Effects of Different Nitrogen and Phosphorus Ratios on the Growth, Nutritional Value, and Nutrient Removal Efficiency of Wolffia globosa
Abstract
1. Introduction
2. Materials and Methods
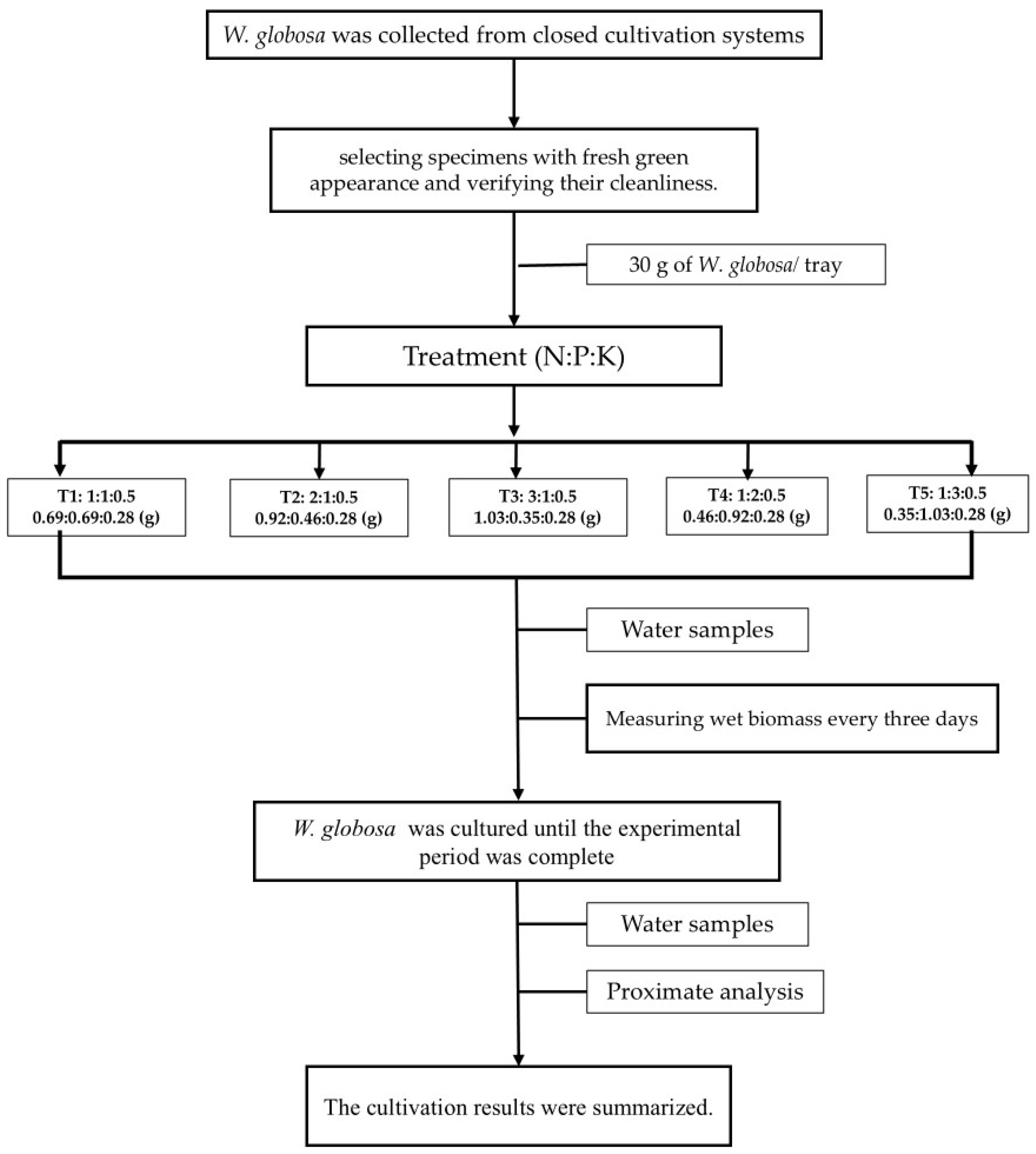
2.1. Plant Material and Experimental Setup
2.2. Preparation of Nutrient Solutions
2.3. Cultivation Method
2.4. Growth Performance of W. globosa
2.5. Nutritional Composition Analysis of W. globosa
2.6. Nutrient Removal Efficiency
2.7. Statistical Analysis
3. Results
3.1. Evaluation of Growth Performance of W. globosa
3.2. Nutritional Composition Analysis
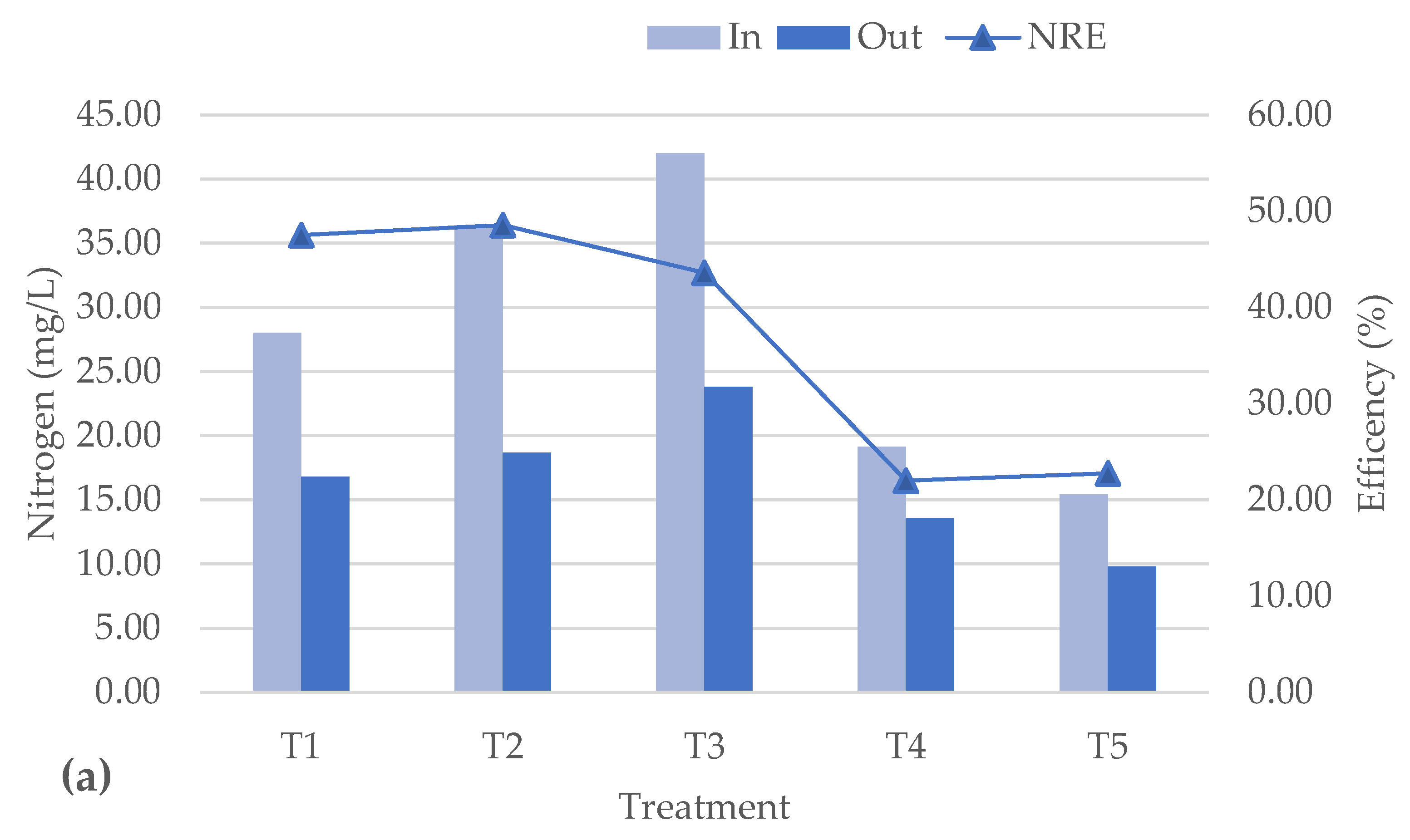
3.3. Analysis of Nutrient Removal Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Wolffia globosa | W. globosa |
| CRD | Completely Randomized Design |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| SGR | Specific Growth Rate |
| Wt | final weight |
| W0 | initial weight |
| NRE | Nitrogen removal efficiency |
| PRE | Phosphorus removal efficiency |
References
- Global Market Insights. Duckweed Protein Market Size & Share Report, 2022–2030. Available online: https://www.gminsights.com/industry-analysis/duckweed-protein-market (accessed on 21 March 2025).
- Thongthung, H.; Chungopast, S.; Petchpoung, K.; Kaewtapee, C. Chemical composition and in vitro protein digestibility of duckweed and feed ingredients. Trends Sci. 2024, 21, 8324. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Meir, A.Y.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant–a randomized controlled trial. Clin. Nutr. 2019, 38, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- On-Nom, N.; Promdang, P.; Inthachat, W.; Kanoongon, P.; Sahasakul, Y.; Chupeerach, C.; Suttisansanee, U.; Temviriyanukul, P. Wolffia globosa-based nutritious snack formulation with high protein and dietary fiber contents. Foods 2023, 12, 2647. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Munilkumar, S.; Patel, A.; Kamilya, D.; Pandey, P.; Sawant, P.B. Lamellidens and Wolffia canopy improves growth, feed utilization and welfare of Labeo rohita (Hamilton, 1822) in integrated multi-trophic freshwater aquaculture system. Aquaculture 2021, 534, 736207. [Google Scholar] [CrossRef]
- Monthakantirat, O.; Chulikhit, Y.; Maneenet, J.; Khamphukdee, C.; Chotritthirong, Y.; Limsakul, S.; Punya, T.; Turapra, B.; Boonyarat, C.; Daodee, S. Total active compounds and mineral contents in Wolffia globosa. J. Chem. 2022, 2022, 9212872. [Google Scholar] [CrossRef]
- Said, D.; Chrismadha, T.; Mayasari, N.; Febrianti, D.; Suri, A. Nutrition value and growth ability of aquatic weed Wolffia globosa as alternative feed sources for aquaculture system. IOP Conf. Ser. Earth Environ. Sci. 2022, 950, 012044. [Google Scholar]
- Calabrese, M.G.; Ferranti, P. Novel Foods: New Food Sources. In Encyclopedia of Food Security and Sustainability; Pasquale, F., Elliot, M.B., Jock, R.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 271–275. [Google Scholar]
- Avelar, Z.; Rodrigues, R.M.; Pereira, R.N.; Vicente, A.A. Future Food Proteins-Trends and Perspectives. In Future Foods; Bhat, R., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2022; pp. 267–285. [Google Scholar]
- Chookhampaeng, S.; Puntura, S.; Chookhampaeng, C. Effect of light, aeration and nutrient concentration on Wolffia globosa growth. Asian J. Plant Sci. 2022, 21, 559–564. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeBusk, W.F. Nutrient removal potential of selected aquatic macrophytes. J. Environ. Qual. 1985, 14, 459–462. [Google Scholar] [CrossRef]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and nutrient removal efficiency of duckweed (Lemna minor) from synthetic and dumpsite leachate under artificial and natural conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef]
- Baek, G.; Saeed, M.; Choi, H.-K. Duckweeds: Their utilization, metabolites and cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef]
- Körner, S.; Vermaat, J. The relative importance of Lemna gibba L., bacteria and algae for the nitrogen and phosphorus removal in duckweed-covered domestic wastewater. Water Res. 1998, 32, 3651–3661. [Google Scholar] [CrossRef]
- Soda, S.; Kawahata, Y.; Takai, Y.; Mishima, D.; Fujita, M.; Ike, M. Kinetics of nutrient removal and biomass production by duckweed Wolffia arrhiza in continuous-flow mesocosms. Ecol. Eng. 2013, 57, 210–215. [Google Scholar] [CrossRef]
- Ruekaewma, N.; Piyatiratitivorakul, S.; Powtongsook, S. Culture system for Wolffia globosa L.(Lemnaceae) for hygiene human food. Songklanakarin J. Sci. Technol. 2015, 37, 575–580. [Google Scholar]
- Lam, T.P.; Lee, T.-M.; Chen, C.-Y.; Chang, J.-S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour. Technol. 2018, 252, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Somboona, S. Cultivation and utilization of duckweed (Wolffia sp.). Kasetsart Ext. J. 2015, 60, 61–74. [Google Scholar]
- Petersen, F.; Demann, J.; Restemeyer, D.; Ulbrich, A.; Olfs, H.-W.; Westendarp, H.; Appenroth, K.-J. Influence of the nitrate-n to ammonium-n ratio on relative growth rate and crude protein content in the duckweeds Lemna minor and Wolffiella hyalina. Plants 2021, 10, 1741. [Google Scholar] [CrossRef]
- Khvatkov, P.; Chernobrovkina, M.; Okuneva, A.; Dolgov, S. Creation of culture media for efficient duckweeds micropropagation (Wolffia arrhiza and Lemna minor) using artificial mathematical optimization models. Plant Cell Tissue Organ Cult. 2019, 136, 85–100. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Phatarpekar, P.; Sreepada, R.; Pednekar, C.; Achuthankutty, C. A comparative study on growth performance and biochemical composition of mixed culture of Isochrysis galbana and Chaetoceros calcitrans with monocultures. Aquaculture 2000, 181, 141–155. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Zygler, A.; Słomińska, M.; Namieśnik, J. 2.04—Soxhlet extraction and new developments such as soxtec. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 65–82. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Bernard, F.A.; Bernard, J.M.; Denny, P. Flower structure, anatomy and life history of Wolffia australiana (Benth.) den Hartog & van der Plas. Bull. Torrey Bot. Club 1990, 117, 18–26. [Google Scholar]
- Sree, K.S.; Maheshwari, S.C.; Boka, K.; Khurana, J.P.; Keresztes, Á.; Appenroth, K.-J. The duckweed Wolffia microscopica: A unique aquatic monocot. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 210, 31–39. [Google Scholar] [CrossRef]
- Kufel, L.; Strzałek, M.; Przetakiewicz, A. Plant response to overcrowding–Lemna minor example. Acta Oecologica 2018, 91, 73–80. [Google Scholar] [CrossRef]
- Edelman, M.; Appenroth, K.J.; Sree, K.S. Editorial: Duckweed: Biological chemistry and applications. Front. Sustain. Food Syst. 2020, 4, 615135. [Google Scholar] [CrossRef]
- Yadav, N.K.; Patel, A.B.; Debbarma, S.; Priyadarshini, M.B.; Priyadarshi, H. Characterization of Bioactive Metabolites and Antioxidant Activities in Solid and Liquid Fractions of Fresh Duckweed (Wolffia globosa) Subjected to Different Cell Wall Rupture Methods. ACS Omega 2024, 9, 19940–19955. [Google Scholar] [CrossRef] [PubMed]
- Sirirustananun, N.; Phimthong, C.; Numpet, T. Utilization of hydroponic fertilizer for watermeal cultivation and an investigation of the suitability of the fresh watermeal (Wolffia arrhiza) supplement for tilapia rearing. Egypt. J. Aquat. Biol. Fish. 2022, 26, 217–228. [Google Scholar] [CrossRef]
- Chikuvire, T.J.; Muchaonyerwa, P.; Zengeni, R. Biomass, nitrogen uptake and content of Wolffia arrhiza depends on strength of swine lagoon water. Water Environ. Res. 2018, 90, 2066–2074. [Google Scholar] [CrossRef]
- Prosridee, K.; Oonsivilai, R.; Tira-Aumphon, A.; Singthong, J.; Oonmetta-Aree, J.; Oonsivilai, A. Optimum aquaculture and drying conditions for Wolffia arrhiza (L.) Wimn. Heliyon 2023, 9, e19730. [Google Scholar] [CrossRef]
- Stomp, A.-M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005, 11, 69–99. [Google Scholar] [CrossRef]
- Hasan, M.R.; Chakrabarti, R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review, 1st ed.; FAO Fisheries and Aquaculture Technical Paper No. 531; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Novák, J.; Hohl, D.; Stuchlík, M.; Hofmann, J.; Tlusty, M.F.; Magalhães, A.L.B.; Maceda-Veiga, A.; Akmal, S.G.; Yue, G.H.; Kumkar, P. Revisiting the History of Ornamental Aquaculture in Europe to Understand the Benefits and Drawbacks of Freshwater Fish Imports. Rev. Aquac. 2025, 17, e13008. [Google Scholar] [CrossRef]

| Treatment | Ratio | Nitrogen (g) | Phosphorus (g) | Potassium (g) |
|---|---|---|---|---|
| T1 | 1:1:0.5 | 0.69 | 0.69 | 0.28 |
| T2 | 2:1:0.5 | 0.92 | 0.46 | 0.28 |
| T3 | 3:1:0.5 | 1.03 | 0.35 | 0.28 |
| T4 | 1:2:0.5 | 0.46 | 0.92 | 0.28 |
| T5 | 1:3:0.5 | 0.35 | 1.03 | 0.28 |
| Treatment | Culture Periods (day) | SGR (g/day) | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 10 | ||
| T1 | 30.21 ± 0.74 | 35.93 ± 4.00 | 47.50 ± 3.18 | 77.03 ± 3.33 a | 98.73 ± 0.81 a | 0.120 |
| T2 | 30.21 ± 0.74 | 35.40 ± 4.16 | 46.47 ± 2.68 | 64.5 ± 3.25 ab | 77.90 ± 2.97 b | 0.095 |
| T3 | 30.21 ± 0.74 | 34.63 ± 4.01 | 41.97 ± 5.36 | 56.40 ± 3.44 b | 71.40 ± 4.93 b | 0.089 |
| T4 | 30.21 ± 0.74 | 36.80 ± 3.14 | 48.47 ± 3.00 | 75.80 ± 6.41 a | 92.37 ± 4.50 a | 0.115 |
| T5 | 30.21 ± 0.74 | 34.70 ± 1.05 | 47.83 ± 5.46 | 71.07 ± 3.25 a | 90.97 ± 3.00 a | 0.113 |
| Proximate Composition (%) | Treatments | ||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | |
| Moisture | 95.51 ± 0.04 ab | 95.34 ± 0.06 a | 95.56 ± 0.08 bc | 95.71 ± 0.06 c | 95.91 ± 0.07 d |
| Dry matter | 4.50 ± 0.04 ab | 4.66 ± 0.06 a | 4.44 ± 0.08 bc | 4.30 ± 0.06 c | 4.09 ± 0.07 d |
| Ash | 19.45 ± 0.07 c | 16.60 ± 0.11 b | 14.66 ± 0.15 a | 22.67 ± 0.20 d | 22.41 ± 0.24 d |
| Protein | 33.36 ± 0.16 c | 35.99 ± 0.62 d | 38.68 ± 0.01 e | 29.33 ± 0.06 b | 27.52 ± 0.19 a |
| Fat | 6.29 ± 0.22 ab | 6.40 ± 0.11 a | 5.60 ± 0.10 c | 5.87 ± 0.03 abc | 5.78 ± 0.09 bc |
| Carbohydrate | 72.81 ± 0.19 | 73.67 ± 0.59 | 72.85 ± 0.30 | 73.84 ± 2.43 | 71.16 ± 1.07 |
| Fiber | 9.21 ± 0.07 | 10.02 ± 1.25 | 9.47 ± 0.44 | 11.68 ± 2.23 | 11.37 ± 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yongyod, R.; Kamolrat, N. Effects of Different Nitrogen and Phosphorus Ratios on the Growth, Nutritional Value, and Nutrient Removal Efficiency of Wolffia globosa. Sci 2025, 7, 53. https://doi.org/10.3390/sci7020053
Yongyod R, Kamolrat N. Effects of Different Nitrogen and Phosphorus Ratios on the Growth, Nutritional Value, and Nutrient Removal Efficiency of Wolffia globosa. Sci. 2025; 7(2):53. https://doi.org/10.3390/sci7020053
Chicago/Turabian StyleYongyod, Rapeepan, and Narong Kamolrat. 2025. "Effects of Different Nitrogen and Phosphorus Ratios on the Growth, Nutritional Value, and Nutrient Removal Efficiency of Wolffia globosa" Sci 7, no. 2: 53. https://doi.org/10.3390/sci7020053
APA StyleYongyod, R., & Kamolrat, N. (2025). Effects of Different Nitrogen and Phosphorus Ratios on the Growth, Nutritional Value, and Nutrient Removal Efficiency of Wolffia globosa. Sci, 7(2), 53. https://doi.org/10.3390/sci7020053







