Genomic Innovations and Marker-Assisted Breeding in Echinacea Species: Insights and Applications
Abstract
1. Introduction
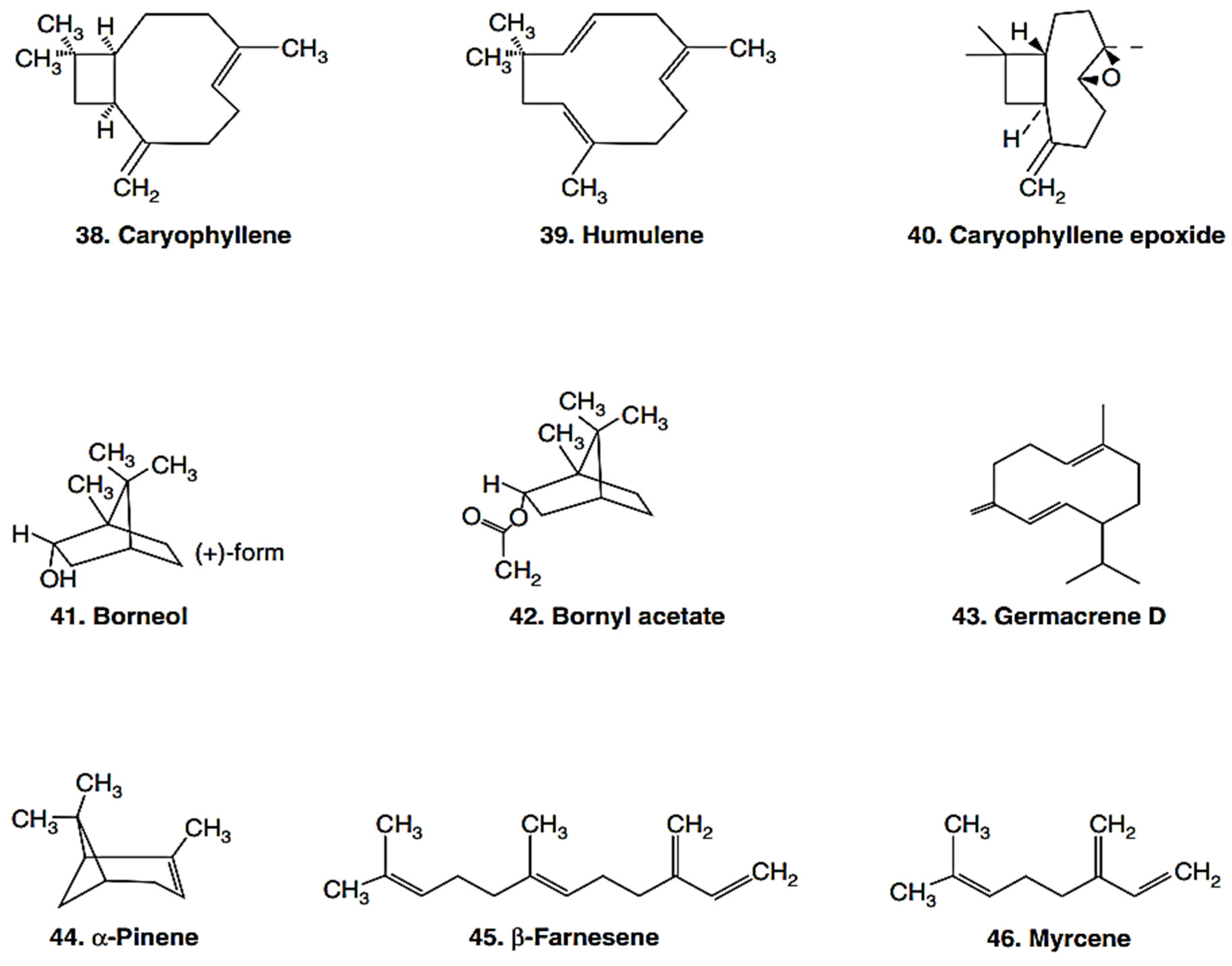
2. Factors That Affect Echinacea Phytochemistry
3. Genetic Engineering and Transgenic Echinacea
4. Molecular Markers and Genomic Resources in Echinacea
5. Advances in Echinacea Breeding Programs
6. Advances in Gene-Editing and Genomic Tools in Echinacea
7. Conclusions and Future Perspective on Echinacea Research
Funding
Data Availability Statement
Conflicts of Interest
References
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [PubMed]
- Huntley, A.L.; Coon, J.T.; Ernst, E. The safety of herbal medicinal products derived from Echinacea species: A systematic review. Drug Saf. 2005, 28, 387–400. [Google Scholar] [PubMed]
- Ault, J.R. Coneflower: Echinacea species. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Springer: Dordrecht, The Netherlands, 2007; pp. 801–824. [Google Scholar]
- Fu, R.; Zhang, P.; Deng, Z.; Jin, G.; Guo, Y.; Zhang, Y. Diversity of antioxidant ingredients among Echinacea species. Ind. Crops Prod. 2021, 170, 113699. [Google Scholar]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Phcog. Rev. 2015, 9, 63. [Google Scholar]
- Vlasheva, M.; Katsarova, M.; Dobreva, A.; Dzhurmanski, A.; Denev, P.; Dimitrova, S. Echinacea species cultivated in Bulgaria as a source of chicoric and caftaric acids. Agronomy 2024, 14, 2081. [Google Scholar] [CrossRef]
- Karadağ, A.E.; Baydar, R.; Kırcı, D. Phytochemical quality analysis of commercial preparations containing Echinacea purpurea. Eur. J. Life Sci. 2024, 3, 45–54. [Google Scholar]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Potassium homeostasis and signaling as a determinant of Echinacea species tolerance to salinity stress. Environ. Exp. Bot. 2023, 206, 105148. [Google Scholar]
- Billah, M.M.; Hosen, M.B.; Khan, F.; Niaz, K. Echinacea. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 205–210. [Google Scholar]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Ferreira, I.C. Echinacea purpurea (L.) Moench: Chemical characterization and bioactivity of its extracts and fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef]
- Zaushintsena, A.V.; Milentyeva, I.; Babich, O.; Noskova, S.Y.; Kiseleva, T.F.; Popova, D.G.; Lukin, A. Quantitative and qualitative profile of biologically active substances extracted from purple echinacea (Echinacea purpurea L.) growing in the Kemerovo region: Functional foods application. Foods Raw Mater. 2019, 7, 84–92. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and pharmacological properties. A review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Salgado-Salazar, C.; Romberg, M.K.; Hudelson, B. Plasmopara echinaceae, a new species of downy mildew affecting cone flowers (Echinacea purpurea) in the United States. Fungal Syst. Evol. 2023, 12, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Choirunnisa, J.P.; Widiyastuti, Y.; Sakya, A.T. Morphological characteristics and flavonoid accumulation of Echinacea purpurea cultivated at various salinity. Biodiversitas J. Biol. Divers. 2021, 22, 53–62. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, H.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Echinacea in hepatopathy: A review of its phytochemistry, pharmacology, and safety. Phytomedicine 2021, 87, 153572. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R. Chemistry, pharmacology and clinical applications of Echinacea products. In Herbs, Botanicals and Teas; Technomic Publishing Company: Lancaster, PA, USA, 2000; Volume 5, pp. 45–73. [Google Scholar]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea reduces the oxidant stress in vitro and in vivo. Int. J. Bio Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef]
- Samuel, D.S.; Priyadarshoni, S.P. Echinacea purpurea—A potent medicinal herb. Drug Invent. Today 2019, 11, 225–232. [Google Scholar]
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxic. 2020, 137, 111125. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Rahimi, A. Improving growth properties and phytochemical compounds of Echinacea purpurea (L.) medicinal plant using novel nitrogen slow-release fertilizer under greenhouse conditions. Sci. Rep. 2020, 10, 13842. [Google Scholar] [CrossRef]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van den Ende, W. Fructans as immunomodulatory and antiviral agents: The case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef]
- Woelkart, K.; Bauer, R. The role of alkamides as an active principle of Echinacea. Planta Med. 2007, 73, 615–623. [Google Scholar] [CrossRef]
- Hinz, B.; Woelkart, K.; Bauer, R. Alkamides from Echinacea inhibits cyclooxygenase-2 activity in human neuroglioma cells. Biochem. Biophys. Res. Commun. 2007, 360, 441–446. [Google Scholar] [CrossRef] [PubMed]
- He, X.G.; Lin, L.Z.; Bernart, M.W.; Lian, L.Z. Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. 1998, 815, 205–211. [Google Scholar] [CrossRef]
- Miller, S.C.; Yu, H.C. Popularity, Diversity, and Quality of Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 143–166. [Google Scholar]
- Chen, Y.L.; Sung, J.M.; Lin, S.D. Effect of extraction methods on the active compounds and antioxidant properties of ethanolic extracts of Echinacea purpurea flower. Am. J. Plant Sci. 2015, 6, 201–211. [Google Scholar] [CrossRef]
- Capek, P.; Šutovská, M.; Kocmálová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Chemical and pharmacological profiles of Echinacea complex. Int. J. Biol. Macromol. 2015, 79, 388–391. [Google Scholar] [CrossRef]
- Yildiz, E.; Karabulut, D.; Yesil-Celiktas, O. A bioactivity based comparison of Echinacea purpurea extracts obtained by various processes. J. Supercrit. Fluids 2014, 89, 8–15. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Park, S.Y.; Paek, K.Y. Biotechnological production of caffeic acid derivatives from cell and organ cultures of Echinacea species. Appl. Microbiol. Biotechnol. 2014, 98, 7707–7717. [Google Scholar] [CrossRef]
- Hudaib, M.; Cavrini, V.; Bellardi, M.G.; Rubies-Autonell, C. Characterization of the essential oils of healthy and virus-infected Echinacea purpurea (L.) Moench Plants. J. Essent. Oil Res. 2002, 14, 427–430. [Google Scholar] [CrossRef]
- Ahmadi, F.; Samadi, A.; Sepehr, E.; Rahimi, A.; Shabala, S. Morphological, phytochemical, and essential oil changes induced by different nitrogen supply forms and salinity stress in Echinacea purpurea L. Biocatal. Agric. Biotechnol. 2022, 43, 102396. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Kirpotina, L.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Lisonbee, B.L.; Black, J.L.; Quinn, M.T. Volatile composition, antimicrobial activity, and in vitro innate immunomodulatory activity of Echinacea purpurea (L.) Moench essential oils. Molecules 2023, 28, 7330. [Google Scholar] [CrossRef]
- Soltanbeigi, A.; Maral, H. Agronomic yield and essential oil properties of Purple Coneflower (Echinacea purpurea L. Moench) with different nutrient applications. Chil. J. Agric. Anim. Sci. 2022, 38, 164–175. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Volatile components of roots, stems, leaves, and flowers of Echinacea species. J. Agric. Food Chem. 1999, 47, 3081–3085. [Google Scholar] [PubMed]
- Bruni, R.; Brighenti, V.; Caesar, L.K.; Bertelli, D.; Cech, N.B.; Pellati, F. Analytical methods for the study of bioactive compounds from medicinally used Echinacea species. J. Pharm. Biomed. Anal. 2018, 160, 443–477. [Google Scholar] [PubMed]
- Hudaib, M.; Fiori, J.; Bellardi, M.G.; Rubies-Autonell, C.; Cavrini, V. GC–MS analysis of the lipophilic principles of Echinacea purpurea and evaluation of cucumber mosaic cucumovirus infection. J. Pharm. Biomed. Anal. 2002, 29, 1053–1060. [Google Scholar]
- Wang, H.G.; Hou, J.Z.; Chen, S.Z.; Ai, T.M. Studies on the chemical constituents of essential oil from Echinacea purpurea. J. Chin. Med. Mater. 2002, 25, 477–478. [Google Scholar]
- Mirjalili, M.H.; Salehi, P.; Badi, H.N.; Sonboli, A. Volatile constituents of the flowerheads of three Echinacea species cultivated in Iran. Flavour Fragr. J. 2006, 21, 355–358. [Google Scholar]
- Murch, S.J.; Peiris, S.E.; Shi, W.L.; Zobayed, S.M.A.; Saxena, P.K. Genetic diversity in seed populations of Echinacea purpurea controls the capacity for regeneration, route of morphogenesis and phytochemical composition. Plant Cell Rep. 2006, 25, 522–532. [Google Scholar]
- Koopman, W.J. Phylogenetic signal in AFLP data sets. Syst. Biol. 2005, 54, 197–217. [Google Scholar]
- Nieri, P.; Adinolfi, B.; Morelli, I.; Breschi, M.C.; Simoni, G.; Martinotti, E. Genetic characterization of the three medicinal Echinacea species using RAPD analysis. Planta Medica 2003, 69, 685–686. [Google Scholar]
- Qu, L.; Chen, Y.; Wang, X.; Scalzo, R.; Davis, J.M. Patterns of variation in alkamides and cichoric acid in roots and aboveground parts of Echinacea purpurea (L.) Moench. Hortscience Publ. Am. Soc. Hortic. Science 2005, 40, 1239. [Google Scholar]
- Coker, P.S.; Camper, N.D. In vitro culture of Echinacea purpurea L. J. Herbs Spices Med. Plants 2000, 7, 1–7. [Google Scholar]
- Perry, N.B.; Burgess, E.J.; Glennie, V.L. Echinacea standardization: Analytical methods for phenolic compounds and typical levels in medicinal species. J. Agric. Food Chem. 2001, 49, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.L.; Wills, R.B.H. Factors affecting the extraction of alkylamides and cichoric acid during ethanolic processing of Echinacea purpurea (L.) Moench. Aust. J. Exp. Agric. 2000, 40, 873–877. [Google Scholar] [CrossRef]
- Caruso, T.J.; Gwaltney Jr, J.M. Treatment of the common cold with echinacea: A structured review. Clin. Infect. Dis. 2005, 40, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Naser, B.; Lund, B.; Henneicke-von Zepelin, H.H.; Köhler, G.; Lehmacher, W.; Scaglione, F. A randomized, double-blind, placebo-controlled, clinical dose–response trial of an extract of Baptisia, Echinacea and Thuja for the treatment of patients with common cold. Phytomedicine 2005, 12, 715–722. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar]
- Hosseinpour, M.; Ebadi, A.; Habibi, H.; Nabizadeh, E.; Jahanbakhsh, S. Enhancing enzymatic and nonenzymatic response of Echinacea purpurea by exogenous 24-epibrassinolide under drought stress. Ind. Crops Prod. 2020, 146, 112045. [Google Scholar] [CrossRef]
- Montanari, M.; Degl’Innocenti, E.; Maggini, R.; Pacifici, S.; Pardossi, A.; Guidi, L. Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food Chem. 2008, 107, 1461–1466. [Google Scholar] [CrossRef]
- Lee, K.M.; Hussaini, Q.; Ke, S.; Lee, C.W. Effects of Salinity, Temperature, and pH on Germination of Echinacea angustifolia Seeds. HortScience 1995, 30, 869F-869. [Google Scholar] [CrossRef]
- Livesey, J.; Awang, D.V.C.; Arnason, J.T.; Letchamo, W.; Barrett, M.; Pennyroyal, G. Effect of temperature on stability of marker constituents in Echinacea purpurea root formulations. Phytomedicine 1999, 6, 347–349. [Google Scholar]
- Li, T.S.; Wardle, D.A. Effects of root drying temperature and moisture content on the levels of active ingredients in Echinacea roots. J. Herbs Spices Med. Plants 2001, 8, 15–22. [Google Scholar] [CrossRef]
- Hevia, F.; Melín, P.; Berti, M.; Fischer, S.; Pinochet, C. Effect of drying temperature and air speed on cichoric acid and alkylamide content of Echinacea purpurea. Acta Hortic. 2001, 576, 321–325. [Google Scholar] [CrossRef]
- Senchina, D.S.; McCann, D.A.; Asp, J.M.; Johnson, J.A.; Cunnick, J.E.; Kaiser, M.S.; Kohut, M.L. Changes in immunomodulatory properties of Echinacea spp. root infusions and tinctures stored at 4 C for four days. Clin. Chim. Acta 2005, 355, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chang, K.F.; Hwang, S.F.; Callan, N.W.; Howard, R.J.; Blade, S.F. Biological control of Pythium damping-off in Echinacea angustifolia with Trichoderma species/Biologische Bekämpfung der Pythium-Umfallkrankheit an Echinacea angustifolia mit Trichoderma-Spezies. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2004, 111, 126–136. [Google Scholar]
- Pavlović, S.; Stojanović, S.; Starović, M. Current study on the parazitic microorganisms of the medicinal and aromatic plants in Serbia. In Proceedings of the 4th Conference on Medicinal and Aromatic Plants of South-East European Countries, Iaşi, Romania, 28–31 May 2006. [Google Scholar]
- Dunich, A.A.; Mishchenko, L.T. Purple coneflower viruses: Species diversity and harmfulness. Biopolym. Cell 2015, 31, 15–28. [Google Scholar] [CrossRef]
- Faehnrich, B.; Franz, C.; Nemaz, P.; Kaul, H.P. Medicinal plants and their secondary metabolites—State of the art and trends in breeding, analytics and use in feed supplementation—With special focus on German chamomile. J. Appl. Bot. Food Qual. 2021, 94, 61–74. [Google Scholar]
- Moraes, R.M.; Sumiyanto, J.; Lata, H.; Tamta, H.; Pugh, N.D.; Wu, X.M.; Pasco, D.S. In vitro Monocyte Activity of Echinacea purpurea: Endophytic Bacteria is Affected by the Host’s Genetic Diversity and Harvest Timing. Planta Med. 2009, 75, P-3. [Google Scholar] [CrossRef]
- Iakab, M.; Domokos, E.; Benedek, K.; Molnár, K.; Kentelky, E.; Buta, E.; Dulf, F.V. The Importance of Mycorrhizal Fungi in the Development and Secondary Metabolite Production of Echinacea purpurea and Relatives (Asteraceae): Current Research Status and Perspectives. Horticulturae 2022, 8, 1106. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, L.P.; Chen, B.D.; Hao, Z.P.; Wang, J.Y.; Huang, L.Q.; Zhang, Y. Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: Current research status and prospectives. Mycorrhiza 2013, 23, 253–265. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Gohari, G.; Fotopoulos, V. Simultaneous application of titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles ameliorates lead (Pb) stress effects in medicinal plant Echinacea purpurea (L.) Moench. Plant Stress 2024, 13, 100546. [Google Scholar] [CrossRef]
- Thomsen, M.O. The impact of cultivation techniques and induced stress on bioactive compounds in Echinacea species. J. Funct. Foods 2012, 5, 125–137. [Google Scholar]
- Sheshbahreh, M.J.; Dehnavi, M.M.; Salehi, A.; Bahreininejad, B. Physiological and yield responses of purple coneflower (Echinacea purpurea (L.) Moench) to nitrogen sources at different levels of irrigation. Physiol. Mol. Biol. Plants 2019, 25, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. Hairy root culture an alternative for bioactive compound production from medicinal plants. Curr. Pharm. Biotechnol. 2021, 22, 136–149. [Google Scholar] [PubMed]
- Bruce, D.; Bruce, A. Engineering Genesis: Ethics of Genetic Engineering in Non-Human Species; Routledge: London, UK, 2014. [Google Scholar]
- Wetzel, R. Applications of Recombinant DNA Technology: The new methods for precise manipulation of genetic material have made possible the transfer of genes between organisms, opening new avenues for basic and applied biochemical research. Am. Sci. 1980, 68, 664–675. [Google Scholar] [PubMed]
- Bradford, K.J.; Van Deynze, A.; Gutterson, N.; Parrott, W.; Strauss, S.H. Regulating transgenic crops sensibly: Lessons from plant breeding, biotechnology and genomics. Nat. Biotechnol. 2005, 23, 439–444. [Google Scholar]
- Lacroix, B.; Citovsky, V. Pathways of DNA transfer to plants from Agrobacterium tumefaciens and related bacterial species. Annu. Rev. Phytopathol. 2019, 57, 231–251. [Google Scholar]
- Setlow, J.K. (Ed.) Genetic Engineering: Principles and Methods; Springer Science & Business Media: New York, NY, USA, 2012; Volume 13. [Google Scholar]
- Potrykus, I. Gene transfer to plants: Assessment of published approaches and results. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 205–225. [Google Scholar] [CrossRef]
- Wang, H.M.; Jeng, S.T.; To, K.Y. In vitro regeneration, Agrobacterium-mediated transformation, and genetic assay of chalcone synthase in the medicinal plant Echinacea pallida. Plant Cell Tissue Organ. Cult. 2017, 130, 117–130. [Google Scholar]
- Koroch, A.; Kapteyn, J.; Juliani, H.R.; Simon, J.E. In vitro regeneration and Agrobacterium transformation of Echinacea purpurea leaf explants. In Trends New Crops New Uses; ASHS Press: Alexandria, VA, USA, 2002; pp. 522–526. [Google Scholar]
- Abbasi, B.H.; Saxena, P.K.; Murch, S.J.; Liu, C.Z. Echinacea biotechnology: Challenges and opportunities. In Vitro Cell. Dev. Biol. Plant 2007, 43, 481–492. [Google Scholar] [CrossRef]
- Saito, K. Genetic engineering in tissue culture of medicinal plants. Plant Tissue Cult. Lett. 1993, 10, 1–8. [Google Scholar]
- Parsons, J.L.; Cameron, S.I.; Harris, C.S.; Smith, M.L. Echinacea biotechnology: Advances, commercialization, and future considerations. Pharm. Biol. 2018, 56, 485–494. [Google Scholar]
- Trypsteen, M.; Van Lijsebettens, M.; Van Severen, R.; Van Montagu, M. Agrobacterium rhizogenes-mediated transformation of Echinacea purpurea. Plant Cell Rep. 1991, 10, 85–89. [Google Scholar] [PubMed]
- Lakshmanan, P.; Danesh, M.; Taji, A. Production of four commercially cultivated Echinacea species by different methods of in vitro regeneration. J. Hortic. Sci. Biotechnol. 2002, 77, 158–163. [Google Scholar]
- Demirci, T.; Akçay, U.Ç.; Göktürk Baydar, N. Physical and biochemical differences in Agrobacterium rhizogenes-mediated transgenic hairy root lines of Echinacea purpurea. In Vitro Cell. Dev. Biol. Plant 2020, 56, 875–881. [Google Scholar]
- Changxing, L.; Galani, S.; Hassan, F.U.; Rashid, Z.; Naveed, M.; Fang, D.; Jianhua, L. Biotechnological approaches to the production of plant-derived promising anticancer agents: An update and overview. Biomed. Pharmacother. 2020, 132, 110918. [Google Scholar]
- Van Etten, M.; Lee, K.M.; Chang, S.M.; Baucom, R.S. Parallel and nonparallel genomic responses contribute to herbicide resistance in Ipomoea purpurea, a common agricultural weed. PLoS Genet. 2020, 16, e1008593. [Google Scholar]
- Choffe, K.L.; Murch, S.J.; Saxena, P.K. Regeneration of Echinacea purpurea: Induction of root organogenesis from hypocotyl and cotyledon explants. Plant Cell Tissue Organ Cult. 2000, 62, 227–234. [Google Scholar]
- Wang, H.M.; To, K.Y. Agrobacterium-mediated transformation in the high-value medicinal plant Echinacea purpurea. Plant Sci. 2004, 166, 1087–1096. [Google Scholar]
- Khalili, S.; Moieni, A.; Abdoli, M. Influence of different strains of Agrobacterium rhizogenes, culture medium, age and type of explant on hairy root induction in Echinacea angustifolia. Iran. J. Genet. Plant Breed. 2014, 3, 49–56. [Google Scholar]
- Demirci, T.; Çelikkol Akçay, U.; Göktürk Baydar, N. Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench. Acta Physiol. Plant 2020, 42, 66. [Google Scholar]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef]
- Tan, R.F.; Tao, J.; Li, L. Genetic transformation of Ipomoea purpurea mediated by Agrobacterium rhizogenes. In Floriculture, Ornamental and Plant Biotechnology; Global Science Books: East Sussex, UK, 2007; Volume 1, pp. 131–135. [Google Scholar]
- Kapteyn, J.; Goldsbrough, P.; Simon, J. Genetic relationships and diversity of commercially relevant Echinacea species. Theor. Appl. Genet. 2002, 105, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vassilevska-Ivanova, R.; Kraptchev, B.; Stancheva, I.; Geneva, M.; Iliev, I.; Georgiev, G. Utilization of related wild species (Echinacea purpurea) for genetic enhancement of cultivated sunflower (Helianthus annuus L.). Turk. J. Agric. For. 2014, 38, 15–22. [Google Scholar]
- Taha, H.S.; Lashin, I.I.; Sharaf, A.M.; Farghal, I.I.; El-Bahr, M.K. In vitro studies and RAPD analysis of Echinacea angustifolia. J. Am. Sci. 2010, 6, 54–61. [Google Scholar]
- Jedrzejczyk, I. Genome size and SCoT markers as tools for identification and genetic diversity assessment in Echinacea genus. Ind. Crops Prod. 2020, 144, 112055. [Google Scholar]
- Wolf, H.T.; Zündorf, I.; Winckler, T.; Bauer, R.; Dingermann, T. Characterization of Echinacea species and detection of possible adulterations by RAPD analysis. Planta Med. 1999, 65, 773–774. [Google Scholar]
- Chen, C.L.; Chuang, S.J.; Chen, J.J.; Sung, J.M. Using RAPD markers to predict polyphenol content in aerial parts of Echinacea purpurea plants. J. Sci. Food Agric. 2009, 89, 2137–2143. [Google Scholar]
- Aziz, A.N.; Sauve, R.J. Genetic mapping of Echinacea purpurea via individual pollen DNA fingerprinting. Mol. Breed. 2008, 21, 227–232. [Google Scholar]
- Still, D.W.; Kim, D.H.; Aoyama, N. Genetic variation in Echinacea angustifolia along a climatic gradient. Ann. Bot. 2005, 96, 467–477. [Google Scholar]
- Ahmad, A.; Wang, J.D.; Pan, Y.B.; Sharif, R.; Gao, S.J. Development and use of simple sequence repeats (SSRs) markers for sugarcane breeding and genetic studies. Agronomy 2018, 8, 260. [Google Scholar] [CrossRef]
- Mechanda, S.M.; Baum, B.R.; Johnson, D.A.; Arnason, J.T. Analysis of diversity of natural populations and commercial lines of Echinacea using AFLP. Can. J. Bot. 2004, 82, 461–484. [Google Scholar]
- Vural, H.C.; Dageri, A. Optimization of DNA isolation for RAPDPCR analysis of selected (Echinaceae purpurea L. Moench) medicinal plants of conservation concern from Turkey. Afr. J. Biotechnol. 2009, 8, 16–19. [Google Scholar]
- Lema-Rumińska, J.; Kulus, D.; Tymoszuk, A.; Varejão, J.M.; Bahcevandziev, K. Profile of secondary metabolites and genetic stability analysis in new lines of Echinacea purpurea (L.) Moench micropropagated via somatic embryogenesis. Ind. Crops Prod. 2019, 142, 111851. [Google Scholar]
- Spooner, D.M.; van Treuren, R.; De Vicente, M.C. Molecular Markers for Genebank Management; Bioversity International: Rome, Italy, 2005; No. 10. [Google Scholar]
- Cui, C.; Shu, W.; Li, P. Fluorescence in situ hybridization: Cell-based genetic diagnostic and research applications. Front. Cell Dev. Biol. 2016, 4, 89. [Google Scholar]
- Pastick, J. Fitness and Morphological Variation in Native, Non-Native, and Hybrid Echinacea Seedlings. Ph.D. Thesis, Lake Forest College, Lake Forest, IL, USA, 2014. [Google Scholar]
- Ganie, S.H.; Upadhyay, P.; Das, S.; Sharma, M.P. Authentication of medicinal plants by DNA markers. Plant Gene 2015, 4, 83–99. [Google Scholar]
- Raclariu, A.C.; Ţebrencu, C.E.; Ichim, M.C.; Ciupercǎ, O.T.; Brysting, A.K.; de Boer, H. What’s in the box? Authentication of Echinacea herbal products using DNA metabarcoding and HPTLC. Phytomedicine 2018, 44, 32–38. [Google Scholar]
- Sharma, N.; Kaur, R. Database Derived Microsatellite Markers (SSRs) of Stevia rebaudiana for Cross-transferability Testing Across Species in Family Asteraceae. Adv. Res. 2017, 9, 1–9. [Google Scholar]
- Chuang, S.J.; Chen, C.L.; Chen, J.J.; Sung, J.M. Using bulked AFLP analysis to assess genetic diversity in Echinacea species. Sci. Hortic. 2010, 124, 400–404. [Google Scholar]
- Lin-na, H.A.N. The morphological markers of different phenotypes Echinacea purpurea. J. Appl. Pharm. Sci. 2013, 3, 078–080. [Google Scholar]
- Korotkikh, I.N.; Baleev, D.N.; Morozov, A.I.; Mizina, P.G.; Sidelnikov, N.I. Breeding of medicinal and essential oil crops in VILAR: Achievements and prospects. Vavilov J. Genet. Breed. 2021, 25, 433. [Google Scholar]
- Ault, J. Breeding and development of new ornamental plants from North American native taxa. Acta Hortic. 2002, 624, 37–42. [Google Scholar]
- Muranty, H.; Jorge, V.; Bastien, C.; Lepoittevin, C.; Bouffier, L.; Sanchez, L. Potential for marker-assisted selection for forest tree breeding: Lessons from 20 years of MAS in crops. Tree Genet. Genomes 2014, 10, 1491–1510. [Google Scholar]
- Sharma, M.; Koul, A.; Sharma, D.; Kaul, S.; Swamy, M.K.; Dhar, M.K. Metabolic engineering strategies for enhancing the production of bioactive compounds from medicinal plants. In Natural Bio-Active Compounds: Volume 3: Biotechnology, Bioengineering, and Molecular Approaches; Springer: Singapore, 2019; pp. 287–316. [Google Scholar]
- Kapteyn, J.; Simon, J.E. The use of RAPDs for assessment of identity, diversity, and quality of Echinacea. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 509–513. [Google Scholar]
- Vural, H.C. Assessment of genetic diversity of echinacea species growing in Turkey using molecular markers. Molecules 2014, 3, 116–124. [Google Scholar]
- Thoen, R.D.; Southgate, A.; Kiefer, G.; Shaw, R.G.; Wagenius, S. The conservation value of small population remnants: Variability in inbreeding depression and heterosis of a perennial herb, the narrow-leaved purple coneflower (Echinacea angustifolia). J. Heredity 2024, 116, 24–33. [Google Scholar]
- Russi, L.; Moretti, C.; Raggi, L.; Albertini, E.; Falistocco, E. Identifying commercially relevant Echinacea species by AFLP molecular markers. Genome 2009, 52, 912–918. [Google Scholar]
- Waananen, A. Fitness Consequences of Pollen Movement and Its Dependence on Spatiotemporal Isolation: Field Studies in Echinacea angustifolia. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2023; pp. 52–63. [Google Scholar]
- Biswas, P.; Kumar, N. Application of molecular markers for the assessment of genetic fidelity of in vitro raised plants: Current status and future prospects. Molecules 2023, 5, 233–256. [Google Scholar]
- Shadab, A.; Saeed, M.; Khan, M.Z.; Parvaiz, A. Use of Molecular Markers for Diagnostic purposes. J. Mol. Biol. Biotechnol. 2018, 5, 132–149. [Google Scholar]
- Baum, B.R.; Mechanda, S.; Livesey, J.F.; Binns, S.E.; Arnason, J.T. Predicting quantitative phytochemical markers in single Echinacea plants or clones from their DNA fingerprints. Phytochemistry 2001, 56, 543–549. [Google Scholar]
- Blasini, D.; Wagenius, S. Assessment of the effects of the introduction of non-native echinacea species in the pollination of native Echinacea angustifolia in Western Minnesota. Assessment 2013, 5, 121–130. [Google Scholar]
- Van Gaal, T.M.; Galatowitsch, S.M.; Strefeler, M.S. Ecological consequences of hybridization between a wild species (Echinacea purpurea) and related cultivar (E. purpurea ‘White Swan’). Sci. Hortic. 1998, 76, 73–88. [Google Scholar]
- Letchamo, W.; Polydeonny, L.V.; Gladisheva, N.O.; Arnason, T.J.; Livesey, J.; Awang, D.V.C. Factors affecting Echinacea quality. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 514–521. [Google Scholar]
- Ng, J.Y.; Chiong, J.D.; Liu, M.Y.M.; Pang, K.K. Characteristics of the Echinacea Spp. research literature: A bibliometric analysis. Eur. J. Integr. Med. 2023, 57, 102216. [Google Scholar] [CrossRef]
- Jonah, P.M.; Bello, L.L.; Lucky, O.; Midau, A.; Moruppa, S.M. The importance of molecular markers in plant breeding programmes. Glob. J. Sci. Front. Res. 2011, 11, 5–12. [Google Scholar]
- Begna, T. Combining ability and heterosis in plant improvement. Open J. Plant Sci. 2021, 6, 108–117. [Google Scholar]
- Devi, B.S.C.; Krishnasreya, M.; Tharani, S.; Elakkiya, M.R. Integrated Molecular Strategies for Enhanced Production of Medicinal Plant-Derived Alkaloids. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 298–313. [Google Scholar]
- Naik, B.J.; Vasamsetti, B.M.K.; Kim, S.C.; Gunti, M.; Swamy, M.K.; Mekapogu, M. Genome editing in medicinal plants using CRISPR/Cas9 tool. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Singapore, 2023; pp. 571–589. [Google Scholar]
- Arun, M.; Halka, J.; Kowsalya, K. CRISPR/Cas9: A novel genetic tool to manipulate plant secondary metabolite pathways. In Genetic Manipulation of Secondary Metabolites in Medicinal Plant; Springer: Singapore, 2023; pp. 45–57. [Google Scholar]
- Bhojiya, A.A.; Joshi, H. Crispr Gene Editing for Secondary Metabolite Production: A Review. In Gene Editing in Plants: CRISPR-Cas and Its Applications; Springer: Singapore, 2024; pp. 437–475. [Google Scholar]
- Borah, A.; Singh, S.; Chattopadhyay, R.; Kaur, J.; Bari, V.K. Integration of CRISPR/Cas9 with multi-omics technologies to engineer secondary metabolite productions in medicinal plant: Challenges and Prospects. Funct. Integr. Genom. 2024, 24, 207. [Google Scholar]
- Chandoliya, R.; Patial, A.; Joshi, S.; Sharma, V.; Joshi, R. Challenges, Advancements, and Opportunities in Genome Editing: A Medicinal Plant Perspective. In Ethnopharmacology and OMICS Advances in Medicinal Plants Volume 2: Revealing the Secrets of Medicinal Plants; Springer Nature Singapore: Singapore, 2024; pp. 403–424. [Google Scholar]
- Hasan, N.; Laskar, R.A.; Farooqui, S.A.; Naaz, N.; Sharma, N.; Budakoti, M.; Bhinda, M.S. Genetic Improvement of Medicinal and Aromatic Plant Species: Breeding Techniques, Conservative Practices and Future Prospects. Crop Des. 2024, 3, 100080. [Google Scholar]
- Rupali, R.; Vidya, N.; Anand, A.V.; Anand, M.; Ramalashmi, K.; Begam, A.K.U.; Arun, M. Enhanced Secondary Metabolite Production by Genome Editing in Medicinal Plants. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 89–100. [Google Scholar]
- Das, S.; Manna, D.; Mondal, T.; Mondal, P. Advanced Systems and Bioreactors for Large-Scale Secondary Metabolite Production in Medicinal Plants. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2024; pp. 341–354. [Google Scholar]
- Bharti, P.K.; Singh, S.K.; Kumari, S. Genetic Manipulation in Medicinal Plants for Enhancement of Secondary Metabolites. In Genetic Manipulation of Secondary Metabolites in Medicinal Plant; Springer: Singapore, 2023; pp. 129–145. [Google Scholar]
- Mehrotra, N.; Khan, S.A. Expanding Horizons: Role of Biotechnology in MAP Research, Production and Utilization. In Medicinal and Aromatic Plants of India Vol. 1; Springer: Cham, Switzerland, 2022; pp. 237–275. [Google Scholar]
- Khan, Z.; Khan, S.H.; Mubarik, M.S.; Sadia, B.; Ahmad, A. Use of TALEs and TALEN technology for genetic improvement of plants. Plant Mol. Biol. Rep. 2017, 35, 1–19. [Google Scholar]
- Dhara, A.K. Role of Herbal Medicines: Management of Lifestyle Diseases; Springer: Singapore, 2023. [Google Scholar]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Salem, M.L.; El-Bahrawy, H.A.; Al-Ashmawy, G.M. In vitro and in vivo studies of the immunomodulatory effect of Echinacea purpurea on dendritic cells. J. Genet. Eng. Biotechnol. 2015, 13, 185–192. [Google Scholar]
- Misra, R.C.; Thimmappa, R.; Bonfill, M. Advances in discoveries of plant phytochemicals. Front. Plant Sci. 2024, 15, 1414150. [Google Scholar]
- McKeown, K.A. A review of preliminary Echinacea genetics and the future potential of genomics. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 29–36. [Google Scholar]
- Pattnaik, S. Next-Generation Sequencing (NGS) for Metabolomics Study in Medicinal Plants Under Stress Condition. In Stress-Responsive Factors and Molecular Farming in Medicinal Plants; Springer: Singapore, 2023; pp. 323–343. [Google Scholar]
- Salami, M.; Heidari, B.; Batley, J.; Wang, J.; Tan, X.L.; Richards, C.; Tan, H. Integration of genome-wide association studies, metabolomics, and transcriptomics reveals phenolic acid-and flavonoid-associated genes and their regulatory elements under drought stress in rapeseed flowers. Front. Plant Sci. 2024, 14, 1249142. [Google Scholar]
- Zhao, J.; Fu, Y.Y.; Park, J.H.; Choi, G.W.; Park, K.Y.; Lee, Y.H.; Chung, I.S. Developing a Dwarf Echinacea purpurea Line by Suppressing DWF3 Expression via RNA Interference. Hortic. Sci. Technol. 2017, 35, 628–636. [Google Scholar] [CrossRef]
- Perry, N.B.; Wills, R.B.; Stuart, D.L. Factors affecting Echinacea quality: Agronomy and processing. In Echinacea; CRC Press: Boca Raton, FL, USA, 2004; pp. 127–142. [Google Scholar]
- Ahmadi, F. Phytochemistry, Mechanisms, and Preclinical Studies of Echinacea Extracts in Modulating Immune Responses to Bacterial and Viral Infections: A Comprehensive Review. Antibiotics 2024, 13, 947. [Google Scholar] [CrossRef]
- Bone, K. Echinacea: What makes it work. Altern. Med. Rev. 1997, 2, 87–93. [Google Scholar]
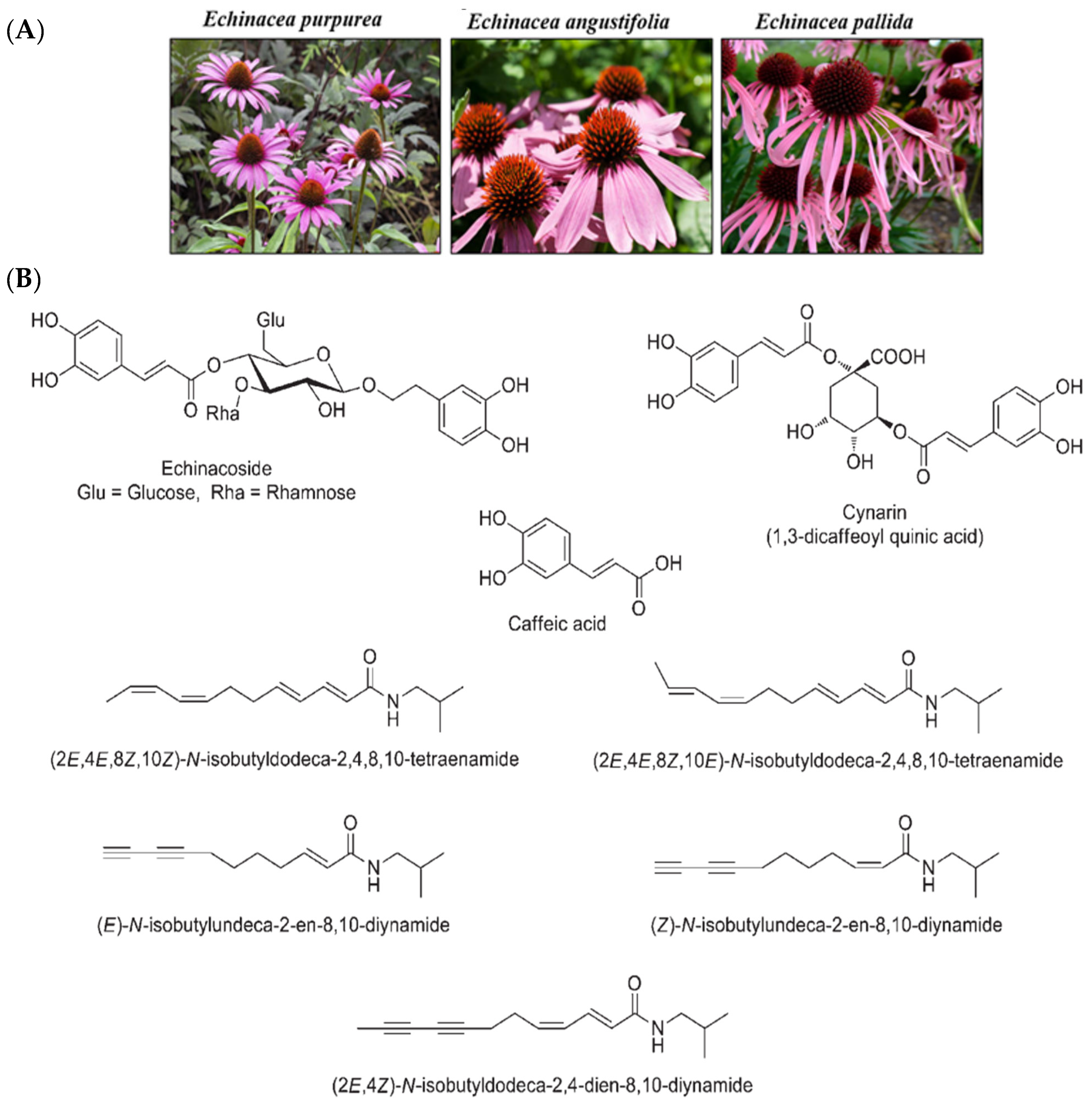
| Alkamide Compounds | Molecular Weight (g/mol) | Echinacea Species |
|---|---|---|
| Undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | 229.32 | E. purpurea, E. angustifolia |
| Undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | 229.32 | E. purpurea |
| Undeca-2E-ene-8,10-diynoic acid isobutylamide | 231.34 | E. purpurea |
| Undeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide | 243.35 | E. purpurea |
| Undeca-2Z,4E-diene-8,10-diynoic acid 2-methylbutylamide | 243.35 | E. purpurea |
| Dodeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | 243.35 | E. purpurea |
| Dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | 243.35 | E. purpurea |
| Dodeca-2E,4E-diene-8,10-diynoic acid isobutylamide | 245.37 | E. purpurea |
| Dodeca-2E,4Z,10E-triene-8-ynoic acid isobutylamide | 245.37 | E. purpurea |
| Dodeca-2E-ene-8,10-diynoic acid isobutylamide | 245.37 | E. angustifolia |
| Dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide | 247.38 | E. angustifolia |
| Dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide | 247.38 | E. purpurea |
| Dodeca-2E,4E,8E,10Z-tetraenoic acid isobutylamide | 249.40 | E. purpurea |
| Dodeca-2E,4E-dienoic acid isobutylamide | 251.41 | E. purpurea |
| Trideca-2E,7Z-diene-8,10-diynoic acid isobutylamide | 257.38 | E. purpurea |
| Dodeca-2E,4Z-diene-8,10-diynoic acid 2-methylbutylamide | 257.38 | E. purpurea |
| Dodeca-2,4,8,10-tetraenoic acid 2-methylbutylamide | 261.41 | E. purpurea |
| Caffeic Acid Conjugates | Flower | Leaf | Rhizome a |
|---|---|---|---|
| Chlorogenic acid (5-O-caffeoylquinic acid) | ++ | ++ | ++ |
| 3,5-O-Dicaffeoylquinic acid | ++ | + | + |
| 4,5-O-Dicaffeoylquinic acid | ++ | + | + |
| Chicoric acid (2,3-O-dicaffeoyltartaric acid) | +++ | +++ | +++ |
| 2-O-Caffeoyl-3-O-feruloyltartaric acid | ? | ++ | ? |
| Caftaric acid (2-O-caffeoyltartaric acid) | ++ | ++ | ++ |
| 2-O-Caffeoyl-3-O-5-[a-carboxy-b-(3,4-dihydroxyphenyl)ethyl]caffeoyltartaric acid | - | ++ | - |
| 2,3-O-Di-5-[a-carboxy-b-(3,4-dihydroxyphenyl)ethyl]caffeoyltartaric acid | - | ++ | - |
| b-(3,4-Dihydroxyphenyl)-ethyl-O-4-O-caffeoyl-b-D-glucopyranoside(desrhamnosylverbascoside) | ++ | + | + |
| b-(3,4-Dihydroxyphenyl)ethyl-O-a-L-rhamnopyranosyl(1Æ3)-4-O-caffeoyl-b-Dglucopyranoside (verbascoside) | ++ | + | + |
| b-(3,4-Dihydroxyphenyl)ethyl-O-a-L-rhamnopyranosyl(1Æ3)-b-Dglucopyranoside(1Æ6)-4-O-caffeoyl-b-D-glucopyranoside (echinacoside) | ++ | + | +++ |
| b-(3,4-Dihydroxyphenyl)ethyl-O-a-L-rhamnopyranosyl(1Æ3)(6-O-caffeoyl-b-Dglucopyranosyl(1Æ6)-4-O-caffeoyl-b-D-glucopyranoside (6-Ocaffeoylechinacoside) | - | - | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, F. Genomic Innovations and Marker-Assisted Breeding in Echinacea Species: Insights and Applications. Sci 2025, 7, 43. https://doi.org/10.3390/sci7020043
Ahmadi F. Genomic Innovations and Marker-Assisted Breeding in Echinacea Species: Insights and Applications. Sci. 2025; 7(2):43. https://doi.org/10.3390/sci7020043
Chicago/Turabian StyleAhmadi, Fatemeh. 2025. "Genomic Innovations and Marker-Assisted Breeding in Echinacea Species: Insights and Applications" Sci 7, no. 2: 43. https://doi.org/10.3390/sci7020043
APA StyleAhmadi, F. (2025). Genomic Innovations and Marker-Assisted Breeding in Echinacea Species: Insights and Applications. Sci, 7(2), 43. https://doi.org/10.3390/sci7020043






