold |
| 2 | Bioresource Technology | Elsevier | United Kingdom | 349 | 2.47 | Q1 | All open access/Green Open Access/Gold Open Access/Bronze Open Access/Hybrid gold |
| 3 | Renewable Energy | Elsevier | United Kingdom | 42 | 1.82 | Q1 | All open access/Green Open Access |
| 4 | Fuel | Elsevier | Netherlands | 40 | 1.38 | Q1 | All open access/Green Open Access/Hybrid gold |
| 5 | Biomass and Bioenergy | Elsevier | United Kingdom | 35 | 1.05 | Q1 | All Open Access/Green Open Access/Bronze Open Access |
| 6 | Journal of Cleaner Production | Elsevier | United Kingdom | 33 | 1.98 | Q1 | All open access/Green Open Access/Hybrid gold |
| 7 | Chemical Engineering Journal | Elsevier | Netherlands | 26 | 2.8 | Q1 | All Open Access/Bronze/Open Access/Green Open Access |
| 8 | Biotechnology For Biofuels | Biomed Central | United Kingdom | 24 | 1.02 | Q1 | All Open Access/Gold Open Access/Green Open Access |
| 9 | Taiyangneng Xuebao Acta Energiae Solaris Sinica | Science Press | China | 21 | 0.24 | Q4 | Open Access |
| 10 | Applied Energy | Elsevier | United Kingdom | 18 | 2.91 | Q1 | All Open Access/Bronze Open Access/Green Open Access/Hybrid Gold Open Access |
| Older Articles | Most-Cited Articles | Topics of Interest for the Period | |
|---|---|---|---|
| P E R I O D I | Using filtrate of waste biosolids to effectively produce byo-hydrogen by anaerobic fermentation [108] | Biohydrogen production: prospects and limitations for practical application [71]. |
|
| Biohydrogen production from starch in wastewater under thermophilic conditions [109]. | Sustainable and efficient production of biohydrogen by electrohydrogenesis [67]. | ||
| P E R I O D II | Simultaneous production of biohydrogen and treatment of starch-containing wastewater in a mixed-culture acid-generating expanded granular sludge bed reactor for long-term operation [70]. | Hydrogen production from agricultural residues by dark fermentation: a review [76]. |
|
| Light fermentation of dark fermentation effluents for biohydrogen production by different Rhodobacter sp. at different initial volatile fatty acid concentrations (VFA) [110]. | Advances in biological hydrogen production processes [68]. | ||
| P E R I O D III | Feasibility study of biohydrogen production from pressed sludge using UASB processes and assessment of operational parameters [111]. | Lignocellulosic materials in biohydrogen and biomethane: impact of structural characteristics and pretreatment [112]. |
|
| Characterization by length heterogeneity-PCR of a hydrogen-producing community derived from obscure fermentation using coastal lake sediments as inoculum [113]. | A biorefinery from Nannochloropsis sp. microalga—Extraction of oils and pigments. Production of biohydrogen from the leftover biomass [114] | ||
| P E R I O D IV | Sustainable bioenergy from residues and waste biofuels [115]. | The waste-energy nexus for the circular economy and environmental protection: recent trends in hydrogen energy [116]. | The aim is to optimize and determine the appropriate conditions for biohydrogen production from biowaste, solid or organic, and organic waste. |
| Enhanced biohydrogen production from a nutrient-free anaerobic fermentation medium with rice straw pretreated with edible fungi [107]. | Free nitrous acid promotes hydrogen production from the dark fermentation of activated sludge [117]. |
| Keywords | Year | Strength | Begin | End | 2003–2024 |
|---|---|---|---|---|---|
| Genetics | 2015 | 12.06 | 2015 | 2017 |  |
| Molecular Biology | 2015 | 4.36 | 2015 | 2015 |  |
| Bioremediation | 2017 | 3.82 | 2017 | 2017 |  |
| Electron Transport | 2021 | 12.84 | 2021 | 2024 |  |
| Pre-Treatments | 2022 | 25.31 | 2022 | 2024 |  |
| Lignin | 2013 | 11.56 | 2021 | 2024 |  |
| Maize | 2021 | 7.79 | 2021 | 2024 |  |
| Lignocellulose | 2013 | 10.23 | 2022 | 2024 |  |
| Iron | 2022 | 6.62 | 2022 | 2022 |  |
| Nanoparticles | 2021 | 11.52 | 2021 | 2024 |  |
| Additives | 2022 | 4.21 | 2022 | 2022 |  |
| Energy Yield | 2023 | 5.57 | 2023 | 2024 |  |
| Sustainable Development | 2022 | 9.49 | 2022 | 2024 |  |
| Cost Effectiveness | 2022 | 7.43 | 2022 | 2024 |  |
| Greenhouse Gases | 2023 | 8.23 | 2023 | 2024 |  |
| Stage of AD-BioH2 | Relevant Sustainable Development Goals | Key Contributions/Benefits |
|---|---|---|
| Waste Collection and Sorting |  | Minimizes landfill waste; promotes recycling and resource efficiency; improves urban waste management. |
| Anaerobic Digestion Process |  | Converts organic waste into renewable energy (biohydrogen); reduces greenhouse gas emissions through waste valorization. |
| Biohydrogen Utilization |  | Provides a clean fuel alternative to fossil fuels; supports sustainable industrial processes and technological innovation. |
| Digestate Utilization |  | Produces nutrient-rich digestate for fertilizer, reducing reliance on chemical fertilizers; enhances soil quality and supports sustainable agriculture. |
| Technology Innovation and Infrastructure Development |  | Encourages research and development; improves process efficiencies; fosters integration of sustainable energy systems into urban infrastructure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, R.; Chicaiza-Ortiz, C.; Herrera-Feijoo, R.J.; Arellano-Yasaca, D.V.; Lee, L.-C.; Supe-Tulcan, R.X.; Marti-Herrero, J. Advancements of Biohydrogen Production Based on Anaerobic Digestion: Technologies, Substrates, and Future Prospects. Sci 2025, 7, 52. https://doi.org/10.3390/sci7020052
Parra R, Chicaiza-Ortiz C, Herrera-Feijoo RJ, Arellano-Yasaca DV, Lee L-C, Supe-Tulcan RX, Marti-Herrero J. Advancements of Biohydrogen Production Based on Anaerobic Digestion: Technologies, Substrates, and Future Prospects. Sci. 2025; 7(2):52. https://doi.org/10.3390/sci7020052
Chicago/Turabian StyleParra, Rossana, Cristhian Chicaiza-Ortiz, Robinson J. Herrera-Feijoo, Diana Victoria Arellano-Yasaca, Lien-Chieh Lee, Roberto Xavier Supe-Tulcan, and Jaime Marti-Herrero. 2025. "Advancements of Biohydrogen Production Based on Anaerobic Digestion: Technologies, Substrates, and Future Prospects" Sci 7, no. 2: 52. https://doi.org/10.3390/sci7020052
APA StyleParra, R., Chicaiza-Ortiz, C., Herrera-Feijoo, R. J., Arellano-Yasaca, D. V., Lee, L.-C., Supe-Tulcan, R. X., & Marti-Herrero, J. (2025). Advancements of Biohydrogen Production Based on Anaerobic Digestion: Technologies, Substrates, and Future Prospects. Sci, 7(2), 52. https://doi.org/10.3390/sci7020052








