In order to facilitate readers’ better understanding, some language descriptions and grammar as well as the layout of some chapters have been modified. The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Missing Acknowledgments
In the original publication [1], the Acknowledgments were not included.
- Acknowledgments: G.T. acknowledges the project “RIPARTI-assegni di RIcerca per riPARTire con le Imprese”, founded by Regione Puglia (Scientific Coordinator: Bianchi). All authors acknowledge NOVUS s.r.l. (Via Enrico Fermi 18, Brindisi, Italy, e-mail: novus@novuscd.it) for providing some of the prototype components and collaborating to its assembly.
Error in Figure/Table
In the original publication, there was a mistake in the legend for Figure 1. It lacked some information for a better comprehension. The correct legend appears below.
- Figure 1. The prototype of the electrolytic generator used for the production of electrolyzed water.
In the original publication, there was a mistake in Figures 3 and 5–8 as published. In Figure 3, some words were not in English. In Figures 5–8, an incorrect standard deviation was reported, and the overall graphic quality could be improved. The corrected Figure 3 and Figure 5, Figure 6, Figure 7 and Figure 8 appear below. Furthermore, Tables 7 and 8 were modified, as in Table 7 incorrect values of EW Generator 1.0 m3/h were reported. In Table 8, incorrect cost data were reported. The corrected Table 7 and Table 8 appear below.
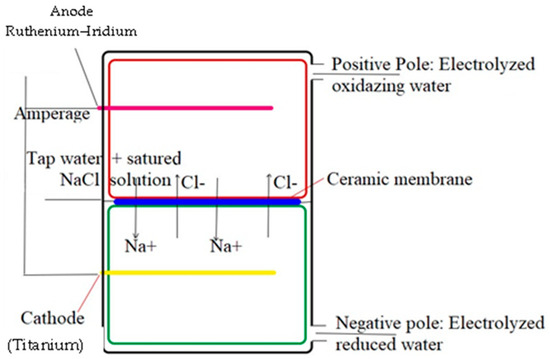
Figure 3.
Schematic representation of the electrochemical activation process of salted water [19].
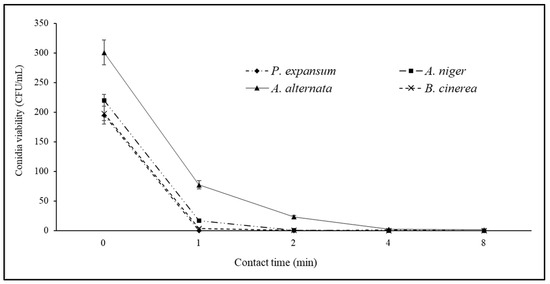
Figure 5.
Viability curves (CFU/mL) of Penicillium expansum, Aspergillus niger, Alternaria alternata, and Botrytis cinerea as a function of contact time (0–8 min) with 6% EW solution at pH 4.56 (see also Table 4).
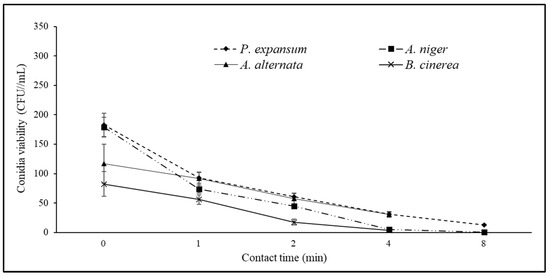
Figure 6.
Viability curves (CFU/mL) of Penicillium expansum, Aspergillus niger, Alternaria alternata, and Botrytis cinerea as a function of contact time (0–8 min) with 6% EW solution at pH 7.80 (see also Table 3).
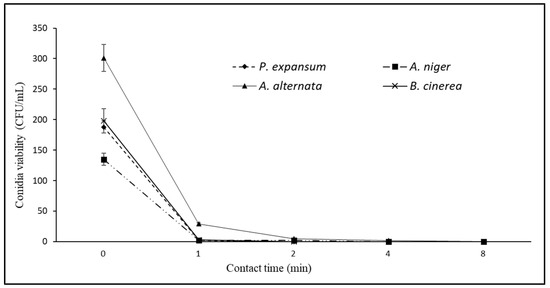
Figure 7.
Viability curves (CFU/mL) of Penicillium expansum, Aspergillus niger, Alternaria alternata and Botrytis cinerea as a function of contact time (0–8 min) with 6% EW solution at pH 4.38 (see also Table 2).
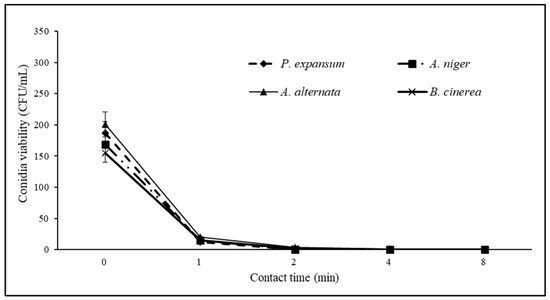
Figure 8.
Viability curves (CFU/mL) of Penicillium expansum, Aspergillus niger, Alternaria alternata and Botrytis cinerea as a function of contact time (0–8 min) with 3% sodium hypochlorite sanitizing solution.

Table 7.
Energy parameters related to the production of: EW, industrial NaClO2, and NaClO2 at 14%.

Table 8.
Energy and economic parameters relating to the dilution in a sanitizing solution of 1.0 m3.
Additional Affiliation
In the published publication, there was an error regarding the affiliation of Simona Marianna Sanzani, Gianluca Tanucci and Biagio Bianchi. In replacement to affiliation 1, the updated affiliation should include: Department of Soil, Plant and Food Sciences, University of Bari Aldo Moro, Via Amendola 165/A, 70126 Bari, Italy. Furthermore, for Simona Marianna Sanzani the e-mail address should be updated to simonamarianna.sanzani@uniba.it.
Addition of an Author
Antonio Ippolito was not included as an author in the original publication. The corrected Author Contributions statement appears here.
- Author Contributions: Conceptualization, S.M.S., A.I., P.C. and B.B.; Data curation, S.M.S., P.C. and G.T.; Formal analysis, S.M.S., P.C. and B.B.; Investigation, S.M.S., P.C., G.T., F.G. and B.B.; Methodology, S.M.S., A.I., P.C. and B.B.; Project administration, B.B.; Resources, S.M.S., A.I. and B.B.; Supervision, B.B.; Validation, S.M.S., A.I., G.T., F.G., P.C. and B.B.; Writing—original draft, G.T.; Writing—Review and Editing, S.M.S., A.I., P.C., F.G. and B.B. All authors have read and agreed to the published version of the manuscript.
Text Correction
In the original publication, the taxonomic name of the phytopathogens was incorrectly reported. Furthermore, they were referred to as bacteria, while they are fungi. This information has been corrected all through the text. Other corrections have been made to reduce typos and improve clarity. A consistent correction has been made to the Material and Methods Section, Section 2.3, the Results Section, Section 3.2, and the Conclusions section.
2.3. Antifungal Assays
EW was tested in vitro against some of the fungal pathogens that cause rot on fruits and vegetables during storage. Strains of Penicillium expansum (Pex04), Aspergillus niger (ASP03), Alternaria alternata (A20), and Botrytis cinerea (Bc28) from the culture collection of the laboratory of Postharvest Pathology of DiSSPA (University of Bari Aldo Moro, Italy) were cultivated on potato dextrose agar (PDA, Oxoid, Milan, Italy) for 7 days at 24 ± 1 °C. The inoculum was prepared by flooding the plates with 5 mL of 0.01% Tween 20 (Merk, Milan, Italy), gently scraping the surface of the colony using a sterile spatula and passing the suspension through two layers of sterile gauze. Conidia concentration was measured using a Thoma counting chamber (HGB Henneberg-Sander GmbH, Lutzellinden, Germany) and adjusted to the required concentration with sterile distilled water.
The effect of electrolyzed solutions on the conidia viability of the different phytopathogens was evaluated by direct contact. Briefly, the spore suspensions were put in contact with EW for a time ranging from 1 to 8 min in a final volume of 1 mL (final conidial concentration 3 × 103 conidia/mL). Sodium hypochlorite at 3% and sterile distilled water were used as controls. For each time point, an aliquot of 100 µL of that contact mix was spread on three semi-selective PDA dishes (amended with ampicillin and streptomycin, 250 mg/L each), then incubated for 2–3 days at 24 ± 1 °C before counting the Colony Forming Units (CFU)/mL.
3.2. Data from Antifungal Assays
Figure 5, Figure 6 and Figure 7 refer to the best results obtained in the fungal viability tests, performed with 6% diluted EW with prototype controller set at pH 1–10, which are related to the contact times and the dilution of EW used, considering the following goals: high inhibition rates of phytopathogen viability, reduced contact times with the sanitizing solution, and minimal volumes of EW to be used in the sanitizing solution.
When the controller was set to pH 5, compared to the control consisting of pathogen conidia incubated in the presence of sterile distilled water, the EW diluted at 6%, corresponding to pH 4.56, EOP 188 mV, and free chlorine 3.40 mg/L (Table 4), allowed, just after 1 min of contact, a reduction in A. alternata viability of 90%, which reached 100% after 2 min of contact (Figure 5). For the other tested fungi, 1 min of contact was sufficient to completely prevent conidia germination (Figure 5).
When the controller was set to pH 10, the EW diluted at 6% with pH 7.80, EOP −41.7 mV, and free chlorine 3.86 mg/L (Table 3) provided a reduction in the pathogen viability up to 80% after 4 min of contact and up to 90% after 8 min of contact (Figure 6).
When the controller was set to pH 1, the EW diluted at 6%, with pH 4.38, EOP 155.7 mV, and free chlorine 2.29 mg/L (Table 2) provided intermediate results: reduction in the viability by 90% for P. expansum, A. niger, B. cinerea, and 74% for A. alternata after 1 min of contact, reduction of 100% after 2 min of contact for P. expansum, A. niger, B. cinerea, and 4 min for A. alternata (Figure 7).
Therefore, the best results were recorded when the controller was set at pH 5 corresponding to an actual pH of 4.56 and a free chlorine value of 3.40 mg/L.
The results may be due to the acidic pH, which caused a greater sensitivity of the cellular membranes of the pathogenic conidia, altering their physiology, hindering replication, and allowing the penetration of acidic compounds [38]. Additionally, a high EOP might influence the production of metabolic compounds such as ATP; the oxidizing compounds could damage the cellular lipid membranes, denature proteins, hinder their reproduction, and degrade DNA, thereby inhibiting enzymatic activity [39].
Therefore, although further large-scale trials are needed, the EW at 6% dilution, with pH 4.56, EOP 188 mV, and free chlorine 3.40 mg/L seems promising against phytopathogens causing rots on fresh fruits and vegetables; this could meet industrial sanitization needs for fresh fruits and vegetables and ensure continuity in washing lines, thanks to contact times ≤ 2 min. This type of EW yielded comparable results to those obtained using a sodium hypochlorite sanitizing solution at 3%; in laboratory tests, this solution proved to be effective against the studied pathogens (Figure 8) and is one of the most used sanitizing solutions in the industrial fruit and vegetable sector.
4. Conclusions
EW demonstrated strong antifungal activity, effectively inhibiting common postharvest fungal pathogens such as P. expansum, A. niger, A. alternata, and B. cinerea. With a pH of 4.56, an EOP of 188 mV, and 3.40 mg/L of free chlorine, EW achieved a 90% reduction in pathogen viability within 1 min and 100% within 2 min, showing comparable or superior efficacy to a 3% sodium hypochlorite solution and making it a viable alternative for sanitizing the fresh produce. The production of EW was found to be less energy-intensive compared to the industrial production of sodium hypochlorite, particularly with the discontinuous operation of EW systems in industrial settings. The specific energy consumption for EW production was 0.11 kWh/L, while the cost of producing EW 2.51 €/m3 compared to sodium hypochlorite 56.05 €/m3. However, the cost is offset by the potential for reusing washing water and the reduced environmental impact. Additionally, EW systems offer flexibility in sanitization processes due to their rapid action and ability to be integrated into existing washing lines. The prototype results suggest that industrial-scale EW production could meet sanitization needs efficiently with lower environmental and health impacts. For example, it could be efficiently coupled with renewable energies such as sunlight. In conclusion, EW is a promising alternative to conventional chemical disinfectants in the food industry, particularly for the postharvest treatment of fresh fruits and vegetables. Its effectiveness, coupled with environmental and potential long-term economic benefits, supports further development and adoption in industrial applications. The results of this study also provide useful insights for the design of machines for EW production, concerning energy usage, control systems, and operating parameters of the electrolytic cell and pumps.
Addition of Citations
Nine references listed below have been added. With this correction, the order of some references has been adjusted accordingly.
Ref. [3], Manzocco, L.; Ignat, A.; Anese, M.; Bot, F.; Calligaris, S.; Valoppi, F.; Nicoli, M.C. Efficient management of the water resource in the fresh-cut industry: Current status and perspectives. Trends Food Sci. Technol. 2015, 46, 286–294.
Ref. [4], Gombas, D.; Luo, Y.; Brennan, J.; Shergill, G.; Petran, R.; Walsh, R.; Hau, H.; Khurana, K.; Zomorodi, B.; Rosen, J.; et al. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Protect. 2017, 80, 312–330. https://doi.org/10.4315/0362-028X.JFP-16-258.
Ref. [5], López-Gálvez, F.; Tudela, J.A.; Gil, M.I.; Allende, A. Use of chlorine dioxide to treat recirculated process water in a commercial tomato packinghouse: Microbiological and chemical risks. Front. Sustain. Food Syst. 2020, 4, 42.
Ref. [14], Tak, S.; Vellanki, B.P.; Ahuja, S. A review on disinfection and disinfection byproducts. In Contaminants in Our Water: Identification and Remediation Methods; ACS Publications: Washington, DC, USA, 2020; pp. 105–117.
Ref. [34], Yan, P.; Daliri, E.B.M.; Oh, D.H. New clinical applications of electrolyzed water: A review. Microorganisms 2021, 9, 136.
Ref. [35], Chisholm, G.; Zhao, T.; Cronin, L. Hydrogen from water electrolysis. In Storing Energy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 559–591.
Ref. [36], Ehyaei, M.A.; Baloochzadeh, S.; Ahmadi, A.; Abanades, S. Energy, exergy, economic, exergoenvironmental, and environmental analyses of a multigeneration system to produce electricity, cooling, potable water, hydrogen and sodium-hypochlorite. Desalination 2021, 501, 114902.
Ref. [40], Li, K.; Fan, Q.; Chuai, H.; Liu, H.; Zhang, S.; Ma, X. Revisiting chlor-alkali electrolyzers: From materials to devices. Trans. Tianjin Univ. 2021, 27, 202–216.
Ref. [41], Cheng, X.; Wang, S.; Huang, W.; Wang, F.; Fang, S.; Ge, R.; Zhang, Q.; Zhang, L.; Du, W.; Fang, F.; et al. Current status of hypochlorite technology on the wastewater treatment and sludge disposal: Performance, principals and prospects. Sci. Total Environ. 2022, 803, 150085.
Reference
- Sanzani, S.M.; Catalano, P.; Tanucci, G.; Giametta, F.; Ippolito, A.; Bianchi, B. Design and Construction and Energy Consumption Study of a New Electrolyzed Water Cell Generator Prototype for Food Disinfection. Sci 2024, 6, 43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).