Abstract
The umbilical cord, comprising three vital blood vessels, serves as the lifeline between mother and fetus. Prenatal care emphasizes detailed ultrasound examinations of the umbilical cord and postnatal inspections of the placenta and cord to preemptively address potential complications. Studies have consistently shown a significant link between a single umbilical artery and unfavorable perinatal consequences, such as mortality and congenital abnormalities. Conversely, the impact of additional vessels remains uncertain. This review is dedicated to enhancing our understanding and refining diagnostic and therapeutic approaches in prenatal healthcare. The objective is to identify knowledge gaps and propose evidence-based solutions to improve care for pregnant women and their unborn babies. The presence of a single umbilical artery in prenatal diagnosis may signify potential risks for fetal anomalies and adverse pregnancy outcomes such as hemodynamic instability, ischemia, and an increased likelihood of intrauterine growth restriction. Additionally, even the presence of supernumerary vessels may be associated with fetal malformations. Serial fetal evaluations are recommended for detecting anomalies and monitoring fetal growth throughout pregnancy. Despite the generally benign nature of isolated SUA and supernumerary vessels, close monitoring and comprehensive prenatal care are essential to ensuring optimal outcomes for both mother and baby.
1. Introduction
The umbilical cord is a deciduous anatomical formation that allows the attachment between mother and fetus, from the center of the placental bulk onto the umbilicus of the fetus [1]. The amniotic membrane crafts a cylindrical casing that houses a pair of umbilical arteries and a single umbilical vein in the normal umbilical cord. This arrangement facilitates the conveyance of oxygen and vital nutrients from the maternal blood supply to the fetal circulatory system [2], simultaneously eliminating waste products from the fetal circulation. The number of vessels in the umbilical cord can be easily evaluated at the site of insertion of the cord in the fetus using color doppler to identify the vessels around the bladder or, alternatively, identifying a free ring of the umbilical cord in cross-section [3]. Irregularities concerning the quantity of vessels can manifest in both the umbilical artery and vein, appearing either in surplus or deficiency. The single umbilical artery is the most common abnormality of the umbilical cord vessels, found in about 1% of single pregnancies [4] and up to 5% in twin gestations [5,6]. The finding of a single umbilical artery is probably a consequence of a primary agenesis or thrombotic atrophy of an umbilical cord artery [7] and is strongly associated with the risk of perinatal death, stillbirth, and fetal malformations [8,9] (mainly in the cardiovascular, gastrointestinal, and genitourinary systems). In addition, if there are diffuse abnormalities in multiple organs, the risk of chromosomal abnormalities increases considerably [10]. The term “supernumerary umbilical vessels” refers to a specific state characterized by the presence of four or more umbilical cord vessels resulting from an additional artery or vein. Meyer et al. [11], despite there being no specific incidence of this rare finding, stated that the likelihood of encountering an additional fourth vessel within the umbilical cord is greater than the likelihood of encountering a solitary umbilical artery. A high frequency of fetal irregularities and unfavorable obstetric results is associated with the occurrence of an extra umbilical vein [12]. However, various studies have documented instances where a supernumerary umbilical cord, comprising two arteries and two veins, was present without any fetal abnormalities [13].
2. Materials and Methods
An initial systematic exploration of databases such as Medline, PubMed, and Scopus was undertaken. Publications without a limit on the timeframe were selected. The following set of search terms were included: Pathological number of vessels in the umbilical cord OR Single umbilical artery OR Four vessels umbilical cord AND Perinatal outcomes OR Fetal malformations OR Chromosomal abnormalities (Title/Abstract). Following the removal of duplicate entries, the authors proceeded with a preliminary review of titles and abstracts to evaluate their alignment with the review’s objectives. This preliminary phase involved sifting through titles and abstracts, culminating in the selection of 21 pertinent articles (Figure 1). These chosen studies form the bedrock of a narrative review designed to dissect and elucidate the nuanced impacts that anomalies in umbilical cord vessel count exert on perinatal outcomes.
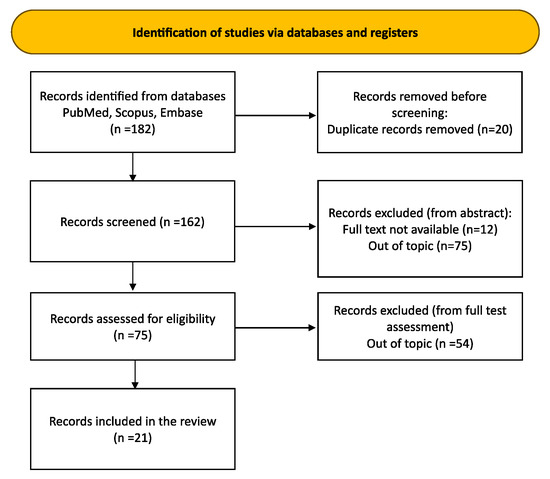
Figure 1.
Article selection flow chart.
3. Results and Discussion
Table 1 summarizes the main results of the included studies.
Clausen et al. [14] conducted a comprehensive review, focusing on the correlation between umbilical cord structural abnormalities and antenatal fetal demise. They looked at 9 studies with a total of over 2500 births and found a strong link between single umbilical artery (SUA) and fetal anomalies. This link accounted for 19.2% of cases and frequently led to perinatal death at a rate of 6.4%. However, there were no notable differences in birth weights or prematurity rates among infants with SUA delivered normally, contradicting some previous claims [15]. The authors highlighted that the presence of SUA in the umbilical cord could impede fetal circulation, leading to blood flow resistance and potential fetal oxygen deprivation. They emphasized the importance of specialized obstetric care for fetuses with SUA to reduce the risk of intrauterine mortality, especially given the early detection capabilities of ultrasound examination [16]. Furthermore, three individual instances of dual umbilical vein cords were documented. While one neonate had an uneventful delivery [17], the others presented with various congenital anomalies [12,18]. However, Meyer et al. [11] and Nadkarni et al. [19] discovered small extra vessels in umbilical cords, unrelated to any problems, suggesting that they could be remnants of early development.
Lei et al. [20] detailed a case involving a four-vessel umbilical cord consisting of two arteries, two veins, and a vein varix supplying the right portal vein. A physical examination revealed no abnormalities in the newborn, a healthy male born at 38 weeks and 5 days gestation, with Apgar scores of 10/10 at both 1 and 5 min. After reviewing the existing literature, the authors discovered severe congenital abnormalities associated with the presence of a supernumerary umbilical cord vessel. Three of the eight cases showed abnormalities in the venous system, and one case had a single umbilical artery. Four of the newborns died at birth, a recorded adverse outcome.
Even though this case had a good outcome, the authors stressed that a supernumerary vein and vein varix are some signs of bad prenatal outcomes, especially when they are present with other congenital abnormalities. Additionally, upon detecting umbilical cord abnormalities, they advocated for a comprehensive prenatal examination and ultrasound follow-up to exclude further congenital anomalies.
Arora et al. [21] presented a case involving a full-term neonate born through spontaneous vaginal delivery at 38 weeks of gestation with a prior prenatal diagnosis of a four-vessel umbilical cord (comprising two arteries and two veins). The infant was admitted to the Neonatal Intensive Care Unit (NICU) due to meconium-stained fluid and respiratory failure, with Apgar scores of 5, 6, and 8 at 1, 5, and 10 min, respectively. An echocardiogram conducted the day after birth revealed a mild hypoplasia of the aortic arch without signs of aortic coarctation, along with a small oval foramen. A month later, a follow-up examination found no abnormalities except for the persistent oval foramen.
The authors underscored the importance of thorough neonatal evaluations, including a detailed examination of the umbilical cord. In cases where pathological abnormalities such as supernumerary vessels are detected, they recommend abdominal and cardiac ultrasound examinations to rule out any associated malformations.
In a case study by Pérez-Cosio et al. [13], a neonate with a prenatal ultrasound diagnosis of a 4-vessel umbilical cord was delivered via cesarean section at 38 weeks and 3 days gestational age. At birth, the infant weighed 3240 g and had normal Apgar scores (8 and 9 at 1 and 5 min), as well as normal cord gas values. Physical examinations during hospitalization and at an 18-month pediatric follow-up revealed no anomalies. Upon reviewing cases from the literature [22,23,24,25,26,27,28,29,30], the authors noted that structural abnormalities were only present in half of the newborns with supernumerary vessels in the umbilical cord. Therefore, the authors emphasized the importance of fetal ultrasound for the early detection of a multivascular umbilical cord, while also acknowledging that it may not always be associated with unfavorable outcomes.
In a case study conducted by Kurakazu et al. [26], a healthy infant girl, weighing 2726 g, was delivered spontaneously by a 37-year-old Japanese woman. The newborn received Apgar scores of 9 at 1 min and 10 at 5 min following birth. Upon an inspection of the umbilical cord, the fetal attachment site revealed four vessels (two arteries and two veins), while the placental attachment site revealed the typical three vessels (two arteries and one vein). Notably, antenatal fetal ultrasound screenings detected no additional congenital abnormalities, and this remained consistent upon birth. The authors stressed the challenge in identifying supernumerary umbilical veins, advocating for comprehensive cord analysis by the second trimester. This should include examinations at key sites to improve anomaly detection and facilitate appropriate management during pregnancy.
Gestational hypertension and oligohydramnios complicated a 27-year-old woman’s pregnancy in Garg et al.’s case study [27], necessitating an emergency cesarean section at 34 weeks due to fetal distress. Despite concerns about potential anomalies associated with umbilical cords featuring five or more vessels, the newborn, weighing 1.6 kg, showed stability without congenital issues. Placental histopathology revealed chorangiosis, characterized by additional blood vessels in the placental tissue. The umbilical cord exhibited four arteries, one vein, and omphalomesenteric duct remnants, consistent with chorangiosis. Clinicians considered the broader clinical context, including the patient’s conditions, in deciding on the cesarean section. This case emphasizes the importance of comprehensive evaluation and personalized management in obstetric care, indicating the need for a nuanced approach beyond singular anatomical findings [28].
In their report, Puvabanditsin and colleagues [29] documented a unique case involving an infant with a four-vessel umbilical cord, comprising two arteries and two veins. The infant, delivered by cesarean section due to fetal distress, weighed 2730 g and was born at 39 weeks of gestation, with Apgar scores of 8 and 9 at 1 and 5 min, respectively. Prenatal sonography conducted at 17 weeks and 2 days revealed multiple fetal abnormalities—notably, cerebellar vermis agenesis, abnormal cardiac anatomy, and heterotaxy syndrome. These problems were confirmed by postnatal sonography, which also found dextrocardia, situs ambiguous, a right-dominant atrioventricular canal, a common atrium, and a hypoplastic left ventricle. Comparing their findings with the existing literature [26,27,28,29], the authors noted varied outcomes. Two of the six analyzed case reports reported serious congenital abnormalities in fetuses, while others either reported no fetal anomalies or lacked comprehensive screening for anomalies. This underscores the significance of comprehensive screening upon identifying umbilical cord abnormalities, which guarantees the prompt detection and suitable handling of potential fetal anomalies.
A study by Aoki et al. [30] at Shimane Medical University Hospital (1993–1995) aimed to explore the correlation between umbilical cord vessel abnormalities and fetal malformations. They examined 444 pregnant Japanese women (24–32 weeks of gestation) using routine obstetric ultrasound. Doppler ultrasonography was employed to detect aberrant umbilical vessels. Prenatal sonography revealed three cases of discordant umbilical artery, two cases of four vessels in the umbilical cord (FVUC), and a single case of a single umbilical artery (SUA), totaling six cases (1.4%). Two (33.3%) of these cases had fetal anomalies. Notably, all infants with aberrant umbilical vessels were female, though the reason remained unexplained, likely due to the limited sample size. The authors concluded that a routine ultrasound screening of the umbilical cord can aid in the prenatal diagnosis of fetal abnormalities.
In a case report by Beck et al. [31], a neonate was delivered vaginally at 42 weeks by an 18-year-old woman after receiving antibiotics for fever. Weighing 4200 g, the newborn received favorable Apgar scores. Post-birth, the infant developed septic shock due to an E. coli infection detected in the blood and cerebrospinal fluid, requiring mechanical ventilation. Despite challenges, physical exams and ultrasounds revealed no abnormalities in the heart or kidneys. The authors thought that the unexpected discovery of a five-vessel umbilical cord might mean that, as the fetus grows, the internal iliac artery might grow extra branches that reach the navel, creating extra umbilical arteries. This hypothesis sheds light on the potential mechanisms behind the observed umbilical cord anomaly.
In an 18-month study by Abuhamad et al. [32], the correlation between specific umbilical artery absence and congenital anomalies in fetuses with a single umbilical artery (SUA) was explored. Initial ultrasounds identified 77 SUA cases, confirmed by a second operator. The ultrasound findings classified them into three categories: no anomalies, additional anomalies, or complex abnormalities. Results showed that 73% had a missing left artery, with 29% anomalies, while 27% had a missing right artery, with 19% anomalies. Among these, 55% had isolated anomalies, and 45% had complex ones, mostly with the left artery missing. Cytogenetic analysis revealed trisomy 18 in four cases, trisomy 4 in one case, and triploidy in one case, all of which were associated with the absence of the left artery. Targeted ultrasonography and genetic counseling were recommended for infants with malformational anomalies showing karyotype alterations, according to the authors.
Cairns et al. [33] conducted a prospective study at Toronto East General Hospital from April to October 1962 to explore the occurrence of a single umbilical artery (SUA) and its link to congenital abnormalities. Out of 2024 deliveries, 20 infants had SUA, but only 2 had accompanying congenital malformations initially overlooked during neonatal examination. Conversely, 31 babies with the normal 2 umbilical arteries displayed congenital malformations. Previous research has linked the association between SUA and congenital anomalies to oxygen deprivation during organ development. However, the study findings suggest that most infants with SUA do not manifest congenital malformations, indicating adequate compensation by the single artery. The study’s results, which revealed a higher incidence of fetal malformations in infants with the typical two umbilical arteries, challenged the hypothesis that suggests SUA as a part of a broader spectrum of organ malformations.
In a retrospective study by Saller et al. [34], conducted at the University of Maryland between 1982 and 1989, the potential link between a single umbilical artery and aneuploidy was investigated. Based on amniocentesis and tissue culture samples, an analysis of chromosomally abnormal pregnancies found 109 cases, but only 53 (48.6%) had full pathological follow-up.
Six (11.3%) of these cases exhibited a significant association (p = 0.033) between a single umbilical artery and specific cytogenetic abnormalities—notably, trisomy 18 in two cases (33.3%) and trisomy 13 in another two cases (33.3%). Interestingly, none of the 18 cases of trisomy 21 within the sample exhibited a single umbilical artery. The authors concluded that prenatal ultrasound examinations could benefit from assessing the number of umbilical vessels when diagnosing congenital malformations, as this information might guide cytogenetic diagnosis effectively.
Battarbee et al. [35] conducted a retrospective cohort investigation at Northwestern Memorial Hospital with the aim of resolving inconsistencies in previous research regarding the potential link between a singular umbilical artery (SUA) and two adverse perinatal outcomes: being small for gestational age (SGA) and preterm birth [36,37,38,39,40,41]. Their study defined SGA as infants born with weights below the 10th percentile and delineated preterm birth as deliveries transpiring prior to 37 completed weeks of gestation due to medical indications [42,43,44,45]. They compared 219 pregnancies with isolated SUA with 219 pregnancies with normal umbilical cords from 2007 to 2014. Exclusion criteria included aneuploidy, multifetal pregnancies, and deliveries outside the hospital. Results showed a significant association (p < 0.001) between SUA and lower birth weight, with a higher incidence of SGA, often linked to pregnancy-induced hypertension. Infants with SUA weighed 284 g less on average than the control group, suggesting potential issues with placental function rather than improved blood flow through the remaining umbilical artery [42,43,44,45].
Predanic et al. [36] conducted a retrospective case–control study to investigate if prenatally diagnosed isolated single umbilical artery (SUA) correlated with a higher incidence of small for gestational age (SGA) at birth. Analyzing 84 SUA pregnancies at Weill Medical College of Cornell University, New York, from July 1999 to September 2004, they compared them with matched infants with confirmed three-vessel umbilical cords. Exclusions included fetal malformations, chromosomal abnormalities, multifetal pregnancies, and the absence of SUA at delivery. Results showed no significant difference in mean birth weights between SUA and control groups, with comparable rates of SGA (7.1% vs. 4.8%). Prenatal ultrasound revealed fetal growth restriction in 50% of SUA cases and 25% of controls.
Johnson et al.’s 2003 review [46] examined two-vessel cords, known as single umbilical arteries (SUA), and their link to genitourinary anomalies. Studies revealed that SUA occurs in approximately 0.63% of singleton pregnancies. Factors like maternal age, neonatal sex, multiple gestations, aneuploidy, race, maternal habits (e.g., smoking, drug use), and ethnicity influence SUA development. While maternal age and neonatal sex show no significant impact, maternal drug use, smoking, and multiple gestations elevate the risk. Ethnicity also plays a role, with Eastern Europeans having higher SUA rates and Japanese having lower rates. Genitourinary anomaly incidence varies widely (0–33%) [41,47,48,49], with vesicoureteral reflux commonly associated, while other defects are typically minor and non-life threatening.
The 2010 retrospective cohort study [41] by Hua et al. looked into whether pregnancies with a single umbilical artery (SUA) had a higher risk of intrauterine growth restriction (IUGR) and kidney and heart problems compared to pregnancies with two umbilical arteries. Analyzing data from Washington University from 1990 to 2007, they found 392 singleton SUA pregnancies, with 281 isolated defects and 111 associated malformations. While maternal age, gravidity, and preeclampsia rates were similar, SUA pregnancies were associated with smoking, chronic hypertension, and pre-existing diabetes. Multivariate analysis revealed a twofold increased risk of IUGR with SUA, even after excluding other anomalies. Due to the heightened IUGR risk, the study recommends serial growth assessments for SUA pregnancies to manage and time delivery appropriately.
In a retrospective clinical trial led by Heifetz et al. [47], the Division of Pathology at Cincinnati Children’s Hospital Medical Center in Cincinnati aimed to compare the clinical and placental characteristics of placentas with a single umbilical artery (SUA) to those with a three-vessel umbilical cord. Histological analysis was conducted on 55 consecutive SUA placentas (Group 1) and 655 3-vessel umbilical cord placentas (Group 2) using hematoxylin and eosin (H&E) staining and CD 34 immunostaining, following Amsterdam criteria. Group 1 exhibited a higher prevalence of variable decelerations, thin umbilical cords, edematous cords, and velamentous insertion compared to Group 2. Group 2 was more likely to have hypercoiled dual umbilical arteries. In Group 1, high-grade distal fetal vascular malperfusion (FVM) was more common. New distal villous malperfusion showed clustered endothelial fragmentation, and established malperfusion showed hyalinized and avascular clusters. SUA was associated with an increased risk of various types of distal villous FVM, suggesting a distinct pathogenesis that could potentially link to fetal issues associated with SUA. The topic of vascular malperfision is recurrent in the included studies and deserves, according to the authors, major attention and further investigation in the case of sovrannumerary vessels in the umbilical cord.
In 1995, Parilla et al. [50] conducted a retrospective cohort study on pregnancies with a single umbilical artery (SUA), analyzing data from January 1991 to June 1994. Among 50 cases, 17 (34%) underwent invasive prenatal diagnosis, with normal findings, except for 1 neonate diagnosed with anomalous pulmonary venous return. Despite complications like placenta previa and preterm labor at 32 weeks and 4 days, this neonate thrived without issues at 3.5 years with a normal karyotype. Although SUA has occasionally been associated with congenital malformations, a consistent pattern has yet to emerge, according to the authors. Thus, detecting isolated SUA should prompt a thorough ultrasound examination to rule out associated anomalies. The authors recommend against genetic testing unless other anomalies are present, as the risk of chromosomal abnormality with isolated SUA cannot be determined. Remarkably, the study’s incidence of small for gestational age (SGA) babies (12%) and preterm delivery (14%) aligned with expected frequencies in the general population.
In their 2010 retrospective analysis, Horton et al. [38] compared 68 pregnancies with an isolated single umbilical artery (SUA) to 68 pregnancies with a standard three-vessel cord (3VC). The study aimed to ascertain if isolated SUA pregnancies carried a higher risk of adverse perinatal outcomes. Neonates with isolated SUA showed significantly lower birth weights (3279 +/− 404 g vs. 3423 +/− 374 g, p = 0.016) and a lower ponderal index (PI) compared to their 3VC counterparts (p = 0.001). Though rates of small for gestational age (SGA) infants did not significantly differ (17.6% vs. 8.8%, p = 0.06), SUA neonates had longer NICU stays. Findings suggest that infants with isolated SUA face heightened risks of prolonged NICU stays and impaired intrauterine growth. The authors recommended serial assessments for fetal growth, despite not finding any disparity in SGA rates. Moreover, the authors propose further studies to explore postnatal catch-up growth and long-term health implications in SUA neonates.
In 2011, Kondi-Pafiti et al. [51] analyzed 1570 stillborn fetuses for a study at the Pathology Laboratory of Aretaieion University of Athens. Of these, 24 cases exhibited a single umbilical artery (SUA), indicating a 1.6% incidence rate. Notably, 21 of these cases presented complex congenital anomalies, with 5 showing chromosomal anomalies. A histological study of the placenta showed important findings, such as large infarcts, signs of prematurity, severe chorioamnionitis, fibrin accumulation, and chorioangiosis. Fetal gestational ages ranged from 15 to 33 weeks, and maternal ages spanned from 17 to 44 years. The authors proposed thrombotic regression as a potential pathogenesis, leading to hemodynamic instability and fetal ischemia. They emphasized the importance of comprehensive fetal evaluation for congenital anomalies in the cases of SUA prenatal diagnosis, recommending thorough ultrasound and karyotyping investigations alongside Doppler ultrasound for detecting intrauterine growth restriction (IUGR) in the third trimester, enabling the early detection and appropriate management of associated fetal complications.
In 1996, Moore et al. [52] documented four cases of intra-abdominal fetal umbilical vein abnormalities at the Hospital for Sick Children, Toronto, Canada. These included diverse anomalies such as a persistent right umbilical vein, an abnormal umbilical vein descending and joining the right iliac vein, an extrahepatic umbilical vein varix, and a dilated intra-abdominal umbilical vein. Based on their characteristics, the authors classified these abnormalities into three groups. Additional cases reported by Jeanty et al. [53] exhibited varying associations with congenital malformations, including fetal mortalities. Understanding these rare abnormalities is crucial due to the differences in fetal risks associated with the timing of detection and the presence of other malformations during pregnancy.
There are some potential constraints to consider regarding the findings of our review on irregularities in the number of vessels in the umbilical cord.
Study Heterogeneity: The review includes 21 studies that may have varying techniques, demographics, and definitions of results. This diversity might result in inconclusive findings and hinder the generalization of conclusions across different contexts. Moreover, in such reviews, if any of the studies included in the analysis are retrospective, there is a possibility of inherent biases, such as selection bias and recall bias, which might impact the reliability of the data.
Population Disparities: The investigations may have been carried out in diverse populations with distinct genetic, environmental, and socio-economic aspects. The variations in these factors can impact the occurrence of single umbilical artery (SUA) and extra blood vessels, as well as the related results, making it difficult to apply the findings uniformly.
The accuracy of finding SUA and supernumerary vessels is contingent upon the quality of prenatal evaluations, including ultrasonography and karyotype analysis, which can vary. Variations in reported incidence and results may arise from differences in technology, operator competence, and diagnostic criteria among studies.
Emphasize Isolated SUA: Although the evaluation acknowledges the significance of isolated SUA, it may not comprehensively cover situations where SUA is accompanied by additional abnormalities. The potential impact of the interplay between SUA and other fetal disorders on risk profiles and outcomes has not been extensively investigated in this research.
The review primarily emphasizes short-term neonatal outcomes, including birth weight and the duration of stay in the Neonatal Intensive Care Unit (NICU). Nevertheless, the comprehensive evaluation of the lasting developmental and health consequences in newborns with SUA or supernumerary arteries may be insufficient, thereby impeding a complete comprehension of the overall effects of these abnormalities.
Clinical Interventions and Management: The review indicates that customized management techniques are crucial; however, it may not thoroughly examine the impact of various management approaches on results. The absence of established protocols for addressing SUA and supernumerary vessels may impact the uniformity of care and results in various healthcare settings.
Potential Confounding Factors: Some of the studies may not have taken into consideration other factors that could have influenced the results, such as maternal health, lifestyle, or pre-existing conditions, which could have potentially distorted the findings.
It is important to take into account these limitations while analyzing the findings of the review and implementing them in clinical settings.

Table 1.
Main findings of the included studies.
Table 1.
Main findings of the included studies.
| Article | Year of Publication | Study Design | Ethnicity and/or Study Place | Results | |
|---|---|---|---|---|---|
| 1 | Clausen et al. [14] | 1989 | Comprehensive Review | Danish Department of Obstetrics and Gynecology, Aarhus Municipal Hospital, University of Aarhus, Denmark | -SUA linked to 19.2% fetal anomalies and 6.4% perinatal death. -No differences in birth weight or prematurity for SUA infants delivered normally. |
| 2 | Lei et al. [20] | 2017 | Case Report And Literature Review | Chinese Department of Ultrasonic Medicine, Fetal Medical Centre, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China | -Supernumerary vein and vein varix signal poor prenatal outcomes, especially with other congenital abnormalities. |
| 3 | Arora et al. [21] | 2022 | Case Report | Indian Paediatrics, Government Medical College & Hospital, Chandigarh, Punjab, India MultiCare Tacoma General Hospital, Tacoma, Washington, USA | -A four-vessel umbilical cord may be linked to multiple congenital malformations. |
| 4 | Pérez-Cosio et al. [13] | 2008 | Case Report And Literature Review | Caucasian Department of Obstetrics and Gynecology, Rush University Medical Center, Chicago, Illinois USA (C.P.-C., E.S., J.S.A.); and Department of Obstetrics and Gynecology, Soroka University Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel (E.S.). | -Structural abnormalities were present in half of the newborns with supernumerary vessels in the umbilical cord. |
| 5 | Kurakazu et al. [26] | 2019 | Case Report | Japanese Department of Obstetrics and Gynecology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan | -Supernumerary vessels of the umbilical can be associated with fetal congenital anomalies. |
| 6 | Garg et al. [27] | 2018 | Case Report | Indian University College of Medical Sciences, Guru Teg Bahadur Hospital, Clinic of Pathology, New Delhi, India | -Most cases of multiple vessels in the umbilical cord involve four vessels due to a persistent right umbilical vein. -Cases with five or more vessels are typically associated with conjoined twins. |
| 7 | Puvabanditsin et al. [29] | 2011 | Case Report And Literature Review | Caucasian Department of Pediatrics, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA; Department of Surgery, Downstate Medical Center, Brooklyn, New York, USA; Department of Pathology, UMDNJ-Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA | -Two out of the six analyzed case reports reported serious congenital abnormalities in fetuses |
| 8 | Aoki et al. [30] | 1997 | Cohort Study | Japanese Department of Obstetrics and Gynecology, Shimane Medical University, Izumo, Japan | -Prenatal sonography revealed three cases of discordant umbilical artery, two cases of four vessels in the umbilical cord (FVUC), and a single case of single umbilical artery (SUA), totaling 6 cases (1.4%). Two (33.3%) of these cases had fetal anomalies. |
| 9 | Beck et al. [31] | 1985 | Case Report | Caucasian Division of Neonatology, Children’s Hospital National Medical Center, Washington, D.C. | -SUA occurs in 0.5% of Black populations and 1.0% of White populations. -4% of SUA survivors have other congenital anomalies, with the rate being twice as high in Black populations compared to White populations. -Multiple artery umbilical cords are linked to infants with multiple abnormalities. |
| 10 | Abuhamad et al. [32] | 1994 | Cohort Study | Caucasian Gynecology, Eastern Virginia Medical School, and the Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Yale Univesity School of Medicine. | In fetuses with a single umbilical artery, the left artery is more often absent than the right. -Cytogenetic and complex fetal anomalies were found only in cases with an absent left artery. -Isolated single umbilical artery cases do not show an increased incidence of small for gestational age fetuses. |
| 11 | Cairns et al. [33] | 1964 | Cohort Study | Caucasian Department of Obstetrics and Gynecology, Toronto East General Hospital | -A higher incidence of fetal malformations is observed in infants with the standard two umbilical arteries. |
| 12 | Saller et al. [34] | 1990 | Cohort Study | Caucasian Divisions of Maternal-Fetal Medicine and Human Genetics, Department of Obstetrics and Gynecology, and the Department of Pathology, University of Maryland School of Medicine | -Six chromosomally (11.3%) abnormal pregnancies exhibited a significant association (p = 0.033) between a single umbilical artery and specific cytogenetic abnormalities—notably, trisomy 18 in two cases (33.3%) and trisomy 13 in another two cases (33.3%). |
| 13 | Battarbee et al. [35] | 2015 | Retrospective Cohort Study | Caucasian The Departments of Obstetrics and Gynecology and Pathology, Northwestern University Feinberg School of Medicine, Chicago, Illinois | -The presence of an isolated single umbilical artery is significantly associated with lower birth weight (3146 g compared to 3430 g) and a higher incidence of small for gestational age (11.9% compared to 2.7%). -The presence of an isolated single umbilical artery is also linked to increased rates of pregnancy-induced hypertension (7.3% compared to 1.8%) and indicated preterm delivery (5.5% compared to 0.9%). |
| 14 | Predanic et al. [36] | 2005 | Retrospective Case–Control Study | Caucasian Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Weill Medical College of Cornell University, New York, New York | -Mean birth weight was similar in both groups (3268 g vs. 3274 g), with a slightly higher prevalence of small for gestational age newborns in the isolated single umbilical artery group (7.1% vs. 4.8%). -Fetal growth restriction was observed in 50% of cases in the isolated single umbilical artery group compared to 25% in the control group. |
| 15 | Johnson et al. [46] | 2003 | Literature Review | Caucasian Department of Urology, Columbia University, New York Presbyterian Hospital, Irving Pavilion, 11th Floor, 161 Fort Washington Avenue, New York, NY 10032, USA | -Factors influencing SUA development include maternal drug use, smoking, multiple gestations, and ethnicity. Maternal age and neonatal sex have no significant impact. -Eastern Europeans have higher SUA rates, while Japanese have lower rates. -Genitourinary anomalies associated with SUA vary widely (0–33%), with vesicoureteral reflux being common, while other defects are usually minor. |
| 16 | Hua et al. [41] | 2010 | Retrospective Cohort Study | Caucasian Department of Obstetrics and Gynecology, Washington University in St. Louis, St. Louis, Missouri. | -Single umbilical artery is associated with a higher risk of renal anomalies (adjusted OR 3.0) and cardiac anomalies (adjusted OR 20.3) compared to double umbilical artery. -Single umbilical artery is also linked to an increased risk of intrauterine growth restriction (IUGR) (adjusted OR 2.1), even when excluding fetuses with known anomalies. |
| 17 | Heifetz et al. [47] | 1984 | Retrospective Clinical Trial | Caucasian Division of Pathology at Cincinnati Children’s Hospital Medical Center in Cincinnati | -SUA was associated with increased risk of various types of distal villous FVM. |
| 18 | Parilla et al. [50] | 1995 | Retrospective Cohort Study | Caucasian Section of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Northwestern University Medical School, Northwestern Memorial Hospital and Evanston Hospital, Chicago, Illinois | -The study’s incidence of small for gestational age (SGA) babies (12%) and preterm delivery (14%) aligned with expected frequencies in the general population. |
| 19 | Horton et al. [38] | 2010 | Retrospective Cohort Study | Caucasian Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, University of North Carolina, Chapel Hill, North Carolina. | -Neonates with isolated SUA had significantly lower birth weights (3279 g vs. 3423 g) and a lower ponderal index compared to those with three-vessel cords. -While the rate of small for gestational age (SGA) infants was not significantly different (17.6% vs. 8.8%), SUA neonates had longer NICU stays. |
| 20 | Kondi-Pafiti et al. [51] | 2011 | Retrospective Cohort Study | Caucasian Pathology Laboratory, 2nd Clinic of Obstetrics and Gynecology, “Aretaieion” University of Athens (Greece) | -Out of 24 stillborns with a single umbilical artery, 21 had significant placental findings such as large infarcts, signs of prematurity, severe chorioamnionitis, fibrin accumulation, and chorioangiosis. |
| 21 | Moore et al. [52] | 1996 | Case Series And Literature Review | Caucasian Department of Radiology and Prenatal Diagnosis Program, The Toronto Hospitals; Department of Pediatrics, Division of Clinical Genetics, The University of Toronto, Ontario, Canada | -Different fetal umbilical vein abnormalities were identified such as persistent right umbilical vein, an abnormal umbilical vein joining the right iliac vein, an extrahepatic umbilical vein varix, and a dilated intra-abdominal umbilical vein. |
4. Conclusions
A diverse range of results emerged from the 21 studies analyzed in this review, which aimed to elucidate the current understanding in the literature regarding the anomalies in the number of vessels in the umbilical cord.
The single umbilical artery (SUA) is caused by thrombotic events that slow down the development of the umbilical artery. This affects both the placenta’s function and the fetus’s circulation. To detect anomalies and intrauterine growth restriction (IUGR), thorough prenatal assessments, including ultrasound and karyotype analysis, are necessary. While SUA pregnancies carry a heightened risk of IUGR, the varying rates of small for gestational age (SGA) highlight the complexity of risk assessment. Isolated SUA increases vulnerability in neonates, leading to prolonged NICU stays and compromised growth. Despite similar SGA rates, neonates with SUA typically have lower birth weights and ponderal indices. The early detection of SUA is crucial for managing fetal risks and optimizing placental function. SUA also raises the likelihood of fetal vascular malperfusion (FVM), necessitating careful monitoring and intervention. In the case of supernumerary vessels in the umbilical cord, given adverse outcomes and associated malformations reported within the living literature, a close prenatal follow-up should be recommended. Tailored management strategies are essential for addressing associated anomalies and placental insufficiencies. Abnormal placental implantation in SUA and supernumerary vessels’ pregnancies contribute to adverse outcomes, underscoring the importance of comprehensive evaluation and targeted interventions to mitigate risks for both mother and fetus.
It is essential to detect single umbilical artery (SUA) and supernumerary arteries early through comprehensive prenatal evaluations in order to effectively manage any dangers to the fetus. Customized management approaches, such as vigilant observation and specific interventions, are crucial in reducing negative consequences associated with placental insufficiency and fetal growth.
Future research should prioritize the standardization of detection methods, the investigation of the long-term outcomes of infants with single umbilical artery (SUA), and the establishment of consistent guidelines for the management of SUA and supernumerary vessels. Further research should explore the correlations between SUA and other prenatal defects in order to have a more comprehensive understanding of their collective influence.
Author Contributions
Conceptualization, A.L.; methodology, L.T. and L.L.; validation, V.R.; formal analysis, A.L. and A.N.; investigation, A.L.; resources, A.L. and A.N.; data curation, V.R.; writing—original draft preparation, V.R., A.N. and A.L.; writing—review and editing, V.R. and A.L.; visualization, L.T. and L.L.; supervision, V.R. and A.L.; project administration, A.L.; funding acquisition, V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All articles included in this review are available in the public databases Scopus and PubMed.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Di Naro, E.; Ghezzi, F.; Raio, L.; Franchi, M.; D’Addario, V. Umbilical cord morphology and pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, V.L.; Dodson, R.B. Bioengineering aspects of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, S108–S113. [Google Scholar] [CrossRef]
- Bosselmann, S.; Mielke, G. Sonographic Assessment of the Umbilical Cord. Geburtshilfe Frauenheilkd. 2015, 75, 808–818. [Google Scholar] [CrossRef]
- Murphy-Kaulbeck, L.; Dodds, L.; Joseph, K.S.; Van den Hof, M. Single Umbilical Artery Risk Factors and Pregnancy Outcomes. Obstet. Gynecol. 2010, 116, 843–850. [Google Scholar] [CrossRef]
- Prabhu, M.; Kuller, J.A.; Biggio, J.R. Ultrasound Markers for Aneuploidy in the Second Trimester. In Perinatal Genetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 95–104. [Google Scholar]
- Bromley, B.; Benacerraf, B.R. Sonographic Fetal Findings with Borderline Significance. In Ultrasound of Fetal Syndromes; Elsevier: Amsterdam, The Netherlands, 2008; pp. 571–602. [Google Scholar]
- Siargkas, A.; Giouleka, S.; Tsakiridis, I.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Prenatal Diagnosis of Isolated Single Umbilical Artery: Incidence, Risk Factors and Impact on Pregnancy Outcomes. Medicina 2023, 59, 1080. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, H.; Rafeei, K.; Maryam Dalili, M.D.; Asadi, N.; Seirfar, N.; Akbarzadeh-Jahromi, M. Prevalence of single umbilical artery, clinical outcomes and its risk factors: A cross-sectional study. Int. J. Reprod. Biomed. IJRM 2021, 19, 441–448. [Google Scholar] [CrossRef]
- Ebbing, C.; Kessler, J.; Moster, D.; Rasmussen, S. Single umbilical artery and risk of congenital malformation: Population-based study in Norway. Ultrasound Obstet. Gynecol. 2020, 55, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Dagklis, T.; Defigueiredo, D.; Staboulidou, I.; Casagrandi, D.; Nicolaides, K.H. Isolated single umbilical artery and fetal karyotype. Ultrasound Obstet. Gynecol. 2010, 36, 291–295. [Google Scholar] [CrossRef]
- Meyer, W.W.; Lind, J.; Moinian, M. An accessory fourth vessel of the umbilical cord. Am. J. Obstet. Gynecol. 1969, 105, 1063–1068. [Google Scholar] [CrossRef]
- Painter, D.; Russell, P. Four-vessel umbilical cord associated with multiple congenital anomalies. Obstet. Gynecol. 1977, 50, 505–507. [Google Scholar]
- Pérez-Cosio, C.; Sheiner, E.; Abramowicz, J.S. Four-Vessel Umbilical Cord. J. Ultrasound Med. 2008, 27, 1389–1391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clausen, I. Umbilical Cord Anomalies and Antenatal Fetal Deaths. Obstet. Gynecol. Surv. 1989, 44, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Cederqvist, L. Die Bedeutung Des Fehlens Einer Arterie in der Nabelschnur. Acta Obstet. Gynecol. Scand. 1970, 49, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.M.; Breckle, R.; Wolfgram, K.R. An Ultrasonic View of the Developing Fetus. Obstet. Gynecol. Surv. 1983, 38, 375–398. [Google Scholar] [CrossRef]
- Murdoch, D.E. Umbilical-Cord Doubling. Obstet. Gynecol. 1966, 27, 555–557. [Google Scholar] [CrossRef]
- Koontz, W.L.; Herbert, W.N.; Seeds, J.W.; Cefalo, R.C. Ultrasonography in the antepartum diagnosis of conjoined twins. A report of two cases. J. Reprod. Med. 1983, 28, 627–630. [Google Scholar]
- Nadkarni, B.B. Congenital anomalies of the human umbilical cord and their clinical significance: A light and electron microscope study. Indian J. Med. Res. 1969, 57, 1018–1027. [Google Scholar]
- Lei, T.; Xie, H.; Feng, J. Prenatal diagnosis of four-vessel umbilical cord with supernumerary vein varix: A case report and literature review. J. Obstet. Gynaecol. Res. 2017, 43, 1200–1204. [Google Scholar] [CrossRef]
- Arora, R.; Arora, P.; Tan-Dy, C. Four-vessel umbilical cord in a neonate with no associated congenital anomalies. BMJ Case Rep. 2022, 15, e251453. [Google Scholar] [CrossRef]
- Jeanty, P. Persistent right umbilical vein: An ominous prenatal finding? Radiology 1990, 177, 735–738. [Google Scholar] [CrossRef]
- Schimmel, M.; Eidelman, A. Supernumerary Umbilical Vein Resulting in a Four-Vessel Umbilical Cord. Am. J. Perinatol. 1998, 15, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A. Four-Vessel Umbilical Cord Without Congenital Abnormalities. South Med. J. 1984, 77, 539. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.L.; Shapiro, M.L.; Haller, J.O.; Schwartz, D. The multivessel umbilical cord: An antenatal indicator of possible conjoined twinning. J. Clin. Ultrasound 1992, 20, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kurakazu, M.; Kurakazu, M.; Murata, M.; Miyamoto, T.; Takahashi, Y.; Hamasaki, M.; Ohta, E.; Yotsumoto, F.; Miyamoto, S. A partial supernumerary umbilical vein: A case report. J. Med. Case Rep. 2019, 13, 149. [Google Scholar] [CrossRef]
- Garg, N.; Diwaker, P.; Aggarwal, S.; Gaur, J.H. Chorangiosis placenta with 5-vessel umbilical cord with omphalomesenteric duct remnant: An unusual association. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 270–272. [Google Scholar] [CrossRef]
- Rao, S.; Sobti, P.; Khurana, N.; Singh, N. Multiple vessels in the umbilical cord: A report of four cases. Indian J. Pathol. Microbiol. 2012, 55, 597. [Google Scholar] [CrossRef] [PubMed]
- Puvabanditsin, S.; Garrow, E.; Bhatt, M.; Kathiravan, S.; Gowda, S.; Wong, R.; Nagar, M. Four-Vessel Umbilical Cord Associated with Multiple Congenital Anomalies: A Case Report and Literature Review. Fetal Pediatr. Pathol. 2011, 30, 98–105. [Google Scholar] [CrossRef]
- Aoki, S.; Hata, T.; Ariyuki, Y.; Makihara, K.; Hata, K.; Kitao, M. Antenatal Diagnosis of Aberrant Umbilical Vessels. Gynecol. Obstet. Investig. 1997, 43, 232–235. [Google Scholar] [CrossRef]
- Beck, R.; Naulty, C.M. A Human Umbilical Cord with Four Arteries. Clin. Pediatr. 1985, 24, 118–119. [Google Scholar] [CrossRef]
- Abuhamad, A.Z.; Shaffer, W.; Mari, G.; Copel, J.A.; Hobbins, J.C.; Evans, A.T. Single umbilical artery: Does it matter which artery is missing? Am. J. Obstet. Gynecol. 1995, 173, 728–732. [Google Scholar] [CrossRef]
- Cairns, J.D.; Mckee, J. Single umbilical artery: A prospective study of 2000 consecutive deliveries. Can. Med. Assoc. J. 1964, 91, 1071–1073. [Google Scholar] [PubMed]
- Sailer, D.N.; Keene, C.L.; Sun, C.C.J.; Schwartz, S. The association of single umbilical artery with cytogenetically abnormal pregnancies. Am. J. Obstet. Gynecol. 1990, 163, 922–925. [Google Scholar] [CrossRef]
- Battarbee, A.N.; Palatnik, A.; Ernst, L.M.; Grobman, W.A. Association of Isolated Single Umbilical Artery with Small for Gestational Age and Preterm Birth. Obstet. Gynecol. 2015, 126, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Predanic, M.; Perni, S.C.; Friedman, A.; Chervenak, F.A.; Chasen, S.T. Fetal Growth Assessment and Neonatal Birth Weight in Fetuses with an Isolated Single Umbilical Artery. Obstet. Gynecol. 2005, 105 Pt 1, 1093–1097. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am. J. Obstet. Gynecol. 2006, 195, 1557–1563. [Google Scholar] [CrossRef]
- Horton, A.; Barroilhet, L.; Wolfe, H. Perinatal Outcomes in Isolated Single Umbilical Artery. Am. J. Perinatol. 2010, 27, 321–324. [Google Scholar] [CrossRef]
- Bombrys, A.; Neiger, R.; Hawkins, S.; Sonek, J.; Croom, C.; McKenna, D.; Ventolini, G.; Habli, M.; How, H.; Sibai, B. Pregnancy Outcome in Isolated Single Umbilical Artery. Am. J. Perinatol. 2008, 25, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.S.; Kurinczuk, J.J.; Konje, J.C. Antenatal detection of a single umbilical artery: Does it matter? Prenat. Diagn. 2003, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Odibo, A.O.; Macones, G.A.; Roehl, K.A.; Crane, J.P.; Cahill, A.G. Single Umbilical Artery and Its Associated Findings. Obstet. Gynecol. 2010, 115, 930–934. [Google Scholar] [CrossRef]
- Alexander, G.; Himes, J.; Kaufman, R.; Mor, J.; Kogan, M. A united states national reference for fetal growth. Obstet. Gynecol. 1996, 87, 163–168. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Xu, Y.; Ren, L.; Zhai, S.; Luo, X.; Hong, T.; Liu, R.; Ran, L.; Zhang, Y. Association Between Isolated Single Umbilical Artery and Perinatal Outcomes: A Meta-Analysis. Med. Sci. Monit. 2016, 22, 1451–1459. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldkrand, J.W.; Moore, D.H.; Lentz, S.U.; Clements, S.P.; Turner, A.D.; Bryant, J.L. Volumetric flow in the umbilical artery: Normative data. J. Matern. Fetal Med. 2000, 9, 224–228. [Google Scholar] [PubMed]
- Johnson, C.W.; Tennenbaum, S.Y. Urologic anomalies and two-vessel umbilical cords: What are the implications? Curr. Urol. Rep. 2003, 4, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, S.A. Single umbilical artery. A statistical analysis of 237 autopsy cases and review of the literature. Perspect. Pediatr. Pathol. 1984, 8, 345–378. [Google Scholar]
- Thummala, M.R.; Raju, T.N.K.; Langenberg, P. Isolated single umbilical artery anomaly and the risk for congenital malformations: A meta-analysis. J. Pediatr. Surg. 1998, 33, 580–585. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Parilla, B.; Tamura, R.; Macgregor, S.; Geibel, L.; Sabbagha, R. The clinical significance of a single umbilical artery as an isolated finding on prenatal ultrasound. Obstet. Gynecol. 1995, 85, 570–572. [Google Scholar] [CrossRef]
- Kondi-Pafiti, A.; Kleanthis, K.C.; Mavrigiannaki, P.; Iavazzo, C.; Bakalianou, K.; Hassiakos, D.; Liapis, A. Single umbilical artery: Fetal and placental histopathological analysis of 24 cases. Clin. Exp. Obstet. Gynecol. 2011, 38, 214–216. [Google Scholar]
- Moore, L.; Toi, A.; Chitayat, D. Abnormalities of the intra-abdominal fetal umbilical vein: Reports of four cases and a review of the literature. Ultrasound Obstet. Gynecol. 1996, 7, 21–25. [Google Scholar] [CrossRef]
- Jeanty, P. Fetal and funicular vascular anomalies: Identification with prenatal US. Radiology 1989, 173, 367–370. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).