Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena
Abstract
1. Introduction
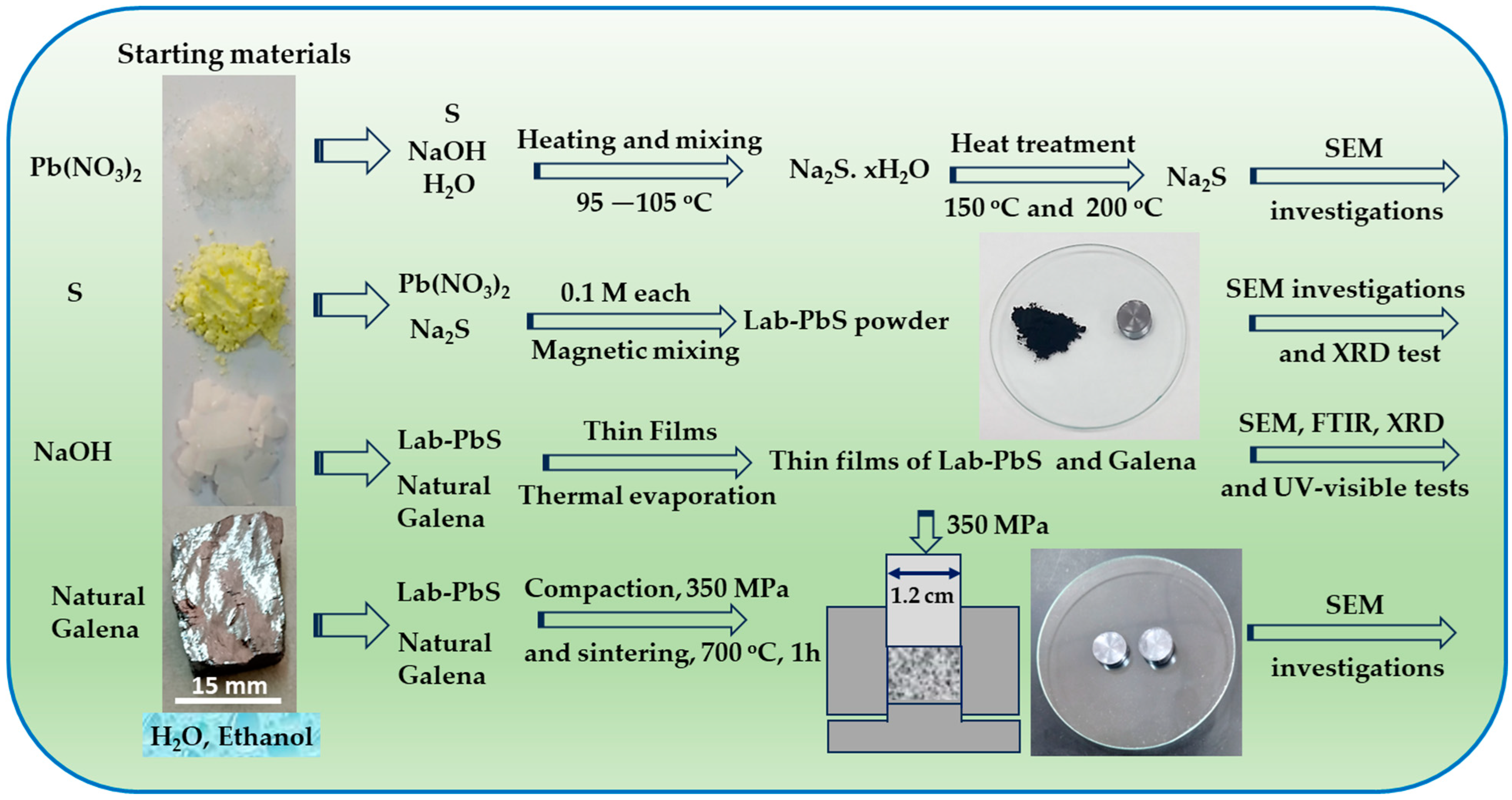
2. Experimental Procedure
2.1. Materials
2.2. Synthesizing Na2S and PbS Compounds
- Exactly 1.56 g of Na2S was weighed using a four-digit analytical balance (Model SEJ-205, Taipei, Taiwan) and then dissolved in 200 mL of distilled water to prepare a 0.1 M light-yellow solution. The dissolving process was quite fast at room temperature.
- A mass of 6.7 g of Pb(NO3)2 was weighed and then dissolved in 200 mL of distilled water on a magnetic stirrer to prepare 0.1 M of a colorless solution.
- The solution of Na2S was then added to the solution of Pb(NO3)2 under continued stirring for 2 h to achieve a better PbS formation. As soon as the sodium sulfide solution was added to the lead nitrate solution, the PbS black powder began to precipitate. The residual sodium nitrate liquid was removed after the PbS powder settled down.
- The formed PbS black powder was then washed four times using distilled water followed by washing with methanol once. The washed PbS powder was left to dry at room temperature in a fume hood. The obtained powder was then heated at approximately 105 °C using a vacuum oven (JEIO TECH, MODEL OV-11, AAH13115K, Seoul, Republic of Korea).
- Another run of PbS precipitation was also conducted, but with ultrasonic agitation using an ultrasonic cleaner (AC ULTRASONIC, 1002. Jeio-Tech Co., Ltd., Seoul, Republic of Korea) at a frequency of 40 kHz to assess the effect of this parameter on the particle size.
- The precipitation was repeated as required to produce the needed amounts of Na2S and PbS.
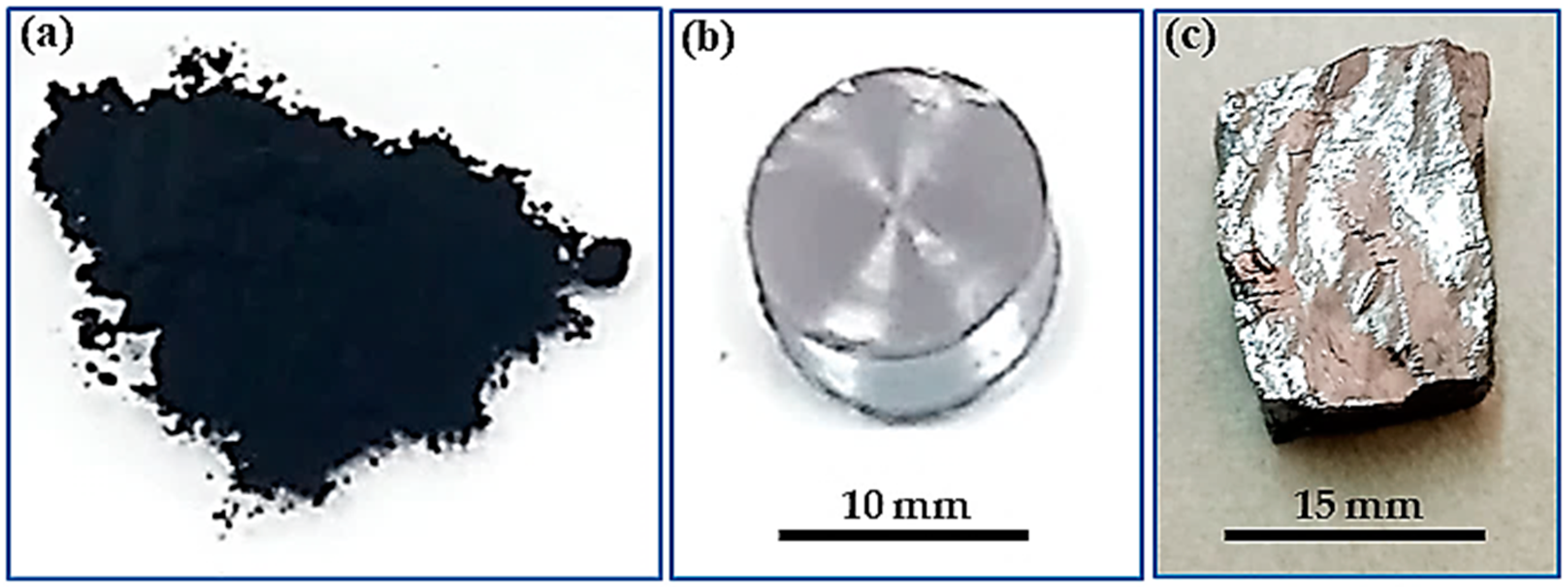
- The produced PbS is denoted as Lab-PbS in the current article. A sample from the Lab-PbS was compacted using a homemade stainless-steel die with a 1.2 cm diameter at a pressure of 350 MPa. The color of the compacted Lab-PbS pellet was similar to that of the PbS enriched-phase galena ore.
2.3. Thin Film Preparation, FTIR, and UV–Visible Spectrophotometry
2.4. XRD Measurements for Lab-PbS, Galena, and the Deposited Thin Films
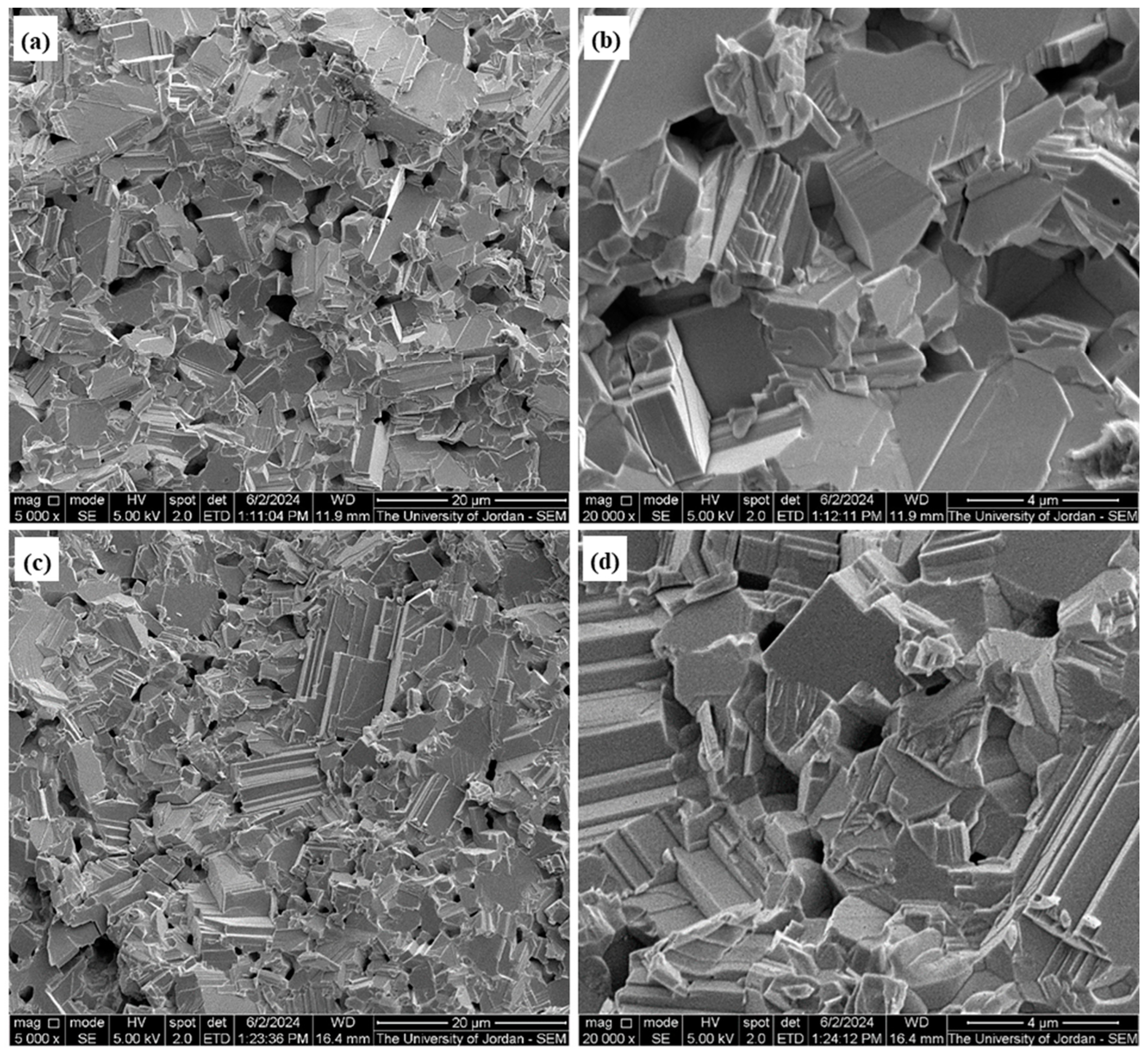
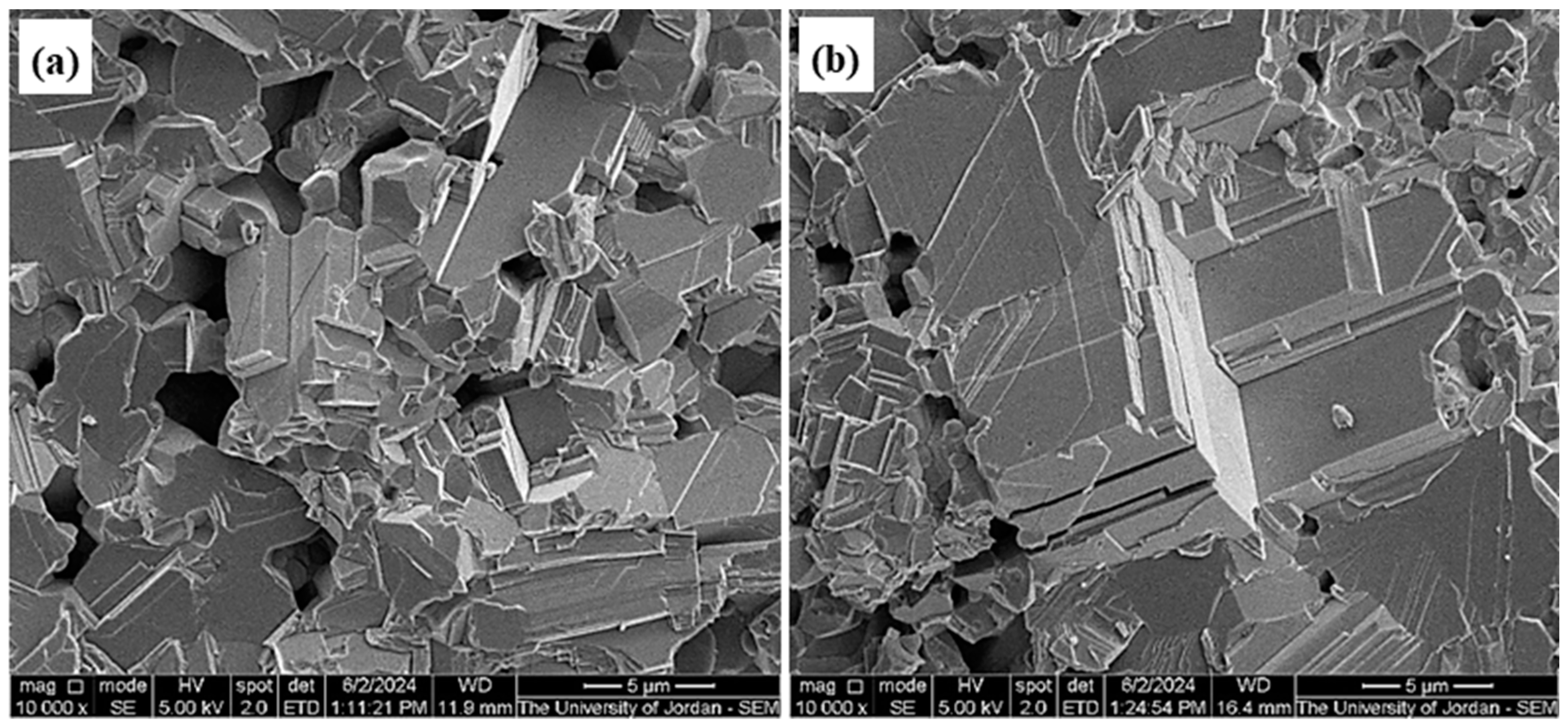
2.5. SEM Product Investigations
2.6. Sintering of Galena and Lab-PbS Powders
3. Results and Discussion
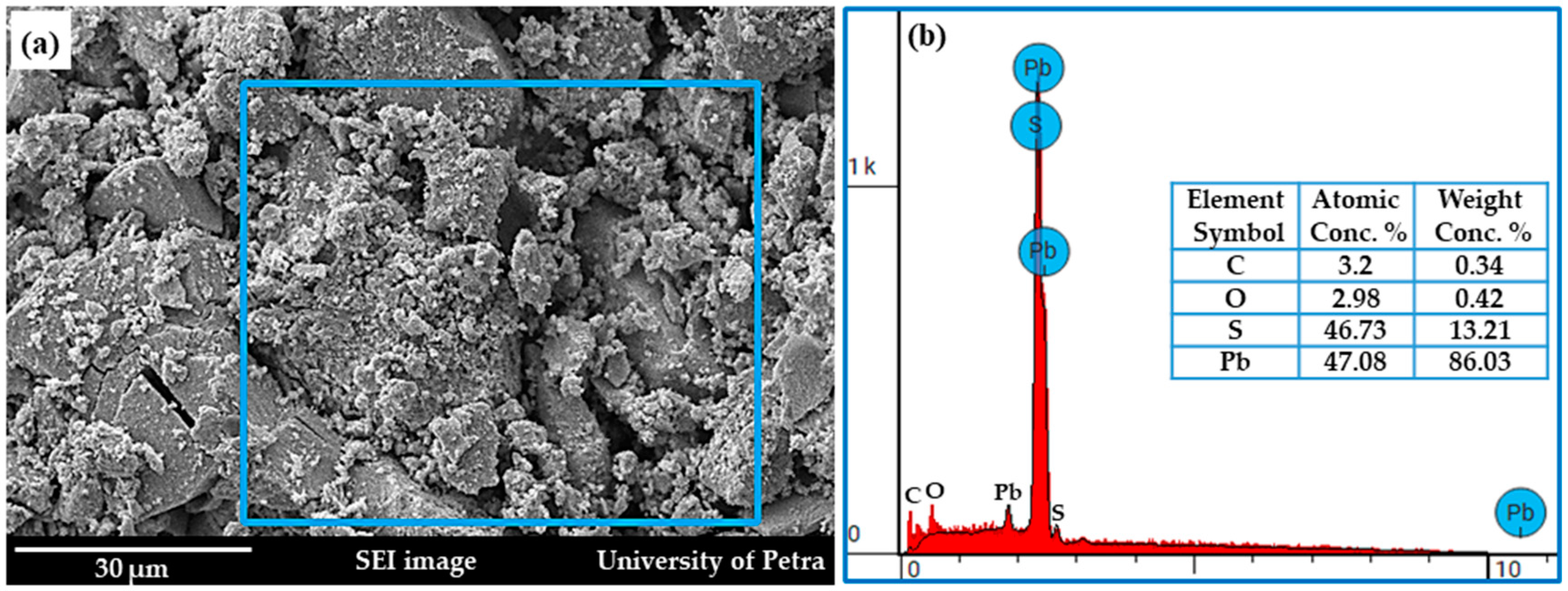
3.1. SEM Investigations of Na2S and Lab-PbS
3.2. XRD
3.3. Microstructure of Thin Films
3.4. UV–Visible and FTIR Spectroscopy Results
3.4.1. UV–Visible of Thin Films
3.4.2. FTIR Analysis
3.5. Sintering Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, S.; Chen, J.; Johansson, E.M.J.; Zhang, X. PbS Colloidal Quantum Dot Inks for Infrared Solar Cells. iScience 2020, 23, 101753. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Saha, S. Fabrication and comparison of photovoltaic devices based on different shape PbS nanoparticles. Mater. Res. Express 2021, 8, 015020. [Google Scholar] [CrossRef]
- Sukharevska, N.; Bederak, D.; Goossens, V.M.; Momand, J.; Duim, H.; Dirin, D.N.; Kovalenko, M.V.; Kooi, B.J.; Loi, M.A. Scalable PbS Quantum Dot Solar Cell Production by Blade Coating from Stable Inks. ACS Appl. Mater. Interfaces 2021, 13, 5195–5207. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Sarkar, D.K.; Shahinuzzaman, M.; Wahab, Y.A.; Khandaker, M.U.; Tamam, N.; Sulieman, A.; Amin, N.; Akhtaruzzaman, M. Green Synthesis of Lead Sulphide Nanoparticles for High-Efficiency Perovskite Solar Cell Applications. Nanomaterials 2022, 12, 1933. [Google Scholar] [CrossRef]
- Mukai, K.; Masuda, I. Theoretical study of multi-band solar cells with a single PbS quantum dot superlattice film as a light absorption layer. Jpn. J. Appl. Phys. 2022, 61, 102005. [Google Scholar] [CrossRef]
- Mukai, K.; Ikeda, S.; Pribyl, I.; Sato, H.; Masuda, I. Improvement of solar cell performance using PbS quantum dot superlattices with iodine ligands. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133285. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Preparation and optical studies of PbS nanoparticles. Opt. Mater. 2015, 46, 522–525. [Google Scholar] [CrossRef]
- Mishra, A.K.; Saha, S. Synthesis and Characterization of PbS Nanostructures to Compare with Bulk. Chalcogenide Lett. 2020, 17, 147–159. Available online: https://chalcogen.ro/147_MishraAK.pdf (accessed on 1 June 2024). [CrossRef]
- Reddy, T.S.; Krishna, S.V.; Kumar, A.V.; Ramanjaneyulu, M.; Sekhar, N.R.; Kumar, M.C.S. Facile synthesis and characterization of PbS thin films doped with various aluminum concentrations for photovoltaic applications. Semicond. Sci. Technol. 2024, 39, 075018. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. PbS nanostructures: A review of recent advances. Mater. Today Sustain. 2023, 21, 100305. [Google Scholar] [CrossRef]
- Meng, W.; Yuan, W.; Wua, Z.; Wang, X.; Xu, W.; Wang, L.; Zhang, Q.; Zhang, C.; Wang, J.; Song, Q. Mechanochemical synthesis of lead sulfide (PbS) nanocrystals from lead oxide. Powder Technol. 2019, 347, 130–135. [Google Scholar] [CrossRef]
- Nnanwube, I.A.; Onukwuli, O.D. Hydrometallurgical Processing of a Nigerian Galena Ore in Nitric Acid: Characterization and Dissolution Kinetics. J. Miner. Mater. Charact. Eng. 2018, 6, 271–293. [Google Scholar] [CrossRef][Green Version]
- Al-Saqarat, B.S.; Al-Mobydeen, A.; AL-Masri, A.N.; Esaifan, M.; Hamadneh, I.; Moosa, I.S.; AlShamaileh, E. Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore. Micromachines 2023, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Kozhevnikova, N.S.; Uritskaya, A.A.; Rempel, A.A. Dependence of the size of nanoparticles of lead sulfide PbS on the chemical affinity of its formation reaction. Dokl. Phys. Chem. 2013, 453, 270–273. [Google Scholar] [CrossRef][Green Version]
- Tshemese, Z.; Khan, M.D.; Mlowe, S.; Revaprasadu, N. Synthesis and characterization of PbS nanoparticles in an ionic liquid using single and dual source precursors. Mater. Sci. Eng. B 2018, 227, 116–121. [Google Scholar] [CrossRef]
- Patel, J.D.; Mighri, F.; Ajji, A.; Chaudhuri, T.K. Morphology and size control of lead sulphide nanoparticles produced using methanolic lead acetate trihydrate–thiourea complex via different precipitation techniques. Martials Chem. Phys. 2012, 132, 747–755. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhya, A. Characterization of PbS nanoparticles synthesized using sodium lauryl sulfate at room temperature. Mater. Today Proc. 2023, 76, 114–119. [Google Scholar] [CrossRef]
- Baláž1, P.; Pourghahramani, P.; Dutková, E.; Fabián, M.; Kováč, J.; Šatka, A. PbS nanostructures synthesized via surfactant assisted mechanochemical route. Cent. Eur. J. Chem. 2009, 7, 215–221. [Google Scholar] [CrossRef]
- Jyothilakshmi, V.P.; Bhabhina, N.M.; Dharsana, M.V.; Swaminathan, S. Wet chemical synthesis of lead sulfide nanoparticles and its application as light harvester in photovoltaic cell. Mater. Today Proc. 2020, 33, 2125–2129. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Guzmán-Caballero, D.E.; Avila-Avendano, J.A.; Pérez-García, C.E.; Quevedo-López, M.A.; Ramírez Bon, R. Morphological, structural, and electrical properties of PbS thin films deposited on HfO2, SiO2, and Al2O3 for TFTs applications. Ceram. Int. 2021, 47, 18898–18904. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, W.; Ning, Z.; Wang, G.; Cheng, X.; Hu, W.; Zhou, W.; Liu, Z.; Yang, S.; Xu, K.; et al. Ambipolar Graphene–Quantum Dot Phototransistors with CMOS Compatibility. Adv. Opt. Mater. 2018, 6, 1800985. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, L.; Ning, Z.; Cheng, Y.; Wang, F.; Xu, K.; Xu, R.; Liu, Z.; Luo, M.; Hu, W.; et al. Silicon: Quantum dot photovoltage triodes. Nat. Commun. 2021, 12, 6696. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, R.D.; Petrova, V.; McDermott, M.J.; Tyler, J.L.; Self, E.C.; Persson, K.A.; Liu, P.; Veith, G.M. Synthesis of model sodium sulfide films. J. Vac. Sci. Technol. A 2021, 39, 053404. [Google Scholar] [CrossRef]
- Roelands, M.; Cuypers, R.; Kruit, K.D.; Oversloot, H.; de Jong, A.; Duvalois, W.; van Vliet, L.; Hoegaerts, C. Preparation & characterization of sodium sulfide hydrates for application in thermochemical storage systems. Energy Procedia 2015, 70, 257–266. [Google Scholar] [CrossRef]
- Du, Z.; Liu, F.; Liu, J.; Pan, F.; Fan, C.; Zhang, J. A green process for producing Na2S from waste Na2SO4 through hydrogen agglomerate fluidized bed reduction of BaSO4. J. Clean. Prod. 2022, 355, 131816. [Google Scholar] [CrossRef]
- Amir Hussain, M.; Rajen Singh, L.; Ranibala Devi, S. Studies ON Structural, Optical and Electrical Properties of Zn-Doped PbS Nanocrystalline Thin Film. Chalcogenide Lett. 2021, 18, 103–111. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 969, 171914. [Google Scholar] [CrossRef]
- Akhtar, S.; Saeed, N.; Hanif, M.B.; Rehman, Z.; Dogar, S.; Mahmood, W.; Mosiałek, M.; Napruszewska, B.D.; Motola, M.A.M.; Khan, A.F. PbS and PbO Thin Films via E-Beam Evaporation: Morphology, Structure, and Electrical Properties. Materials 2022, 15, 6884. [Google Scholar] [CrossRef]
- Sahadevan, J.; Muthu, S.E.; Kulathuraan, K.; Arumugam, S.; Kim, I.; Sri Pratha, G.B.; Sivaprakash, P. Structural, morphology and optical properties of PbS (Lead Sulfide) thin film. Mater. Today Proc. 2022, 64, 1849–1853. [Google Scholar] [CrossRef]
- Rajathi, S.; Kirubavathi, K.; Selvaraju, K. Preparation of nanocrystalline Cd-doped PbS thin films and their structural and optical properties. J. Taibah Univ. Sci. 2017, 11, 1296–1305. [Google Scholar] [CrossRef]
- Zaragoza-Palacios, B.G.; Torres-Duarte, A.R.; Castillo, S.J. Synthesis and characterization of nanoparticles and thin films of PbS by a high-performance procedure using CBD. J. Mater. Sci. Mater. Electron. 2021, 32, 22205–22213. [Google Scholar] [CrossRef]
- Donato, D.P.; Cases, J.M.; Kongolo, M.; Michot, L.; Burneau, A. Infrared Investigation of amylxanthate Adsorption on Galena: Influence of oxidation, pH, and Grinding. Colloids Surf. 1990, 44, 207–228. [Google Scholar] [CrossRef]
- Chernyshova, I.V. An in situ FTIR study of galena and pyrite oxidation in aqueous solution. J. Electroanal. Chem. 2003, 558, 83–98. [Google Scholar] [CrossRef]
- Nabiyouni, G.; Moghimi, E.; Hedayati, K.; Jalajerdi, R. Room temperature synthesis of lead sulfide nanoparticles. Main Group Met. Chem. 2012, 35, 173–178. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Ali, H.M.; Adam, A.G.; Shokr, E.K. Vacuum-evaporated PbS:0.03 Zn thin films with varying thicknesses for environmental applications. Opt. Mater. 2024, 148, 114885. [Google Scholar] [CrossRef]
- Chirita, P. Galena Oxidation in Oxygen-Bearing Acidic Solutions. ACS Earth Space Chem. 2019, 3, 2593–2600. [Google Scholar] [CrossRef]
- Jameel, M.H.; Saleem, S.; Hashim, M.; Roslan, M.S.; Somaily, H.H.N.; Hessen, M.M.; El-Bahy, Z.M.; Ashiq, M.G.B.; Hamzah, M.Q.; Jabbar, A.H.; et al. A comparative study on characterizations and synthesis of pure lead sulfide (PbS) and Ag-doped PbS for photovoltaic applications. Nanotechnol. Rev. 2021, 10, 1484–1492. [Google Scholar] [CrossRef]
- Rosly, N.Z.; Abdullah, A.H.; Kamarudin, M.A.; Ashari, S.E.; Ahmad, S.A.A. Adsorption of Methylene Blue Dye by Calix [6] Arene-Modified Lead Sulphide (Pbs): Optimisation Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2021, 18, 397. [Google Scholar] [CrossRef]
- Yepseu, P.Y.; Girardet, T.; Nyamen, L.D.; Fleutot, S.; Ketchemen, K.I.Y.; Kun, W.N.; Cleymand, F.; Ndifon, P.T. Synthesis and Photocatalytic Activity of High-Quality Lead (II) Sulfide Nanoparticles from Lead (II) Thiosemicarbazone Complexes as Single Source Precursors. J. Nanomater. 2024, 2024, 9932000. [Google Scholar] [CrossRef]
- Al-Saqarat, B.S.; Al-Mobydeen, A.; Al-Dalahmeh, Y.; AL-Masri, A.N.; Altwaiq, A.M.; Hamadneh, I.; Abu-Afifeh, Q.; Zoubi, M.M.; Esaifan, M.; Moosa, I.S.; et al. Study of Galena Ore Powder Sintering and Its Microstructure. Metals 2024, 14, 439. [Google Scholar] [CrossRef]




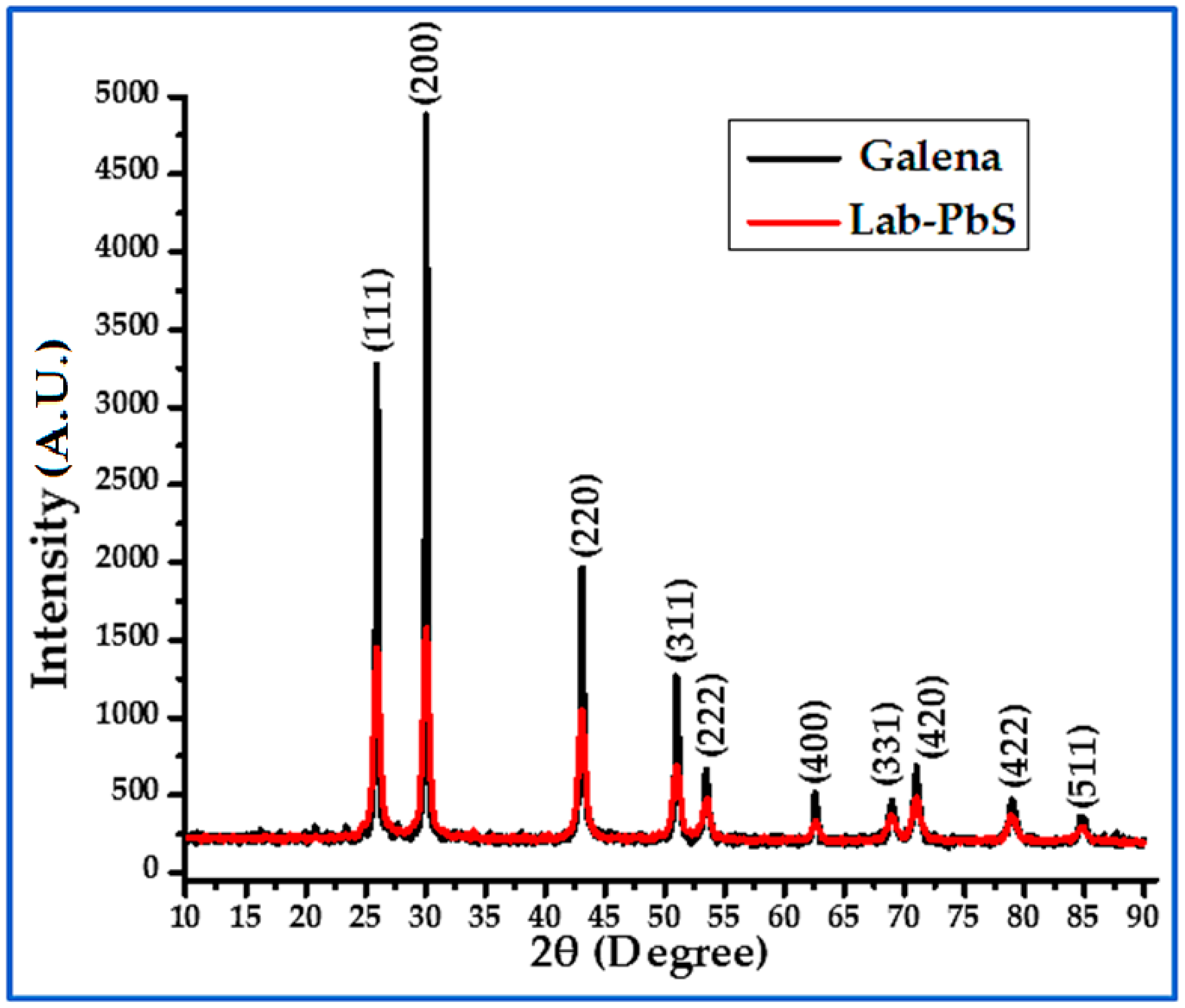
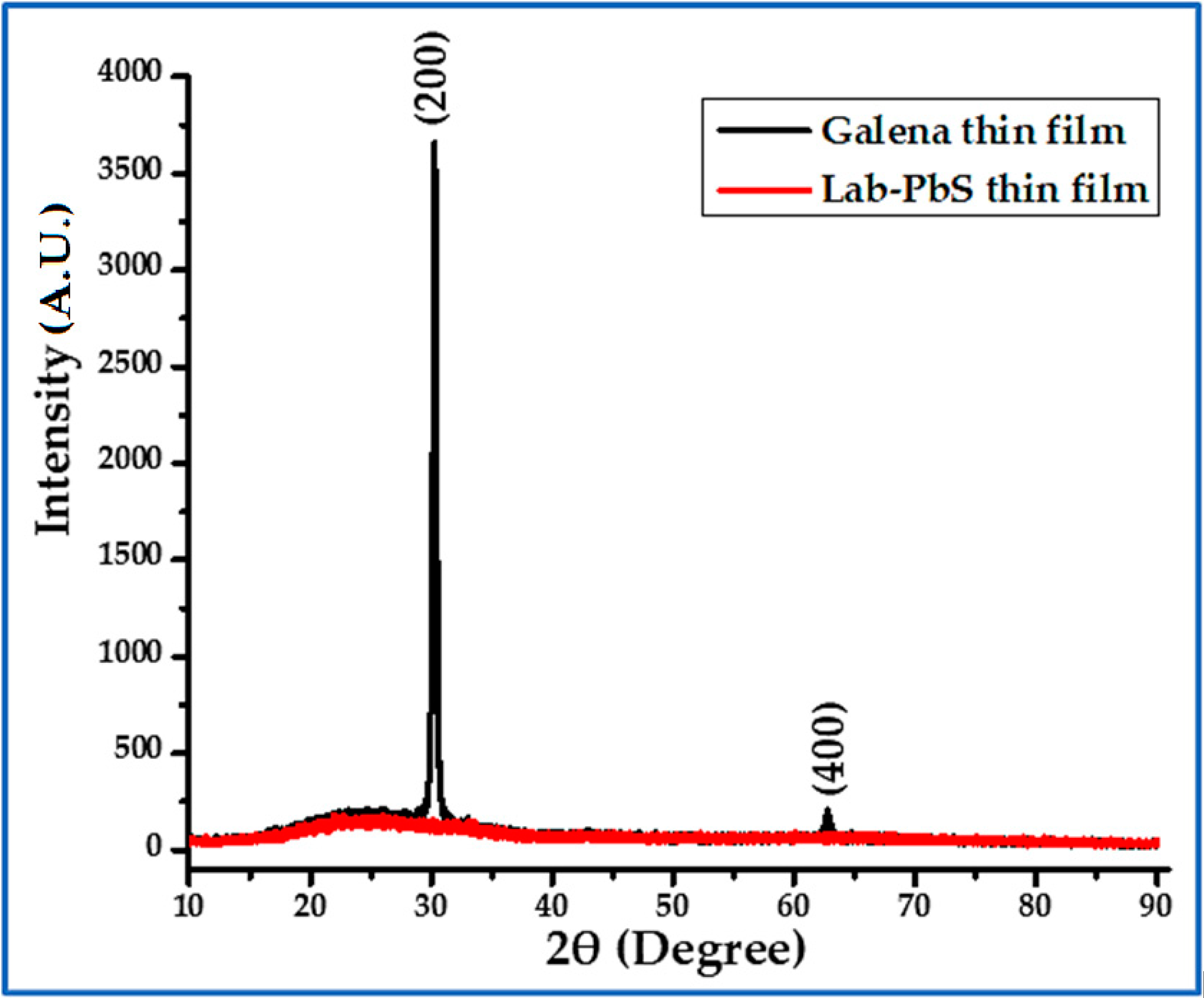
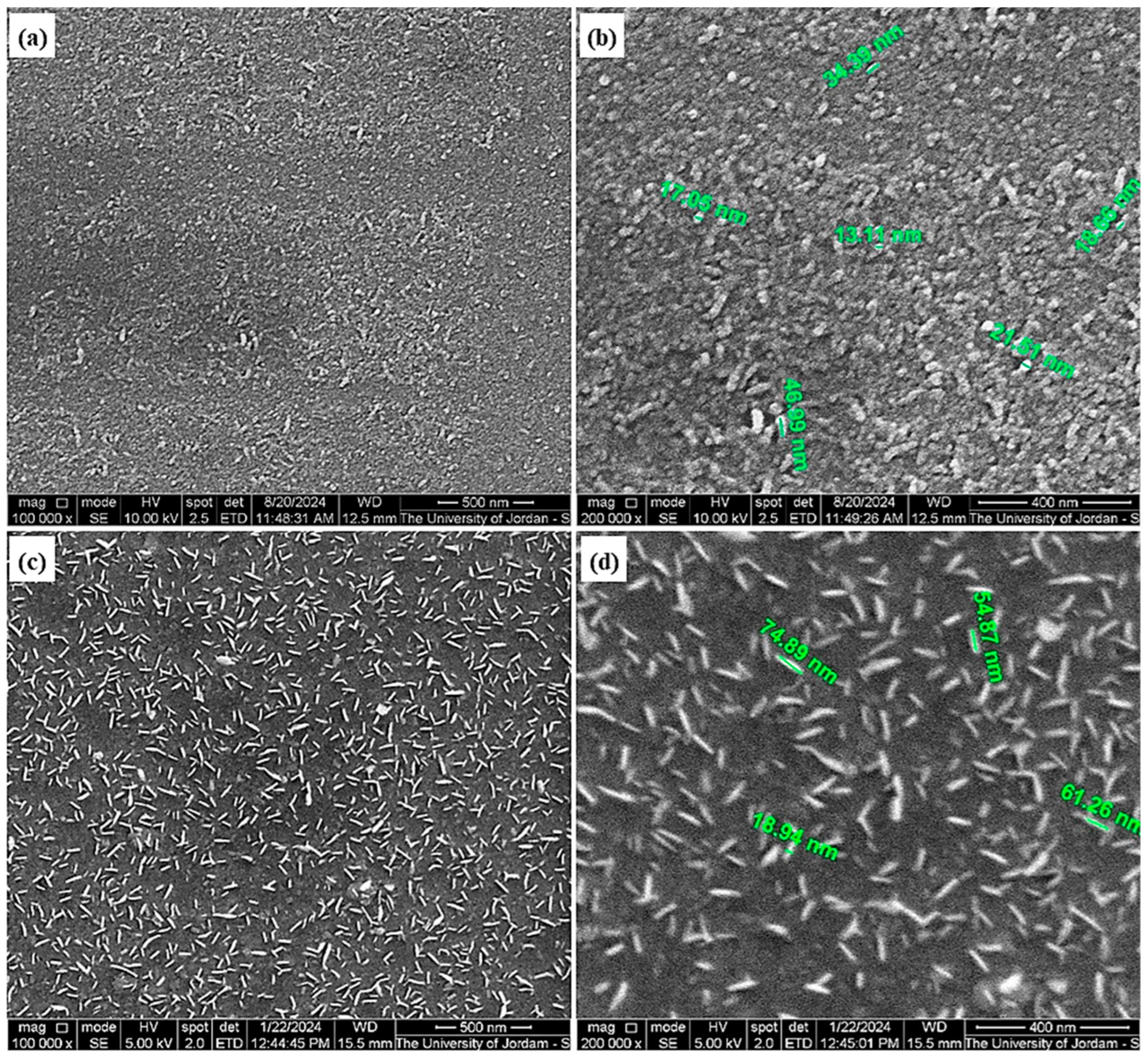
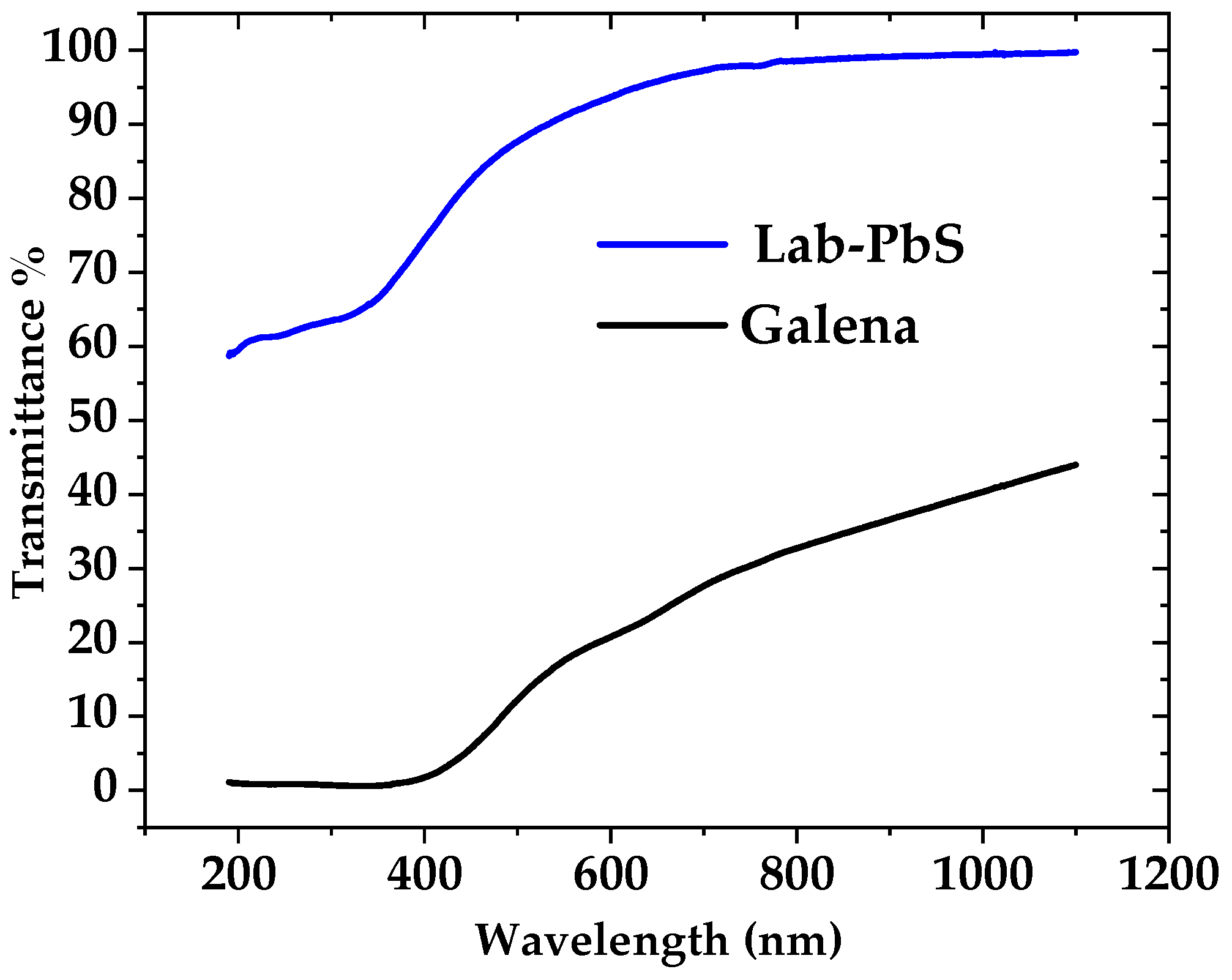
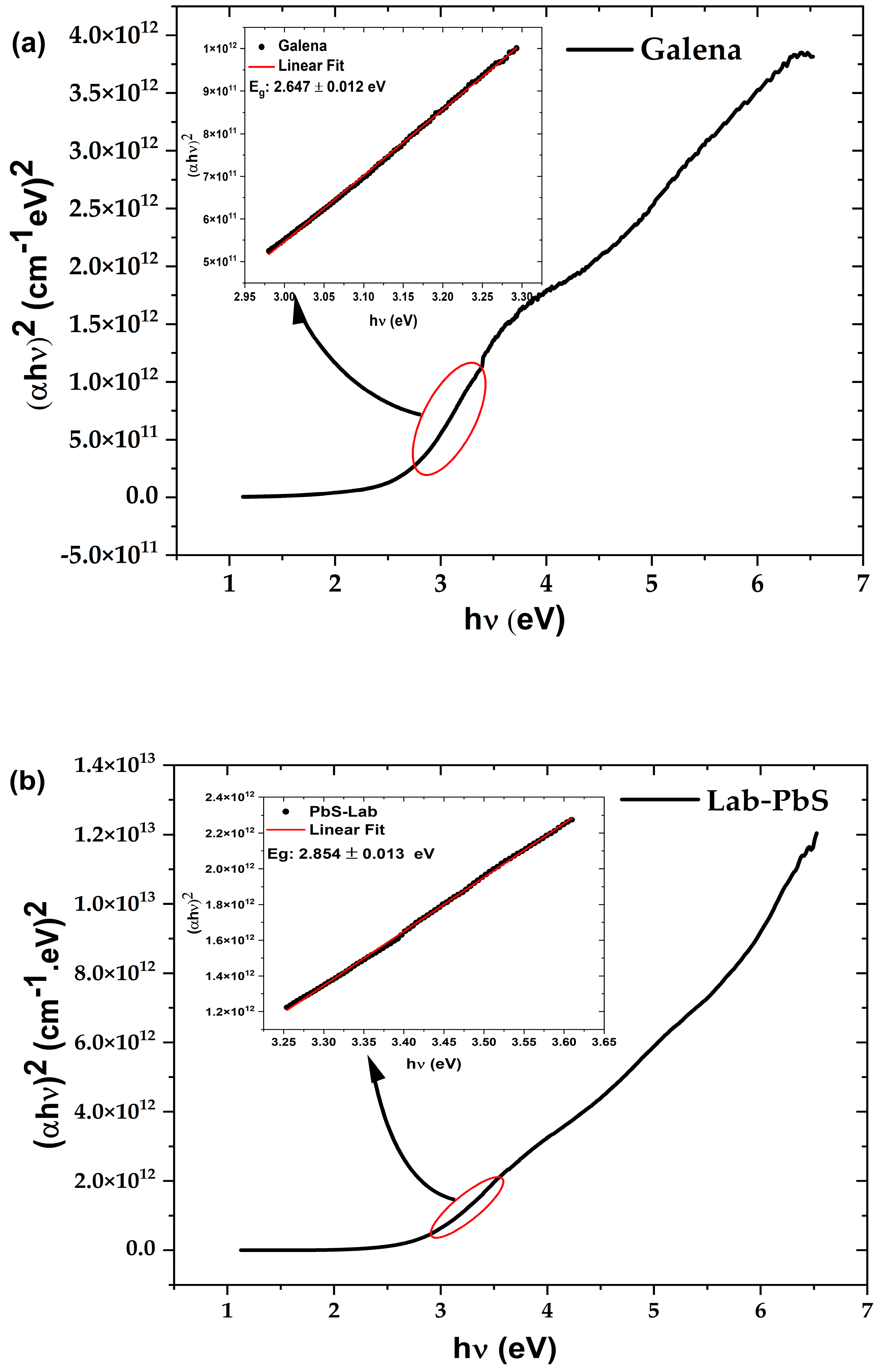

| Sample | Phase | Crystal Structure | Unit Cell (Lattice), Measured | Unit Cell Lattice, Standard | Space Group |
|---|---|---|---|---|---|
| Lab-PbS | PbS, Ref (COD 96-901-3404) | Cubic | a = 5.9367628 | a = 5.932 | Fm-3m (225) |
| Galena | PbS, Ref (COD 69-901-3404) | Cubic | a = 5.9360250 | a = 5.932 | Fm-3m (225) |
| Parameters | Peaks Position (°) | β (FWHM) (°) | D (nm) | Av. D (nm) | |
|---|---|---|---|---|---|
| K | λ (Å) | 30.2635 | 0.3243 | 26.52 | 26.56 |
| 0.94 | 1.54178 | 62.7296 | 0.3658 | 26.59 | |
| Sample | Comp. Pressure | Average Green Density (g/cm3) | Average Sintered Density (g/cm3) | RD% | Sintering Condition |
|---|---|---|---|---|---|
| Galena | 350 MPa | 6.27 | 7.36 | 99.2 | 700 °C, 1 h in vacuum, heating rate 10 °C/min from room temperature to 700 °C, furnace cool. |
| Lab-PbS | 350 MPa | 5.30 | 7.28 | 98.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShamaileh, E.; Lahlouh, B.; AL-Masri, A.N.; Al-Qderat, M.; Mahmoud, W.; Alrbaihat, M.; Moosa, I.S. Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena. Sci 2024, 6, 61. https://doi.org/10.3390/sci6040061
AlShamaileh E, Lahlouh B, AL-Masri AN, Al-Qderat M, Mahmoud W, Alrbaihat M, Moosa IS. Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena. Sci. 2024; 6(4):61. https://doi.org/10.3390/sci6040061
Chicago/Turabian StyleAlShamaileh, Ehab, Bashar Lahlouh, Ahmed N. AL-Masri, Mariam Al-Qderat, Wadah Mahmoud, Mohammad Alrbaihat, and Iessa Sabbe Moosa. 2024. "Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena" Sci 6, no. 4: 61. https://doi.org/10.3390/sci6040061
APA StyleAlShamaileh, E., Lahlouh, B., AL-Masri, A. N., Al-Qderat, M., Mahmoud, W., Alrbaihat, M., & Moosa, I. S. (2024). Comparative Microstructure Characteristics of Synthesized PbS Nanocrystals and Galena. Sci, 6(4), 61. https://doi.org/10.3390/sci6040061








