Promising Catalyst for Chlorosilane Dismutation
Abstract
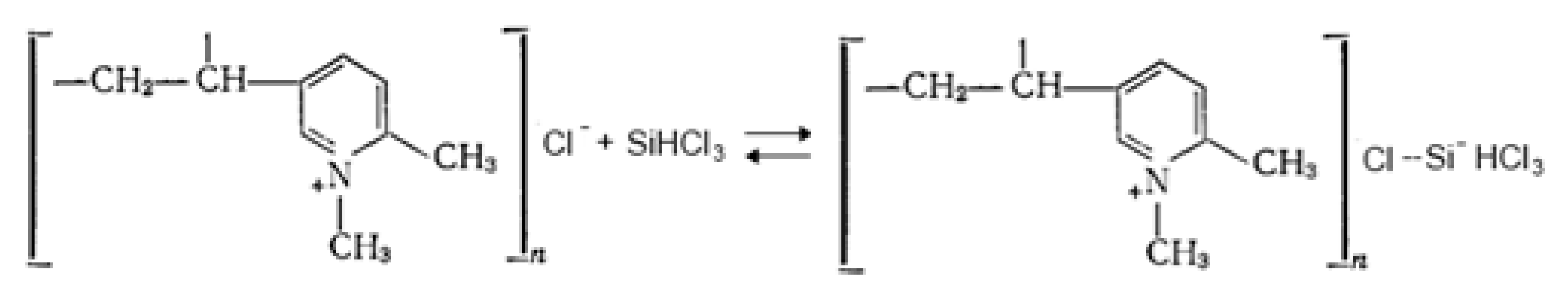
1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setty, H.S.N.; Yaws, C.L.; Martin, B.R.; Wangler, D.J. Method of Operating a Quartz Fluidized Bed Reactor for the Production of Silicon. U.S. Patent 3,963,838, 15 June 1976. [Google Scholar]
- Iya, S.K.; Flagella, R.N.; Dipaolo, F.S. Heterogeneous decomposition of silane in a fixed bed reactor. J. Electrochem. Soc. 1982, 129, 1531–1535. [Google Scholar] [CrossRef]
- Eaglesham, D.J.; Cerullo, M. Dislocation-free Stranski-Krastanow growth of Ge on Si(100). Phys. Rev. Lett. 1990, 64, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bathey, B.R.; Cretella, M.C. Solar-grade silicon. J. Mater. Sci. 2005, 17, 3877–3896. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, W. CFD–PBM coupled simulation of silicon CVD growth in a fluidized bed reactor: Effect of silane pyrolysis kinetic models. Chem. Eng. Sci. 2015, 127, 84–94. [Google Scholar] [CrossRef]
- Niu, J.; Dai, Y.; Yin, L.; Shang, J.; Crittenden, J.C. Photocatalytic reduction of triclosan on Au–Cu2O nanowire arrays as plasmonic photocatalysts under visible light irradiation. Phys. Chem. Chem. Phys. 2015, 17, 17421–17428. [Google Scholar] [CrossRef] [PubMed]
- Tirumala, R.T.A.; Dadgar, A.P.; Mohammadparast, F.; Ramakrishnan, S.B.; Mou, T.; Wang, B.; Andiappan, M. Homogeneous versus heterogeneous catalysis in Cu2O-nanoparticle-catalyzed C–C coupling reactions. Green Chem. 2019, 21, 5284–5290. [Google Scholar] [CrossRef]
- Bailey, D.L.; Shafer, P.W.; Wagner, G.H. Disproportionation of Chlorosilanes Employing Amine-Type Catalysts. U.S. Patent 2,834,648, 13 May 1958. [Google Scholar]
- Jung, I.N.; Cho, K.D.; Lim, J.C.; Yoo, B.R. Redistribution Catalyst and Methods for Its Preparation and Use to Convert Chlorosilicon Hydrides to Silane. U.S. Patent 4,613,491, 23 September 1986. [Google Scholar]
- Erickson, C.E.; Wagner, G.H. Disproportionation of Silane Derivatives. U.S. Patent 2,627,451, 3 February 1953. [Google Scholar]
- Bakay, C.J. Verfahren zur Herstellung von Silan. DE Patent 2507864A1, 28 August 1975. [Google Scholar]
- Litteral, C.J. Disproportionation of Chlorosilane. U.S. Patent 4,113,845, 12 September 1978. [Google Scholar]
- Litteral, C.J. Verfahren zur Herstellung von Disproportionierungsprodukten von Chlorsilanverbindungen. U.S. Patent 2,162,537, 13 July 1972. [Google Scholar]
- Seth, K.K. Chlorosilane Disproportionation Process. U.S. Patent 4,395,389, 26 July 1983. [Google Scholar]
- Grishnova, N.D.; Gusev, A.V.; Moiseev, A.N.; Mochalov, G.M.; Balanovsky, N.V.; Kharitonov, T.N. Catalytic Activity of Anion-Exchange Resins in the Trichlorosilane Disproportionation Reaction. J. Appl. Chem. 1999, 72, 1667–1672. [Google Scholar]
- Зyбaкoвa, Л.Б. Cинтeтичecкиe иoнooбмeнныe мaтepиaлы; Зyбaкoвa, Л.Б., Teвлинa, A.C., Дaвaнкoвa, A.Б., Eds.; Xимия: Mocквa, Russia, 1978. [Google Scholar]
- Mochalov, G.; Stolmakov, Y.; Zhuchok, O. Thermodynamics and Kinetics of the Reaction of Catalytic Dismutation of Chlorosilanes in the Vapor Phase in the Temperature Range of 353–393 K. Chemengineering 2023, 7, 13–27. [Google Scholar] [CrossRef]
- Zou, N.; Lin, X.; Li, M.; Li, L.; Ye, C.; Chen, J.; Qiu, T. Ionic Liquid@Amphiphilic Silica Nanoparticles: Novel Catalysts for Converting Waste Cooking Oil to Biodiesel. ACS Sustain. Chem. Eng. 2020, 8, 18054–18061. [Google Scholar] [CrossRef]
- Union Carbide Corporation. Low-Cost Solar Array Project: Feasibility of the Silane Process for Producing Semiconductor-Grade Silicon; Final Report (Phases I and II); Union Carbide Corp.: New York, NY, USA, 1979. [Google Scholar]
- Vorotyntsev, V.M.; Mochalov, G.M.; Kolotilova, M.A. Liquid-vapor equilibria in systems based on dichlorosilane. Russ. J. Phys. Chem. A 2005, 79, 16–19. [Google Scholar]
- Yungman, V.S. (Ed.) Thermal Constants of Substances; Wiley: New York, NY, USA, 1999; Volumes 1–8, Available online: https://www.chem.msu.ru/cgi-bin/tkv.pl?show=welcome.html/welcome.html (accessed on 18 September 2023).










| DCS | MCS | ||
|---|---|---|---|
| T, K | P, bar | T, K | P, bar |
| 273 | 0.81 | 253 | 1.43 |
| 283 | 1.15 | 263 | 1.99 |
| 293 | 1.60 | 273 | 2.71 |
| 303 | 2.18 | 283 | 3.61 |
| 313 | 2.90 | 293 | 4.71 |
| 323 | 3.80 | 303 | 6.03 |
| 333 | 4.90 | 313 | 7.62 |
| 343 | 6.22 | 323 | 9.47 |
| 353 | 7.79 | --- | --- |
| 363 | 9.64 | --- | --- |
| 373 | 11.79 | --- | --- |
| TCS dismutation kinetics 1 bar total pressure | |||||
| τ (s) | Mole Fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.00000 | 0.000347 | 0.0459 | 0.878 | 0.0466 |
| 0.000027 | 0.00110 | 0.0729 | 0.822 | 0.0752 | |
| 1.75 | 0.000089 | 0.00190 | 0.0900 | 0.785 | 0.0941 |
| 2.5 | 0.000126 | 0.00223 | 0.0955 | 0.773 | 0.100 |
| 3 | 0.000136 | 0.00231 | 0.0968 | 0.770 | 0.102 |
| 4 | 0.000135 | 0.00230 | 0.0968 | 0.768 | 0.104 |
| TCS dismutation kinetics 2 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 0.8 | 0.000025 | 0.00108 | 0.0717 | 0.809 | 0.0740 |
| 0.000076 | 0.00176 | 0.0866 | 0.777 | 0.0904 | |
| 1 | 0.000110 | 0.00201 | 0.0911 | 0.767 | 0.0954 |
| 1.3 | 0.000126 | 0.00221 | 0.0942 | 0.760 | 0.0990 |
| 1.5 | 0.000134 | 0.00227 | 0.0952 | 0.758 | 0.100 |
| 2 | 0.000134 | 0.00227 | 0.0952 | 0.758 | 0.100 |
| 2.5 | 0.000145 | 0.00238 | 0.0977 | 0.768 | 0.103 |
| 3.5 | 0.000144 | 0.00239 | 0.0978 | 0.767 | 0.103 |
| TCS dismutation kinetics 3 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 0.7 | 0.000067 | 0.00165 | 0.0836 | 0.768 | 0.0871 |
| 0.000089 | 0.00188 | 0.0878 | 0.759 | 0.0919 | |
| 0.8 | 0.000113 | 0.00209 | 0.0913 | 0.751 | 0.0958 |
| 1 | 0.000126 | 0.00219 | 0.0930 | 0.747 | 0.0978 |
| 2 | 0.000128 | 0.00220 | 0.0928 | 0.746 | 0.0980 |
| TCS dismutation kinetics 4 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 0.7 | 0.000110 | 0.00196 | 0.0882 | 0.742 | 0.0924 |
| 0.000116 | 0.00210 | 0.0905 | 0.737 | 0.0950 | |
| 0.9 | 0.000126 | 0.00218 | 0.0917 | 0.734 | 0.0965 |
| 2 | 0.000125 | 0.00216 | 0.0918 | 0.735 | 0.0964 |
| TCS dismutation kinetics 5 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.000134 | 0.00220 | 0.0905 | 0.710 | 0.0954 |
| 0.000135 | 0.00221 | 0.0904 | 0.711 | 0.0953 | |
| TCS dismutation kinetics 6 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.000132 | 0.00216 | 0.0890 | 0.700 | 0.0938 |
| 0.000130 | 0.00217 | 0.0891 | 0.699 | 0.0937 | |
| DCS dismutation kinetics 1 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 0.6 | 0.0840 | 0.126 | 0.468 | 0.291 | 0.00184 |
| 0.0866 | 0.127 | 0.460 | 0.295 | 0.00219 | |
| 0.7 | 0.0882 | 0.127 | 0.455 | 0.298 | 0.00251 |
| 2 | 0.0881 | 0.128 | 0.456 | 0.298 | 0.00252 |
| DCS dismutation kinetics 2 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.0891 | 0.125 | 0.441 | 0.296 | 0.00340 |
| 0.0890 | 0.125 | 0.440 | 0.297 | 0.00341 | |
| DCS dismutation kinetics 3 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.0894 | 0.125 | 0.442 | 0.298 | 0.00342 |
| 0.0893 | 0.126 | 0.442 | 0.298 | 0.00341 | |
| DCS dismutation kinetics 4 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.0866 | 0.122 | 0.429 | 0.289 | 0.00331 |
| 0.0866 | 0.122 | 0.429 | 0.288 | 0.00332 | |
| DCS dismutation kinetics 5 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.0867 | 0.121 | 0.429 | 0.289 | 0.00332 |
| 0.0867 | 0.122 | 0.430 | 0.289 | 0.00332 | |
| DCS dismutation kinetics 6 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.0853 | 0.121 | 0.422 | 0.284 | 0.00327 |
| 0.0854 | 0.120 | 0.423 | 0.284 | 0.00326 | |
| MCS dismutation kinetics 1 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.413 | 0.213 | 0.275 | 0.0682 | 0.000290 |
| 0.413 | 0.214 | 0.276 | 0.0682 | 0.000288 | |
| MCS dismutation kinetics 2 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.413 | 0.213 | 0.276 | 0.0682 | 0.000288 |
| 0.413 | 0.214 | 0.276 | 0.0682 | 0.000290 | |
| MCS dismutation kinetics 3 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.408 | 0.211 | 0.272 | 0.0673 | 0.000284 |
| 0.407 | 0.211 | 0.273 | 0.0673 | 0.000285 | |
| MCS dismutation kinetics 4 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.409 | 0.211 | 0.273 | 0.0674 | 0.000285 |
| 0.408 | 0.211 | 0.273 | 0.0674 | 0.000286 | |
| MCS dismutation kinetics 5 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.402 | 0.208 | 0.268 | 0.0664 | 0.000280 |
| 0.402 | 0.208 | 0.269 | 0.0664 | 0.000281 | |
| MCS dismutation kinetics 6 bar total pressure | |||||
| τ (s) | Mole fraction | ||||
| S | MCS | DCS | TCS | STC | |
| 0.5 1 | 0.396 | 0.204 | 0.264 | 0.0653 | 0.000277 |
| 0.396 | 0.205 | 0.265 | 0.0654 | 0.000276 | |
| Molar Concentration (mmol/L) | ||||||
|---|---|---|---|---|---|---|
| Total Pressure, bar | Mole Fraction | |||||
| S | MCS | DCS | TCS | STC | τ (s) | |
| 1 | 0.00481 | 0.0791 | 3.25 | 25.5 | 3.42 | 3 |
| 2 | 0.00963 | 0.158 | 6.49 | 51.0 | 6.84 | 2.5 |
| 3 | 0.0145 | 0.238 | 9.77 | 76.7 | 10.3 | 1 |
| 4 | 0.0194 | 0.318 | 13.1 | 103 | 13.7 | 0.9 |
| 5 | 0.0242 | 0.398 | 16.3 | 128 | 17.2 | 0.5 |
| 6 | 0.0291 | 0.478 | 19.6 | 154 | 20.6 | - |
| Total Pressure, bar | Mole Fraction | ||||
|---|---|---|---|---|---|
| S | MCS | DCS | TCS | STC | |
| 1 | 3.01 | 4.23 | 14.9 | 10.0 | 0.115 |
| 2 | 6.01 | 8.46 | 29.8 | 20.0 | 0.230 |
| 3 | 9.05 | 12.7 | 44.8 | 30.1 | 0.346 |
| 4 | 12.1 | 17.0 | 59.8 | 40.2 | 0.462 |
| 5 | 15.1 | 21.3 | 74.8 | 50.4 | 0.579 |
| 6 | 18.2 | 25.6 | 89.9 | 60.5 | 0.695 |
| Total Pressure, bar | Mole Fraction | ||||
|---|---|---|---|---|---|
| S | MCS | DCS | TCS | STC | |
| 1 | 13.7 | 7.10 | 9.16 | 2.26 | 0.00955 |
| 2 | 27.4 | 14.2 | 18.3 | 4.53 | 0.0191 |
| 3 | 41.3 | 21.3 | 27.6 | 6.81 | 0.0288 |
| 4 | 55.1 | 28.5 | 36.8 | 9.10 | 0.0384 |
| 5 | 69.0 | 35.7 | 46.1 | 11.4 | 0.0481 |
| 6 | 82.8 | 42.8 | 55.3 | 13.7 | 0.0577 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuchok, O.; Stolmakov, Y.; Kalinina, A.; Medov, V.; Belousova, D.; Maleev, N.; Mochalov, G. Promising Catalyst for Chlorosilane Dismutation. Sci 2024, 6, 38. https://doi.org/10.3390/sci6030038
Zhuchok O, Stolmakov Y, Kalinina A, Medov V, Belousova D, Maleev N, Mochalov G. Promising Catalyst for Chlorosilane Dismutation. Sci. 2024; 6(3):38. https://doi.org/10.3390/sci6030038
Chicago/Turabian StyleZhuchok, Olesya, Yegor Stolmakov, Alexandra Kalinina, Vitaly Medov, Darya Belousova, Nikita Maleev, and Georgy Mochalov. 2024. "Promising Catalyst for Chlorosilane Dismutation" Sci 6, no. 3: 38. https://doi.org/10.3390/sci6030038
APA StyleZhuchok, O., Stolmakov, Y., Kalinina, A., Medov, V., Belousova, D., Maleev, N., & Mochalov, G. (2024). Promising Catalyst for Chlorosilane Dismutation. Sci, 6(3), 38. https://doi.org/10.3390/sci6030038






