1. Introduction
According to statistics from the World Health Organization (WHO), cancer ranks as the second leading cause of death worldwide [
1]. It is estimated that approximately 10 million deaths from cancer occur each year [
1,
2]. Over the past century, external beam energy for cancer treatment has steadily advanced, resulting in the development of two primary approaches. The first, radiotherapy, uses X-ray, gamma, and proton beams to induce ionizing damage [
1,
2,
3,
4,
5,
6,
7,
8,
9]. The second, photodynamic therapy, relies on photons to activate photosensitizers, producing reactive oxygen species that cause cytotoxic effects [
10,
11,
12,
13]. For decades, these two therapies evolved separately: photodynamic therapy has been applied to treat surface tumors, while radiotherapy has been directed toward deeper tumors [
1]. In recent years, efforts to combine radiotherapy and photodynamic therapy have intensified, resulting in the development of innovative techniques such as X-ray photodynamic therapy [
14] and proton-induced photodynamic therapy [
15]. For example, Grigalavicius et al. [
15] demonstrated that proton beams can activate fluorescence and singlet oxygen
1O
2 in photosensitizers for proton-induced photodynamic therapy (PrDT). It has been suggested that the photosensitizers increased the interaction cross-section, leading to accelerated high-energy deposition [
15]. Therefore, monitoring the charged particles traveling within the tissue is crucial for classic radiotherapy approaches and new cutting-edge therapeutic procedures.
It is important to note that one of the key challenges associated with the medical procedures mentioned above is effectively modeling tissue-particle interactions and the computational resources required for this task. Accurate modeling of radiation interactions is essential for various applications. To fully harness the potential of charged particles, a thorough understanding of energy deposition across different media is critical. Monte Carlo techniques are commonly used to achieve this [
16]. Monte Carlo particle transport packages are highly advanced tools capable of simulating the interaction of particle radiation with matter as it passes through a medium [
17,
18,
19,
20]. These approaches can capture system behavior across all scales, including secondary and higher-order interactions, but they are computationally expensive with significant evaluation times [
20]. In other words, although Monte Carlo methods have been highly successful in modeling these more complex target materials, they come with a significant computational cost, particularly when the problem is time-dependent [
20]. The goal of this work is to develop a new simple model that accurately captures the general behavior of charged particles within the energy ranges relevant to therapy (up to 350 MeV). It is important to note that approximate analytical solutions to this problem have been proposed previously [
20,
21]. Grimes et al. [
20] provided an analytical solution for the velocities of charged particles with respect to the distance traveled in a medium, expressed in terms of the inverse exponential function with relativistic coordinate transformations. This is not a standard function, and no closed-form expression exists for it [
20]. The solution is perfectly executed and accurate, as it is valid for relativistic velocities; however, its use requires a strong mathematical and physical background.
An alternative solution has also been presented by Martinez et al., who provided a series solution (based on regular perturbation methods) for the range and dose distribution that does not include the inverse exponential function [
21]. This solution is accurate when considering small relativistic effects. By including a large number of terms in the series solution, the application of the results can be extended to protons with energies up to 200 MeV.
Both the results of Grimes et al. and Martinez et al. are based on solving the Bethe equation below:
where
n is the electron density of the material,
e is the electron charge,
me is the electron mass,
I is the mean excitation potential,
z is the multiple of the electron charge, and
, where
is the speed of the particle and
is the speed of light in a vacuum. It is important to highlight that a particle’s energy depends on its speed, which, in turn, is a function of distance
. Since the energy levels relevant to radiotherapy are considered, the relativistic definition of particle kinetic energy should be used. This definition is expressed in terms of the Lorentz factor [
20]:
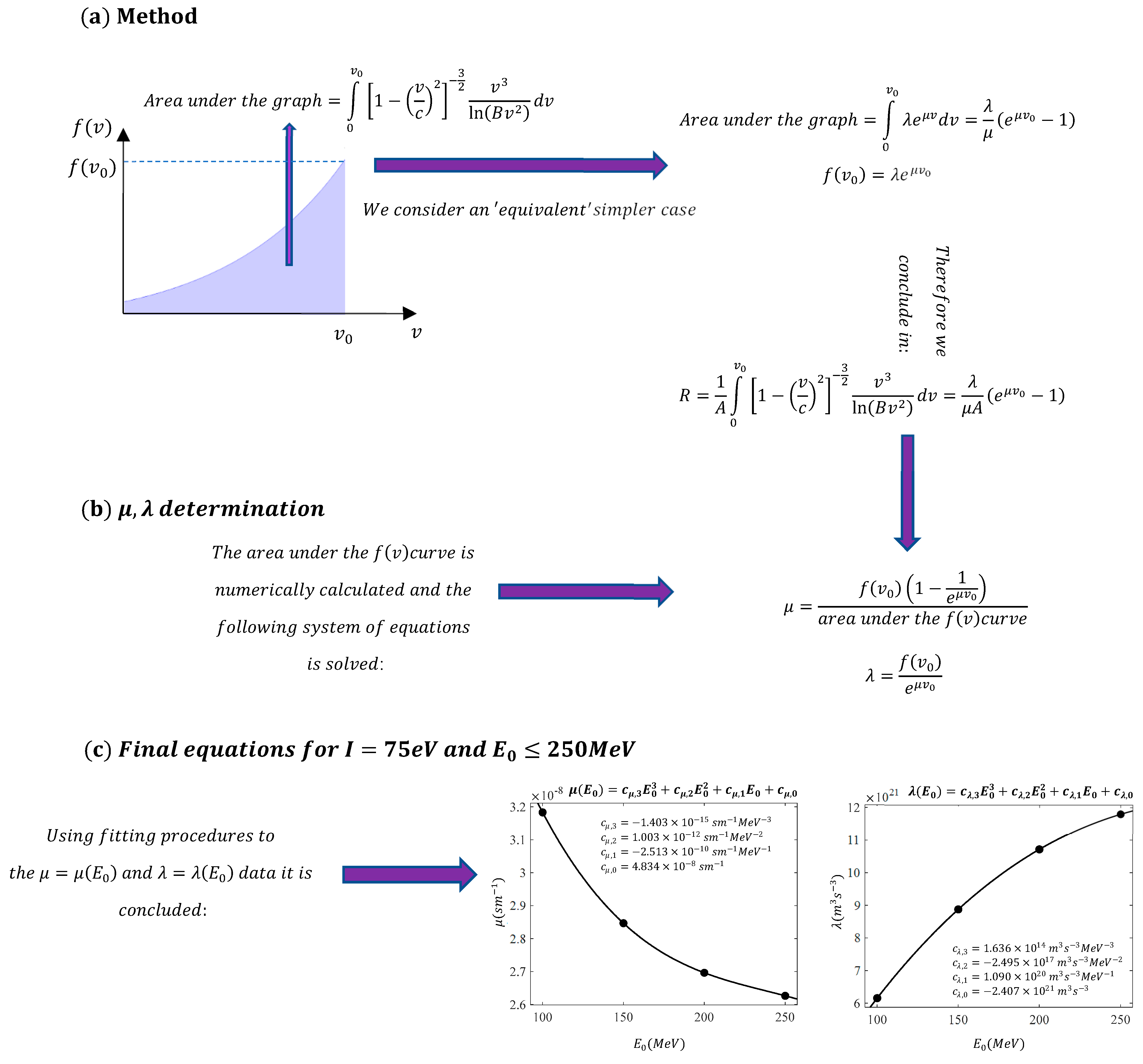
According to Grimes et al. [
20], Equation (1) can be simplified as follows:
where
and
. The approximate Equation (3) results in negligible error when applied to energies relevant to radiotherapy. In particular, when using Equation (3) instead of (1), there is an error of approximately 1% for high-energy protons (250 MeV) [
1]. The procedure employed by Grimes et al. [
20] to derive the function
is described as follows: using the chain rule, they resulted in
Subsequently, by substituting Equation (4) into Equation (3), it is easily concluded that
By solving the above differential equation, the range of the charged particles can be determined [
20]. However, the strategies employed in previous publications [
20,
21] require advanced mathematical tools, special functions [
20], or include series solutions, with results limited to protons with energies up to 200 MeV [
21]. While these approaches are of significant mathematical interest from both theoretical and applied physics, as well as biomedical engineering perspectives, they may have limited practical applicability for radiotherapy professionals or young researchers in medicine and biomedical technology who lack experience with advanced mathematical procedures.
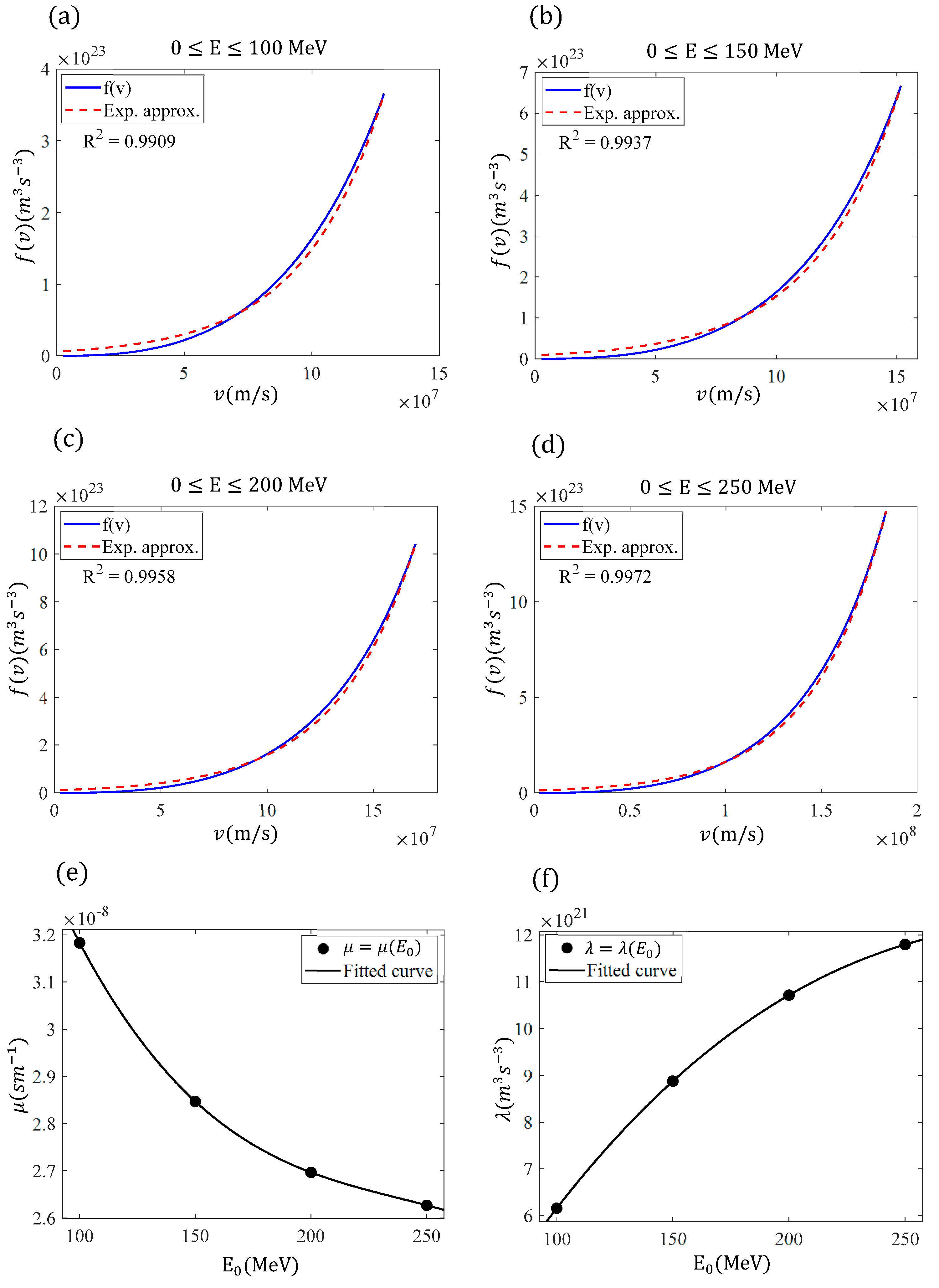
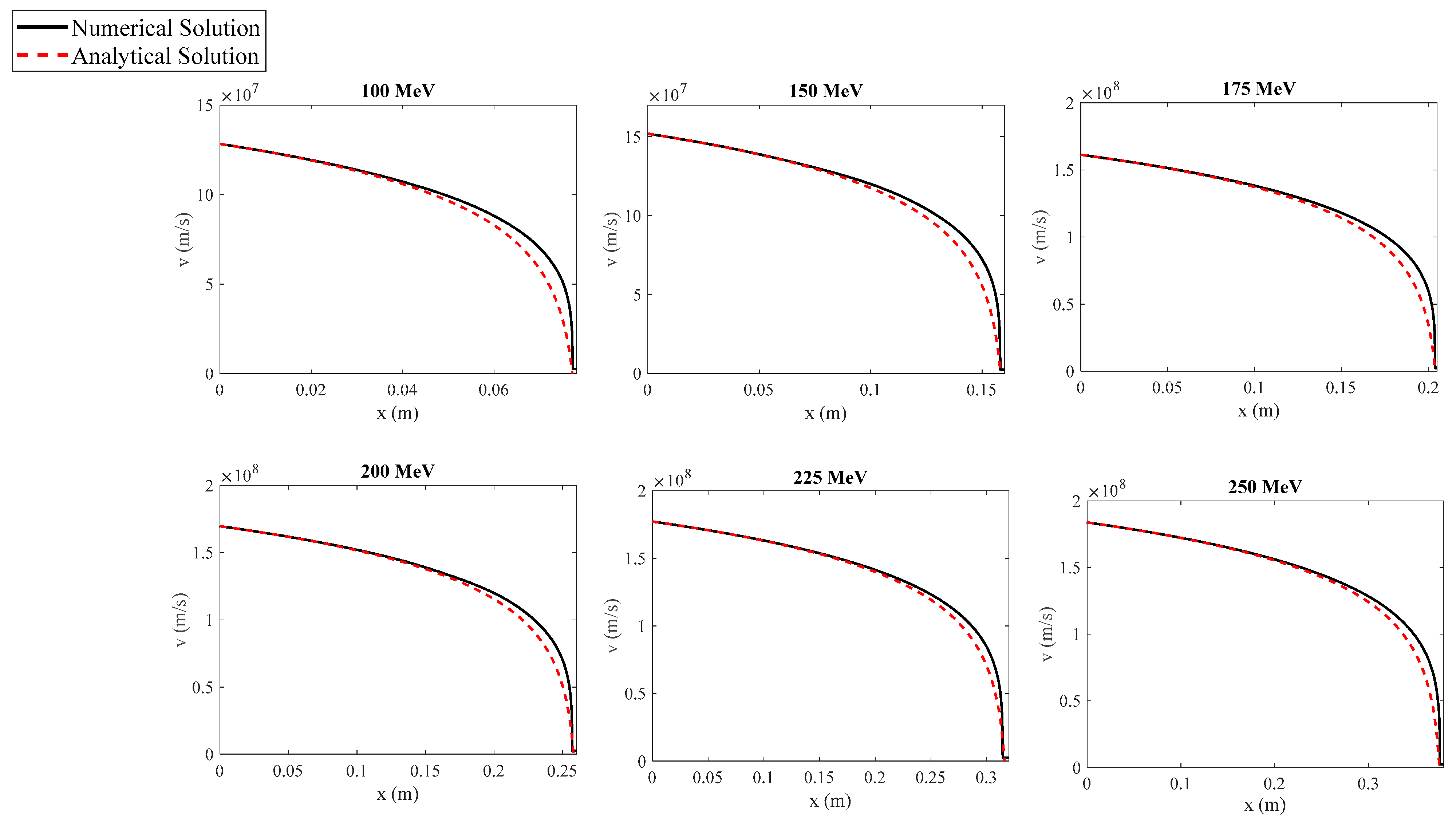
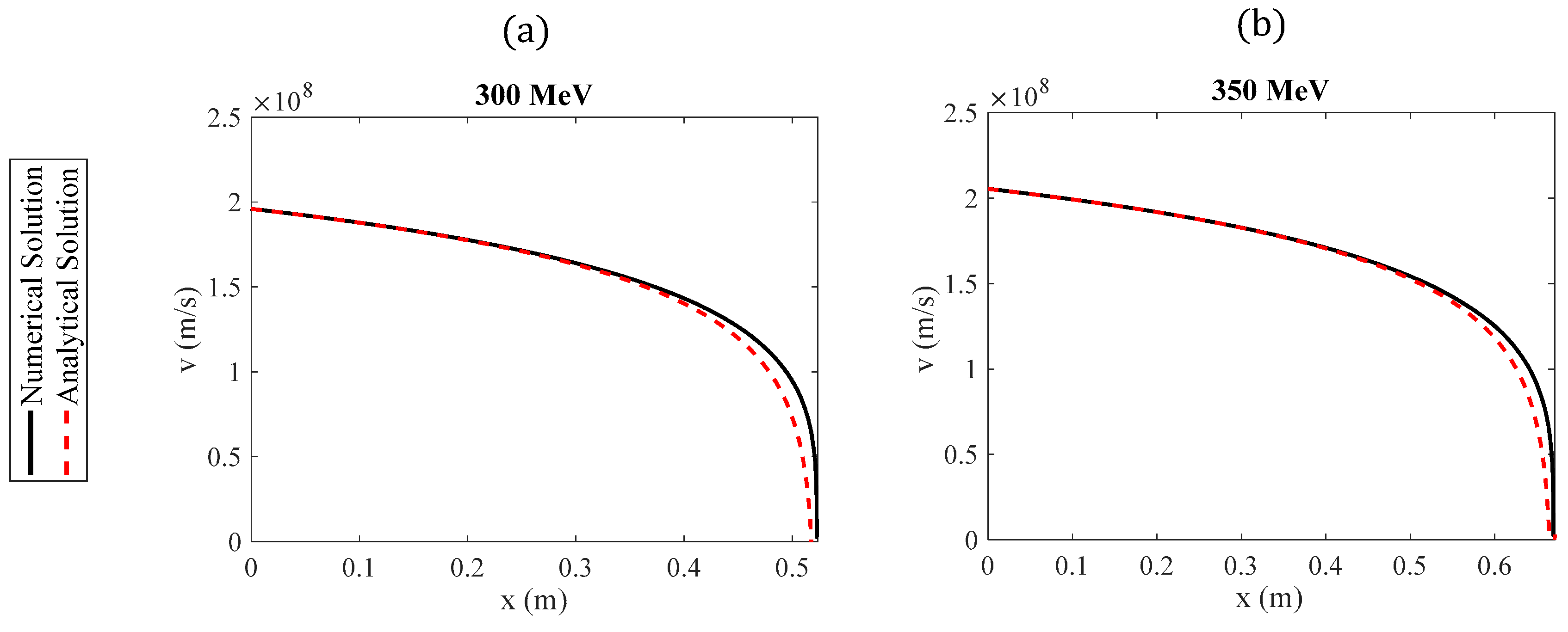
The goal of this paper is to simplify the procedure for approximately determining the function while simultaneously deriving straightforward, easily adaptable solutions for the range of particles in a medium. We demonstrate that the range of charged particles within a medium and their velocity as a function of distance can be described by elementary functions, with appropriate coefficients depending on the particles’ initial energy. The analysis presented in this paper focuses on protons, as they are used in both radiotherapy and new combined therapeutic practices, as previously mentioned. However, these methods can equally be applied to any charged particle.
This paper is organized as follows: In
Section 2 (Materials and Methods), we derive a simple equation for the range of the charged particles. The approach to avoiding the extreme mathematical complexity of this problem is to identify an ‘equivalent’ simpler problem using fitting methods that employ basic exponential functions. In
Section 3 (Results), the newly derived equation is tested, and the results are compared with other analytical and experimental findings from the literature, as well as with exact numerical results. In addition, the function
is derived based on the simple exponential approximation introduced in
Section 2, where
is the velocity of the protons with respect to the distance traveled
. Furthermore, the case of protons with very large initial energies (>250 MeV) is considered. In
Section 4 (the Discussion Section), further insights regarding small initial energies and the uncertainties in the associated variables are also presented, emphasizing their impact on the derived equations and the analytical results.
4. Discussion and Further Considerations
In this paper, we revisited a classic problem: the calculation of the range and velocities of protons traveling through a medium for biomedical applications. Radiation therapy is a powerful cancer treatment used alone or in combination with other methods. During the therapy, high-energy photon or proton beams deliver energy to tissues through atomic or nuclear interactions [
26]. Therefore, proton interactions with matter are of tremendous importance in medicine and biomedical technology.
As mentioned in the introduction, several approximate mathematical approaches have been previously derived to solve Equation (1) and avoid computationally expensive methods [
20,
21,
27]. However, despite providing solutions with excellent accuracy, these approaches are either based on advanced mathematical and physical methods [
20] or focus on cases where relativistic effects are small [
21]. Therefore, the aim of this paper is to find a simpler solution that will significantly assist practitioners in the field of radiotherapy. The idea was to avoid complex mathematical procedures by considering a ‘similar’ but much simpler problem using the approximate Equation (9). It is also worth noting for readers who are not familiar with these mathematical procedures that ‘considering similar problems’ and approximating functions with simpler ones are widely used in other fields of nonlinear science, particularly in studies related to oscillations and periodic phenomena [
28,
29,
30,
31]. This is how the idea for deriving this simple solution was conceived. Despite providing equations that yield excellent results compared to other studies and numerical results, there are still some points that require further clarification. The first issue is related to the ionization potential, which was assumed to be 75 eV. The value of 75 eV used is consistent with the guidelines set by the International Commission on Radiation Units and Measurements (ICRU) [
32]. Another report recommends a lower value of 67 eV [
33]. Other authors have suggested a significantly higher value of 80.2 ± 2 eV [
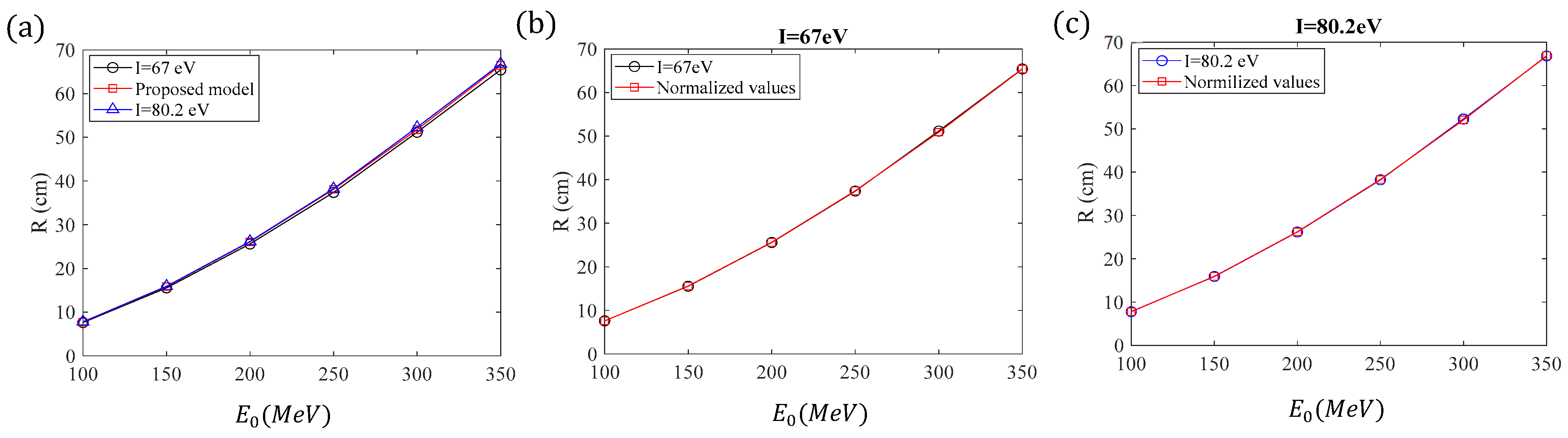
34], based on experimental range measurements. It is also important to note that one of the main advantages of deriving mathematical models to describe the motion of protons within a medium is that it is straightforward to observe the impact of different parameters (such as the ionization potential) on the results.
To assess the influence of the ionization potential, we will calculate the exact range of protons using Equation (18) for
and
and for initial energies ranging from 100 MeV to 350 MeV. The results will be compared to those derived using Equations (12), (16) and (17) (and the limit values of
Table 3 for high proton energies). The results are presented for comparison in
Table 5.
If considering
, the exact range is slightly smaller than the one obtained using Equations (12), (16) and (17) and
Table 3 (since these were derived by assuming
). On the other hand, when using
, the exact range is slightly larger than the one obtained using Equations (12), (16) and (17) and
Table 3. The exact range for
= 67 eV and
= 80.2 eV, along with the values derived by the model presented in this paper, are shown for comparison in
Figure 6a. It is evident from
Table 5 and
Figure 6a that the errors related to the uncertainty of the ionization potential are small. In addition, it is straightforward to normalize the results concerning the range derived by the model proposed in this paper for the cases with ionization potentials of 67 eV and 80.2 eV. According to
Table 5, the average error when
is 1.63% while the average error when
is 0.63%.
Therefore, for an ionization potential of 67 eV, we can normalize the model presented in this paper by reducing the values by 1.63%, and for an ionization potential of 80.2 eV, we can normalize them by increasing the values by 0.63%. In other words, for
:
whereas, for
:
Another critical issue, as already mentioned in the introduction, is the case of very small ranges, since these applications are related to new methods that combine radiotherapy with photodynamic therapy. In reference [
15], proton energies between 15 MeV and 16 MeV were used, achieving a small penetration into matter of approximately 2 mm. For this case, we will repeat the procedure presented in
Section 2. Since the initial energy range is small, we will consider constant
and
values for initial energies between 15 MeV and 16 MeV. The exact values of the initial velocities,
and
,
parameters are presented in
Table 7. This approximation yields excellent results, as shown in
Table 8. Therefore, for applications that combine radiotherapy with photodynamic therapy (PDT) we can safely use the following values:
and
.
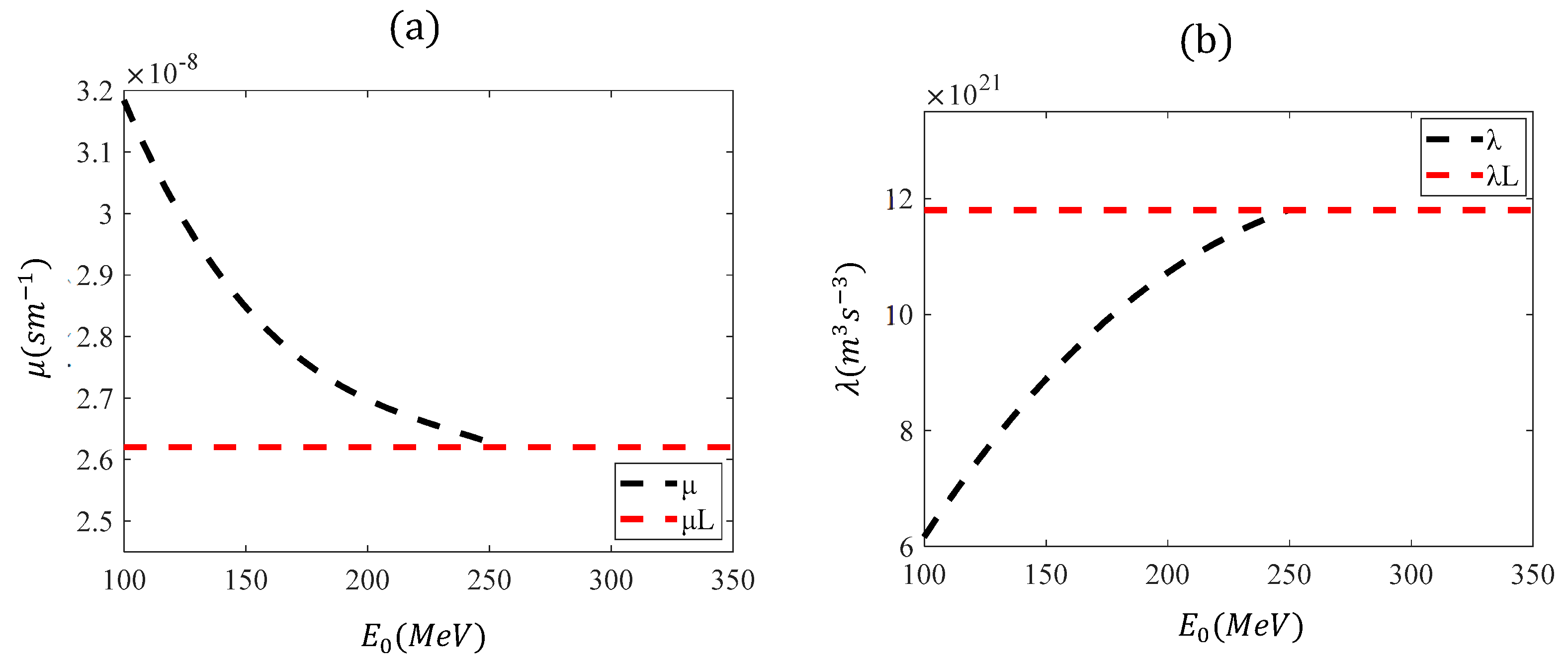
The reason for repeating the procedure to calculate the appropriate
μ and
λ parameters is that Equations (16) and (17) were determined by fitting a polynomial function to the data in the range
. For small initial energies, these equations are not applicable. For example, when using Equations (16) and (17) for
,
and
. The negative value in the λ-parameter results in a negative range, which has no physical significance. In
Figure 7, the functions
and
, based on Equations (16) and (17), are presented in the range
. The range of their applicability is also indicated (
).
Another important point to address is how closely water represents tissue in terms of mean excitation potential. Water is often used as a surrogate for tissue due to its well-known properties, making it a reasonable approximation for soft tissue in many cases. However, it is important to acknowledge that tissues do vary in their composition and structure, which can lead to differences in their mean excitation potential compared to water. While water provides a good approximation for many soft tissues, particularly for general radiotherapy calculations, specific tissues like bone, fat, or muscle may have different mean excitation potentials. This variation can be important when high precision is required. In other words, the I-value is either overestimated or underestimated in many cases, depending on the tested tissue. Despite the fact that these errors are small, they may influence dose delivery, increasing the risk of radiation-induced side effects due to imperfect targeting of the tumor. This means there is a possibility of exposing surrounding healthy tissues to radiation.
Nonetheless, for most practical purposes, water remains a valid and widely accepted reference for the mean excitation potential in tissue modeling. Further discussions on this topic could indeed provide valuable insights, especially in contexts where tissue-specific properties are critical. Useful tables for the
I-values of various biological materials are presented in [
35].
It is also important to note that the use of Monte Carlo simulations leads to a more accurate solution compared to the numerical integration of the Bethe equation. This is because Monte Carlo simulations can account for proton-nucleus scattering events that the Bethe equation does not consider. For these reasons, small deviations in range calculations may arise when using our model compared to Monte Carlo simulations or real experiments. However, for a Monte Carlo solution to be truly effective, it requires an acceptable definition of the Monte Carlo range, as well as a deep understanding of the Monte Carlo method and the nuances of setting all necessary parameters. On the other hand, the proposed approach, based on solving the Bethe equation, provides valuable insights into the physical behavior of the system, particularly given the complexities associated with achieving a professional and realistic Monte Carlo solution. Therefore, despite not providing the ‘exact’ solution, the error is minor, and the method is extremely simple and easily applicable by practitioners in radiotherapy.
In addition, an interesting question that arises is why polynomial fittings were used for the
and
parameters, as described in Equations (16) and (17). Given the approximation of a limiting value for energies greater than 250 MeV, a more rational approach would be to invoke exponential functions combined with a constant term in both cases. The reason for not employing the latter approach is that only third-degree polynomials provided a perfect fit to the data within the range of 100 MeV to 250 MeV. The errors if using exponential fits would be larger than the ones presented in
Section 2 and
Section 3. Therefore, using the approach presented in this paper, we successfully achieved precise and straightforward solutions for determining the range of protons in a medium. Additionally, it is important to note that this paper focused on protons traveling through a medium across the entire range of energies relevant to biomedical applications. The same methods can also be applied to other charged particles, such as carbon ions [
36]. For the case of carbon ions, the atomic number z is 6, and the particle’s mass is
[
20]. Therefore, the parameter
A in Equation (3) is equal to:
. To further test the model proposed in this paper, we present
Table 9, which compares range calculations in H
2O for carbon ions with experimental results and results for the PSTAR database.
Future research will focus on further experimental validation in clinical settings, such as proton therapy centers. Since the model proposed in this paper has been proven to be accurate, simple, and free from computational demands or high processing times, it is likely to become a valuable tool in real clinical applications.