Abstract
The Bryonia genus (Cucurbitaceae) is divided into 13 plants considered medicinal species with a significant pharmacological value fortreating as well as preventing various ailments. The current systematic review aims to present useful and updated findings published onthis genus inthe last two decades. Based on PubMed, Science Direct, JSTOR, and Google Scholar, 42 of the available previous studies on Bryonia have been selected from 2000 to 2022. Thereafter, these studies were analyzed, summarized, and separately recorded according to the topic or section, adding some comments foreach. Our review provided a botanical description of the genus, followed by itsindigenous uses. Furthermore, more than 150 reported phytochemical compounds were grouped into families such as terpenoids, alkaloids, flavonoids, glycosides, saponins, and volatile oils. Hereby, thebiological activities part of this genus wereexposed, including itsantimicrobial, antioxidant, antidiabetic, antinociceptive, and anti-inflammatory functions, along with an interesting anticancer efficiency. Overall, our findings could contribute to forthcoming investigations that may lead to determining the responsible phytoconstituents for Bryonia’s efficiency.
1. Introduction
Sinceantiquity, the world population has resorted to plants for medical purposes in order to provide for their healthcare needs [1]. Today, about 80% of itrelies on folk phytotherapy involving indigenous knowledge, traditional culture, and medicinal herbs from itssurroundings [2]. Certain plant families offer a highly curative effect with a worldwide distribution and large diversity, such as Cucurbitaceae. This family covers almost 100 genera spread across tropical and subtropical areas [3,4,5]. Among these genera that have shown significant importance as medicinal plants is the Bryonia genus. It comprises 10 direct children (B. cretica L., B. multiflora Boiss. and Heldr., B. monoica Aitch. and Hemsl., B. aspera Steven ex Ledeb. and B. alba L., B. lappifolia Vassilcz., B. melanocarpa Nabiev, B. syriaca Boiss., B. verrucosa Aiton, and B. flexuosa Yıld.) and 3 accepted subspecies (B. dioica Jacq., B. marmorata (E. Petit) Jauzein, and B. acuta Desf) across dioecious and monoecious types [6,7]. Some species are widely distributed with slight morphological dissimilarities from one to another, especially in the fruits, while others have a limited range. Additionally, all Bryonia species have an Irano-Turanian origin, prospering in many Mediterranean regions [8]. Bryonia was well known in ancient times for it shealing value. In fact, various diseases were traditionally treated using Bryonia species, for example, infectious diseases, tissue inflammation, cough, influenza, lung disease, cancers, hemorrhoids, diabetes, peritonitis, jaundice, typhoid, bone pain, and nervous and cardiac disorders [9,10,11,12]. These diversified therapeutic uses and medicinal properties of the genus are due to interesting phytochemical components such as polyphenols, flavonoids, triterpene, glycosides, sterols, saponins, and alkaloids [13,14]. Nonetheless, a few Bryonia species have a significant toxic potentiality, where the most poisonous part is the roots [15,16]. Furthermore, in this smallest genus of the Cucurbitaceae family, B. alba Linn.and B. dioica Jacq. are the common species used in folk medicine as valuable remedies against serious ailments [11,17]. Despite this phytochemical and historical medical distinction of Bryonia, the species from this genus still face scientific deficiency, with slight interest shown in recent studies. Therefore, the present study aimed to systemically summarize the botanical, ethnobotanical, biological, and phytochemical properties of species belonging to the genus Bryonia. Moreover, this systematic review ended the controversy over the exact number of Bryonia species.
2. Methodology
In accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), the current systematic review was designed and reported.
2.1. Search Strategy
The search was conducted inthe following databases: the JSTOR “www.search.scielo.org (accessed on 30 December 2022)”, PubMed® “www.pubmed.ncbi.nlm.nih.gov (accessed on 30 December 2022)”, ScienceDirect® “www.sciencedirect.com/search (accessed on 30 December 2022)”, and Google Scholar “www.scholar.google.com (accessed on 30 December 2022)” databases. The term “Bryonia” was the main word used in the searches individually or in association with other keywords such as “botany”, “phytochemistry”, “GC-MS”, “HPLC”, “pharmacology”, “biological activity”, “ethnomedicine”, “traditional use”, “cancer”, “anticancer”, “antiproliferative”, “antitumor”, “cytotoxicity”, and “toxicity”. The accepted species of this genus were obtained from thedatabases The Plant List (TPL) (www.theplantlist.org/, accessed on 30 December 2022), World Flora Online (WFO) (www.worldfloraonline.org/, accessed on 30 December 2022), and Plants of the World Online (POWO) (www.powo.science.kew.org/, accessed on 30 December 2022), validating their scientific names. Moreover, we adopted the Boolean operator “AND” in the searches that contained two or more of these keywords.
2.2. Study Selection
With the use of only verified accepted species names and selected keywords in the search and analysis, articles published in the last 22 years (2000–2022) reporting specifically botanical, phytochemical, pharmacological, and toxicological data on Bryonia species were included, following the following eligibility criteria:
- (1)
- Validated source of material: plant materials were identified to the taxonomic levels of genus and species.
- (2)
- Appropriate methodology: standard methods for pharmacological assays both in vitro and in vivo were employed, along with phytochemical investigation.
- (3)
- Access to the full-text article in the English language: articles published only in non-English languages were excluded.
On the other hand, review articles, e-books, book chapters, and doctoral theses were excluded. Moreover, articles with titles containing the word Bryonia as synonyms of other species not referring to the genus Bryonia or even unverified names, not dealing with the previous search terms, and not available in the full text were excluded.
2.3. Data Extraction
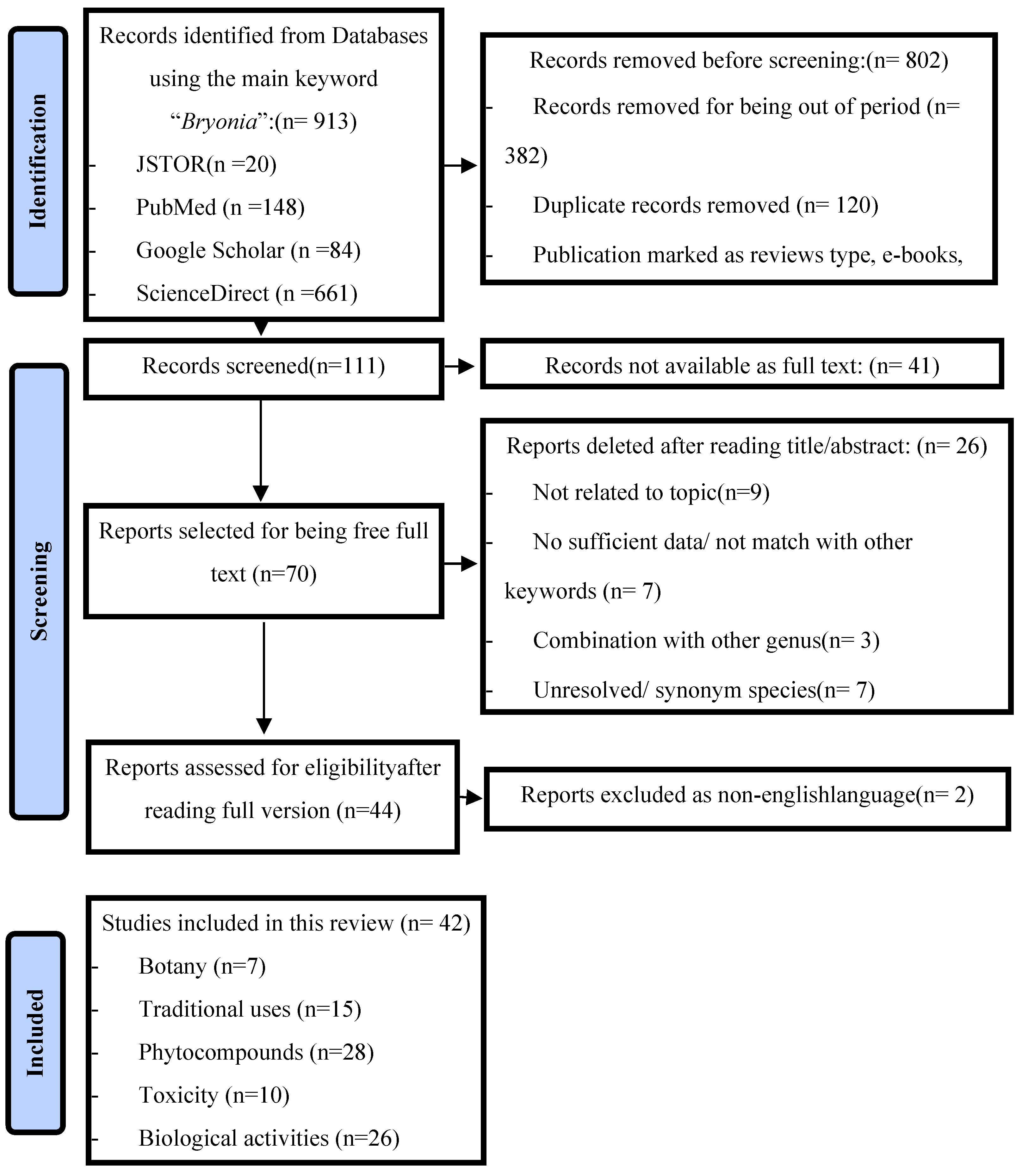
Overall, 111 scientific documents were selected from the databases and screened. After reading the title and/or abstract, 26 documents that did not fit into the scope of this review were excluded. A total of 44 articles had their full text analyzed, being screened by the title, abstract, and specific information throughout the text. Consequently, 42 articles with data on the traditional uses, pharmaceutical activities, phytochemicals, and toxicity of Bryonia species were included in this study. The available information on Bryonia was categorized into many sections: botanical description, phytochemicals and bioactivecompounds, traditional uses of Bryonia, in vivo and in vitro pharmacological studies, and the anticancer and toxicity potential of Bryonia. The identification and selection process isillustrated in Figure 1.
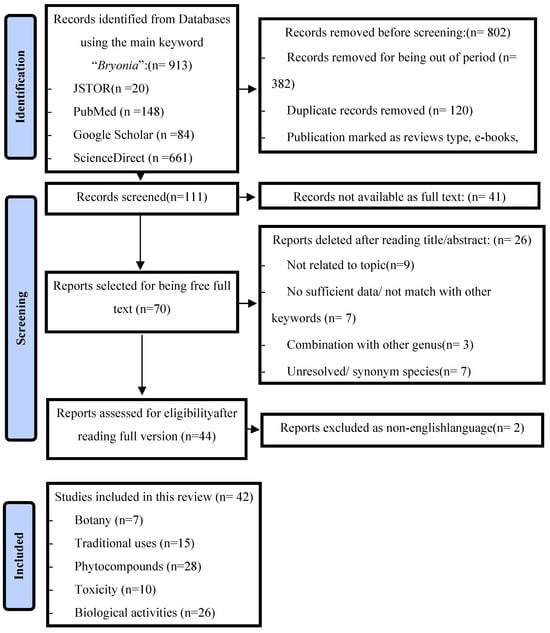
Figure 1.
Identification and selection process of the bibliographic sources based on a PRISMA 2020 flow diagram.
3. Results and Discussion
3.1. Botany (Taxonomy, Description, and Distribution)
The Bryonia genus belongs to the Cucurbitaceae family and comprises 13 known taxa with accepted names (Table 1). Previously, B. alba, B. verrucosa, B. syriaca, B. lappifolia, B. cretica, B. multiflora, B. monoica, B. melanocarpa, and B. aspera were the only accepted species included in the Bryonia genus, while the status of the 10th species (B. flexuosa Yıld) was reported as unresolved [18]. According to the recent articles and updated taxonomy (POWO and WFO), the genus Bryonia includes a total of 10 accepted direct species and 3 subspecies, namely B. acuta, B. marmorata, and B. dioica, considered B. cretica subspecies [6,7,19,20,21]. In the same line, Tutin et al. (2010) reported the presence of three subspecies belonging to the B. cretica species [4].

Table 1.
Bryonia accepted species and subspecies according to WFO and POWO databases [6,7].
Bryonia species are flowering annual herbs defined by tuberous roots, palmate-lobed leaves (about 3–5 at sharp angles), small flowers with threestamens, and several types of spherical berries with oblate–ovoid seeds. On the contrary, these bryonies show non-similar external morphological characteristics differing from one species to another.
As shown in Table 2, almost all Bryonia species flower in spring (March to May), while the flowering of other species may extend into summer (B. alba and B. cretica). The flowers are green or yellow or even a mixed color between the two, together forming a tiny greenish yellow mass of 3–4 flowers on the stem, which climbs in threes due to itslong tendrils. On the other hand, B. dioica has blue or white flowers [11]. With the exception of B. alba, B. monoica, and B. aspera., all Bryonia species are dioecious, and their ripe fruits are often red, having a similar shape. Moreover, the macroscopy details are still poorly described for B. melanocarpa, B. marmorata, and B. lappifolia besides the anatomical structures of all species.

Table 2.
Description and distribution of Bryonia species.
Owing to their adaptability, Bryonia species are widely dispersed all over the world and cultivated in different environmental conditions, which may explain their distribution in different regions such as the Mediterranean to Central European territories, Northern Africa, and Central Asia [25]. Nonetheless, some species are considered native to specific regions such as B. dioica and B. cretica from North Africa (Algeria, Morocco) and Western Europe and B. multiflora, B. aspera, and B. alba from Turkey [3,26,27].
3.2. Traditional Uses
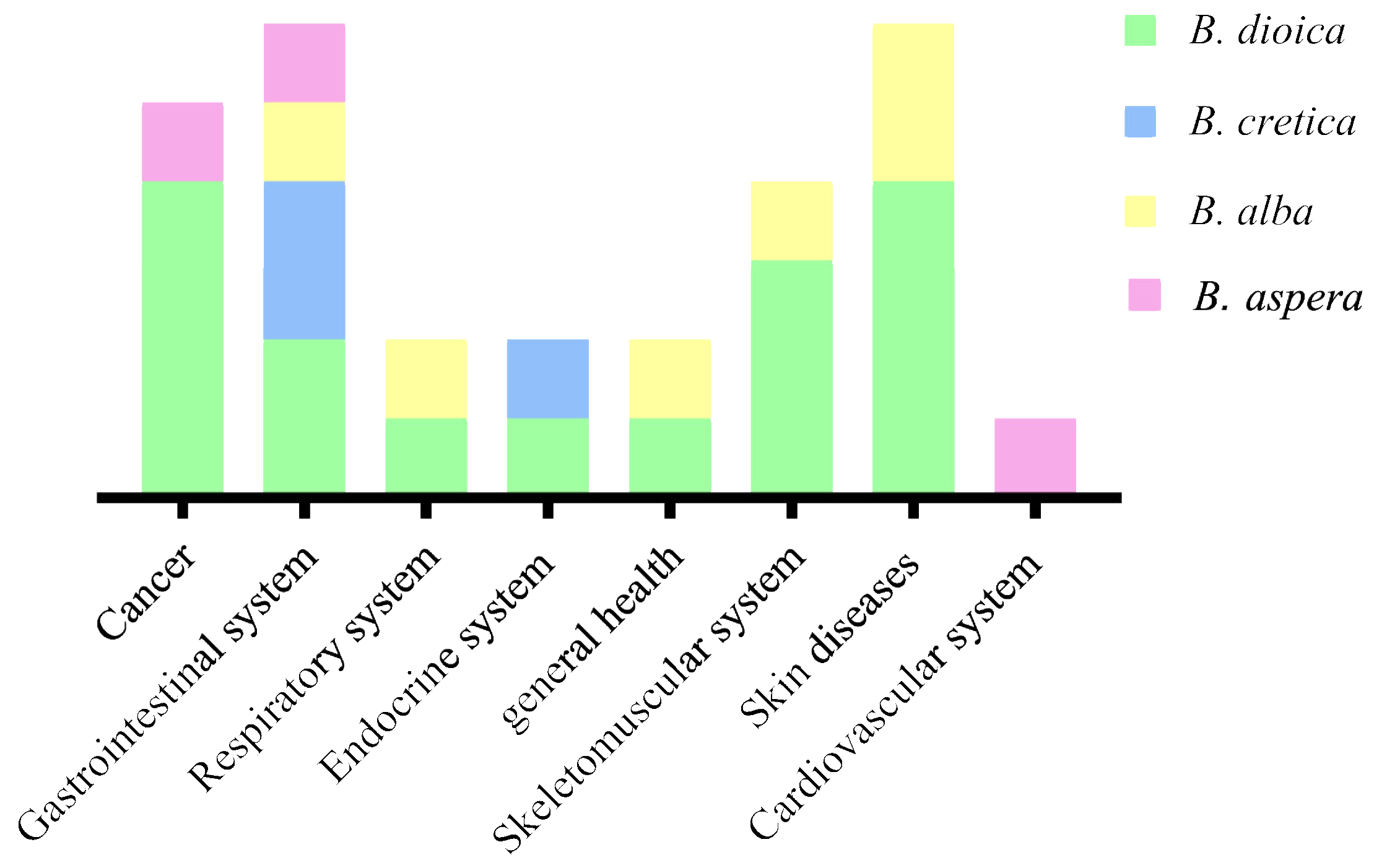
Bryonia species have been used for centuries to treat different diseases or manage a variety of disorders with a focus on theirtuberous roots’ proprieties (bitter, toxic…) [8,25]. Figure 2 illustrates different disease categories treated traditionally using Bryonia species. Ethnobotanical studies showed that B. dioica, B. aspera, B. alba, and B. cretica are the most involved species. Nonetheless, B. dioica seems to be more known than others species in Northern Africa due to its anticancer property (Algeria, Morocco, and Tunisia) [2,11,28]. B. dioica is used to treat breast cancer, nose cancer, skin cancer, and uterus tumors as well asbruises, rheumatic pains, arthritis, minor wounds, and lesions. Other investigations reported that theroots of B. dioica are used as remedies for liver failure, stomachache, ulcers, and diabetes [11,15]. Furthermore, the species is used in Iraq to treat bronchitis, while the leaves and seeds are used to cure fevers using internal or external administration [29].
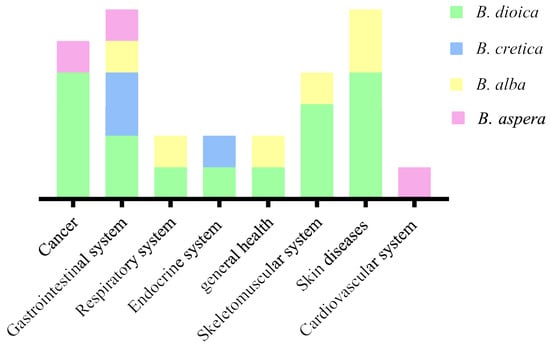
Figure 2.
Bryonia species traditionally used to treat several ailments.
In eastern and northern Europe, the B. alba root has been used in diverse systems including the gastrointestinal, respiratory, and skeletomuscular systems to treat intestinal worms, convulsions, peritonitis, jaundice, headaches, and pneumonia [13]. The root extract is applied torheumatic and joint pain in Turkish folk medicine, whereas white bryony is commonly applied for the treatment of skin diseases, edemas, and bruises [21]. On the other hand, B. aspera was reported to be commonly used in the Turkmen Sahara region (northeast of Iran) to treat gastrointestinal and cardiac diseases and some cancer types [24,30]. Likewise, the roots of B. cretica were used by Egyptian healers to treat gastric disorders and diabetes [31].
3.3. Pharmacological Proprieties (In Vivo/In Vitro Application)
Different Bryonia species have shown interesting biological properties including antioxidant, anti-inflammatory, antibacterial, anti-parasitic, anticancer, etc. (Table 3).
- Antioxidant
Gholivand and Piryaei (2012) investigated the antioxidant ability of the methanolic extract of B. dioica (leaves, stems, and flowers) using DPPH scavenging, β-carotene bleaching, and reducing power tests. The polar subfraction of the flowers provided the highest radicalscavenging activity with the lowest IC50 value of 23.17 ± 4.24 μg/mL. Despite this result, both the polar and nonpolar subfractions of all parts provide a high radical scavenging inhibition. In the β-carotene bleaching assay, the flowers’ polar subfraction and leaves’ nonpolar subfraction had a notable result against linoleic acid oxidation, with inhibition values of 98.35 ± 8.7 and 83.62 ± 5.91%, respectively [32]. Likewise, a promising antioxidant potential has been demonstrated for the methanolic extract of leaves of the Iraqi B. dioica [33]. In another study on immature and ripened fruits of Portuguese B. dioica, it has been found that immature fruit extract exhibited the lowest antioxidant activity with an EC50 value ranging from 1.07 to 18.01 mg/mL due to its lower phenolic and flavonoid contents (33.37 and10.32 mg CE/g extract, respectively). On the other hand, the fruit extract showed an important antioxidant potential at the maturity stage (EC50: 0.22–1.21 mg/mL) [34].
InRomania, more studies have focused on the antioxidation potential of B. alba species using different approaches (cell-free systems and cellular assays) to evaluate the peroxidase-inhibiting effect, as well as justify the antioxidant/antiradical activity of the leaf extract and its isolated flavonoids. An important inhibition of myeloperoxidase and horseradish peroxidase has been reported and shown according to its active molecules lutonarin and saponarin (IC50 = 0.92 ± 0.55 µg/mL). Moreover, the best inhibition of the ROS produced by neutrophils and macrophages was obtained using the isolated compounds isoorientin and lutonarin [35]. Similarly, Lelciu et al. (2019) investigatedthe antioxidant capacity of methanolic extract of B. alba’s aerial parts using six in vitro assays. A significant antioxidant capacity related to the flavonoid content has been demonstrated (DPPH: IC50 = 99.8 ± 0.92 µg/mL; TEAC: IC50 = 19.9 ± 0.89 µg/mL; CUPRAC: IC50 = 238 ± 2.24; FRAP: IC50 = 217 ± 2.45; SNPAC: IC50 = 217 ± 2.45; and EPR: IC50 = 427 ± 2.46 µg/mL) [14]. Similar results have been reported for B. alba from Turkey [13].
- Anti-inflammatory
Based on the ethnomedicinal uses of some Bryonia species to treat inflammatory disorders, several in vitro and in vivo investigations have been carried out to evaluate the anti-inflammatory potential of Bryonia species, especially B. alba and B. dioica (Table 3).
Ukiya et al. (2002) evaluated the anti-inflammatory activity of MeOH extract of the B. dioica roots and its fractions (n-hexane, EtOAc, n-butanol, and H2O) using an in vivo TPA-induced inflammation assessment in mice. Among the four fractions, a remarkable inhibition effect of the EtOAc-soluble fraction (inhibitory ratio 90% at 1 mg/ear) was pointed out. Moreover, the same test was further used to assess the inhibitory effects of six pure triterpene glycosides (four new compounds, bryoniosides 2–5, with two known compounds, cabenoside D and bryoamaride) isolated from the EtOAc-soluble fraction. A strong anti-inflammatory effect was shown (ID50 = 0.2–0.7 mg/ear) against TPA-induced inflammation comparedto that of a reference drug (1.6 mg/ear) [36]. In the same line asthese findings, n-hexane, EtOAc, and MeOH extracts of B. alba exhibited promising in vivo anti-inflammatory effects [13]. Likewise, an extract of B. alba leaves and its flavonoids showed anti-inflammatory effects through the inhibition of the pro-oxidant enzyme myeloperoxidase [17].
- Antibacterial
As shown in Table 3, the antibacterial activity of B. dioica was tested on nine bacterial strains. Significant antimicrobial activity has been reported against three of them: E. coli, K. pneumonieae, and P. valgaris [33]. Furthermore, Dhouioui et al. (2016) stated that Gram-positive bacteria were more susceptible to the lipid fraction of B. dioica roots than that of the fruits. However, the lipid fractions extracted from both plant parts were less active than antibiotics [37].
- Antiparasitic
By using two parasitic strains 3D7 (chloroquine sensitive) and W2 (chloroquine resistant), an extract B. alba’s aerial partswas tested for its anti-plasmodialpotential, showing no cellular toxicity inthese Plasmodium falciparum strains [14].
- Anti-infection
In a study carried out by Goswami et al. (2022), a 14-day-old chick embryo (Gallus gallus domesticus) inoculated with recombinant Delta SARS-CoV-2 spike RBD protein was injected with a diluted ethanolic extract of B. alba before the inoculation as pre-treatment and after the inoculation as post-treatment. The results showed that the B. alba extract was able to upregulate IFN-α and IL-10, especially in post-treatment, indicating an immunomodulatory effect through a pro-inflammatory cytokine decrease [38].
- Antidiabetic
Chekroun et al. (2017) used astreptozotocin (STZ)-induced diabetic rat model to establish the antidiabetic effect of B. dioica root aqueous extract. After 21 days of daily treatment using the B. dioica extract (20 mg/kg i.p), the B. dioica extract exhibited a similar effect to the standard drug inreducingtheblood glucose levels by −59% and −51%, respectively, restoring the normal biochemical parameters levels, as well as reversing the reduction in body weight of the rats [39]. Likewise, Uyar et al. (2017) investigated the protective effects of B. multiflora extract on pancreatic, liver, and kidney cells. The findings showed a significant increase in the insulin antibody immune-positive areas for thetreated groups with extract doses of 100, 200, and 400 mg/kg BW/day, inducing a significant improvement in the beta cells’ function. Besides this effect, B. multiflora extract was found to inhibit the damage formed in the liver, kidneys, and pancreas; hence, the ability of the plant inthe treatment of diabetes and its complications has been connected to its antioxidant activity [40].
- Hepatoprotective effect
Kadhim (2014) investigated the hepatoprotective action of anethanolic extract of B. dioica leaves on rats with CCl4-induced hepatotoxicity. This assay was based on examination of the transaminase (ALT and AST) activity and the histopathological changes in the rats’ livers. The results confirmed the protective capacity of B. dioica against the toxic effects of CCL4 since the extract showed the recovery of the hepatic architecture from CCL4-induced necrosis and of the decline in the ALT and AST activities to a normal level [29].
- Antinociceptive
The antinociceptive ability of ethyl acetate extract obtained from B. alba roots was assessed using p-benzoquinone-induced abdominal constriction and tail flick tests in mice. The first assay showed a significant antinociceptive effect with the lowest writhing number of 26.0 ± 4.3 compared to those of the other extracts (n-hexane and methanolic) and identical to that of the reference drug (aspirin). However, none of the extracts prepared from B. alba roots showed any activity in the second assay [13].
- Anti-polycystic ovary syndrome
In a recent experiment by Tahvilian et al. (2022), a B. dioica root methanolic extract was evaluated against polycystic ovary syndrome in female rats receiving subcutaneous injections of testosterone enanthate. After 28-day treatment, the hormone (FSH and LH) and glucose levels were significantly normalized besides a decrease in theLDL level and LDL/HDL ratio in the B. dioica groups, showing an ameliorative as well as a preventive effect on PCOS-induced rats [41].
- Anticancer
Increasing evidence emphasizes the anticancer activity of Bryonia species, especially that of its cucurbitacin constituents, given an interesting anticancer profile to this genus. These triterpene-type compounds, mainly obtained from the root parts, present potential as biomarkers in herbal therapy, showing particular promise as anticancer agents, with a general mechanism based on cell cycle blockage and programmed death, like apoptosis or autophagy [42]. Cucurbitacins exert their cytotoxic effects against cancer cells by inhibiting their proliferation, invasion, and migration [43].
Anethanolic extract of B. cretica roots was found to exhibit important antiproliferative action againsthuman leukemia U937 cells. Furthermore, two cucurbitane-type triterpenes (cucurbitacins B and E) isolated from the same extract displayed a comparable effect oncell growth inhibition with IC50 values of 9.2 and 16 nM after 72 h, respectively, along with that of camptothecin (8.6 nM) [31]. Likewise, cucurbitacin E showed a strong cytotoxic activity (IC50 = 40 nM after 72 h) on HT1080 cells dissimilar to that of isocucurbitacin D (0.71 uM) [44]. Moreover, Pourgonabadi et al. (2017) reported thesignificant inhibitory activity and apoptotic effects exerted by a hydro-alcoholic root extract of B. aspera against Hela and HN-5 cell lines [30]. In another study, B. aspera methanolic extract induced thecell death and apoptosis of B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL) [45]. Similar results were reported for achloroform extract of B. aspera roots and two of its identified compounds (cucurbitacin L, and neocucurbitacin C) [46]. Significant cytotoxic activity has been exerted by B. aspera species against different tumor cells, including MCF7 (human breast adenocarcinoma), HepG2 (hepatocellular carcinoma), and WEHI (mouse fibrosarcoma) [45].
B. dioica has also been studied for its anticancer effects. Indeed, several studies have demonstrated that different extracts of the species were able to inhibit the growth of cancer cells, induce apoptosis, and result in cell cycle arrest through a variety of molecular mechanisms. Benarba et al. (2012) demonstrated that anaqueous extract of B. dioica roots significantly inhibited the cell growth of the Burkitt’s lymphoma BL41 cell line by inducing apoptosis through the mitochondrial pathway (activation of caspase-3 and caspase-9, PARP cleavage, and loss of the mitochondrial membrane potential) [28]. In another study, Benarba et al. (2019) found that the same extract at a lower concentration (50 μg/mL) was able to induce both apoptosis and G2/M cell cycle arrest in MDA-MB 231 breast cancer cells. This anticancer activity was attributed to its identified major phenolic compound myricetin [47]. Furthermore, amethanol extract of B. dioica showed in vitro and in vivo anticancer effects against B16F10 melanoma cells, in which the cucurbitacins obtained from this extract were able to induce apoptosis, as well as arrest cell cycle progression [48].
Other studies based on anti-inflammatory analysis have demonstrated that the triterpenes from B. dioica roots areexpected to have a high anti-tumor-promoting role inmice. Likely, a compound isolated from B. dioica, namely bryoside, has been noticed as an anti-inflammatory and anti-tumor agent [36].

Table 3.
Bryonia species in vivo/in vitro applications.
Table 3.
Bryonia species in vivo/in vitro applications.
| Ailments/Activities | Bryonia Species (Family/Part) | Product | A Model/Strains | B Inhibitory Assay | Dosage | Control (Negative ⊖/Positive ⊕) | Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| In vitro assays | ||||||||
| Anti-Oxidant | B. dioica fruits, leaves, stems, flowers, roots | Methanolic/ethanolic extract | DPPH, RP, inhibition of B-carotene bleaching, inhibition of lipid peroxidationTBARS | 1 mg/mL | Extract | An antioxidant effect | [32,34] | |
| B. alba roots | N-hexane, ethylacetate (EtOAc), methanol (MeOH) | DPPH, ABTS, FRAP, and hydroxyl radicalscavenging assay | 0.01 mg dw/mL | EtOAc extract showed strong DPPH and ABTS radicalscavenging activity | [13] | |||
| B. alba leaves, aerial parts | Flavonoids (lutonarin, saponarin, isoorientin, isovitexin) | L012 substrate (probe) | Horseradish peroxidase (HRP)-catalyzed oxidation assay | 2 and 100 lg/mL | Enzyme alone enzyme and probe | Inhibition ofthe peroxidase-catalyzed reactions showed significant antioxidant activity, proved to pass through cell membranes and to exhibit their antioxidant/antiradical effect | [14,17] | |
| Myeloperoxidase | Direct MPO assay | Buffer DMSO | ||||||
| Neutrophils | Effects on the total ROS produced by PMA-activated neutrophils | Non-PMA-activated cells | ||||||
| HL-60 | Effects on the ROS produced by PMA-activated HL-60 monocytes | Non-activated cells | ||||||
| SNPAC EPR | 1 mg/mL | An antioxidant effect | ||||||
| Antibacterial | B. dioica roots, fruits | Lipid fraction | E. coli, S. typhimurium E. faecium, S. agalactiae, S. aureus | Disk diffusion method according to NCCLS method inhibition zone | 15 uL | Ampicillin | Inhibited the growth of all the test bacterial strains | [37] |
| B. dioica leaves | Ethanolic extract | E. coli, S. aureas, K. pneumoniea, S. typhi, P. aeruginosa, P. valgaris | Well diffusion assay | 200 (mg mL−1) | Antimicrobial activity against three Gram-negative microorganisms E. coli, K. pneumoniea, and P. valgaris. | [33] | ||
| Anti-Plasmodial | B. alba aerial parts | Methanolic extract | Plasmodium falciparum strains: 3D7 and W2 | Activity of the plasmodial lactate dehydrogenase (pLDH) at 630 nm | 0.8 and 100 ug/mL | Infected and uninfected erythrocytes | No cellular toxicity on the parasitic strains used | [14] |
| Anti-proliferative | B. aspera roots | Hydro-ethanolic | HN-5 and Hela cells | MTT assay | 12.5 to 500 μg/mL | Decreased cell viability in Hela and HN-5 cell lines in a concentration- and time-dependent manner | [30] | |
| B. cretica roots | Triterpene glycosides, bryoniosides A and B | Humanleukemia U937 cells | Cytotoxicity assay | 9.2 and 16 nM 72 h | Inhibition of cell proliferation | [31] | ||
| B. cretica roots | Isocucurbitacin D (MeOH) | HT1080 cells | MTT assay | 1 uM | Positive control Cucurbitacin E | Cytotoxic effects with the disruption of the target protein cofilin | [31] | |
| Cancer/Tumor | B. alba roots | 23, 24-dihydrocucurbitacin D (dhc D) Ethanol | Cancer cells A-549, COLO 205, SK-MEL-2, L121O | Cytotoxicity test | Antitumor effect on tumor cells | [49] | ||
| B. dioica roots | Bryoniosides, cucurbitacins, cucurbitacin A, cucurbitacin G methanolic extract | In vitro B16F10 melanoma cancer cells | MTT assay Apoptotic effects | Increasing number of cells that express a block in cell cycle progression in the sub-G1phase, followed by cell death induced by apoptosis, anti-melanoma effects by inhibiting cell migration, and invasion through the FAK and Srcsignaling pathways | [48] | |||
| B. dioica roots | Methanolic extract Cucurbitaneglycosides | EBV-EA activation Rajicells (virus nonproducer) | Method of probit-graphic interpolation | 1 × 10 mol ratio/TPA | MeOH-CHCl3-H2O | Deglycosylation enhances the inhibitory effects on EBV-EA activation (anti-tumor) | [36] | |
| B. dioica roots | Aqueous extract | Burkitt’s lymphoma BL41 cells Propidium iodide (PI) staining of cell DNA | Corroborative assays Flow cytometry analysis of dot-blot light scatter profiles | 250 to 500 ug/mL | Untreated cells | Inducesapoptosis in Burkitt’s lymphoma cells line BL41 by triggering the mitochondria-mediated pathway (exp: activation of caspase-9 and -3 the cleavage of PARP and degradation of PUMA) | [28] | |
| B. aspera aerial parts | Methanolic | NALM-6 and REH cell lines | MTT assay | 200–300 uL/mL | Cytotoxic effect causing apoptosis induction | [45] | ||
| B. aspera roots | Hydro-ethanolic extract | HN-5 and Hela cell lines | Apoptosis assay | 12.5–100 μg/mL 48 h | Cells + Dulbecco’s modified Eagle’s medium (DMEM) Normal cells | Cell death is involved in B. aspera-induced toxicity in Hela and HN-5 cell lines | [30] | |
| B. aspera roots | Neocucurbitacin C and 7β-hydroxy dihydrocucurbitacin D chloroform extract | Cancer cell lines (MCF7, HepG2, and WEHI) and normal cells MDBK | MTT assay | <50 μg/mL | 5-fluorouracil and tamoxifen Non-treated cells | Strongly reduced growth of cancer cells | [46] | |
| Cytotoxicity activity | B. alba leaves | Flavonoids (lutonarin, saponarin, isoorientin, isovitexin) | A549, HeLa, WI38, neutrophils, HL-60 cells | Cell viability | Non-treated cells | Very low toxicity or absence of toxicity of the tested samples | [17] | |
| B. alba aerial parts | Methanolic extract | A549, HeLa, WI38 | Cytotoxicity assay | 100 ug/mL | -Non-treated cells + Camptothecin | No cellular toxicity | [14] | |
| B. alba roots | Aqueous and methanol extracts | Human normal (lymphocytes) (HeLa and Caco-2) cells | Comet assay | No genotoxic effects | [50] | |||
| B. dioica | Aqueous extract | BL41 cells | MTT assay | 250–500 ug/mL | Cytotoxicity effect | [28] | ||
| In vivo assays | ||||||||
| Inflammation | B. dioica roots | Glycosides | Female ICR mice | TPA-Induced Inflammation | 1 mg per ear | MeOH-CHCl3-H2O | Inhibitory effects against TPA-induced inflammation Anti-tumor | [36] |
| B. alba roots | N-hexane, ethylacetate (EtOAc), methanol (MeOH) | Swiss albino mice | Carrageenan-induced hind paw edema model | 0.1 mg/kg | 25 μL of saline PC | EtOAc extract showed a statistically significant anti-inflammatory activity in a carrageenan-induced hind paw edema model and an acetic-acid-induced increase in capillary permeability | [13] | |
| Acetic-acid-induced increase in capillarypermeability (Whittle method) | 0.2 mL/20 g | |||||||
| Diabetes | B. dioica roots | Aqueous extract | n5-STZ diabetic rats (Wismar) | Acute toxicity | 30 mg/kg, i.p/21 days | Non-diabetic (5 mL/kg b.w./day of saline solution NaCl 9‰; i.p.); diabetic (5 mL/kg b.w./day of saline solution NaCl 9‰; i.p.) | Significantly decreased the level of serum glucose with 64% reductions. The body weight as well as serum levels of total cholesterol, triglycerides, and urea were markedly reversed secondary to subacute administrationof the aqueous extract. Changes in the weight of internal organs were restored to normal by the prolonged effect of the BDRaq extract treatment. The BDRaq extract appeared to have maximum antidiabetic activity in normalizing all studied parameters during the acute and subacute treatments | [39] |
| B. multiflora | Wistar albino male rats | Acute toxicity test | 100, 200 and 400 mg/kg | Citrate buffer STZ | Prevented damage withliver, kidney, and pancreasamelioration in the functioning of the betacells. | [40] | ||
| hepatotoxicity | B. dioica Leaves | Ethanolic extract | Albino male rats | CCL4-induced hepatic damage | 250 mg/kg | Saline Ccl4 | Protection against the toxic effects of CCL4, hepatoprotective effect | [29] |
| Anti-nociceptive | B. alba Roots | N-hexane, ethylacetate (EtOAc), methanol (MeOH) | Swiss albino mice | P-benzoquinone-induced abdominal constriction Tail flick test | 0.1 mg/kg | Vehicle/ aspirin Morphine | EtOAc extract displayed antinociceptive activity in the p-benzoquinone-induced writhing mouse model Marked anti-inflammatory effects, with 50% inhibitory doses (ID50) of 0.2–0.6 mg None of the extracts showed any activity in the tail flick test | [13] |
| SARSCoV-2 infections. | Bryonia alba | Ethanolic extract | Gallus gallus embryo | Induced pathogenesis assay | 10 μg/mL | Alcohol (70%) | Upregulation of IFN-α, IFN-ß, and TGF-ß by Delta SARS-CoV-2 spike protein RBD antigen | [38] |
| Polycystic ovary syndrome | B. dioica | Methanolic extract | Immature female Wistar rats | DHEA antiandrogenic assay | 30 mg/kg/day for 28 days | Saline Metformin | Protective effect on PCOS rats and normalized the hormones, glucose, LDL, and LDL/HDL ratio, improvement effect on the symptoms and markers of PCOS and fertility | [41] |
| Toxicity | B. dioica Roots | Aqueous extract | Male mice | Acute/subacute oral toxicity | 250–1000 mg/kg 62.5–250 mg/kg | Distilled water | Dose higher than 250 mg/kg was shown to be toxic for mice in acute toxicity Dose up to 250 mg/kg was shown to be safe for animals in subacute toxicity | [15] |
| B. alba Leaves | Flavonoids (lutonarin, saponarin, isoorientin, isovitexin) | Zebrafish larvae | In vivo toxicity assay | 25 embryos not treated | No changes in the parameters were noticed within the 72 h, similar to the ones of the negative control Absence of zebrafish toxicity was confirmed | [17] | ||
| B. alba Aerial parts | Methanolic extract | Zebrafish (Danio rerio) | In vivo acute toxicity | 0.1–100 ug/mL | Non-treated larvae | Lack of toxicity | [14] | |
| Cancer | B. alba Roots | 23, 24-dihydrocucurbitacin D (dhc D) Ethanol | ICR mice sarcoma 180 ascites tumor cells | Antitumoric test | 5 to 10 mg/kg 60 days | 0.2% ethanol + saline Sarcoma 180 + saline | Antitumor effect | [49] |
| B. dioïca Root | Bryoniosides, cucurbitacins, cucurbitacin A, cucurbitacin G Methanolic extract | Balb/c in mice | Apoptotic effect | 50 mg M/kg/d (for 28 days) | B16F10-injected mice treated only with PBS | Cell death induced by apoptosis anti-melanoma effects in vivo by inhibiting cell migration and invasion through the FAK and Srcsignaling pathways | [48] | |
A HN-5: head and neck squamous cell carcinoma, Hela: cervix adenocarcinomacell lines, ICRmice: mice from the Institute of Cancer Research, HL-60: human leukemia cells, HT1080: fibrosarcoma cells, A549: lung cancer cells, WI38: fetal lung fibroblast cells, NALM6: human B-cell precursor leukemia cell line, REH: non-B acute lymphoblastic leukemia cell line, MDBK: Madin–Darby bovine kidney, EBV-EA: Epstein–Barr virus earlyantigen. B DPPH: diphenyl-2-picrylhydrazyl radical scavenging, RP: reducing power, TBARS: inhibition of lipid peroxidation using thiobarbituric-acid-reactive substancesin brain tissue homogenates, ABTS: [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate)] radicalscavenging assay, FRAP: ferric-reducing antioxidant power, PMA: phorbol-12-myristate-13-acetate, SNPAC: silver nanoparticle antioxidant capacity, EPR: electron paramagnetic resonance, TPA: 12-O-tetradecanoylphorbol-13-acetate, DHEA: dehydroepiandrosterone, CCl4: carbon tetrachloride.
3.4. Phytochemistry and Bioactive Compounds
Phytochemical studies on Bryonia species have led to the isolation and identification of approximately 150 primary and secondary metabolites to date (Figure 3). Table 4 shows their names, types, corresponding plant sources, and the nature of the extracts, with an illustration of some of the main compound structures in Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8.
As primary metabolites, elements have the highest percentage with 19 compounds, followed by fatty acids with 15 compounds, and sugars with 12 compounds (Figure 3). In fact, at the time of writing this review, the presence ofboth sugars (8%) and vitamins (5%) has been reported in only two species of Bryonia: B. dioica and B. alba. Meanwhile, elements (12%) were particularly detected in B. dioica. Likewise, a few proteins (four compounds) such as lectin, bryodiofin, and bryodin were found in the B. dioica roots.
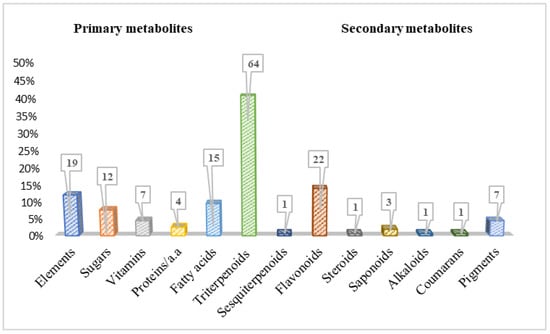
Figure 3.
Phytoconstituent classes found in Bryonia species.
Figure 3.
Phytoconstituent classes found in Bryonia species.
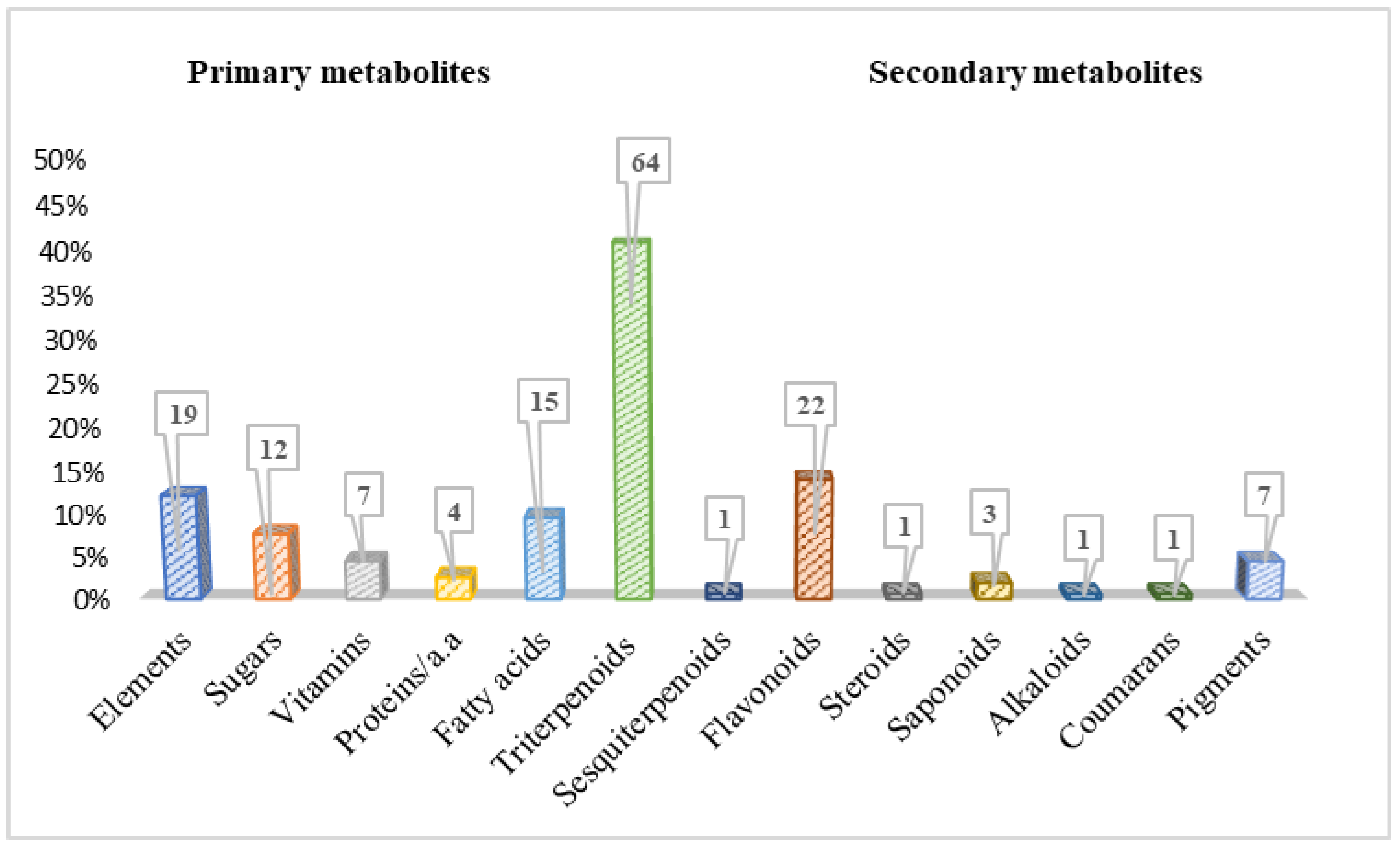
Considering secondary metabolites, this review reports a total of 100 compounds identified in this genus from 2000 to the present day (Table 4). Predominantly, the terpenoidclass was detected in Bryonia species, including triterpenoids as the main type, with 64 compounds; sesquiterpenoids; and tetraterpenoids. Moreover, flavonoids were remarkably revealed with 22 compounds. However, chemicals such as alkaloids, saponins, steroids, and coumarans were marginally reported (Figure 3).

Table 4.
Phytochemical compounds identified in Bryonia species.
Table 4.
Phytochemical compounds identified in Bryonia species.
| Compounds | Species | Plant Part | Area | Extract 1 | Analysis 2 | Ref. |
|---|---|---|---|---|---|---|
| Primary metabolites | ||||||
| Elements | ||||||
| Fe, Si, P, Al, Mn, Mg, Pb, Ni, Mo, Ca, Cu, Zn, Na, K, Sr, Co, Cd, As, and Hg | B. dioica B. alba | Root | Ukraine | Ash | AAS | [51] |
| Sugars | ||||||
| Fructose | B. dioica | Fruit | Portugal | MeOH | [34] | |
| Glucose | ||||||
| Sucrose | ||||||
| Trehalose | ||||||
| Raffinos | B. alba B. multiflora | Root | Ukraine | dH2O | GC/MS | [52] |
| Ribose | ||||||
| Rhamnose | ||||||
| Arabinose | ||||||
| Xylose | ||||||
| Fucose | ||||||
| Mannose | ||||||
| Galactose | ||||||
| Vitamins | ||||||
| α-Tocopherol | B. dioica | Fruit | Portugal | MeOH | [34] | |
| β-Tocopherol | ||||||
| γ-Tocopherol | ||||||
| δ-Tocopherol | ||||||
| Ascorbic acid | ||||||
| Proteins/a.a | ||||||
| Lectin | B. dioica | Root | ||||
| Bryodiofin | ||||||
| Bryodin | ||||||
| N4-(2-hydroxyethyl)-L-asparagine | B. dioica | |||||
| Other a.a | B. alba B. mutiflora | Root | Ukraine | dH2O, MeOH | HPLC | [34,53] |
| Fatty acids | ||||||
| Methyl jasmonate | B. dioica | |||||
| α-linolenic acid | B. dioica | |||||
| Trihydroxyocdecadeinoic acids | B. multiflora B. alba | Root | ||||
| Bryonolicacid (Ba) 3β-Hydroxy-D: C-Friedoolean-8en-29-Oic acid | [38] | |||||
| Other essential oils | B. dioica | Fruit, aerial parts, root | Tunisia | Oil | GC-FID/GC–MS | [37] |
| Secondary metabolites | ||||||
| Terpenoids (Triterpenoid) | ||||||
| Dihydrocucurbitacin B (DHCB) | B. cretica | Root | Egypt | EtOH | HPLC | [31] |
| B. aspera | Iran | TCM | [46] | |||
| Dihydrocucurbitacin D (DHCD) | B. alba | Root | Armenia | MeOH | H/C-NMR, UV, MS | [46,49] |
| B. aspera | Iran | TCM | ||||
| Dihydrocucurbitacin E (DHCE) | B. cretica | Root | Egypt | EtOH | HPLC | [31] |
| Hexanorcucurbitacin D | B. cretica | Root | Egypt | EtOH | HPLC | [31] |
| Cucurbitacin B(CuB) (Amarine) | B. multiflora | Root | Turkey | MeOH | HPLC | [54] |
| B. verrucosa | TCM | |||||
| B. cretica | ||||||
| Cucurbitacin G | B. cretica | Root | Egypt | MeOH | NMR, X-ray crystallography | [27] |
| Cucurbitacin H | B. cretica | Root | Egypt | MeOH | NMR, X-ray crystallography | [27] |
| Cucurbitacin I (Elatericin B) | B. alba | Root | Turkey | MeOH | HPLC | [27,31,36,54] |
| B. multiflora | TCM | |||||
| B. dioica | MeOH | |||||
| B. verrucosa | Germany | EtOH | C-Sephadex LH-20 | |||
| B. cretica | Egypt | MeOH | HPLC | |||
| Cucurbitacin D (Elatericin A) | B. alba | Root | EtOH | HPLC | [31,54] | |
| B. verrucosa | ||||||
| B. cretica | Egypt | MeOH | ||||
| Cucurbitacin E (α-Elaterin) | B. alba | Root | HPLC | [31,54] | ||
| B. verrucosa | ||||||
| B. cretica | Root | Egypt | EtOH | |||
| Cucurbitacin J | B. alba | Root | HPLC | [31,54] | ||
| B. dioica | ||||||
| B. cretica | Egypt | EtOH | ||||
| Cucurbitacin K | B. alba | Root Root | [36,54] | |||
| B. dioica | ||||||
| Cucurbitacin L | B. alba | Root | MeOH | H/C-NMR TLC, C- Sephadex LH-20 | [23,36,46,54,55] | |
| B. melanocarpa | Uzbekistan | |||||
| B. dioica | Germany | |||||
| B. cretica | ||||||
| B. aspera | Iran | TCM | ||||
| Cucurbitacin S | B. dioica | |||||
| Bryoamaride | B. melanocarpa | Root | Uzbekistan | MeOH | H/C-NMR TLC, C/H NMR | [23,36,55] |
| B. dioica | Japan | |||||
| Isomultiflorenol | B. melanocarpa | |||||
| bryocoumaricacid | B. dioica | |||||
| 3a-hydroxy-multiflora-7, 9 (11)-dien-29a-oic acid | B. dioica | |||||
| Arvenin IV | B. alba | |||||
| Isocucurbitacin G | B. cretica | Root | Egypt | MeOH | NMR, X-ray crystallography | [27] |
| Isocucurbitacin H | B. cretica | Root | Egypt | MeOH | NMR, X-ray crystallography | |
| Isocucurbitacin D | B. cretica | Root | Egypt | MeOH | NMR, X-ray crystallography | |
| Iso Dihydrocucurbitacin D | B. aspera | Root | Iran | TCM MeOH | 2D NMR | [26,46] |
| Epi-Iso Dihydrocucurbitacin B | ||||||
| 7β-hydroxy dihydrocucurbitacin D | ||||||
| 25-Oglucosyl Dihydrocucurbitacin D | ||||||
| 2-O-Glucosyl Dihydrocucurbitacin D | ||||||
| 4-Hydroxy-N-(2-hydroxyethyl)-benzamide (bryonamide A) | ||||||
| 4-Hydroxy-3-methoxy-N-(2-hydroxyethyl)-benzamide (bryonamide B) | ||||||
| Bryonolic acid | B. aspera | Root | Iran | TCM | 2D NMR | [26,46] |
| B. melanocarpa | MeOH | |||||
| Tirucalla-5, 24-dien-3b-ol | B. dioica | Root | MeOH | [36] | ||
| 25-O-acetyl bryoamaride | B. dioica | Root | Germany | MeOH | C-Sephadex LH-20 | |
| Bryodiosides A | B. dioica | Root | Japan | EtOH | C-Sephadex LH-20 | |
| Bryodiosides B | ||||||
| Bryodiosides C | ||||||
| Bryodulcoside | B. dioica | Root | Germany | MeOH | ||
| Bryonoside | B. dioica | Root | Japan | EtOH | C-Sephadex LH-20 FDMS, H/C NMR | |
| Bryoside | B. dioica | Root | Chemical, FDMS, H/C NMR | |||
| Cucurbitacin I 2-O-â-D-glucopyranoside | B. dioica | Root | Germany | MeOH | C-Sephadex LH-20 | |
| 10α-cucurbitadienol | B. dioica | Root | Japan | ACE -MeOH | HPLC | |
| (24R)-24-ethyl-5a-cholest-7-en-3b-ol | B. melanocarpa | |||||
| B-sitosterol-3-O-glucoside | B. cretica | |||||
| Stigmasta-7E, 24 (28)-dien-3b-ol | B. melanocarpa | Root | ||||
| 4a-methyl stigmasta-7E, 24 (28)-dien-3b-ol | ||||||
| (24R)-24-ethyl-5a-cholest-7-en-3b-ol 3-O-b-D-glucopyranoside | ||||||
| 10 Is 2-O-B-D-glucopyranoside | ||||||
| Elaterinide | B. dioica | Root | Germany Algeria | MeOH | C-Sephadex LH-20 | [28,36] |
| Tetrahydrocucurbitacin | B. dioica | Root | Algeria | [36] | ||
| Bryonioside A | B. dioica B. cretica | Root | Japan Egypt | EtOAc EtOH | HPLC | [26,36] |
| Bryonioside B | B. dioica B. cretica | Root | Japan Egypt | EtOAc EtOH | HPLC | [26,36] |
| Bryonioside C | B. dioica | Root | Japan | EtOAc of MeOH | HPLC | [36] |
| Bryonioside D | ||||||
| Bryonioside E | ||||||
| Bryonioside F | ||||||
| Bryonioside G | ||||||
| Cabenoside D | B. dioica | Root | Japan | EtOAc | C/H NMR | [36] |
| Bryodulcosigenin | B. dioica | Root | Japan | EtOAc | C/H NMR | |
| Bryosigenin | B. dioica | Root | Japan | EtOAc | C/H NMR | |
| Bryogenin | B. dioica | |||||
| 22-deoxocucurbitosides A | B. alba B. multiflora | Root | EtOH | |||
| 22-deoxocucurbitosides B | B. alba | EtOH | ||||
| 22-deoxocucurbitosides D | B. multiflora | Root | ||||
| 22-deoxocucurbitacin D | B. alba | EtOH | ||||
| Neocucurbitacin C | B. aspera | Root | Iran | TCM | 2D NMR | [26,46] |
| Terpenoids (Sesquiterpenoids) | ||||||
| Maaliol | B. dioica | Root | Morocco | dH2O | GC–MS | [15] |
| Saponins | ||||||
| Brydiosides A | B. dioica | Root | Algeria | [26,28,49] | ||
| Brydiosides B | ||||||
| Brydiosides C | ||||||
| Steroids | ||||||
| Delta7-stigmastenol | B. dioica | |||||
| Flavonoids | ||||||
| Saponarin | B. dioica B. alba | Root Leaves/aerial part | Algeria Romania | MeOH | HPLC–DAD, MS/NMR | [14,17,28,39] |
| Isovitexin | B. alba | Aerial parts | Romania | MeOH | HPLC–DAD | [14,17] |
| Vitexin | ||||||
| Vicenin | B. dioica | [11] | ||||
| Lutonarin | B. alba | Leaves/aerial parts | Romania | MeOH | HPLC–DAD, MS/NMR | [14,17] |
| Isoorientin | B. alba | Leaves/aerial parts | Romania | MeOH | HPLC–DAD, MS/NMR | [14,17] |
| 5, 7, 4′-trihydroxy flavone 8-Cglucopyranoside | B. alba | |||||
| Alliaroside | B. dioica | |||||
| Apigenin-C-Hexoside-O-Hexoside | B. dioica | Fruit | Portugal | EtOH | HPLC–DAD–ESI/MS | [56] |
| Apigenin-6-C-Glucoside-8-C-Glucoside | ||||||
| Apigenin-6-C-Glucoside-7-O-Glucoside | ||||||
| Apigenin-C-Hexoside-O-Hexoside | ||||||
| Apigenin-6-C-Glucoside (Isovitexin) | ||||||
| Quercetin-3-O-Neohesperidoside | ||||||
| Quercetin-O-Rhamnosyl-Pentoside | ||||||
| Quercetin-O-Rhamnosyl-Rhamnoside | B. dioica | Root | [12] | |||
| Quercetin-O-Hexoside 8 | ||||||
| Kempferol 3,7-Di-O-Rhamnoside | ||||||
| Kaempferol-O-Rhamnosyl-Hexoside-O-Rhamnoside | ||||||
| Kaempferol-3-O-Neohesperidoside | ||||||
| Kaempferol-O-Pentosyl-Rhamnoside | ||||||
| Kaempferol-3,4′-Di-O-Rhamnoside | ||||||
| Alkaloid | ||||||
| Bryonicine | B. alba | Leaves | EtOH | [38,57] | ||
| Coumarans | ||||||
| Coumaran | B. dioica | Root | Morocco | dH2O | GC–MS | [15] |
| Pigments | ||||||
| Chlorophyll A | B. dioica | Fruit | Portugal | MeOH | [34] | |
| Chlorophyll B | ||||||
| Lycopene | ||||||
| β-carotene | B. dioica | Fruit/shoot | Portugal Spain | MeOH | [34,58] | |
| Lutein | B. dioica | Shoot | Spain | HPLC–PDA | [58] | |
| Neoxanthin | ||||||
| Violaxanthin | ||||||
1 EtOH: ethanolic extract, EtOAc: ethyl acetate extract, MeOH: ethanol extract, TCM: chloroform extract, dH2O: aqueous extract; 2 HPLC: high-performance liquid chromatography, GC–MS: gas chromatography–mass spectrometry, H/C-NMR: proton/carbon nuclear magnetic resonance, GC-FID: gas chromatography with flame ionization detection, HPLC–PDA: high-performance liquid chromatography–photodiode array detection, HPLC–DAD–ESI/MS: high-performance liquid chromatography–diode array detection–electro-spray ionization mass spectrometry, 2D NMR: two-dimensional nuclear magnetic resonance spectroscopy, FDMS: field desorption mass spectrometry, TLC: thin-layer chromatography, UV: ultraviolet, MS: mass spectrometry.
- Terpenoids
Terpenoids are considered the largest phytochemical class widely distributed in almost all plants. According to their carbon units, terpenoids include monoterpenes, diterpenes, triterpenes, sesquiterpenes, and sesterpenes [59]. Triterpenoids were found to be abundant in Bryonia species. Among them, cucurbitacins are considered the most important triterpenoids of the Bryonia species. Cucurbitacins, highly oxygenated triterpenoids with a 19-(10→9β)–abeo–10α–lanost–5–ene ring skeleton and 5,(6) –double bond, are often tetracyclic and highly unsaturated [60]. Actually, cucurbitacins E, B, I, D, J, K, and L, dihydrocucurbitacins E and B, and tetrahydrocucurbitacin I were identified in Bryonia roots, with some differences due to the harvest period, geographical location of the plant, and extraction methods [61]. Sallam et al. (2010) isolated two cucurbitacins, isocucurbitacins G and H, from Bryonia roots [27]. Earlier, other triterpenoids, namelybryonoside and bryoside, were isolated [62]. Ukiya et al. (2002) isolated ninetriterpene glycosides: bryoniosides A−G, cabenoside, and bryoamaride [36] (Figure 4).
On the other hand, three tetraterpenoids (lycopenetetra, chlorophyll A, and β-carotene) along with a sesquiterpenoid (Maaliol) have been reported in methanolic and aqueous extracts from B. dioica species (Figure 5).
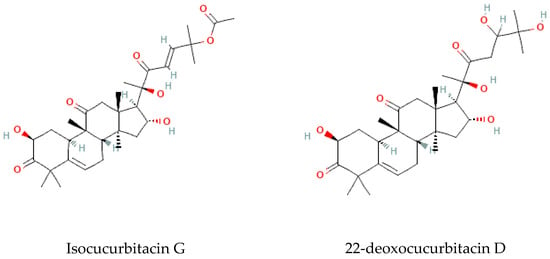
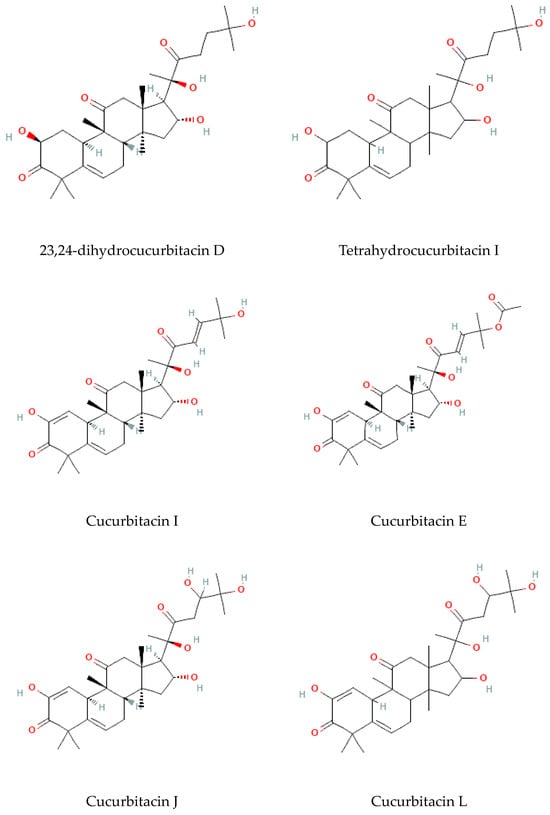
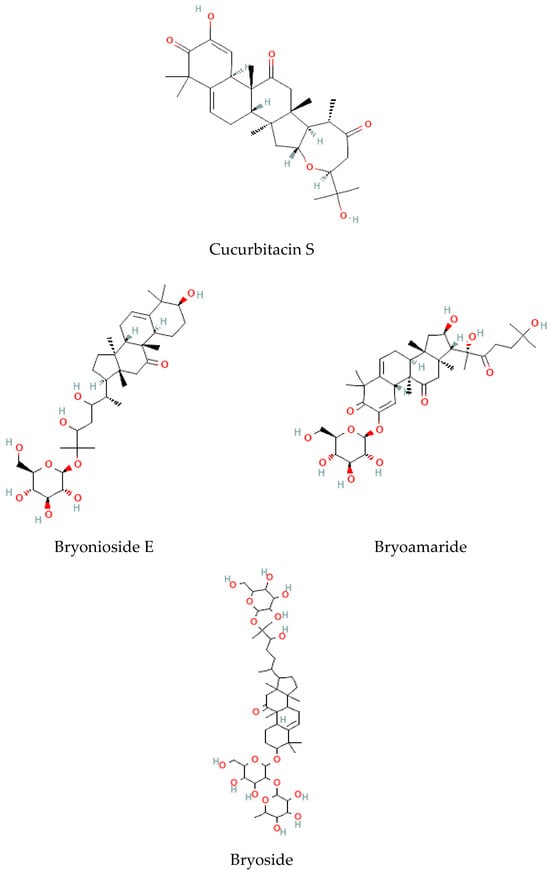
Figure 4.
Triterpenoid-type molecules isolated from Bryonia genus.
Figure 4.
Triterpenoid-type molecules isolated from Bryonia genus.
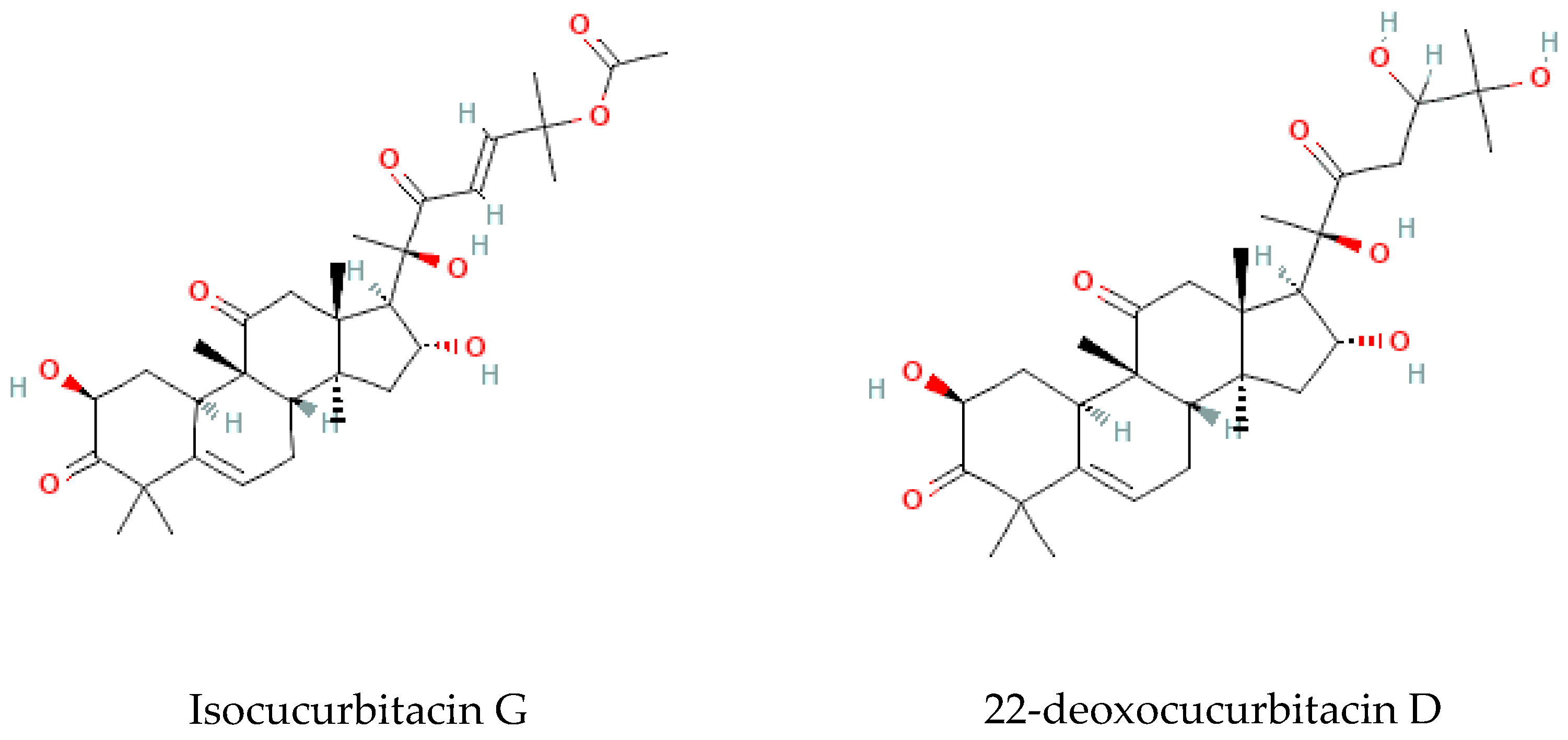
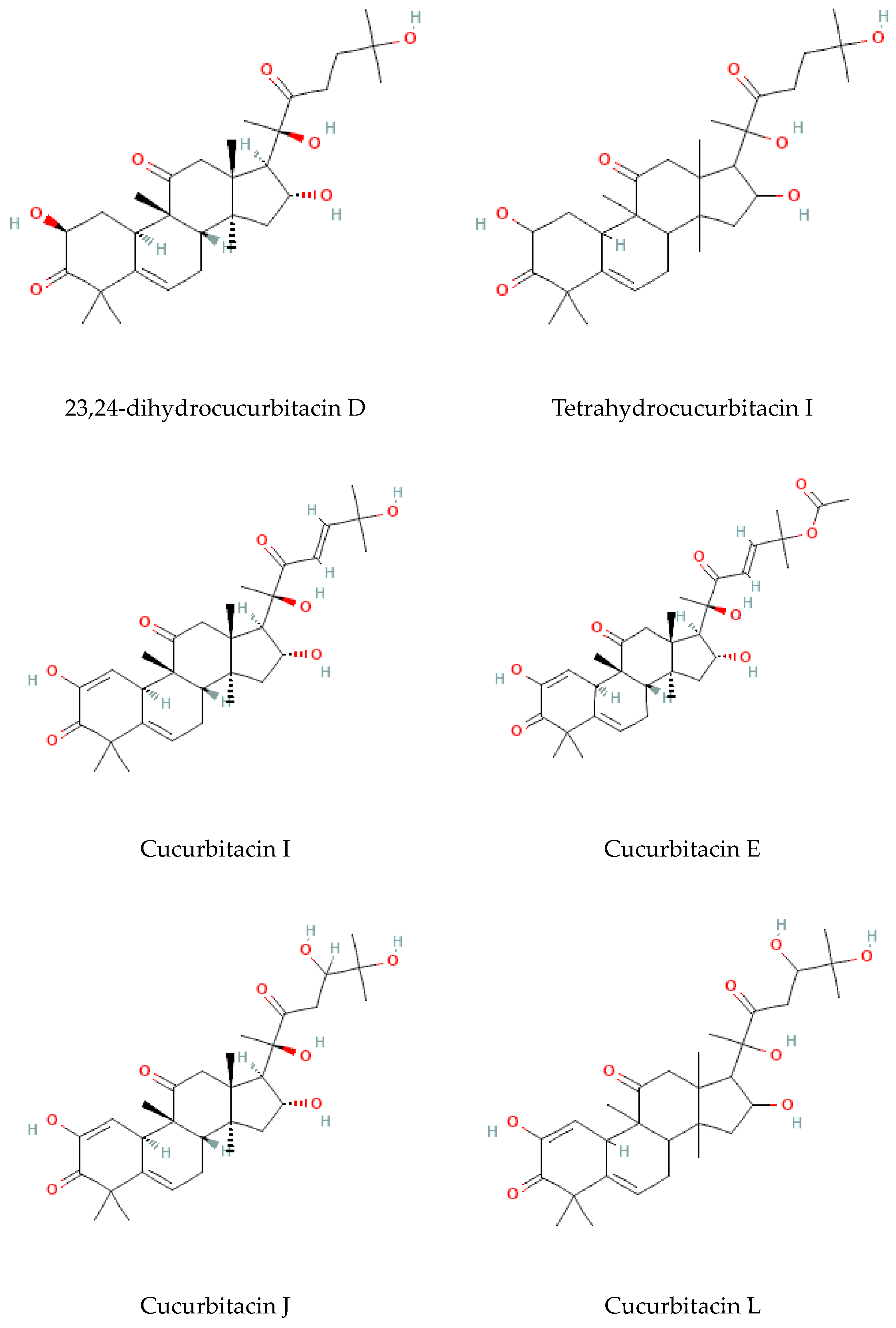
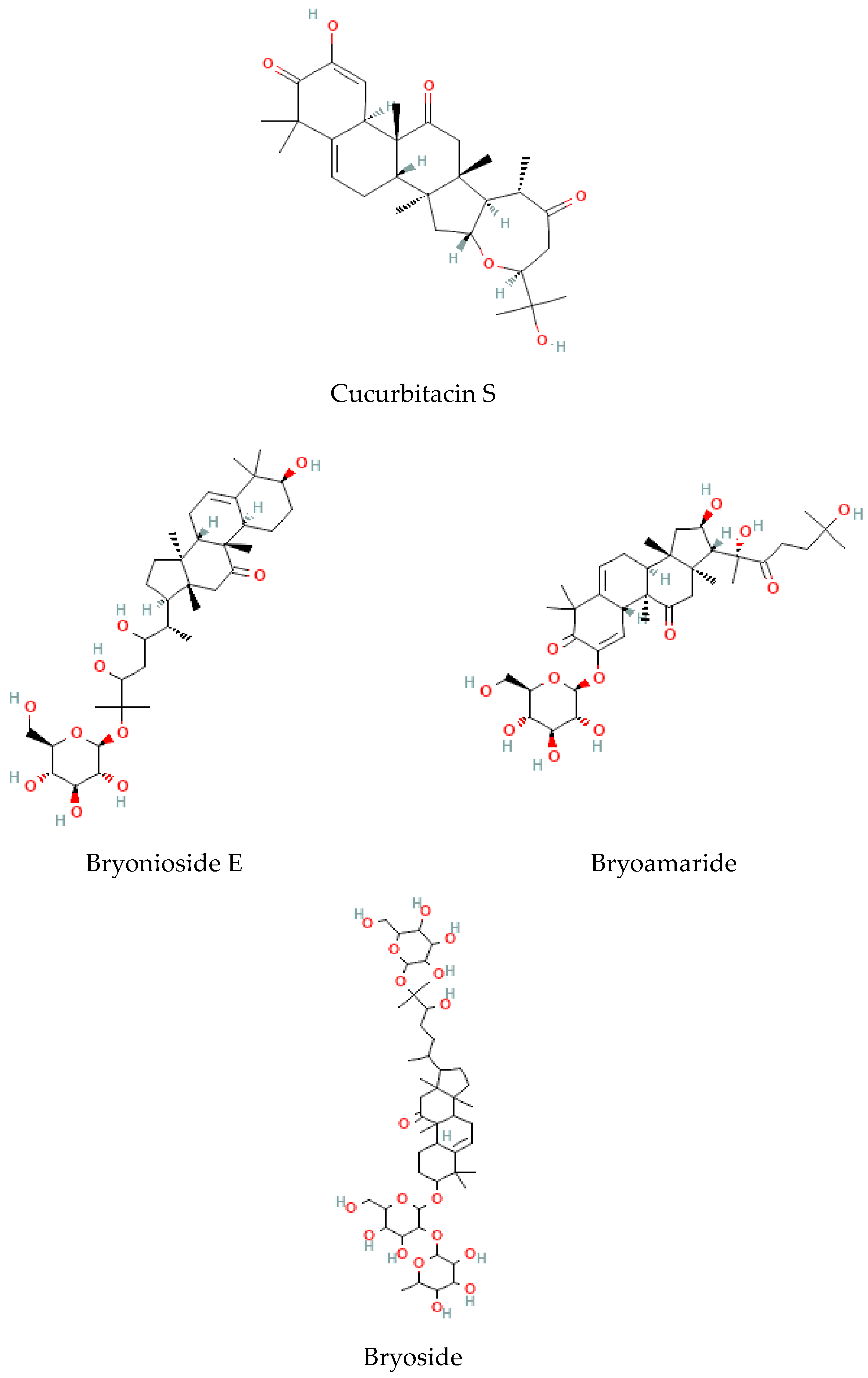
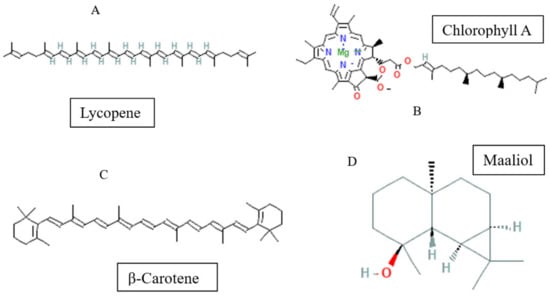
Figure 5.
Tetraterpenoid/sesquiterpenoid-type molecules isolated from Bryonia genus ((A–C) tetraterpenoids and (D) sesquiterpenoid).
Figure 5.
Tetraterpenoid/sesquiterpenoid-type molecules isolated from Bryonia genus ((A–C) tetraterpenoids and (D) sesquiterpenoid).
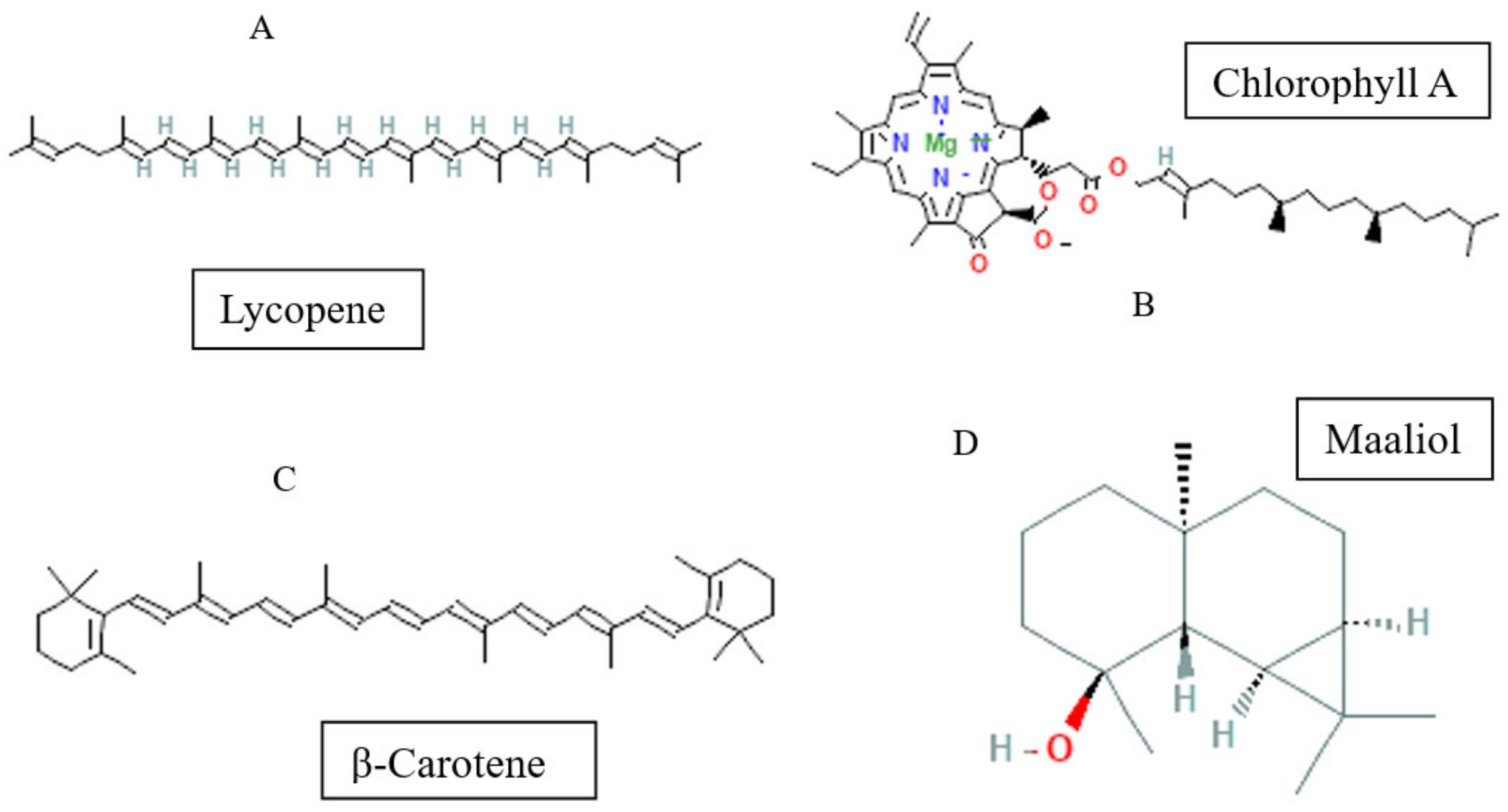
- Flavonoids
Considered the second major phytochemical group in the genus Bryonia, 23 flavonoids (Table 4, Figure 6) have been isolated from this genus, including 9 flavanols and 11 flavones, during the latest 22 years. Lelciu et al. (2019) identified four flavonoids in the crude extract of the aerial parts of B. alba [17]. These flavonoids were lutonarin, saponarin, isoorientin, and isovitexin. Earlier, saponarin and vicenin-2 were reported in B. dioica [63]. Moreover, Barreira et al. (2013) reported the presence of both O- and C-glycosides of flavonoids in the methanolic extract of B. dioica fruits. Actually, they identified O- and C-glycosides of two flavonols—quercetin (Quercetin-3-O-neohesperidoside, Quercetin-O-rhamnosyl-pentoside, Quercetin-O-rhamnosyl-rhamnoside, Quercetin-O-hexoside) and kaempferol (Kaempferol-O-rhamnosyl-hexoside-O-rhamnoside, Kaempferol-3-O-neohesperidoside, Kaempferol-O-pentosyl-rhamnoside, Kaempferol-O-pentosyl-rhamnoside, Kaempferol-3,4′-di-O-rhamnoside)—and one flavone: apigenin (Apigenin-6-C-glucoside, Apigenin-C-hexoside-O-hexoside, Apigenin-6-C-glucoside-8-C-glucoside, Apigenin-6-C-glucoside-7-O-glucoside, Apigenin-C-hexoside-O-hexoside) [56].
Among the flavonols, five kaempferol and four quercetin flavonols were revealed completely in the B. dioica roots with no reports onthe extraction method or even the detection method. Nevertheless, Quercetin-3-O-Neohesperidoside and Quercetin-O-Rhamnosyl-Pentoside were found in an ethanol extract of B. dioica fruit using HPLC–DAD–ESI/MS. Likewise, the flavone apigenin (Apigenin-C-Hexoside-O-Hexoside, Apigenin-6-C-Glucoside-8-C-Glucoside, Apigenin-6-C-Glucoside-7-O-Glucoside, Apigenin-C-Hexoside-O-Hexoside, Apigenin-6-C-Glucoside) was detected. In addition, both B. alba and B. dioica leaves showed other type of flavonoids such as saponarin, lutonarin, and isoorientin via different methods (HPLC–DAD and MS/NMR). Gholivand and Piryaei (2012) reported that flavonoids were obtained from B. dioica flowers, leaves, and stems using several extracts (polar and nonpolar) [32]. Similarly, amethanolic extract of B. alba’s aerial parts showed the presence of lutonarin, saponarin, isoorientin, and isovetexin [14].
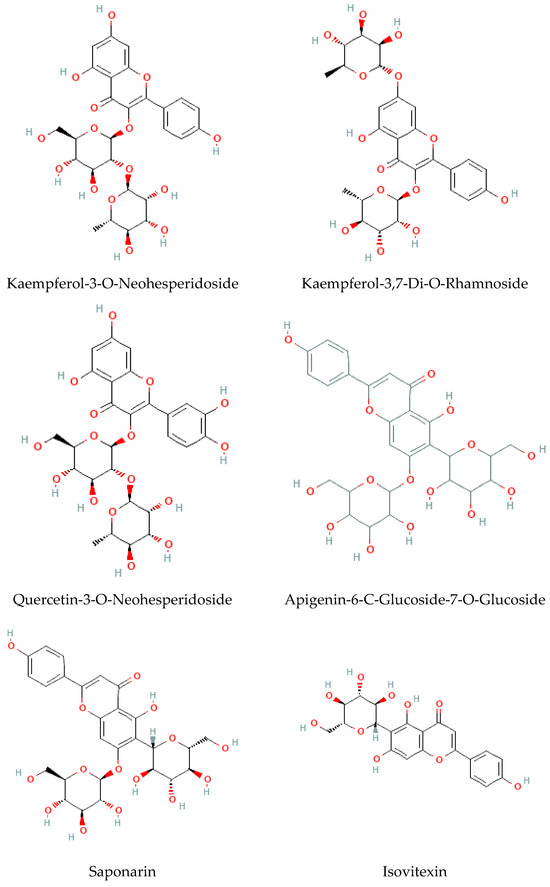
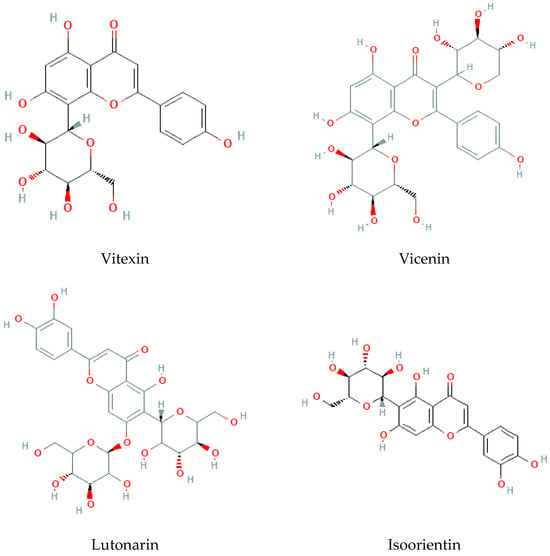
Figure 6.
Flavonoid-type molecules isolated from Bryonia genus.
Figure 6.
Flavonoid-type molecules isolated from Bryonia genus.
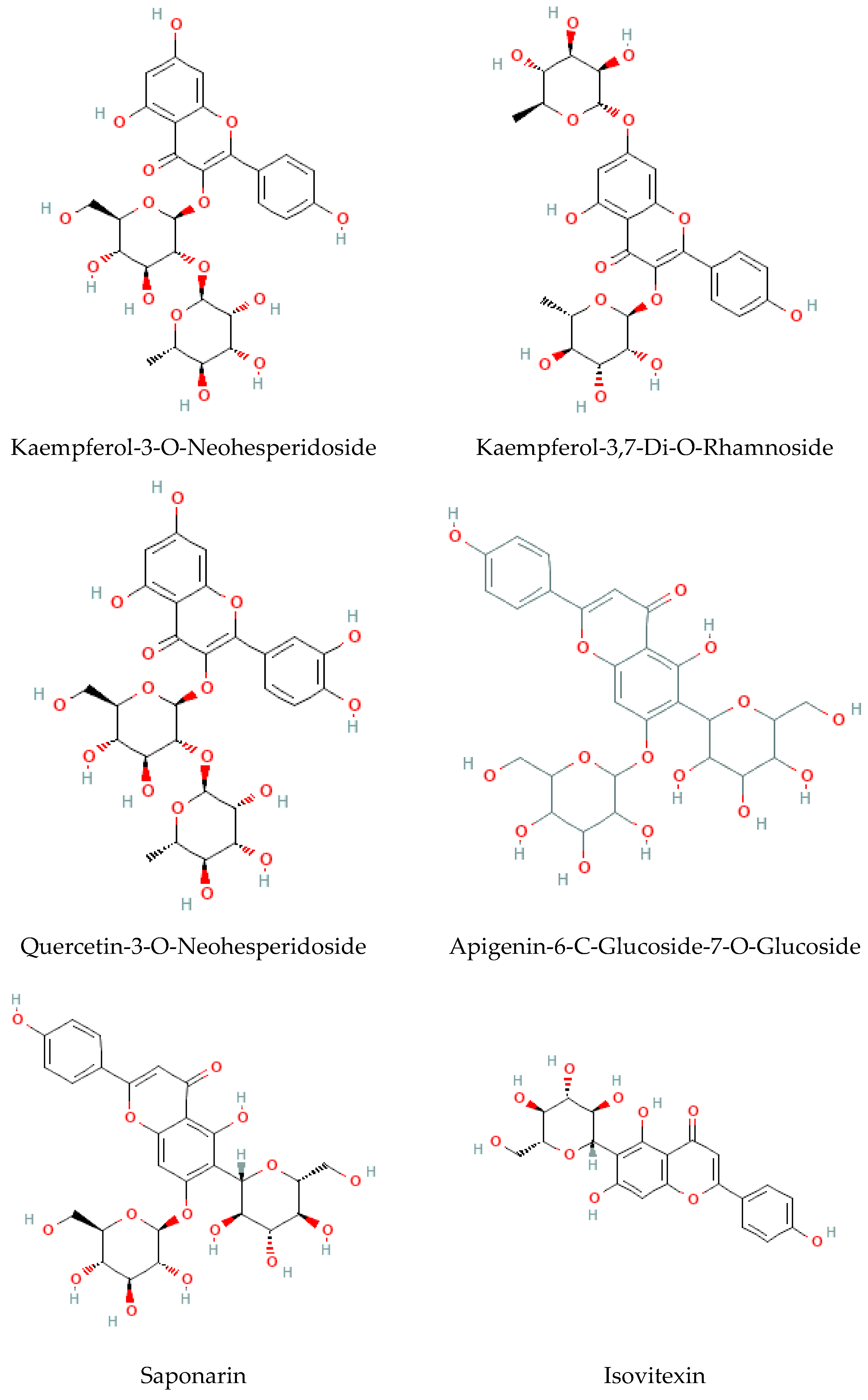
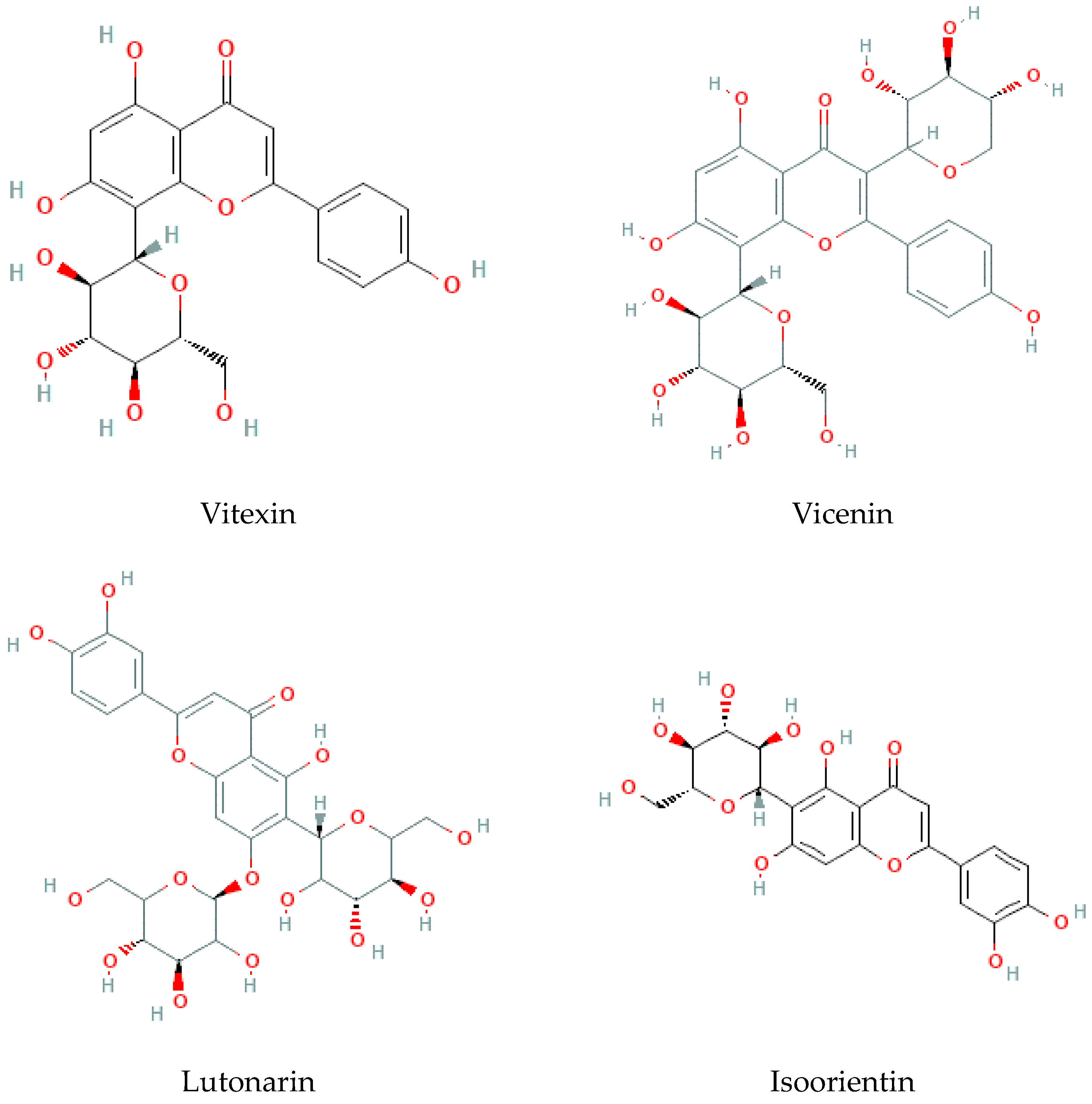
- Alkaloids
Few data on the alkaloidcomposition of Bryonia L. have been reported. One of the alkaloid compounds mentioned was bryonicine, extracted from the leaves of B. alba through maceration with ethanol [38,57].
- Fatty acids
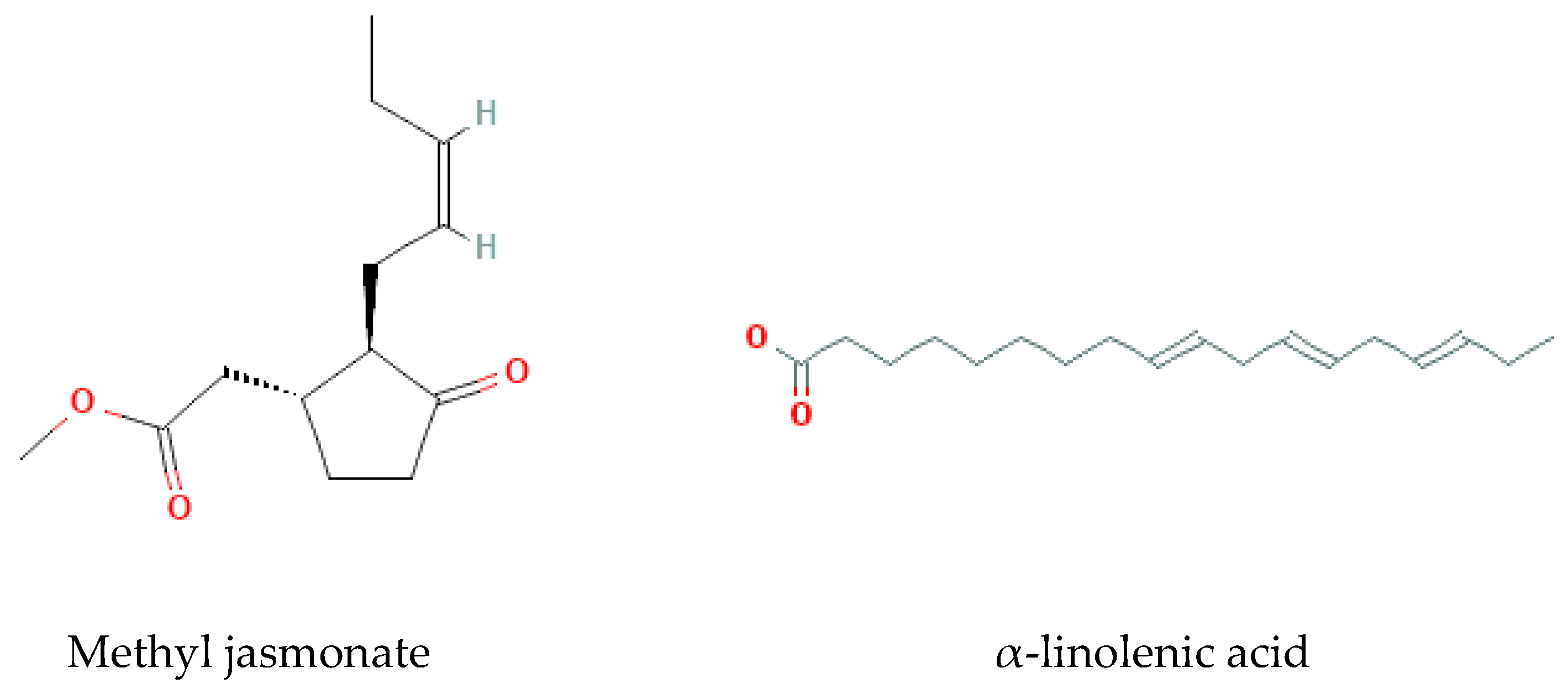
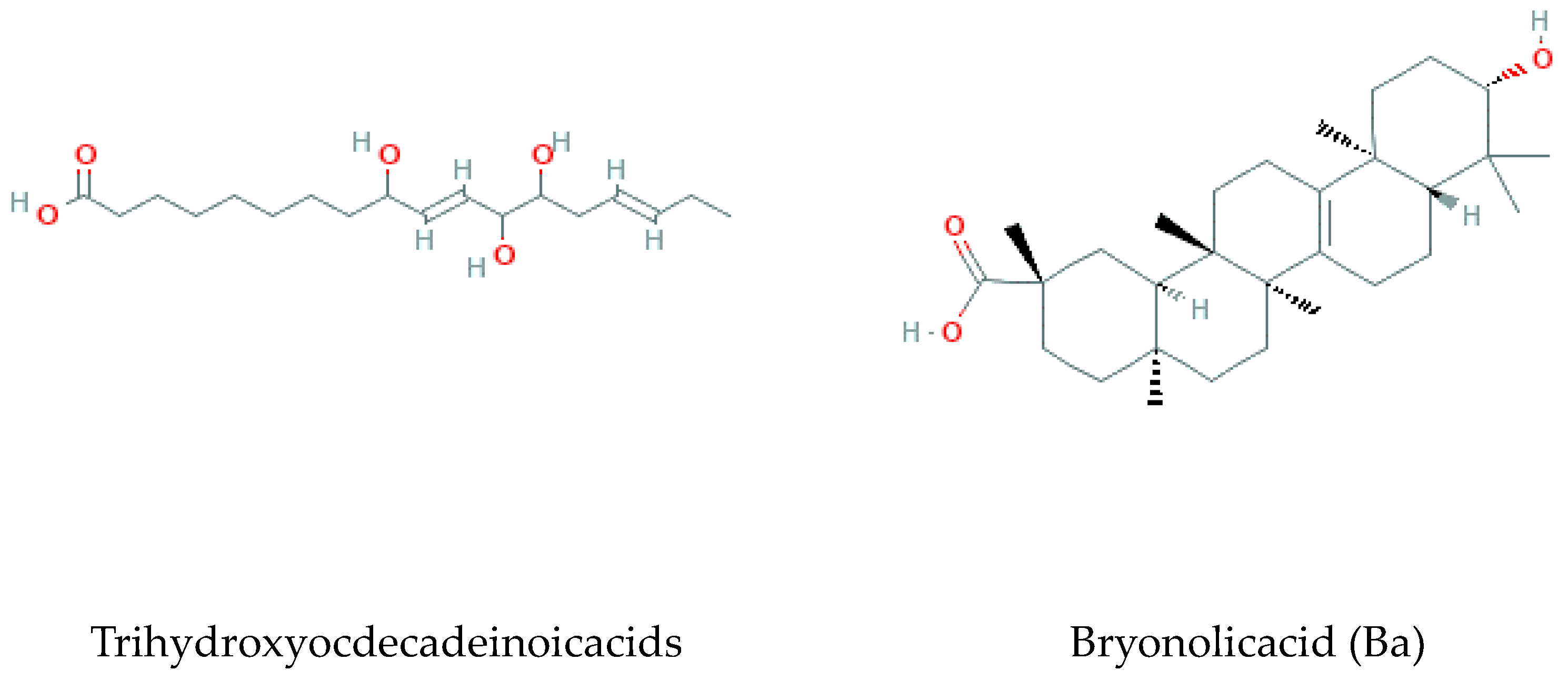
Methyl jasmonate, α-linolenic acid, trihydroxyocdecadeinoicacids, and Bryonolic Acid (Ba) 3β-Hydroxy-D:C-Friedoolean-8en-29-Oic Acid are the fatty acids found and reported in previous studies on B. multiflora, B. alba, and B. dioica [37] (Figure 7).
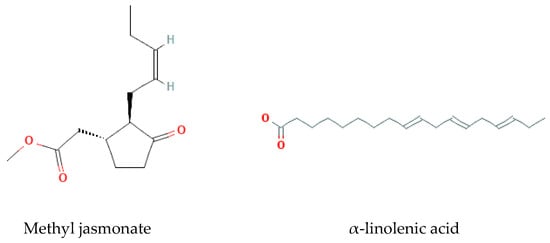
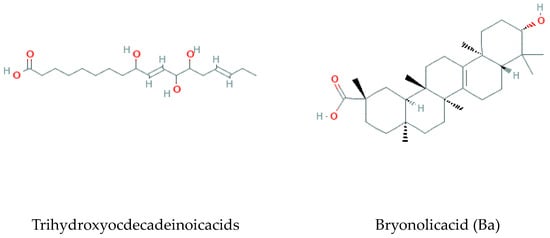
Figure 7.
Fatty acids isolated from Bryonia genus.
Figure 7.
Fatty acids isolated from Bryonia genus.
- Other compounds:
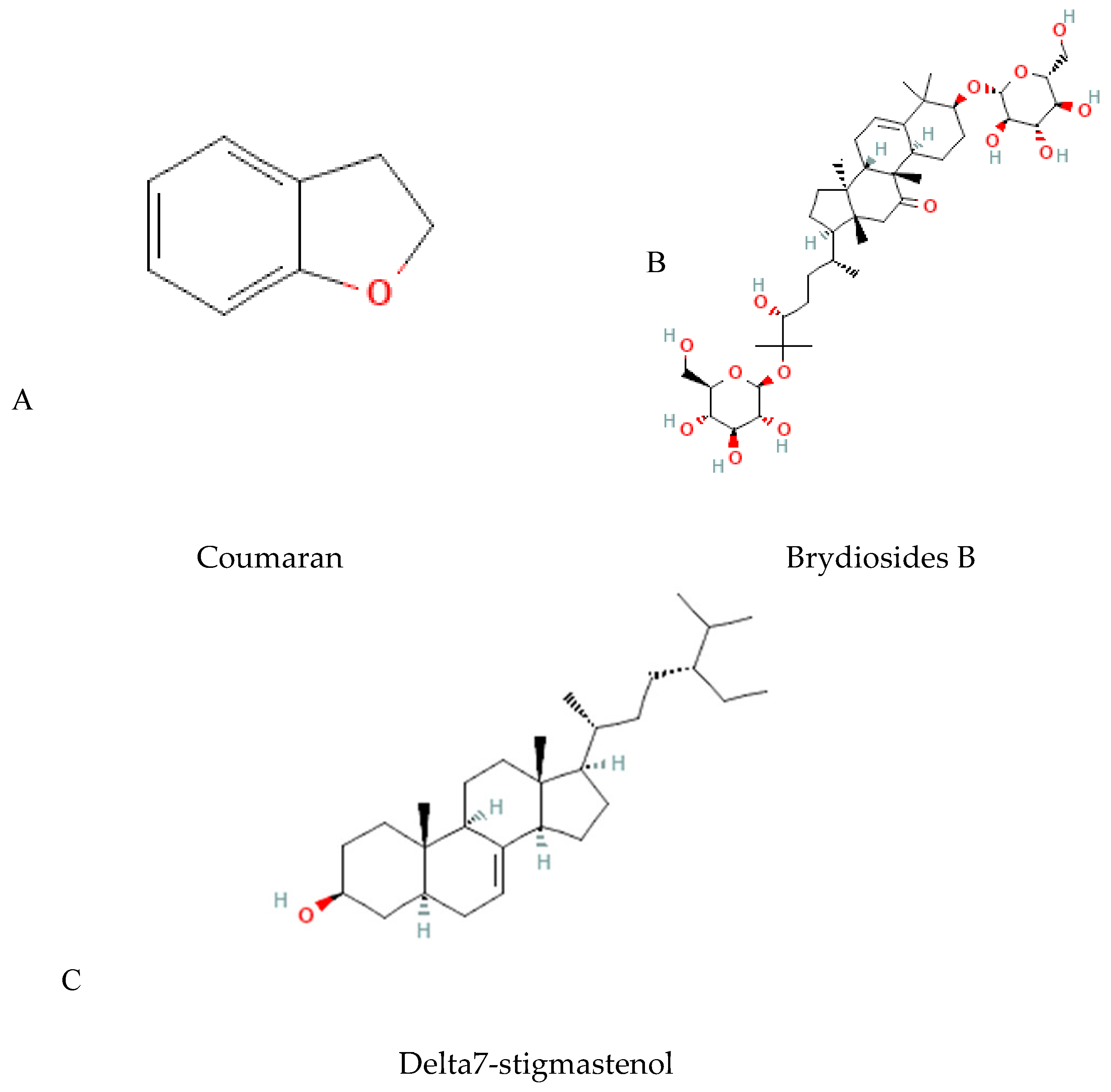
In particular, B. dioica roots were studied and revealed the identification of coumaran in their aqueous extract, beside saponins (brydiosides) and a steroid (Delta7-stigmastenol), for which the details are still unknown (Figure 8). Furthermore, other compounds were reported in the B. dioica, B. alba, and B. cretica species, especially terpenoids as mainly bioactive compounds (Table 4).
Figure 8.
Other molecules isolated from Bryonia genus. ((A) Coumaran, (B) saponin, and (C) steroid).
Figure 8.
Other molecules isolated from Bryonia genus. ((A) Coumaran, (B) saponin, and (C) steroid).
In this review, other compounds, namely C-glycosides, emerged as significant building blocks for many naturally occurring triterpenoids, bryoniosides (Figure 4), and the flavonoid apigenin-6-C-glucoside-7-O-glucoside (Figure 6) [64].These compounds have attracted plenty of attention as bioactive agents, flavor precursors, and detergents, which are either extracted from plant materials or chemically synthesized [65]. However, these methods suffer from low yields as a matter of their stability and functionality. Thus, various approaches have been developed for the formation of C-glycosidic usingimproved biocatalytic processes involving GT enzymes [65]. A previous study focused on developing efficient protocols for the synthesis of 1,2-annulated glycosides. The study reported the synthesis of sugar-fused indolines via C(sp2)–H/NeH activation starting from 2-nitroglycals using palladium-catalyzed CeH amination reactions [66]. Moreover, another study adopted the Prins cyclization approach using pivaloyl protection to form 2-deoxy-3,4-fused C-aryl/alkyl glycosides [67]. These strategies succeeded in synthesizing a protected C-glycoside compound viadecomposition from the first step.
Furthermore, among the identified phytochemicalsin Bryonia, a few compounds were isolated as pure bioactive molecules from different genera and investigated for their biological effects (Table 5). Most of them, such as isoorientin, isovitexin, cucurbitacin A, B, C, and D, and 23,24-dihydrocucurbitacin D, exhibited anticancer activity against gastric cancer, lung cancer, and ovarian cancer [68,69,70,71,72,73,74]. Moreover, bryoniosides A and B and isocucurbitacin D showed an antiproliferation capacity [31]. Besides their antioxidant properties, other flavonoid compounds, namely lutonarin and saponarin, were considered anti-inflammation agents due to theirinhibition of the phosphorylation of signaling effectors and the expression of inflammatory mediators, especially tumor necrosis factor (TNF)-α and the inflammatory enzyme cyclooxygenase-2 (COX-2) [75,76].

Table 5.
The main bioactive compounds identified in Bryonia genus with important bioactivities.
4. Limitations
Although a systematic process was applied to the research, selection, and analysis of the included studies, there are several limitations to point out. First, the small number of studies deserves consideration. Also, the selected studies focused on certain species, providing, in general, some biological data. Moreover, most of the included investigations were conducted on species extracts as sources of pharmacological agents and did not consider the isolation of active compounds, whereas some of the selected studies recorded the detected compounds without experimental applications. On the other hand, the lack of in vivo assessments of the toxicity profiles remains a challenge when interpreting the Bryonia research. Although previous studies reported the significant anticancer potential of compounds such as cucurbitacins, the molecular mechanisms involved and signaling pathways targeted remain unclear and need to be further investigated. Importantly, in spite of the significant biological activities reported here, no clinical trials have been undertaken to demonstrate the clinical usefulness of such products. However, this systematic review allowed us to summarize the research on particular compounds and identify important pharmacological activities that could be addressed in future research projects.
5. Conclusions
This systematic review documented interesting findings showing the diversity of Bryonia species spread across the world, isolated molecules from their different parts, and their therapeutic potential. Here, we found that some species such as B. dioica, B. aspera, B. alba, and B. cretica were more studied than other species in the genus due to their traditional uses. These plants maypossess important economic value aseasily cultivable species that represent a large reservoir of chemical components, principally triterpenoids (cucurbitacins and dihydrocucurbitacins). According to most of the previously published papers, this major class, besides flavonoids, especially lutonarin, saponarin, isoorientin, andisovitexin, showed remarkable pharmaceutical properties as antioxidant, anti-inflammatory, and mainly anticancer agents.Although the current report provides a detailed account of the updated information in terms ofvaluable results on taxonomy, geographic distribution, ethnopharmacology, and more on the phytochemical and therapeutic aspects, some limitations regarding the isolation of active compounds, clinical usefulness, and pharmaceutical application of Bryonia species should be considered. Therefore, further studies should be focused on identification and clarification of the molecular mechanisms of the biological activities of phytochemicals isolated from Bryonia species, in particular from the root parts of both B. alba and B. dioica. The genus needs more in-depth research to reveal the molecules responsible for these biological activities, which may lead to discovering novel and unique therapeutic molecules.
Author Contributions
B.B. and K.B. contributed equally to the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Assefa, B.; Glatzel, G.; Buchmann, C. Ethnomedicinal Uses of Hagenia abyssinica (Bruce) J.F. Gmel. Among Rural Communities of Ethiopia. J. Ethnobiol. Ethnomedicine 2010, 6, 20. [Google Scholar] [CrossRef]
- Belhouala, K.; Benarba, B. Medicinal Plants Used by Traditional Healers in Algeria: A Multiregional Ethnobotanical Study. Front. Pharmacol. 2021, 12, 760492. [Google Scholar] [CrossRef]
- Kocyan, A.; Zhang, L.-B.; Schaefer, H.; Renner, S.S. A Multi-Locus Chloroplast Phylogeny for the Cucurbitaceae and Its Implications for Character Evolution and Classification. Mol. Phylogenetics Evol. 2007, 44, 553–577. [Google Scholar] [CrossRef] [PubMed]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europea; Cambridge University Press: Cambridge, UK; London, UK; New York, NY, USA; Melbourne, Australia, 2010; Volume 3, pp. 297–299. [Google Scholar]
- Schaefer, H.; Renner, S.S. Phylogenetic Relationships in the Order Cucurbitales and a New Classification of the Gourd Family (Cucurbitaceae). Taxon 2011, 60, 122–138. [Google Scholar] [CrossRef]
- WFO. Available online: http://www.worldfloraonline.org (accessed on 30 December 2022).
- POWO. Available online: https://powo.science.kew.org (accessed on 30 December 2022).
- Volz, S.; Renner, S.S. Phylogeography of the ancient Eurasian medicinal plant genus Bryonia (Cucurbitaceae) inferred from nuclear and chloroplast sequences. Taxon 2009, 58, 550–560. [Google Scholar] [CrossRef]
- Patel, S.; Showers, D.; Vedantam, P.; Tzeng, T.-R.; Qian, S.; Xuan, X. Microfluidic Separation of Live and Dead Yeast Cells Using Reservoir-Based Dielectrophoresis. Biomicrofluidics 2012, 6, 34102. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, M.; Thacker, M.; Kadhim, A.; Holmes, L. Treatment of Unicameral Bone Cyst: Systematic Review and Meta Analysis. J. Child. Orthop. 2014, 8, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Benarba, B. Ethnomedicinal study of Bryonia dioica, a plant used as anti-breast cancer herbal therapy in North West Algeria. J. Med. Herbs Ethnomed. 2015, 1, 113. [Google Scholar]
- Jasiem, T.M.; Eldalawy, R.; Alnaqqash, Z.A.E. Pharmacological Activities and Chemical Constituents and of Bryonia dioica L.: A Review. Indian J. Public Health Res. Dev. 2020, 11, 2189. [Google Scholar] [CrossRef]
- Ilhan, M.; Dereli, F.T.G.; Tümen, I.; Akkol, E.K. Anti-inflammatory and antinociceptive features of Bryonia alba L.: As a possible alternative in treating rheumatism. Open Chem. 2019, 17, 23–30. [Google Scholar] [CrossRef]
- Ielciu, I.; Frédérich, M.; Hanganu, D.; Angenot, L.; Olah, N.-K.; Ledoux, A.; Crișan, G.; Păltinean, R. Flavonoid Analysis and Antioxidant Activities of the Bryonia alba L. Aerial Parts. Antioxidants 2019, 8, 108. [Google Scholar] [CrossRef]
- Bourhia, M.; Bari, A.; Ali, S.W.; Benbacer, L.; Khlil, N. Phytochemistry and Toxicological Assessment of Bryonia dioica Roots Used in North-African Alternative Medicine. Open Chem. 2019, 17, 1403–1411. [Google Scholar] [CrossRef]
- Neogi, S.B.; Roy, D.K.; Sachdeva, A.K.; Sharma, R.; Gupta, R.; Ganguli, A. Evidence of prenatal toxicity of herbal based indigenous formulations for sex selection in rat models. J. Tradit. Complement. Med. 2021, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G.; et al. Flavonoid composition, cellular antioxidant activity and (myelo)peroxidase inhibition of a Bryonia alba L. (Cucurbitaceae) leaves extract. J. Pharm. Pharmacol. 2018, 71, 230–239. [Google Scholar] [CrossRef]
- TPL. Available online: www.theplantlist.org (accessed on 30 December 2022).
- Rus, L.M.; Ielciu, I.I.; Păltinean, R.; Vlase, L.; Ştefănescu, C.; Crişan, G.C. Morphological and Histo-Anatomical Study of Bryonia alba L. (Cucurbitaceae). Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 47–52. [Google Scholar] [CrossRef]
- Volz, S.M.; Renner, S.S. Hybridization, polyploidy, and evolutionary transitions between monoecy and dioecy in Bryonia (Cucurbitaceae). Am. J. Bot. 2008, 95, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Svanberg, I. From Medicinal Plant to Noxious Weed: Bryonia alba L. (Cucurbitaceae) in Northern and Eastern Europe. J. Ethnobiol. Ethnomedicine 2019, 15, 22. [Google Scholar] [CrossRef]
- Baars, E.W.; Zoen, E.B.-V.; Willcox, M.; Huber, R.; Hu, X.-Y.; van der Werf, E.T. CAM treatments for cough and sore throat as part of an uncomplicated acute respiratory tract infection: A systematic review of prescription rates and a survey among European integrative medical practitioners. Eur. J. Integr. Med. 2020, 39, 101194. [Google Scholar] [CrossRef]
- Isaev, M.I. Bryonia isoprenes. II. Cucurbitacin L and bryoamaride from Bryonia melanocarpa. Chem. Nat. Compd. 2000, 36, 292–294. [Google Scholar] [CrossRef]
- Ghorbani, A. Studies on Pharmaceutical Ethnobotany in the Region of Turkmen Sahra, North of Iran. J. Ethnopharmacol. 2005, 102, 58–68. [Google Scholar] [CrossRef]
- Renner, S.S.; Scarborough, J.; Schaefer, H.; Paris, H.S.; Janick, J.; Pitrat, M. Dioscorides’s Bruonia Melaina Is Bryonia alba, Not Tamus communis, and an Illustration Labeled Bruonia Melaina in the Codex Vindobonensis Is Humulus lupulus Not Bryonia dioica. In Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008; pp. 273–280. [Google Scholar]
- Sahranavard, S.; Naghibi, F.; Siems, K.; Jenett-Siems, K. New Cucurbitane-Type Triterpenoids from Bryonia aspera. Planta Medica 2010, 76, 1014–1017. [Google Scholar] [CrossRef]
- Sallam, A.A.; Hitotsuyanagi, Y.; Mansour, E.-S.S.; Ahmed, A.F.; Gedara, S.; Fukaya, H.; Takeya, K. Cucurbitacins from Bryonia cretica. Phytochem. Lett. 2010, 3, 117–121. [Google Scholar] [CrossRef]
- Benarba, B.; Meddah, B.; Aoues, A. Bryonia dioica Aqueous Extract Induces Apoptosis through Mitochondrial Intrinsic Pathway in BL41 Burkitt’s Lymphoma Cells. J. Ethnopharmacol. 2012, 141, 510–516. [Google Scholar] [CrossRef]
- Kadhim, E.J. Phytochemical investigation and hepato-protective studies of Iraqi Bryonia dioica (Family Cucurbitaceae). Int. J. Pharm. Pharm. Sci. 2014, 6, 187–190. [Google Scholar]
- Pourgonabadi, S.; Amiri, M.S.; Mousavi, S.H. Cytotoxic and Apoptogenic Effects of Bryonia aspera Root Extract against Hela and HN-5 Cancer Cell Lines. DOAJ Dir. Open Access J. 2017, 7, 66–72. [Google Scholar]
- Matsuda, H.; Nakashima, S.; Abdel-Halim, O.B.; Morikawa, T.; Yoshikawa, M. Cucurbitane-Type Triterpenes with Anti-proliferative Effects on U937 Cells from an Egyptian Natural Medicine, Bryonia cretica: Structures of New Triterpene Glycosides, Bryoniaosides A and B. Chem. Pharm. Bull. 2010, 58, 747–751. [Google Scholar] [CrossRef][Green Version]
- Gholivand, M.B.; Piryaei, M. The antioxidant activity, total phenolics and total flavonoids content of Bryonia dioica Jacq. Biologija 2012, 58, 3. [Google Scholar] [CrossRef]
- Khamees, A.H.; Kadhim, E.J.; Sahib, H.B.; Mutlag, S.H. In vitro analysis of antioxidant and antimicrobial activity of Iraqi Bryonia dioica. Int. J. Pharm. Sci. Rev. Res. 2017, 43, 248–252. [Google Scholar]
- Rafael, M.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Topical anti-inflammatory plant species: Bioactivity of Bryonia dioica, Tamus communis and Lonicera periclymenum fruits. Ind. Crop. Prod. 2011, 34, 1447–1454. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Toriumi, M.; Koike, K.; Kimura, Y.; Nikaido, T.; Aoi, W.; Nishino, H.; et al. Anti-Inflammatory and Anti-Tumor-Promoting Effects of Cucurbitane Glycosides from the Roots of Bryonia dioica. J. Nat. Prod. 2002, 65, 179–183. [Google Scholar] [CrossRef]
- Dhouioui, M.; Boulila, A.; Jemli, M.; Schiets, F.; Casabianca, H.; Zina, M.S. Fatty Acids Composition and Antibacterial Activity of Aristolochia longa L. and Bryonia dioïca Jacq. Growing Wild in Tunisia. J. Oleo Sci. 2016, 65, 655–661. [Google Scholar] [CrossRef]
- Goswami, P.; Chatterjee, D.; Ghosh, S.; Paira, K.; Das, S. Balanced cytokine upregulation by diluted ethanolic extract of Bryonia alba in Delta SARS-CoV-2 Spike protein RBD-induced pathogenesis in Gallus gallus embryo. Bull. Natl. Res. Cent. 2022, 46, 169. [Google Scholar] [CrossRef]
- Chekroun, E.; Bechiri, A.; Azzi, R.; Adida, H.; Benariba, N.; Djaziri, R. Antidiabetic Activity of Two Aqueous Extracts of Two Cucurbitaceae: Citrullus Colocynthis and Bryonia dioica. Phytotherapie 2016, 15, 57–66. [Google Scholar] [CrossRef]
- Uyar, A.; Yaman, T.; Kele, O.; Alkan, E.; Celik, I.; Yener, Z. Protective Effects of Bryonia Multiflora Extract on Pancreatic Beta Cells, Liver and Kidney of Streptozotocin-Induced Diabetic Rats: Histopathological and Immunohistochemical Investigations. Indian J. Pharm. Educ. Res. 2017, 51, s403–s411. [Google Scholar] [CrossRef]
- Tahvilian, R.; Gravandi, M.M.; Noori, T.; Papzan, A.; Jamshidi, N.; Iranpanah, A.; Afsaneh, M.; Shirooie, S. The therapeutic effect of methanolic extract Bryonia dioica Jacq. in a female rat model of polycystic ovary syndrome. J. Rep. Pharm. Sci. 2022, 11, 79. [Google Scholar]
- Hussain, H.; Green, I.R.; Saleem, M.; Khattak, K.F.; Irshad, M.; Ali, M. Cucurbitacins as Anticancer Agents: A Patent Review. Recent Patents Anti-Cancer Drug Discov. 2019, 14, 133–143. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, B.; Sharma, U.; Parashar, G.; Parashar, N.C.; Rani, I.; Ramniwas, S.; Kaur, S.; Haque, S.; Tuli, H.S. Apoptotic and antimetastatic effect of cucurbitacins in cancer: Recent trends and advancement. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1867–1878. [Google Scholar] [CrossRef]
- Nakashima, S.; Oda, Y.; Morita, M.; Ohta, A.; Morikawa, T.; Matsuda, H.; Nakamura, S. Analysis of Active Compounds Using Target Protein Cofilin―Cucurbitacins in Cytotoxic Plant Bryonia cretica. Toxins 2022, 14, 212. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Esmaeili, S.; Bashash, D.; Nayeri, N.D.; Farahani, M.E.; Gharehbaghian, A. Cytotoxic and Apoptogenic Activity of Bryonia aspera Extract on Pre-B Acute Lymphoblastic Leukemia Cell Lines. DOAJ Dir. Open Access J. 2018, 12, 204–212. [Google Scholar]
- Sahranavard, S.; Naghibi, F.; Ghaffari, S. Cytotoxic activity of extracts andpure compounds of Bryonia aspera. Int. J. Pharm. Pharm. Sci. 2012, 4, 541–543. [Google Scholar]
- Benarba, B.; Elmallah, A.; Pandiella, A. Bryonia dioica Aqueous Extract Induces Apoptosis and G2/M Cell Cycle Arrest in MDA-MB 231 Breast Cancer Cells. Mol. Med. Rep. 2019, 20, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Abdessamad, I.B.; Bouhlel, I.; Chekir-Ghedira, L.; Krifa, M. Antitumor Effect of Bryonia Dioïca Methanol Extract: In Vitro and In Vivo Study. Nutr. Cancer 2019, 72, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.O.; Lee, Y.G.; Lim, H.B.; Kwon, N.S.; Aprikian, G.V.; Lee, D.W. Antitumor Activity of 23, 24-dihydrocucurbitacin D IsoIated from Bryonia alba L. Toxicol. Res. 2000, 16, 263–267. [Google Scholar]
- Nersesyan, A.k.; Ar, C. Possible Genotoxic Activity of Extracts of Bryonia alba Roots on Human Lymphocytes and Transformed Cells. Neoplasma 2002, 49, 114–116. [Google Scholar] [PubMed]
- Karpiuk, U.V.; Al Azzam, K.M.; Abudayeh, Z.H.M.; Kislichenko, V.; Naddaf, A.; Cholak, I.; Yemelianova, O. Qualitative and Quantitative Content Determination of Macro-Minor Elements in Bryonia alba L. Roots using Flame Atomic Absorption Spectroscopy Technique. Adv. Pharm. Bull. 2016, 6, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Karpyuk, U.V.; Kislichenko, V.S.; Gur’eva, I.G. Carbohydrate Composition of Bryonia alba. Chem. Nat. Compd. 2016, 52, 672–673. [Google Scholar] [CrossRef]
- Karpyuk, U.V.; Kislichenko, V.S.; Gur’eva, I.G. HPLC Determination of Free and Bound Amino Acids in Bryonia alba. Chem. Nat. Compd. 2015, 51, 399–400. [Google Scholar] [CrossRef]
- Toker, G.; Erdemoğlu, N.; Toker, M.C. High performance liquid chromatographic analysis of cucurbitacins in some Bryonia species. FABAD J. Pharm. Sci. 2000, 25, 153–156. [Google Scholar]
- Khan, M.T.H.; Choudhary, M.I.; Rahman, A.U.; Mamedova, R.P.; Agzamova, M.A.; Sultankhodzhaev, M.N.; Isaev, M.I. Tyrosinase inhibition studies of cycloartane and cucurbitane glycosides and their structure–activity relationships. Bioorganic Med. Chem. 2006, 14, 6085–6088. [Google Scholar] [CrossRef]
- Barreira, J.C.; Pereira, E.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Bryonia dioica, Tamus communis and Lonicera periclymenum fruits: Characterization in phenolic compounds and incorporation of their extracts in hydrogel formulations for topical application. Ind. Crop. Prod. 2013, 49, 169–176. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Yek, S.M.; Motahharifar, N.; Gorab, M.G. Recent Developments in the Plant-Mediated Green Synthesis of Ag-Based Nanoparticles for Environmental and Catalytic Applications. Chem. Rec. 2019, 19, 2436–2479. [Google Scholar] [CrossRef] [PubMed]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J.; Olmedilla-Alonso, B. Carotenoid content of wild edible young shoots traditionally consumed in Spain (Asparagus acutifolius L., Humulus lupulus L., Bryonia dioica Jacq. and Tamus communis L.). J. Sci. Food Agric. 2014, 94, 1914–1916. [Google Scholar] [CrossRef]
- Zhang, T.-L.; Yu, H.; Ye, L. Metabolic Engineering of Yarrowia Lipolytica for Terpenoid Production: Tools and Strategies. ACS Synth. Biol. 2023, 12, 639–656. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, J. Die Cucurbitacine in Bryonia alba Und Bryonia dioica. Phytochemistry 1975, 14, 1587–1589. [Google Scholar] [CrossRef]
- Hylands, P.J.; Kosugi, J. Bryonoside and bryoside—New triterpene glycosides from Bryonia dioica. Phytochemistry 1982, 21, 1379–1384. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Cisowski, W. High-performance liquid chromatographic determination of flavone C-glycosides in some species of the Cucurbitaceae family. J. Chromatogr. A 1994, 675, 240–243. [Google Scholar] [CrossRef]
- Parida, S.P.; Das, T.; Ahemad, M.A.; Pati, T.; Mohapatra, S.; Nayak, S. Recent advances on synthesis of C-glycosides. Carbohydr. Res. 2023, 530, 108856. [Google Scholar] [CrossRef]
- Schwab, W.; Fischer, T.; Wüst, M. Terpene Glucoside Production: Improved Biocatalytic Processes Using Glycosyltransferases. Eng. Life Sci. 2015, 15, 376–386. [Google Scholar] [CrossRef]
- Verma, A.K.; Chennaiah, A.; Dubbu, S.; Vankar, Y.D. Palladium catalyzed synthesis of sugar-fused indolines via C(sp2)–H/N H activation. Carbohydr. Res. 2019, 473, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Dubbu, S.; Chennaiah, A.; Verma, A.K.; Vankar, Y.D. Stereoselective synthesis of 2-deoxy-β-C-aryl/alkyl glycosides using Prins cyclization: Application in the synthesis of C-disaccharides and differently protected C-aryl glycosides. Carbohydr. Res. 2018, 468, 64–68. [Google Scholar] [CrossRef]
- Huang, H.-K.; Lee, S.-Y.; Huang, S.-F.; Lin, Y.-S.; Chao, S.-C.; Lee, S.-C.; Cheng, T.-H.; Loh, S.-H.; Tsai, Y.-T. Isoorientin Decreases Cell Migration via Decreasing Functional Activity and Molecular Expression of Proton-Linked Monocarboxylate Transporters in Human Lung Cancer Cells. Am. J. Chin. Med. 2020, 48, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Z.; Huang, P.; Sun, C.; Yu, W.-Y.; Zhang, H.; Yu, C.; He, J. Isovitexin Potentiated the Antitumor Activity of Cisplatin by Inhibiting the Glucose Metabolism of Lung Cancer Cells and Reduced Cisplatin-Induced Immunotoxicity in Mice. Int. Immunopharmacol. 2021, 94, 107357. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Ma, W.; Kou, W.; Li, C.; Zhao, J. Anticancer Activity of Cucurbitacin-A in Ovarian Cancer Cell Line SKOV3 Involves Cell Cycle Arrest, Apoptosis and Inhibition of MTOR/PI3K/Akt Signaling Pathway. J. BUON 2018, 23, 124–128. [Google Scholar]
- Xu, J.; Chen, Y.; Yang, R.; Tong, Z.; Wei, K.; Su, Y.; Yang, S.; Zhang, T.; Li, X.; Zhang, L.; et al. Cucurbitacin B Inhibits Gastric Cancer Progression by Suppressing STAT3 Activity. Arch. Biochem. Biophys. 2020, 684, 108314. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, Z.; Lin, M.; Shang, Y.; Wang, F.; Zhou, J.; Huang, W. In vitro and in vivo antitumor activity of cucurbitacin C, a novel natural product from cucumber. Front. Pharmacol. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Sikander, M.; Bin Hafeez, B.; Malik, S.; Alsayari, A.; Halaweish, F.T.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. Cucurbitacin D exhibits potent anti-cancer activity in cervical cancer. Sci. Rep. 2016, 6, 36594. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Dong, A.; Huo, X.; Wang, H.; Wang, J.; Si, J. The Inhibition of Gastric Cancer Cells’ Progression by 23,24-Dihydrocucurbitacin E through Disruption of the Ras/Raf/ERK/MMP9 Signaling Pathway. Molecules 2022, 27, 2697. [Google Scholar] [CrossRef]
- Yang, J.Y.; Woo, S.-Y.; Lee, M.J.; Kim, H.Y.; Lee, J.H.; Kim, S.-H.; Seo, W.D. Lutonarin from Barley Seedlings Inhibits the Lipopolysacchride-Stimulated Inflammatory Response of RAW 264.7 Macrophages by Suppressing Nuclear Factor-κB Signaling. Molecules 2021, 26, 1571. [Google Scholar] [CrossRef]
- Min, S.-Y.; Park, C.-H.; Yu, H.-W.; Park, Y.-J. Anti-Inflammatory and Anti-Allergic Effects of Saponarin and Its Impact on Signaling Pathways of RAW 264.7, RBL-2H3, and HaCaT Cells. Int. J. Mol. Sci. 2021, 22, 8431. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zhang, X.; Li, J.; Xi, C.; Wang, W.; Lu, Y.; Xuan, L. 23,24-Dihydrocucurbitacin B Promotes Lipid Clearance by Dual Transcriptional Regulation of LDLR and PCSK9. Acta Pharmacol. Sin. 2019, 41, 327–335. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wei-Tan, H.; Hu, C.Y.; Wang, W.Q.; Chu, G.H.; Wei, L.H.; Chen, L. Anticancer activity of 23, 24-dihydrocucurbitacin B against the HeLa human cervical cell line is due to apoptosis and G2/M cell cycle arrest Retraction in/10.3892/etm. 2021.10033. Exp. Ther. Med. 2018, 15, 2575–2582. [Google Scholar]
- Soh, D.; Bakang, B.T.; Tchouboun, E.N.; Nganso, Y.O.D.; Defokou, U.D.; Sidjui, L.S.; Nyassé, B. New cucurbitane type triterpenes from Momordica foetida Schumach.(Cucurbitaceae). Phytochem. Lett. 2020, 38, 90–95. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).