Abstract
Phytophthora capsici is a phytopathogen that affects postharvest bell peppers, as it causes rotting and decreases their commercial value. This study evaluates the efficacy of chitosan as a biocontrol agent against P. capsici on bell peppers using in vitro and in vivo assays. The antifungal activity of chitosan was evaluated at four concentrations (0.5, 1.0, 1.5, and 2.0 g L−1). Its effect on mycelial growth inhibition, sporangial germination, disease incidence and severity, and fruit weight loss was determined. The results show that concentrations of 1.0 g L−1 or higher completely inhibited P. capsici growth and sporangial germination in vitro and reduced disease incidence and severity to 0% in treated fruit. Furthermore, chitosan treatments preserved the fresh and dry weight of the fruit, which prevented postharvest deterioration. This study demonstrates that chitosan is an effective and environmentally friendly alternative for the management of postharvest diseases in bell peppers. This could reduce consumer dependence on synthetic fungicides and preserve fruit quality.
1. Introduction
Bell peppers (Capsicum annuum L.) are of high economic and nutritional value, rich in vitamins, antioxidants, and bioactive compounds, and are widely consumed in fresh and processed forms [1]. However, their high susceptibility to postharvest diseases represents a critical challenge because it negatively affects product quality, shelf life, and economic profit [2,3]. Postharvest diseases can limit the storage period, shelf life, and commercial life of a variety of horticultural products [4]. These diseases cause greater economic losses than those that occur during the growing stage [5].
Phytophthora capsici is a cosmopolitan and hemibiotrophic phytopathogen capable of infecting a wide variety of horticultural crops at different phenological stages, particularly those belonging to the Solanaceae family [6]. This oomycete causes various symptoms, such as root, stem, fruit, and crown rot, in vegetables [7].
The control of P. capsici has historically involved the application of synthetic fungicides including metalaxyl, mefenoxam, cymoxanil, dimethomorph, fluazinam, oxathiapiprolin, zoxamide, and azoxystrobin [3,8]. But P. capsici has adapted to these fungicides [9,10]. Furthermore, application of these products has been shown to cause damage to the environment and human health [11].
In this context, there has been increasing interest in developing more sustainable and environmentally friendly postharvest disease management strategies, among which the use of edible coatings stands out. Edible coatings contain natural antimicrobials that aim to preserve the quality and extend the shelf life of food products [12].
Among the materials used as edible coatings, chitosan has been employed in the postharvest of agricultural products [13,14]. Chitosan has the potential to interfere with spore germination, germ tube elongation, and the mycelial growth of phytopathogenic fungi [14,15]. In addition, chitosan induces defence responses: it activates lytic enzymes, produces pathogenesis-related proteins (PR), generates reactive oxygen species, and synthesises phytoalexins, among other mechanisms [16,17]. Chitosan coatings on fruit reduce respiration rates, prolong storage periods, maintain firmness, and control microbial growth [12,18].
Previous studies have reported that the application of chitosan achieved inhibitions of 90 and 95% in the mycelial growth of P. capsici in vitro [19,20]. In addition, its ability to induce defence mechanisms in fruits has been reported in tomatoes, where it increased the enzymatic activity of peroxidases and polyphenol oxidases, reducing the incidence of postharvest diseases [21]. It has been observed that the application of chitosan in fruits, such as strawberries and apples, reduces weight loss and maintains firmness during storage [22,23]. In the case of bell peppers, the application of 1% chitosan reduced the incidence of Colletotrichum capsici by 73.12% [24]. Similarly, its application in bananas and apples has shown a significant reduction in the incidence of postharvest diseases [17,25].
Despite the demonstrated potential of chitosan in the postharvest stage, few studies have evaluated its effectiveness in the management of phytopathogens, such as Phytophthora capsici in bell pepper fruits. This natural biopolymer presents antimicrobial and protective properties that could contribute significantly to the reduction in diseases and the preservation of fruit quality throughout storage. This study aims to determine the effect of chitosan in vitro and in vivo on the growth and development of P. capsici, as well as its impact on fruit preservation.
2. Materials and Methods
2.1. Isolation of Phytophthora spp. in Bell Peppers
The samples of bell peppers with symptoms of rot were taken from crops at La María Experimental Campus, which belongs to the Facultad de Ciencias Agrarias y Forestales at the Universidad Técnica de Quevedo, Ecuador. The site is located 7.5 kilometres along the Quevedo–El Empalme road, with geographical coordinates 01°06′24″ S, 79°29′70″ W, and an altitude of 75 m above sea level.
The bell peppers were stored in sterile plastic bags before they were transported in refrigerated conditions to the microbiology laboratory at La María Experimental Campus for analysis. The bell peppers were disinfected with 2% sodium hypochlorite for 1 min, then rinsed three times with sterile distilled water and dried on absorbent paper under sterile conditions. Subsequently, tissue fragments (5 mm) were taken from the advancing zone of the lesion and were seeded in Petri dishes with V8-agar (V8) selective medium supplemented with ampicillin (0.25 g L−1) and rifampicin (0.01 g L−1) to promote the growth of Phytophthora spp. and prevent bacterial growth. Plates were incubated at 25 °C for 5 days in the dark.
2.2. Morphological Identification of Phytophthora spp.
Phytophthora spp. was identified by assessing colony morphology on V8 plates incubated at 25 °C for 5 days. Colony morphology, such as colony shape, texture, and colour, was determined, as were the characteristics of structures, such as the shape of sporangia, chlamydospores, pedicels, papillae, and sporangiophore, using an Olympus microscope (Olympus Corporation, Tokyo, Japan) at 40× magnification [26].
2.3. Pathogenicity Test of Phytophthora spp.
The most virulent isolate of P. capsici was determined from the isolates obtained. The bell peppers were disinfected with 1% sodium hypochlorite for 3 min, then rinsed with sterile distilled water and dried. Then, a superficial 2 mm diameter incision was made in the epidermis, where 20 μL of each fungus was placed at a concentration of 1 × 106; zoospores mL−1. The control was inoculated with sterile distilled water. The samples were placed in humid chambers in the form of 18 × 26 cm, 0.05 mm thick plastic bags. These bags were placed in corrugated cardboard boxes that measured 30 × 25 × 15 cm and were stored at 26 °C with 60% relative humidity for 7 days. The damage diameter (cm) was determined and measured using a digital calliper (Traceable® Digital Calipers, Control Company, Friendswood, TX, USA). Additionally, the fruits were weighed to record the fresh weight. Subsequently, they were dried in an oven at 65 °C until a constant weight was reached to determine the dry weight. A severity index of 0 to 5 was considered according to the modified scale of Ro et al. [27], where 0 = no symptoms, 1 = symptoms with <10% disease incidence, 2 = symptoms with 11–20%, 3 = symptoms with 21–40%, 4 = symptoms with 41–70%, and 5 = 71–100% disease incidence. Bell peppers with severity indices of 2 or higher were considered to be of no commercial value. For each treatment, six replicates (six fruits) were used in a completely randomised design (CRD).
2.4. Molecular Identification and Phylogenetic Analysis of Phytophthora spp.
For DNA extraction of Phytophthora spp., the methodology of Raeder and Broda [28] was modified and used. The ITS1-5.8S-ITS2 region of the rDNA was amplified with primers ITS1 (5′ TCCGTAGGTGAACCCTGCGG 3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′). The PCR programme for Phytophthora spp. included an initial denaturation at 98 °C for 30 s, followed by 35 cycles at 98 °C for 10 s, 54 °C for 20 s, 72 °C for 35 s, and a final extension step at 72 °C for 10 min. The amplified sequences were analysed via a comparison with the NCBI GenBank database by the local alignment of nucleotide sequences with the BLAST program (version 2.16.1+).
A phylogenetic analysis was performed with MEGA 12 software (MEGA Software, Tempe, AZ, USA) [29]. To generate the phylogenetic tree, the ITS sequence obtained was aligned with other species available from NCBI. The sequences were selected by means of a search for nucleotide sequences homologous to the obtained ITS sequence. The sequences were aligned with MUSCLE [30] using the Gblocks programme [31] within the Phylogeny.fr platform [32]. Segments with variable positions or gaps were discarded, and only the conserved blocks of the multiple alignment were kept. With the curated sequences, the best-fit DNA evolutionary model was identified using MEGA 7.0.21 [29]. A phylogenetic analysis of Phytophthora spp. was performed. For this, the maximum parsimony (MP) criterion was applied by means of the Kimura-2 parameter model [33] and Gama distribution using 10,000 replicates (Bootstrap).
2.5. Antifungal Activity of Chitosan on the Mycelial Growth of Phytophthora capsici
The mycelial growth of P. capsici was quantified on V8 agar supplemented with different concentrations of low-molecular-weight chitosan (0.5, 1, 1.5, and 2 g L−1) (160 kDa, 85% DD). A 5 mm diameter disc that contained an active colony of P. capsici was inoculated in each treatment, and the Petri dishes were kept at 25 °C for 7 days. Petri dishes containing only the phytopathogen were used as the control [34]. Six replicates per treatment were used under a completely randomised experimental design. The percentage of mycelial growth inhibition (PMGI, %) was determined by measuring colony diameter in two perpendicular directions to assess the effect of different concentrations of chitosan on P. capsici development.
where R1 represents the mycelial growth of Phytophthora capsici on the control plate and R2 is the mycelial growth of Phytophthora capsici on plates with the chitosan treatments.
PMGI (%) = [(R1 − R2)/R1] × 100
2.6. Effect of Chitosan on Sporangial Germination
A suspension of P. capsici sporangia was prepared and adjusted to a concentration of 105 sporangia mL−1. The suspensions were treated with different concentrations of chitosan (0.5, 1, 1.5, and 2 g L−1), and sterile distilled water was used as a control. Samples were incubated in the dark at 28 °C for 12 h, and then sporangial germination was assessed using microscopic observation [35]. Germination was considered apparent when the sporangium released zoospores or when a germinating structure was observed. The formula used to calculate the germination percentage was as follows:
2.7. Postharvest Treatments and Experimental Conditions
Five treatments were used: four chitosan treatments (0.5, 1, 1.5, 1.5, and 2 g L−1) [13] and one control (where only P. capsici was applied to the bell peppers). The peppers were disinfected with 1% sodium hypochlorite for 3 min and the coatings were applied. Fruits were then rinsed, air-dried for 1 h, randomly divided into five batches, and immersed for 1 min in the chitosan concentrations (0.5, 1, 1.5, 1.5, and 2 g L−1). All of the bell peppers were dried for 2 h at 26 °C with 60% relative humidity. Twenty-four hours before inoculation with P. capsici, chitosan treatments were applied. P. capsici inoculation was performed by means of a zoospore suspension with a concentration of 1 × 106; zoospores mL−1. To facilitate infection, superficial wounds were created in the epidermis of the fruits with a sterile needle. Each bell pepper received 20 µL of the suspension directly onto its wounds and was placed in a humid chamber in the form of an 18 × 26 cm, 0.05 mm thick plastic bag. The bags were placed in 30 × 25 × 15 cm corrugated cardboard boxes. The boxes were stored at 26 °C and 60% relative humidity for 7 days [13]. For each treatment, six replicates (six fruits) were used in a CRD.
2.8. Determination of Lesion Area Using Image Analysis
The lesion area of P. capsici on bell peppers was determined using ImageJ® 1.54g software (National Institutes of Health, Bethesda, MD, USA). High-resolution digital images of inoculated fruit were captured under controlled lighting conditions to minimise shadows and reflections. Each image included a millimetre-scale reference in order to calibrate the measurements in ImageJ. Upon opening the images in the software, the line tool was used to measure the scale reference and set the pixel-to-measurement unit ratio. ImageJ automatically calculated the area of each lesion in centimetres squared, based on the previously set scale [36].
2.9. Effect of Chitosan on the Incidence of Phytophthora capsica in Bell Peppers
The disease incidence (DI) of chitosan-treated fruits was assessed as the percentage of bell peppers with rot symptoms according to the methodology of Khaliq et al. [37], with modifications. The incidence was calculated by dividing the number of infested fruits with symptoms by the total number of fruits assessed. Six replicates (six bell peppers) were used for this assessment per treatment with a CRD. The percentage of disease incidence was determined using the following equation:
where IF is the number of infected fruits and TF is the total number of fruits.
DI (%) = (IF/TF) × 100
2.10. Effect of Chitosan on the Severity of Phytophthora capsica in Bell Peppers
Disease severity (DS) in bell peppers after chitosan application was determined according to the procedure described by Long et al. [24] with modifications. Six replicates (six peppers) were used for each treatment. The severity index used in the pathogenicity test in Section 2.3 was also employed here. Disease severity was determined via the following equation:
where ni = number of fruits at severity stage DS, sti = value assigned to the severity index (0–5), N = total number of fruits assessed, and K = highest severity index level (5).
2.11. Effect of Chitosan on the Fresh and Dry Weight of Bell Peppers Inoculated with Phytophthora capsici
The effect of chitosan on the fresh and dry weight of bell pepper fruits infested with P. capsici was determined. Fruit fresh weight (g) was determined using a technical balance (Sartorius Entris 2202-1S, Sartorius AG, Göttingen, Germany, 2 kg ± 1 g) for all treatments. Subsequently, the dry weight was obtained after subjecting the bell peppers to an oven-drying process at 65 °C until a constant weight was reached, which ensured the complete elimination of residual moisture [13].
2.12. Determination of Weight Loss in Bell Peppers
The bell peppers’ weight loss was determined by the difference between the initial and final weight of each sample after the storage period [38]. The initial fresh weight of each bell pepper was recorded before the application of chitosan treatments and the inoculation with P. capsici. Subsequently, the bell peppers were stored at 26 °C with 60% relative humidity for 9 days, with intermediate evaluations at 3 and 6 days. At each evaluation, the bell peppers were individually weighed using a precision analytical balance (Sartorius Entris 2202-1S, Sartorius AG, Göttingen, Germany, 2 kg ± 1 g). Weight loss was calculated as the percentage reduction from the initial weight via the following formula:
where IW (g) = initial weight of the bell pepper before storage and FW (g) = final weight of the bell pepper at each evaluation interval (3, 6, and 9 days).
Weight loss (%) = (IW − FW/IW) × 100
Six replicates per treatment were carried out, considering the bell peppers treated with different concentrations of chitosan (0.5, 1.0, 1.5, and 2.0 g L−1) and a control inoculated with P. capsici but no chitosan.
2.13. Efficiency of Chitosan Treatments for the Control of Phytophthora capsici
The efficiency of the four chitosan treatments (0.5, 1, 1.5, 1.5, and 2 g L−1) in the control of P. capsici was determined with the formula suggested by Abbott [39].
where E = Efficiency (%), FIWoQ = disease severity in the control (without chitosan) and FIWQ = Disease severity with chitosan application.
E = [(FIWoQ − FIWQ)/FIWoQ] × 100
2.14. Statistical Analysis
Data were subjected to a one-way analysis of variance (ANOVA) using R Studio software (R version 4.4.2). Tukey’s test (p < 0.05) was used for mean separation. Prior to the ANOVA, the normality of the data was verified with the Shapiro–Wilk test and the homogeneity of variances with Bartlett’s test. In cases where the data did not meet these assumptions, logarithmic, arcsine, or square root transformations were applied. When, despite the transformations, the data did not fit the criteria of normality and homogeneity, a non-parametric analysis was performed with the Kruskal–Wallis test, followed by Dunn’s comparison test (p < 0.05) for the separation of medians.
3. Results
3.1. Morphological Characterisation of Phytophthora spp.
Two isolates of Phytophthora spp. were obtained from bell peppers, which were labelled as Phy1 and Phy2. Figure 1 shows the macro- and microscopic morphology of the two Phytophthora spp. isolates (Phy1 and Phy2). Figure 1A–D correspond to isolate Phy1 and Figure 1E–H to isolate Phy2. For both isolates, ovoid-shaped sporangia were identified with the presence of papillae (Figure 1A,B,G), which are reproductive structures that contain zoospores and facilitate the dissemination of the phytopathogen. In both isolates, sporangia with short pedicels were observed, and the sporangiophores had a simple sympodial arrangement. The hyphae are coenocytic without transverse septa (Figure 1C,E), a distinctive feature of oomycetes. The isolate Phy2 has abundant chlamydospores (Figure 1F), which are resistance structures that allow for the persistence of the phytopathogen under adverse conditions. Figure 1D,H shows the colony morphology of the isolate on V8 culture medium. A spongy growth and whitish colour were observed.
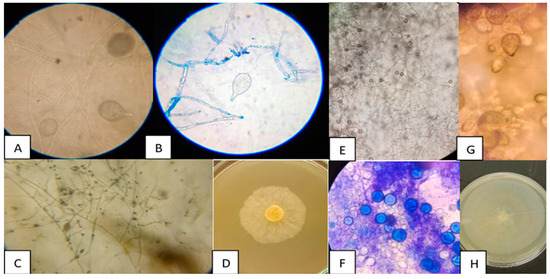
Figure 1.
Macroscopic and microscopic morphology of two Phytophthora spp. isolates obtained from bell peppers, identified as Phy1 and Phy2. Phy1: (A,B) ovoid sporangia, (C) coenocytic hyphae without transverse septa, and (D) colony morphology on V8 medium; Phy2: (E) coenocytic hyphae without transverse septa, (G) ovoid sporangia, (F) chlamydospores, and (H) colony morphology on V8 medium.
3.2. Pathogenicity of Phytophthora spp. Isolates
The isolate Phy1 presented the highest disease severity with a value of 5. No statistically significant differences (p > 0.05) in damage diameter were observed between Phy1 and Phy2. In terms of fresh and dry weight losses, bell peppers inoculated with Phy1 presented significantly (p < 0.05) lower values (139.24 g fresh weight and 13.66 g dry weight) compared to Phy2 (149.32 g fresh weight and 14.90 g dry weight). This indicates that the bell peppers inoculated with Phy1 lost more fresh and dry weight, which implies a greater impact on quality compared to Phy2. On the other hand, the non-inoculated control bell peppers showed no disease symptoms (severity = 0), with the highest values for fresh weight (160.03 g) and dry weight (16.15 g). The isolate Phy1 was selected for further analysis due to its higher virulence on the bell peppers, evidenced by the maximum severity recorded (5) and the highest damage diameter (13.17 cm) (Table 1).

Table 1.
Severity and damage caused by Phytophthora spp. on bell peppers.
3.3. Molecular Identification of Phytophthora capsici
Molecular identification analysis confirmed that isolate Phy1 (indicated by a black diamond) was Phytophthora capsici, which showed 100% coverage with 100% identity to the sequence registered in the NCBI/GenBank database under accession number MT678669.1. Bootstrap values are indicated at each node of the tree. They are high (>90), which suggested high reliability in the phylogenetic relationships established. The isolate Phytophthora sp. clusters within the Phytophthora capsici clade, with high phylogenetic support (100), and this confirms its identity as P. capsici (Figure 2).
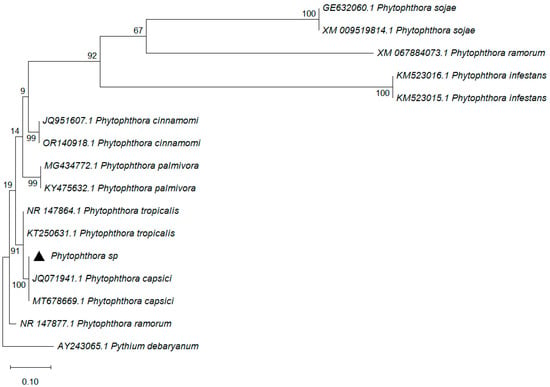
Figure 2.
Phylogenetic tree of Phytophthora sp. from the ITS1-5.8s-ITS2 region. Bootstrap values are given as percentages over nodes (10,000 bootstrap). GenBank accession numbers precede the names of each species. The black triangle (▲) indicates the isolate of Phytophthora sp. sequenced and characterised in the present study.
3.4. Mycelial Growth Inhibition of Phytophthora capsici
Figure 3 shows the percentage of radial mycelial growth inhibition (PMGI) of P. capsici. Treatments of 1, 1.5, and 2 g L−1 of chitosan achieved total (100%) inhibition of P. capsica mycelial growth. All chitosan treatments exerted an inhibitory effect on P. capsici, with significant differences (p ≤ 0.05) compared to the control treatment. These effects are attributed to chitosan’s ability to alter the integrity of the fungal plasma membrane, which interferes with metabolic processes essential for its development, such as energy production and protein biosynthesis and redox balance [16,40].
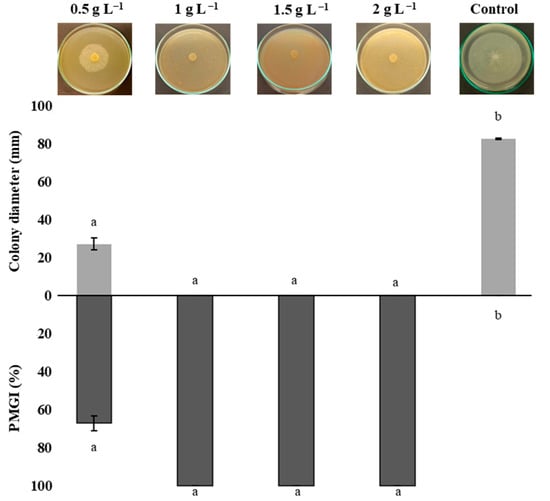
Figure 3.
The effects of chitosan on colony diameter and percentage of mycelial growth inhibition of Phytophthora capsici. Means with equal letters in columns (a, b) do not differ significantly according to Dunn’s test (p ≤ 0.05). ± Standard deviation.
3.5. Inhibition of Sporangium Formation
The control treatment showed the highest percentage of germinated sporangia, with a value of 71%. The treatments with the highest concentrations of chitosan (1, 1.5, and 2 g L−1) showed no significant differences (p > 0.05) and recorded the lowest germination values. In contrast, the 0.5 g L−1 treatment showed significant differences (p ≤ 0.05) with respect to the other chitosan treatments. All chitosan treatments were able to inhibit sporangium formation compared to the control treatment (Figure 4).
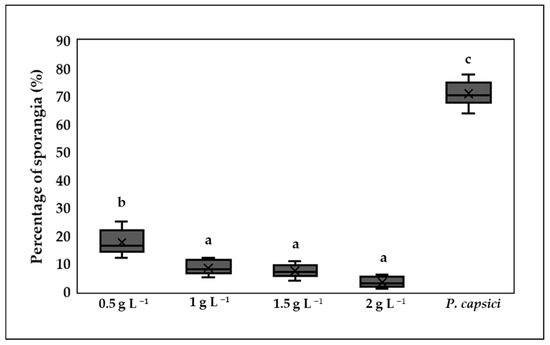
Figure 4.
The effects of chitosan on sporangial germination. Means with equal letters (a, b, c) do not differ significantly according to Tukey’s test (p ≤ 0.05). ± Standard deviation.
3.6. Lesion Area Measurement in Phytophthora capsica-Infected Bell Peppers
Chitosan treatment reduced the lesion area caused by P. capsici in bell peppers. Concentrations of 1, 1.5, and 2 g L−1 showed the best fruit condition, with a healthy appearance and no visible signs of injury (0%) and no statistical differences (p > 0.05). The 0.5 g L−1 treatment showed symptoms of fruit rot (20.33% lesion); however, its lesion area was significantly reduced (p ≤ 0.05) compared to the control. Fruit exposed only to P. capsici without any chitosan treatment exhibited significant damage (32.39% lesion) and had no commercial value (Figure 5).
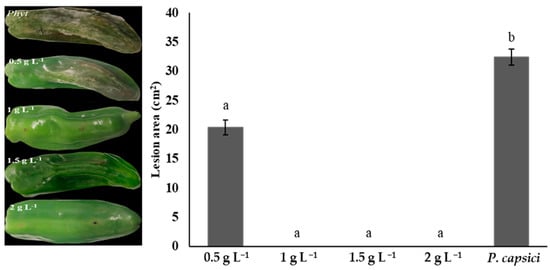
Figure 5.
The effects of chitosan on lesion areas of bell peppers infected with Phytophthora capsici. Means with equal letters in the columns (a, b) do not differ significantly according to Dunn’s test (p ≤ 0.05). ± Standard deviation.
3.7. Disease Incidence and Severity
Bell peppers treated with chitosan at concentrations of 1.0, 1.5, and 2.0 g L−1 showed no statistical differences (p > 0.05). These treatments had no symptoms of infection and a 0% incidence. The lowest-concentration treatment (0.5 g L−1) had an incidence of 55.56%, but this was lower than the control, which had the highest disease incidence (91.67%). These results demonstrate chitosan’s ability to prevent the establishment of this phytopathogen and maintain fruit quality (Figure 6). The concentration of 1 g L−1 is sufficient to inhibit P. capsici infection, as the same results were observed with higher concentrations.
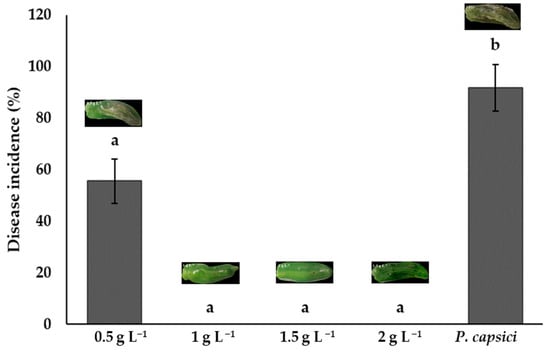
Figure 6.
The effects of chitosan on Phytophthora capsici disease incidence. Means with equal letters in the columns (a, b) do not differ significantly according to Dunn’s test (p ≤ 0.05). ± Standard deviation.
Bell peppers treated with chitosan at concentrations of 1.0, 1.5, and 2.0 g L−1 showed no visible signs of infection, with 0% disease severity and no statistical differences (p > 0.05) between them. Nonetheless, the concentration of 0.5 g L−1 showed a disease severity of 34.44%. The positive control (phytopathogen without chitosan) presented the highest severity at 86.11%, which demonstrates the high virulence of P. capsici. These results demonstrate the potential for the use of chitosan as a biological control strategy to inhibit the development of P. capsici in bell peppers (Figure 7).
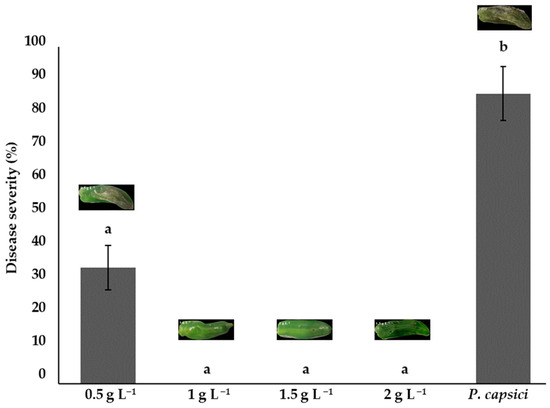
Figure 7.
The effects of chitosan on Phytophthora capsici disease severity. Means with equal letters in the columns (a, b) do not differ significantly according to Dunn’s test (p ≤ 0.05). ± Standard deviation.
3.8. Fresh and Dry Weight of Fruits
The bell peppers treated with chitosan at concentrations of 1, 1.5, and 2 g L−1 did not show significant differences (p > 0.05) between them in fresh and dry weight, but were statistically superior (p ≤ 0.05) to the P. capsici treatment. On the other hand, the 0.5 g L−1 treatment had significantly lower values than the treatments with higher concentrations. The control showed the highest losses in fresh and dry weight, which reflects the damage caused by the infection. These results highlight the ability of chitosan, particularly at concentrations of 1 g L−1, to preserve both the fresh and dry weight of the fruit, minimise damage caused by P. capsici, and maintain postharvest quality (Figure 8).
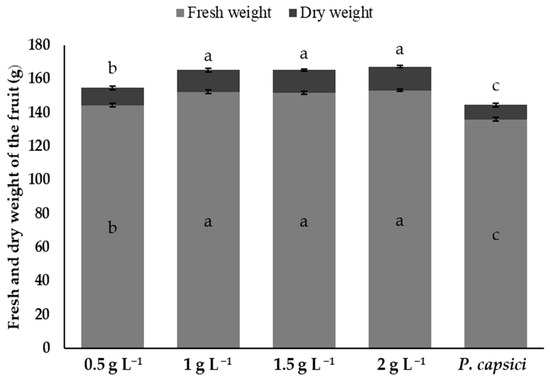
Figure 8.
The effects of chitosan on the fresh and dry weight of bell peppers inoculated with Phytophthora capsici. Means with equal letters (a, b, c) do not differ significantly according to Tukey’s test (p ≤ 0.05). ± Standard deviation.
3.9. Weight Loss in Bell Peppers During Storage
The results obtained show that the highest weight loss in bell peppers at the three evaluation times (3, 6, and 9 days) was observed in the control: 23.7% at 9 days of storage. This treatment showed significant differences (p ≤ 0.05) compared to the chitosan treatments. Fruit treated with chitosan at concentrations of 1, 1.5, and 2 g L−1 showed the lowest weight losses (p > 0.05) on day 9, 11.77, 11.51, and 11.48%, respectively, which indicates a greater efficiency in the conservation of fruit quality. The lowest chitosan concentration (0.5 g L−1) recorded a weight loss of 15.23% at day 9, a value significantly higher (p ≤ 0.05) than that obtained with the highest chitosan concentration, but lower than that observed in the control (Figure 9).
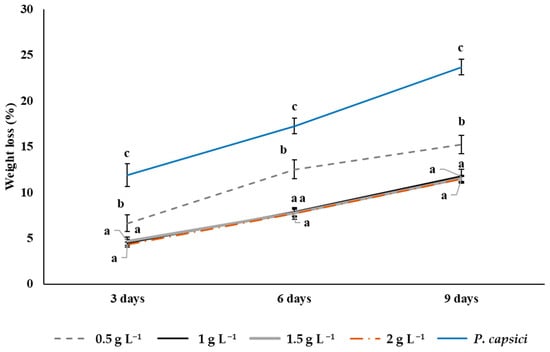
Figure 9.
The effects of chitosan on weight loss (%) in bell peppers at 3, 6, and 9 days of postharvest storage. Means with equal letters (a, b, c) do not differ significantly according to Tukey’s test (p ≤ 0.05). ± Standard deviation.
3.10. Efficacy of Chitosan in the Control of Phytophthora capsici
Treatments with chitosan at 1, 1.5, and 2 g L−1 showed 100% efficiency, with no significant differences (p > 0.05) between them, which evidences their high effectiveness in preventing the disease in fruit. In contrast, the chitosan treatment at 0.5 g L−1 showed a significantly lower efficiency, with an average of 60%, suggesting a limited efficacy compared to the higher concentrations. The concentration of 1 g L−1 is sufficient to achieve the maximum protective effect; thus, the use of higher concentrations does not confer an additional benefit in terms of efficiency (Figure 10).
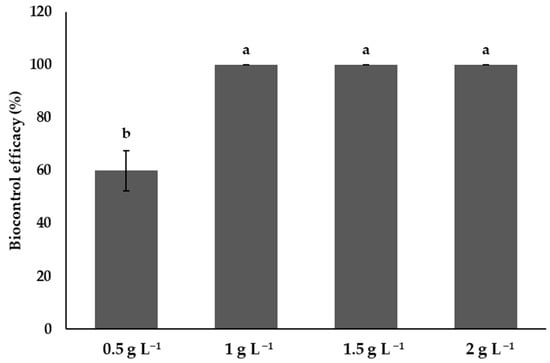
Figure 10.
The effects of chitosan on the efficiency of Phytophthora capsici control. Means with equal letters in the columns (a, b) do not differ significantly according to Dunn’s test (p ≤ 0.05). ± Standard deviation.
4. Discussion
The morphological characterisation of Phytophthora spp. isolates confirmed the presence of sporangia and chlamydospores, key structures in their infection cycle and dissemination. The shape of the sporangia recorded in the present study is consistent with the description of P. capsici, which can adopt various shapes, e.g., ovoid, sub-globose, ellipsoid, globose, fusiform, pyriform, and irregular shapes [41,42]. These morphological characteristics, combined with molecular identification, allow for a precise differentiation of P. capsici from other species within the genus [43,44].
In our study, the sporangia presented an ovoid morphology with short pedicels, results that coincide with those obtained by Van Tran et al. [45]. The identification of simple sympodial sporangiophores in all isolates is in agreement with that reported by Kuswinanti et al. [46]. Furthermore, Karyath Palliyath et al. [47] found the presence of intercalary chlamydospores in isolates of Phytophthora palmivora, which is similar to what was observed in this work. Reyes-Tena et al. [48] morphologically characterised isolates of P. capsica, most of which had sympodial simple sporangiophores and ovoid sporangia with long pedicels, and the presence of chlamydospores was not obtained in all isolates. The isolate with the greatest impact on weight loss (Phy1) could be associated with greater virulence or greater alteration of the cellular integrity of the fruit, accelerating decay and senescence processes.
The accurate identification of phytopathogens is essential in order to develop effective postharvest management strategies [49], especially in the implementation of biocontrol agents. In this context, molecular analysis provides a reliable tool in order to differentiate P. capsici from other species of the genus and allows for a targeted approach in its control.
Previous studies have used ITS1-5.8S-ITS2 sequencing for the identification of Phytophthora species. Abdul Haq et al. [50] applied this technique for the molecular identification of Phytophthora spp. isolates, while Seddaiu and Linaldeddu [51] phylogenetically characterised several species of the genus with the same gene region. They obtained results that agree with those of the present study. In the latter, the concordance between the phylogenetic analysis and the closest BLAST matches supports the identification of the isolates, evidenced by the high bootstrap support at the terminal nodes of the phylogenetic tree.
Chitosan, in general, is a natural polymer recognised for its low toxicity, high bio-degradability, and safety in food applications [16]. Furthermore, it has been approved by international regulatory agencies, such as the US Food and Drug Administration (FDA), which classifies it a Generally Recognised as Safe (GRAS) substance for direct human consumption [52].
The polycationic property of chitosan is key to its antifungal activity, as the extension of its polymer chain influences its effectiveness [53]. Inhibition of mycelial growth in the presence of chitosan could be related to the electrostatic interaction of the amino groups of this biopolymer with fungal cell wall components (negatively charged phospholipids), which leads to structural destabilisation, loss of cytoplasmic contents, and, finally, cell death [16,54].
Chitosan has been shown to interfere with spore germination, germ tube elongation and mycelial development of phytopathogenic fungi [15]. The results obtained here are in agreement with those of Meng et al. [40], who reported that chitosan significantly reduced the spore germination and mycelial growth of Aspergillus ochraceus. Moreover, they observed morphological alterations in the hyphae, characterised by wrinkling, abnormal branching, and vacuolation.
The results of this study are consistent with previous research where chitosan significantly inhibited the mycelial development of P. capsici [19]. Also, in vitro chitosan application significantly reduced P. capsici growth, where concentrations of 100 and 500 μg mL−1 achieved 90 and 95% inhibition of mycelial growth, respectively [20].
Recent investigations have shown that chitosan induces modifications in cell morphology, as well as structural disruptions and alterations in the molecular organisation of fungal cells [55]. In addition, the free amino groups of chitosan show a strong ability to chelate metal ions on the microbial cell surface, which results in the formation of stable complexes and limits microorganisms’ nutrient uptake [56,57].
Chitosan has been shown to induce significant structural damage in Botrytis cinerea in that it causes alterations to its surface morphology and compromises cell integrity. This effect includes the presence of deformed and damaged hyphae, together with the formation of an outer layer on the cell surface of the fungus, which inhibits its growth [58]. Vitti et al. [59] reported a significant reduction in the fungal growth of Botrytis cinerea and alterations in its morphology. The concentration of 0.5 mg mL−1 resulted in a 70% inhibition of fungal growth, which is in line with what was observed in the present study. What is more, Oliveira Junior et al. [60] found that the application of chitosan caused a reduction in the size of the hyphae of Alternaria alternata, B. cinerea, and Rhizopus stolonifer fungi. The mycelia also showed abnormal shapes and swelling, which demonstrates the impact of chitosan on the cell structure of various phytopathogens.
Chitosan has been shown to induce spore dehydration, an effect that could be associated with loss of cell contents and vacuole rupture [61]. Similar results to those obtained in this study were observed in P. infestans, where chitosan significantly inhibited both mycelial growth and spore germination under in vitro conditions [62]. In another study, chitosan application (0.5 g L−1) was shown to reduce the number of oospores and sporangia of P. nicotianae and chitosan-treated zoospores formed cysts that did not germinate [35].
Chitosan has been shown to cause damage to the vacuole system and plasmalemmasomes of P. capsici hyphae, since plasmalemmasome structures play a crucial role in cell wall synthesis. These results suggest that chitosan may inhibit mycelial growth and sporangial germination by interfering with cell wall biogenesis processes [63].
Chitosan can play a dual role by directly inhibiting fungal growth and, at the same time, activating defence mechanisms in the fruit. Chitosan applied to fruit has been shown to induce the expression of defence-related genes, including those encoding PR such as chitinases and β-1,3-glucanases, which improves fruit resistance against postharvest phytopathogens [64]. In addition, it enhances the activities of protective enzymes, such as superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia lyase-1 (PAL) [55,65].
Chitosan, due to its mechanisms of action (such as its direct inhibition of mycelial growth, interference with spore germination, structural alteration of the cell membrane, and activation of defensive mechanisms in fruits), presents a significantly lower risk of inducing resistance in phytopathogens compared to synthetic fungicides, which usually act on specific molecular targets [16,57]. However, their comparison in terms of cost and effectiveness with synthetic fungicides depends on multiple factors, such as scale of production, method of application, and frequency of treatment [66,67].
Safari et al. [21] applied chitosan to tomato fruits that were infected with Fusarium oxysporum. They found that it reduced the disease incidence and severity by more than 70% and prolonged shelf life by up to 15 days by maintaining the activity of PAL, POD, and PPO enzymes. Meng et al. [68] obtained similar findings in pears, where chitosan treatment stimulated the enzyme activity of POD, PPO, chitinases, and β-1,3-glucanases, which could favour fruit resistance against phytopathogen infections.
Chitosan coatings generate a semi-permeable film around the fruit, which can inhibit the growth of phytopathogens by altering the integrity of cell membranes [69]. In the present study, the barrier generated by chitosan concentrations (1, 1.5 and 2 g L−1) could have inhibited the growth of P. capsici and delayed the ripening and senescence process of the bell peppers, reducing disease incidence and severity. In addition, chitosan coatings help to strengthen the epidermal structure of the fruit, which protects against infection by phytopathogens [21].
Chitosan can exert an effect on the microbiota present on the fruit surface; however, our results indicate that its application was essential for the control of P. capsici. In the control treatment, where chitosan was not applied, the fruit showed a high rate of severity and incidence of the disease, completely losing its commercial value.
In apples, the application of chitosan reduced the incidence of blue rot disease caused by Penicillium expansum by more than 50% [17]. Similarly, Sikder and Islam [25] observed that bananas treated with chitosan exhibited a reduction in disease incidence and severity, with the most prominent effect at the highest chitosan concentration (1%).
The formation of a continuous film on the fruit surface, induced by chitosan, contributes to the inhibition of P. capsici because it creates a physical barrier that prevents direct contact between the phytopathogen and the fruit tissue and decreases the gas exchange necessary for its growth [12].
This biopolymer forms a semi-permeable layer on the surface of fruits, which modifies the permeability to oxygen (O₂) and carbon dioxide (CO₂), decreases respiration rates, and enables the increase in antioxidant activity. Additionally, it limits the disease incidence and severity, as it creates a less favourable environment for the development of phytopathogens [70].
Yunita et al. [71] reported that a 0.75% chitosan coating was effective in reducing disease infestation and severity of damage, as well as delaying the ripening of papayas for six days. In bell peppers, chitosan coating suppressed the development of A. alternata during the cold storage stage and preserved physicochemical qualities [72].
Chitosan application showed significant inhibition of Colletotrichum capsici incidence and severity in postharvest bell peppers. At a concentration of 1%, a reduction in disease incidence of up to 73.12% was observed and the diameter of lesions on fruits was 0.34 cm, significantly lower than the 1.28 cm recorded in the control. These results indicate a progressive decrease in symptoms with increasing chitosan concentrations [24], and are similar to the results obtained in this work.
Recent studies have explored various strategies for the post-harvest management of bell pepper phytopathogens, highlighting the potential of chitosan-based coatings and their combination with bioactive compounds. Coatings based on chitosan nanoparticles (CSNP) and their combination with bioactive compounds, such as thyme essential oil (CSTEO-NP) and aqueous extract of propolis (CSEP-NP), improve the postharvest preservation of bell pepper. CSNP reduces bacterial growth, while CSTEO-NP minimises fungal growth. These coatings help to reduce weight loss and preserve the nutritional quality of the fruit, standing out as a natural alternative to prolong its shelf life [73]. In another study, it was shown that the application of polyamines (spermidine and spermine) in combination with 1% chitosan coating prolonged the quality and postharvest shelf life of bell pepper stored at 10 °C for 56 days, reducing weight loss by 47.6% [74].
In addition to chitosan, other biopolymers have been studied for their efficacy in postharvest preservation and phytopathogen control. Alginates, derived from seaweed, have been used to form coatings that provide excellent moisture retention and barrier properties, reducing weight loss in fruits such as papaya and mango [75,76]. Also, methylcellulose-based coatings have been effective in reducing weight loss and delaying senescence in Capsicum annuum L., prolonging its shelf life in ambient storage [77]. The application of guar gum on tomatoes reduced the rate of respiration and weight loss, delaying ripening and maintaining fruit firmness during storage [78]. These results reinforce the role of biopolymers as sustainable alternatives for postharvest disease management and fruit quality preservation.
Fruit weight is closely related to the respiration rate and moisture evaporation from the fruit tissue to the environment, factors that depend on the postharvest treatment applied and the storage temperature [79]. In the present investigation, chitosan-treated bell peppers had higher fresh and dry weights than the control fruits, suggesting a positive effect of chitosan in preserving fruit quality.
In line with our results, Bhanushree et al. [80] reported that fruits treated with 3% (w/v) chitosan showed higher water retention and higher fresh weight compared to untreated fruits. Likewise, Torres-Rodriguez et al. [13] showed that the highest concentration of chitosan (3 g L−1) maintained the fresh and dry weight in tomatoes inoculated with Fusarium oxysporum without significant differences in relation to the treatment based on synthetic fungicides. This effect is attributed to the formation of a semi-permeable film on the fruit surface, which reduces water loss (by limiting moisture and gas exchange), decreases transpiration rate, and delays physiological deterioration [81].
The higher weight retention observed in chitosan treatments could be associated with the induction of defence responses in the fruit, which contributes to the maintenance of cell wall integrity and turgor [70].
Chitosan coatings improve the postharvest quality and shelf life of fruits during the storage period by minimising physicochemical changes and microbial growth [12]. Chitosan acts not only as an antifungal agent, but also helps to preserve essential bioactive compounds, such as antioxidants, because it decreases the rate of respiration and oxidative stress, which contributes to the maintenance of the postharvest quality of the fruit.
In strawberries, chitosan coatings reduced weight loss in comparison to the control (without chitosan) over nine days of storage [38]. In apples, the application of chitosan (2%) reduced weight loss, whereby a weight loss of 2.66% during the storage period was recorded. In contrast, uncoated apples showed a higher weight loss (8.50%) after 35 days [23].
Chitosan coatings increase the activity of antioxidant enzymes, which is related to a lower rate of decay and a reduction in weight loss compared to uncoated fruit [38]. Moreover, chitosan-based coatings promote an increase in dry matter and a decrease in water activity, which contributes to reduced weight loss and microbial spoilage [12]. Chitosan also functions as a semi-permeable barrier to oxygen, carbon dioxide and moisture, which helps to reduce respiration and water loss and in turn limits fruit dehydration and shrinkage [22,82].
In the present study, the efficiency of chitosan treatments in the management of P. capsici in bell peppers could be associated with the activation of induced defence mechanisms, such as systemic acquired resistance (SAR) [16,38]. This mechanism involves the pre-activation of fruit defences by inducing the expression of defence-related genes, which increases resistance to subsequent infections. It has been shown that chitosan can induce the expression of key genes, such as chitinase, peroxidase, β-1,3-glucanase, XET, PR8, and PAL1, which play a key role in cell wall fortification and the degradation in cell wall components of phytopathogenic fungi [64].
Regarding the efficiency of the treatments, the concentration of 1 g L−1 of chitosan proved to be the most effective in reducing the incidence and severity of P. capsici, maintaining the postharvest quality of the bell pepper. Although higher concentrations (1.5 and 2 g L−1) also showed efficacy (100%), the increase in concentration implies a higher input cost without a proportional improvement in the antifungal effect. Chitosan may be an option for the postharvest control of phytopathogens. However, the implementation of chitosan on an industrial scale in postharvest handling faces key challenges, such as formulation stability, the optimisation of large-scale application techniques, and the development of automated spraying or dipping systems in postharvest processing facilities. Future research should therefore focus on optimising these strategies.
5. Conclusions
Chitosan proved to be highly effective both under in vitro conditions and in the postharvest preservation of bell peppers inoculated with Phytophthora capsici. During in vitro tests, concentrations of 1, 1.5, and 2 g L−1 completely inhibited mycelial growth and sporangial germination of the phytopathogen, which demonstrates its antifungal potential. In fruit, these treatments reduced disease incidence and severity to 0% and minimised fresh and dry weight loss during storage in the presence of Phytophthora capsici. The concentration of 1 g L−1 was sufficient for maximum efficacy, as higher concentrations did not produce significantly greater effects. These results support the use of chitosan as a sustainable and effective alternative for the postharvest control of Phytophthora capsici, as it reduces dependence on synthetic fungicides and extends the shelf life of the fruit. However, future research should focus on evaluating the application of chitosan-based coatings under commercial storage conditions.
Author Contributions
Conceptualization, J.A.T.-R. and J.J.R.P.; methodology, J.A.T.-R., J.J.R.P. and L.T.L.R.; software, E.O.R.-P. and L.G.H.-M.; validation, J.J.R.P., L.G.-M. and E.O.R.-P.; formal analysis, J.A.T.-R., L.G.-M. and J.J.R.P.; investigation, J.A.T.-R., J.J.R.P. and L.T.L.R.; resources, J.A.T.-R. and L.T.L.R.; writing—original draft preparation, J.A.T.-R., J.J.R.P. and E.O.R.-P.; writing—review and editing, E.O.R.-P., L.G.-M. and L.G.H.-M.; visualisation, J.J.R.P., L.G.H.-M. and L.T.L.R.; supervision, J.J.R.P., L.G.-M. and J.A.T.-R.; project administration, J.J.R.P.; funding acquisition, J.A.T.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
To the Universidad Técnica Estatal de Quevedo for the support provided through the Fondo Concursable para la Investigación Científica y Tecnológica (FOCICYT) 10th Call, through the project “Quitosano: Una Solución Biodegradable para el Control de Fitopatógenos en Postcosecha”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ivan, I.M.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Olaru, O.T.; Nițulescu, G.M.; et al. Phytochemical Profile, Antioxidant and Cytotoxic Potential of Capsicum annuum (L.) Dry Hydro-Ethanolic Extract. Pharmaceutics 2024, 16, 245. [Google Scholar] [CrossRef]
- Tahboub, M.B.; Sanogo, S.; Bosland, P.W.; Murray, L. Heat Level in Chile Pepper in Relation to Root and Fruit Infection by Phytophthora capsici. HortScience 2008, 43, 1846–1851. [Google Scholar] [CrossRef]
- Ávila-Oviedo, J.L.; Méndez-Inocencio, C.; Rodríguez-Torres, M.D.; Angoa-Pérez, M.V.; Chávez-Avilés, M.N.; Martínez-Mendoza, E.K.; Oregel-Zamudio, E.; Villar-Luna, E. Antagonistic Effects and Volatile Organic Compound Profiles of Rhizobacteria in the Biocontrol of Phytophthora capsici. Plants 2024, 13, 3224. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ranjbar, A. Advances in Postharvest Diseases Management of Fruits and Vegetables: A Review. Horticulturae 2023, 9, 1099. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of Microbial Antagonists for the Control of Postharvest Diseases of Fruits: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Volynchikova, E.; Kim, K.D. Biological Control of Oomycete Soilborne Diseases Caused by Phytophthora capsici, Phytophthora infestans, and Phytophthora nicotianae in Solanaceous Crops. Mycobiology 2022, 50, 269–293. [Google Scholar] [CrossRef]
- Dunn, A.R.; Lange, H.W.; Smart, C.D. Evaluation of Commercial Bell Pepper Cultivars for Resistance to Phytophthora Blight (Phytophthora capsici). Plant Health Prog. 2014, 15, 19–24. [Google Scholar] [CrossRef]
- Liu, P.; Cai, Y.; Zhang, J.; Wang, R.; Li, B.; Weng, Q.; Chen, Q. Antifungal Activity of Liquiritin in Phytophthora capsici Comprises Not Only Membrane-Damage-Mediated Autophagy, Apoptosis, and Ca2+ Reduction but Also an Induced Defense Responses in Pepper. Ecotoxicol. Environ. Saf. 2021, 209, 111813. [Google Scholar] [CrossRef]
- Lu, X.H.; Hausbeck, M.K.; Liu, X.L.; Hao, J.J. Wild Type Sensitivity and Mutation Analysis for Resistance Risk to Fluopicolide in Phytophthora capsici. Plant Dis. 2011, 95, 1535–1541. [Google Scholar] [CrossRef]
- Barchenger, D.W.; Lamour, K.H.; Bosland, P.W. Challenges and Strategies for Breeding Resistance in Capsicum Annuum to the Multifarious Pathogen, Phytophthora capsici. Front. Plant Sci. 2018, 9, 628. [Google Scholar] [CrossRef]
- Burandt, Q.C.; Deising, H.B.; von Tiedemann, A. Further Limitations of Synthetic Fungicide Use and Expansion of Organic Agriculture in Europe Will Increase the Environmental and Health Risks of Chemical Crop Protection Caused by Copper-Containing Fungicides. Environ. Toxicol. Chem. 2024, 43, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Carranza-Patino, M.S.; Gaibor-Fernández, R.R.; Rivas-García, T.; Rueda-Puente, E.O. Chitosan: Biocontrol Agent of Fusarium oxysporum in Tomato Fruit (Solanum lycopersicum L.). Emir. J. Food Agric. 2024, 36, 1–9. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, W.; Yu, L.; Shi, Y.; Deng, Y.; Wang, D.; Zhong, Y. Chitosan Coatings with Different Degrees of Deacetylation Regulate the Postharvest Quality of Sweet Cherry through Internal Metabolism. Int. J. Biol. Macromol. 2024, 254, 127419. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Zhu, Y.; Hu, H.; Jia, Q.; Sun, C.; Zhu, X.; Liu, Y. Antifungal Activity and Mechanism of Chitosan against Fusarium solani Caused Ginger Soft Rot during Postharvest Storage. Postharvest Biol. Technol. 2024, 208, 112680. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Castellanos, T.; Angulo, C.; Quiñones-Aguilar, E.E.; Hernandez-Montiel, L.G. A Biopolymer with Antimicrobial Properties and Plant Resistance Inducer against Phytopathogens: Chitosan. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12231. [Google Scholar] [CrossRef]
- Gong, W.; Sun, Y.; Tu, T.; Huang, J.; Zhu, C.; Zhang, J.; Salah, M.; Zhao, L.; Xia, X.; Wang, Y. Chitosan Inhibits Penicillium expansum Possibly by Binding to DNA and Triggering Apoptosis. Int. J. Biol. Macromol. 2024, 259, 129113. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf Life Extension of Fresh Fruit and Vegetables by Chitosan Treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Le, V.T.; Bach, L.G.; Pham, T.T.; Le, N.T.T.; Ngoc, U.T.P.; Tran, D.-H.N.; Nguyen, D.H. Synthesis and Antifungal Activity of Chitosan-Silver Nanocomposite Synergize Fungicide against Phytophthora capsici. J. Macromol. Sci. Part A 2019, 56, 522–528. [Google Scholar] [CrossRef]
- Héctor, L.M.; Elizabeth, V.O.; José, A.J.Z.; Gabriel, L.U. Treatment with chitosan protects habanero pepper against the infection with Phytophthora capsici. Isr. J. Plant Sci. 2010, 58, 61–65. [Google Scholar] [CrossRef]
- Safari, Z.S.; Ding, P.; Nakasha, J.J.; Yusoff, S.F. Controlling Fusarium Oxysporum Tomato Fruit Rot under Tropical Condition Using Both Chitosan and Vanillin. Coatings 2021, 11, 367. [Google Scholar] [CrossRef]
- Petriccione, M.; De Sanctis, F.; Pasquariello, M.S.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Mencarelli, F. The Effect of Chitosan Coating on the Quality and Nutraceutical Traits of Sweet Cherry During Postharvest Life. Food Bioprocess Technol. 2015, 8, 394–408. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Preserving Apple (Malus domestica var. Anna) Fruit Bioactive Substances Using Olive Wastes Extract-Chitosan Film Coating. Inf. Process. Agric. 2017, 4, 90–99. [Google Scholar] [CrossRef]
- Long, L.T.; Tan, L.V.; Boi, V.N.; Trung, T.S. Antifungal Activity of Water-Soluble Chitosan against Colletotrichum capsici in Postharvest Chili Pepper. J. Food Process. Preserv. 2018, 42, e13339. [Google Scholar] [CrossRef]
- Sikder, M.B.H.; Islam, M.M. Effect of Shrimp Chitosan Coating on Physico-Chemical Properties and Shelf Life Extension of Banana. Int. J. Eng. Technol. Sci. 2019, 6, 41–54. [Google Scholar] [CrossRef]
- Drenth, A.; Sendall, B. Practical Guide to Detection and Identification of Phytophthora. Trop. Plant Prot. 2001, 1, 32–33. [Google Scholar]
- Ro, N.-Y.; Sebastin, R.; Hur, O.-S.; Cho, G.-T.; Geum, B.; Lee, Y.-J.; Kang, B.-C. Evaluation of Anthracnose Resistance in Pepper (Capsicum spp.) Genetic Resources. Horticulturae 2021, 7, 460. [Google Scholar] [CrossRef]
- Raeder, U.; Broda, P. Rapid Preparation of DNA from Filamentous Fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of Phylogenies after Removing Divergent and Ambiguously Aligned Blocks from Protein Sequence Alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.Fr: Robust Phylogenetic Analysis for the Non-Specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.-B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and Antifungal Activities of a Low Molecular Weight Chitosan and Its N-/2(3)-(Dodec-2-Enyl)Succinoyl/-Derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- González-Peña Fundora, D.; Falcón-Rodríguez, A.B.; Costales Menéndez, D.; Foroud, N.A.; Vaillant Flores, D.; Aispuro-Hernández, E.; Martínez-Téllez, M.Á. Chitosan Induces Tomato Basal Resistance against Phytophthora Nicotianae and Inhibits Pathogen Development. Can. J. Plant Pathol. 2022, 44, 400–414. [Google Scholar] [CrossRef]
- Cui, Q.; Li, X.; Hu, S.; Yang, D.; Abozeid, A.; Yang, Z.; Jiang, J.; Ren, Z.; Li, D.; Li, D.; et al. The Critical Role of Phenylpropanoid Biosynthesis Pathway in Lily Resistance Against Gray Mold. Int. J. Mol. Sci. 2024, 25, 11068. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, G.; Muda Mohamed, M.T.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of Gum Arabic Coating Combined with Calcium Chloride on Physico-Chemical and Qualitative Properties of Mango (Mangifera indica L.) Fruit during Low Temperature Storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal Activity of Chitosan against Aspergillus ochraceus and Its Possible Mechanisms of Action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef]
- Li, Z.; Long, W.; Zheng, J.; Lei, J. Isolation and Identification of Phytophthora capsici in Guangdong Province and Measurement of Their Pathogenicity and Physiological Race Differentiation. Front. Agric. China 2007, 1, 377–381. [Google Scholar] [CrossRef]
- Soto-Plancarte, A.; Rodríguez-Alvarado, G.; Fernández-Pavía, Y.L.; Pedraza-Santos, M.E.; López-Pérez, L.; Díaz-Celaya, M.; Fernández-Pavía, S.P. Protocolos de aislamiento y diagnóstico de Phytophthora spp. enfoque aplicado a la investigación. Rev. Mex. Cienc. Agrí. 2017, 8, 1867–1880. [Google Scholar] [CrossRef]
- Meyer, M.D.; Hausbeck, M.K. Age-Related Resistance to Phytophthora Fruit Rot in ‘Dickenson Field’ Processing Pumpkin and ‘Golden Delicious’ Winter Squash Fruit. Plant Dis. 2013, 97, 446–552. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Hausbeck, M.K. Resistance of Pepper to Phytophthora Crown, Root, and Fruit Rot Is Affected by Isolate Virulence. Plant Dis. 2010, 94, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, Q.; Ha, C.V.; Vvedensky, V.V.; Han, V.-C. Current Status and Characterization of Phytophthora Species Associated with Gummosis of Citrus in Northern Vietnam. J. Phytopathol. 2023, 171, 478–488. [Google Scholar] [CrossRef]
- Kuswinanti, T.; Patandjengi, B.; Melina; Hardina, N. Morphological and Molecular Characterization of Phytophthora Palmivora Isolates and Their Virulence Test on Cocoa Clone Sulawesi 2. J. Fitopatol. Indones. 2023, 19, 145–155. [Google Scholar] [CrossRef]
- Karyath Palliyath, G.; Kilingar Subrahmanya, M.; Antony, G.; Binod Bihari, S.; Hegde, V.; Muliyar Krishna, R. A Rapid in Vitro Leaf Inoculation Assay to Investigate Phytophthora palmivora–Coconut Interactions. J. Phytopathol. 2021, 169, 316–328. [Google Scholar] [CrossRef]
- Reyes-Tena, A.; Rodríguez-Alvarado, G.; Fernández-Pavía, S.P.; Pedraza-Santos, M.E.; Larsen, J.; Vázquez-Marrufo, G.; Reyes-Tena, A.; Rodríguez-Alvarado, G.; Fernández-Pavía, S.P.; Pedraza-Santos, M.E.; et al. Caracterización morfológica de aislados de Phytophthora capsici provenientes de Jalisco y Michoacán, México. Rev. Mex. Fitopatol. 2021, 39, 75–93. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phillips, A.J.L.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of Molecular Data to Identify Fungal Plant Pathogens and Guidelines for Pathogenicity Testing Based on Koch’s Postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef]
- Abdul Haq, M.; Shahzad, S.; Lodhi, A.M.; Rajput, A.Q. Morphological and Molecular Characterization of Four Phytophthora Species with the First Report of Phytophthora Lacustris from Pakistan. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2024, 158, 457–463. [Google Scholar] [CrossRef]
- Seddaiu, S.; Linaldeddu, B.T. First Report of Phytophthora acerina, P. plurivora, and P. pseudocryptogea Associated with Declining Common Alder Trees in Italy. Plant Dis. 2020, 104, 1874. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Tan, W.; Li, Q.; Gu, G.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Activity of Pyridine-Based Triple Quaternized Chitosan Derivatives. Molecules 2018, 23, 2604. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, Mode of Action, and in Vivo Activity of Chitosan and Its Micro- and Nanoparticles as Antimicrobial Agents: A Review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Carranza-Patiño, M.S.; Herrera-Feijoo, R.J.; Preciado-Rangel, P.; Hernandez-Montiel, L.G. Biocontrol of Fusarium solani: Antifungal Activity of Chitosan and Induction of Defence Enzymes. Plants 2025, 14, 431. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Silva Júnior, S.; Stamford, N.P.; Lima, M.A.B.; Arnaud, T.M.S.; Pintado, M.M.; Sarmento, B.F. Characterization and Inhibitory Activity of Chitosan on Hyphae Growth and Morphology of Botrytis Cinerea Plant Pathogen. Int. J. Appl. Res. Nat. Prod. 2014, 7, 31–38. [Google Scholar]
- Vitti, A.; Coviello, L.; Triunfo, M.; Guarnieri, A.; Scieuzo, C.; Salvia, R.; Falabella, P.; Nuzzaci, M. Actividad Antifúngica in Vitro y Eficacia Del Recubrimiento Comestible in Vivo Del Quitosano Derivado de Insectos Contra Botrytis cinerea En Fresa. Int. J. Biol. Macromol. 2024, 279, 135158. [Google Scholar] [CrossRef]
- Oliveira Junior, E.N.D.; Melo, I.S.D.; Franco, T.T. Changes in Hyphal Morphology Due to Chitosan Treatment in Some Fungal Species. Braz. Arch. Biol. Technol. 2012, 55, 637–646. [Google Scholar] [CrossRef]
- Jiménez-Mejía, R.; Arceo-Martínez, M.T.; Loeza-Lara, P.D. Quitosano: Actividad antimicrobiana y mecanismos de acción. E-CUCBA 2018, 9, 17–23. [Google Scholar]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Actividad Antifúngica Del Quitosano Contra Phytophthora infestans, Patógeno Del Tizón Tardío de La Papa. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Han, X.; Du, Y. Antifungal Activity of Oligochitosan against Phytophthora capsici and Other Plant Pathogenic Fungi in Vitro. Pestic. Biochem. Physiol. 2007, 87, 220–228. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Monir, G.A.; Hassan, M.S.S.; Ahmed, Y.; Refaat, M.H.; Ismail, I.A.; El-Garhy, H.A.S. Exogenously Applied Chitosan and Chitosan Nanoparticles Improved Apple Fruit Resistance to Blue Mold, Upregulated Defense-Related Genes Expression, and Maintained Fruit Quality. Horticulturae 2021, 7, 224. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-Based Coating Enriched with Hairy Fig (Ficus hirta Vahl.) Fruit Extract for “Newhall” Navel Orange Preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan Antimicrobial and Eliciting Properties for Pest Control in Agriculture: A Review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Shcherban, A.B. Chitosan and Its Derivatives as Promising Plant Protection Tools. Vavilov J. Genet. Breed. 2023, 27, 1010–1021. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of Chitosan and Oligochitosan on Growth of Two Fungal Pathogens and Physiological Properties in Pear Fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Abebe, Z.; Tola, Y.B.; Mohammed, A. Effects of Edible Coating Materials and Stages of Maturity at Harvest on Storage Life and Quality of Tomato (Lycopersicon esculentum Mill.) Fruits. Afr. J. Agric. Res. 2017, 12, 550–565. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–Aloe Vera Gel Coating Delays Postharvest Decay of Mango Fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Yunita, R.; Amin, N.N.; Damayanti, T.A. Pemanfaatan Kitosan Untuk Mengendalikan Antraknosa Pada Pepaya (Colletotrichum gloeosporioides) Dan Meningkatkan Daya Simpan Buah. J. Fitopatol. Indones. 2012, 8, 97. [Google Scholar] [CrossRef]
- Hernández-López, G.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Barrera-Necha, L.L. Recubrimiento Comestible de Quitosano Nanoestructurado Cargado Con α-Pineno Para La Preservación de La Calidad Poscosecha de Capsicum annuum L. y El Control de Alternaria alternata. Int. J. Biol. Macromol. 2020, 165, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Barrera-Necha, L.L.; Cuevas-Gómez, K.D. Effect of Chitosan-Based Natural Products Nanocoatings on Green Bell Peppers During Storage. Food Bioprocess Technol. 2023, 16, 1703–1715. [Google Scholar] [CrossRef]
- Sharma, S.; Krishna, H.; Barman, K.; Kole, B.; Singh, S.K.; Behera, T.K. Synergistic Effect of Polyamine Treatment and Chitosan Coating on Postharvest Senescence and Enzyme Activity of Bell Pepper (Capsicum annuum L.) Fruit. S. Afr. J. Bot. 2022, 151, 175–184. [Google Scholar] [CrossRef]
- Narsaiah, K.; Wilson, R.A.; Gokul, K.; Mandge, H.M.; Jha, S.N.; Bhadwal, S.; Anurag, R.K.; Malik, R.K.; Vij, S. Effect of Bacteriocin-Incorporated Alginate Coating on Shelf-Life of Minimally Processed Papaya (Carica papaya L.). Postharvest Biol. Technol. 2015, 100, 212–218. [Google Scholar] [CrossRef]
- Rastegar, S.; Hassanzadeh Khankahdani, H.; Rahimzadeh, M. Effectiveness of Alginate Coating on Antioxidant Enzymes and Biochemical Changes during Storage of Mango Fruit. J. Food Biochem. 2019, 43, e12990. [Google Scholar] [CrossRef]
- Chaple, S.; Vishwasrao, C.; Ananthanarayan, L. Edible Composite Coating of Methyl Cellulose for Post-Harvest Extension of Shelf-Life of Finger Hot Indian Pepper (Pusa jwala). J. Food Process. Preserv. 2017, 41, e12807. [Google Scholar] [CrossRef]
- Ruelas-Chacon, X.; Contreras-Esquivel, J.C.; Montañez, J.; Aguilera-Carbo, A.F.; Reyes-Vega, M.L.; Peralta-Rodriguez, R.D.; Sanchéz-Brambila, G. Guar Gum as an Edible Coating for Enhancing Shelf-Life and Improving Postharvest Quality of Roma Tomato (Solanum lycopersicum L.). J. Food Qual. 2017, 2017, 8608304. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of Calcium Dips and Chitosan Coatings on Postharvest Life of Strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Bhanushree, L.S.; Vasudeva, K.R.; Suresha, G.J.; Sadananda, G.K.; Mohamad Tayeebulla, H.; Halesh, G.K. Influence of Chitosan on Postharvest Behavior of Papaya (Carica papaya L.) Fruits under Different Storage Conditions. J. Pharmacogn. Phytochem. 2018, 7, 2010–2014. [Google Scholar]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of Edible Chitosan Coating on Quality and Shelf Life of Sliced Mango Fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria ananassa cv Camarosa) under Commercial Storage Conditions. LWT-Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).