Abstract
Apis mellifera L. is considered one of the most important pollinators in nature. Unfortunately, in addition to other insect species, honey bee populations are decreasing at an alarming rate, urging researchers to investigate the causes and stressors that precipitated this decline. This study focuses on chemical stressors that are found to affect bee populations. We used pollen and honey samples to examine the variations in pesticides, selenium, and heavy metals in two different landscapes: urban and agricultural areas of northeastern Colorado, USA. Subsequently, we extrapolated the risks of these toxins’ residues to Apis spp. Based on the current literature, we found no spatial variations in metal and selenium concentrations in the pollen and honey samples collected from urban and agricultural areas. Moreover, we observed no spatial variations in pesticide concentrations in pollen and honey samples. Based on the previous literature and a comparison of the residues of heavy metals, selenium, and pesticides in our pollen and honey samples, we found that the heavy metal and selenium residues in some honey and pollen likely pose a severe health risk to honey bees. Although the levels of pesticide residues were below the documented thresholds of risk, we consider the possibility of synergistic chemical impacts. Our findings support future efforts to investigate the health risks associated with multiple-factor combinations.
1. Introduction
As pollinators, Apis mellifera Linnaeus (honey bees) are a vital part of the ecosystem, visiting more than 90% of the 107 leading global crop plants [1]. However, the number of managed honey bee hives has decreased, and this reduction has become an international issue over the past two decades. Managed hives have reduced by 25% in Europe over the last 20 years and by 59% in North America over the previous 58 years [2]. This is supported by further evidence documenting the decline of European honey bee colonies since at least 1972 [1]. In addition, the Food and Agriculture Organization of the United Nations (FAO) documents a broader frame of reference, including the years 1961–2007, during which honey bee colonies decreased in both Europe and North America (−49.5%) [3]. Growing concern about the declining number of bees worldwide has prompted scientists and researchers to investigate the factors contributing to their demise.
One phenomenon associated with bee population decline is called colony collapse disorder. This phenomenon is defined as a dead colony in which most worker bees inexplicably disappear from the colony, leaving behind the queen, a few immature bees, and plentiful food [4]. Many stressors were found to escalate this phenomenon and have been classified into different categories based on their nature and origin: (1) biological stressors, including pathogens and parasites, such as deformed wing viruses and Varroa mites [5,6]; (2) physical stressors, including habitat fragmentation and the decline of foraging resources [7], as well as climate change [8]; (3) chemical stressors, including pesticides [9,10,11,12], fertilizers [13], and heavy metals [14,15]; and (4) nutritional stressors, including a poor diet and inadequate beekeeping practices [6,16].
Pesticides have been extensively investigated and documented as the primary stressor affecting honey bees. Chief among them include neonicotinoids which, as neurotoxins, have a wide range of effects on pollinators, including (1) the impairment of foraging behavior [10]; (2) the impairment of colony reproduction [17]; (3) lethal damage to the nervous system [18]; and (4) the inhibition of immunity [19].
Another stressor apart from pesticides is heavy metals, although less is known about their effects on bee species relative to those of pesticides. However, an increasing number of studies have reported the relationship between the increase in heavy metal concentrations in soil and plants and the decline in bee species’ diversity, richness, health, and foraging behavior [20,21,22]. Heavy metals are ubiquitous in the environment and are often amplified in the environment as a result of either natural events, such as forest fires, volcanic emissions, and sea spray [23], or through human activities, such as industrial emissions, hydraulic fracturing, and coal-burning power plants [24].
Selenium (Se) is a trace mineral that occurs naturally in certain alkaline soils [25]. The amount of selenium in soil varies with soil type and texture, organic matter content, and rainfall [26]. In addition, how a plant assimilates selenium is influenced by the physicochemical factors of the soil, such as redox status, pH, and microbiological activity [27]. For bees, selenium is lethal at high concentrations; sublethal exposure impairs honey bees’ learning and long-term memory and reduces their foraging efficiency [28], and a direct proportion was found between bee mortality rate and the presence of selenium in their diet [29].
Generally, bees can encounter toxins by consuming contaminated nectar or pollen and/or exposure to contaminated dust from direct sprays or contacting contaminated surfaces [11]. Most research has focused on honey bee exposure to pesticides in agricultural settings because of the associated pesticide applications [30,31,32,33]. However, recent studies also document the exposure of honey bees to pesticides in urban areas [34,35]. A few studies have considered the significance of the combinatorial implications of both pesticides and heavy metal residues in honey bees and their products [36].
This study will assess the spatial variations in pesticide and heavy metal residues in pollen and honey. It will ascertain if there is a significant difference in the pesticide and heavy metal contents in honey bee products collected from agricultural versus urban areas. It will also address the combinatorial risk to honey bees when exposed to these contaminants through pollen and nectar consumption by relating our findings with previously documented toxicological evidence related to the isolated effects of pesticides and heavy metals.
2. Materials and Methods
2.1. Study Sites and Site Selection
We surveyed 24 hives distributed in seven counties in Northern Colorado. Pollen and honey samples were collected from 10 hives during the summer of 2019 and 14 hives during the summer of 2020. A total of 13 hives were in urban areas, while 11 hives were located in agricultural areas, as shown in Figure 1.
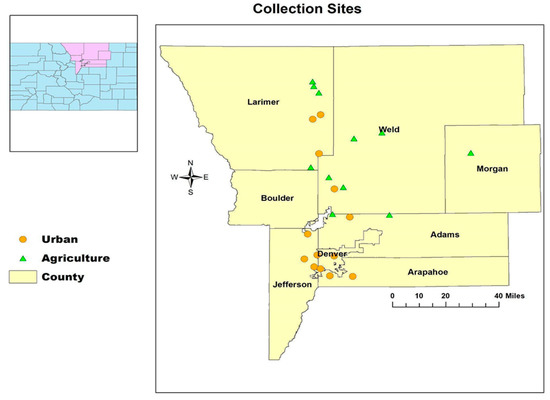
Figure 1.
Hive sampling sites for pollen and honey in northern Colorado, USA, 2019–2020.
Sampling sites were selected based on how urban and agricultural areas are classified. According to the United States Census Bureau, urban areas are defined as continuously built-up areas with populations of 2500–50,000 or more and average densities of at least 1000 inhabitants per square mile [37], while the Colorado General Assembly defines agricultural land as “A parcel of land, whether located in an incorporated or unincorporated area and was used the previous two years and presently is used as a farm or ranch” [38]. We had to identify the landscape type using Google Earth, taking into consideration the observation that honey bees can fly for more than 3 km when searching for food [39].
2.2. Sample Collection, Preparation, and Analysis
Pollen samples were collected using pollen traps (Bee Flower, Gyengbuk High-tech village, South Korea) at the entrance of each hive. These traps force forager bees to enter the hive through a screen where they drop their pollen loads, which fall into the trap box. The pollen samples were collected from the trap boxes and stored in the laboratory at −20 °C until analysis. Two to three samples were collected from each hive between the months of June and September in the years 2019 and 2020. Honey samples were collected from each site between September and October (during the harvest season) and stored in the laboratory at −20 °C until analysis [40].
2.3. Selenium and Heavy Metals Analysis
A total of 59 pollen samples and 21 honey samples were weighed (five grams of pollen and ten grams of honey for each sample), arranged in a box at room temperature, and then submitted to the Soil, Water, and Plant Testing Laboratory, Colorado State University, for analysis [41]. An analysis of heavy metals was performed to detect the residues of Arsenic (As), Cadmium (Cd), Lead (Pb), and Selenium (Se), using the Nitric and Perchloric Acids method [42].
2.3.1. Chemicals and Reagents
Concentrated nitric acid and Perchloric acid (60–70%) were purchased from Fisher Scientific. Deionized water was obtained from a filtration system.
2.3.2. Analysis
Five grams of ground pollen or honey were transferred into a calibrated digest tube. Then, 5 mL of HNO3 and 5 mL of HC1O4 were added, and the samples were then left to digest overnight without heat in a hood. The pollen or honey samples were then heated on the digestion block at 125–130 °C for 48 h; the temperature was increased over the next 6–8 h up to 200 °C. After the samples cooled, 10–20 mL of deionized water was added to rehydrate the samples. Next, more deionized water was added to reach a volume of 50 mL. The samples were then mixed thoroughly until homogenized. The samples were left to settle overnight and were then analyzed with an atomic absorption spectrometer.
2.4. Pesticide Analysis
We gathered a total of 61 pollen samples and 21 honey samples. Five grams of pollen and ten grams of honey were taken from each sample. The samples were then arranged in a cooler box at ~4 °C [40] and shipped overnight to the Chemical Ecology Core Facility at Cornell University (Ithaca, NY, USA). A pesticide analysis was performed to detect the residues of 92 types of pesticides, including some metabolites and breakdown products using the EN 15662 QuEChERS procedure [43] via liquid chromatography–mass spectrometry (LC-MS/MS), as shown in Appendix A, Table A1.
2.4.1. Chemicals and Reagents
Acetonitrile and HPLC-grade water were purchased from EMD Millipore (Billerica, MA, USA). LC-MS-grade formic acid was purchased from Thermo Scientific (Waltham, MA, USA). The 5M ammonium formate solution, the QuEChERS extraction packets (4 g MgSO4; 1 g NaCl; 1 g sodium citrate tribasic dihydrate; 0.5 g sodium citrate dibasic sesquihydrate), and the d-SPE kits (150 mg MgSO4, 25 mg PSA and 25 C18EC) were purchased from Agilent Technologies (Santa Clara, CA, USA). The deuterated internal standards were purchased from Sigma-Aldrich International (Saint Louis, MO, USA).
2.4.2. Pollen Samples
A total of 5 g of pollen was mixed with 10 mL of acetonitrile and 5 mL of water and then homogenized for 1 min using ceramic beads (2.8 mm diameter) and a Bead Ruptor 24 (OMNI International, Kennesaw, GA, USA). After homogenization, 6.5 g of EN 15662 salts was added (4 g MgSO4; 1 g NaCl; 1 g sodium citrate tribasic dihydrate; 0.5 g sodium citrate dibasic sesquihydrate). The samples were then thoroughly vortexed and centrifuged at 7300× g for 5 min. One mL of supernatant was collected and transferred into a d-SPE (dispersive solid phase extraction) tube containing 150 mg MgSO4 and 25 mg PSA. After the d-SPE step, 496 µL of supernatant was collected, and 4 µL of internal standard solution (d4-fluopyram 0.15 µg/mL; d3-pyraclostrobin 0.3 µg/mL; 13C6-metalxyl 0.3 µg/mL) was added. The samples were filtered through a 0.22 µm PTFE and analyzed immediately afterward.
2.4.3. Honey Samples
First, 7 g of honey was mixed with 3 mL of water and then 10 mL of acetonitrile. The samples were vortexed for 1 min and mixed with 6.5 g of EN 15662 salts (4 g MgSO4; 1 g NaCl; 1 g sodium citrate tribasic dihydrate; 0.5 g sodium citrate dibasic sesquihydrate). The samples were thoroughly vortexed again and centrifuged at 7300× g for 5 min. Then, 1 milliliter of supernatant was collected and transferred into a d-SPE (dispersive solid phase extraction) tube containing 150 mg MgSO4 and 25 mg PSA. After the d-SPE step, 496 µL of supernatant was collected, and 4 µL of internal standard solution (d4-fluopyram 0.15 µg/mL; d3-pyraclostrobin 0.3 µg/mL; 13C6-metalxyl 0.3 µg/mL) was added. The samples were filtered through a 0.22 µm PTFE and analyzed immediately thereafter.
2.4.4. Analysis
The analysis was performed with a Vanquish Flex UHPLC system (Dionex Softron GmbH, Germering, Germany) coupled with a TSQ Quantis mass spectrometer (Thermo Scientific, San Jose, CA). The UHPLC was equipped with an Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 µm particle size). The mobile phase consisted of (A) water with 2 mM ammonium formate and 0.1% formic acid and (B) acetonitrile/water (98:2, v/v) with 2 mM ammonium formate and 0.1% formic acid. The temperature of the column was set at 40 °C, and the flow rate of the LC was 300 µL/min. The elution program was the following: 1.5 min equilibration (2% B) prior to injection, 0–0.5 min (2% B, isocratic), 0.5–15 min (2→70% B, linear gradient), 15–17 min (70→100% B, linear gradient), 17–20 min (100% B, column wash), 20–20.2 min (100%→2% B, linear gradient), 20.2–23 min (2% B, re-equilibration). The flow from the LC was directed into the mass spectrometer through a heated electrospray probe (H-ESI). The settings of the H-ESI were spray voltage, 2000 V for positive mode and 2000 V for negative mode; sheath gas, 55 (arbitrary unit), auxiliary gas, 25 (arbitrary unit), sweep gas, 2 (arbitrary unit); ion transfer tube temperature, 325 °C; vaporizer temperature, 350 °C.
MS/MS detection was carried out using the selected reaction monitoring (SRM) mode. Two transitions were monitored for each compound: one for quantification and the other for confirmation. The SRM parameters for each individual compound are summarized in Table 1. The resolutions of both Q1 and Q3 were set at 0.7 FWHM, the cycle time was 0.4 s, and the pressure of the collision gas (argon) was set at 2 mTorr.
2.5. Statistical Analysis
The spatial differences in pesticides and heavy metal concentrations were assessed by comparing the mean values of the entire study period between locations. Descriptive statistics (means and standard means of errors) were calculated from all analyzed samples. When a compound was below the limit of detection (<LOD), the concentration of half of the LOD was used for statistical analysis [44]. A multiple t-test analysis was performed to compare samples from agricultural settings versus urban ones using GraphPad Prism 9.3.1 (GraphPad Software, San Diego, CA, USA). p-values < 0.05 were considered to be statistically significant.
3. Results
3.1. Selenium and Heavy Metals
Differences were observed in the concentrations of the heavy metals (As, Pb, and Cd) and Se detected in pollen samples collected from the agricultural versus urban areas. The mean concentrations of Se and As detected in the pollen samples were 1.16 and 1.08 times higher in agricultural sites than they were in urban sites. The mean concentrations of Cd and Pb detected in pollen samples were 1.04 and 1.62 times higher in urban sites than they were in agricultural sites. Table 1 summarizes the statistical data of the heavy metal concentrations in pollen samples collected from hives in agricultural and urban areas.

Table 1.
Statistical data summary of heavy metals and selenium detected in pollen samples.
Table 1.
Statistical data summary of heavy metals and selenium detected in pollen samples.
| Heavy Metal | Agriculture | Urban | Multiple t-Test | |||
|---|---|---|---|---|---|---|
| Mean (ppb) | Mean (ppb) | ± SEM * | p-Value | t | df | |
| As | 377 | 347 | 159.5 | 0.99 | 0.009 | 20 |
| Cd | 174 | 182 | 21.91 | 0.99 | 0.012 | 20 |
| Pb | 333 | 540 | 144.8 | 0.16 | 1.432 | 21 |
| ** Se | 1307 | 1124 | 378.4 | 0.63 | 0.483 | 21 |
* SEM: standard error of the mean difference between the two averages; ** Se: is a trace mineral.
Figure 2 presents boxplots with Tukey whiskers showing the concentrations (ppb) of As, Cd, Pb, and Se detected in pollen samples collected in urban or agricultural locations. Overall, no significant variations were observed for these metals in pollen samples from all sites (all with p > 0.05).
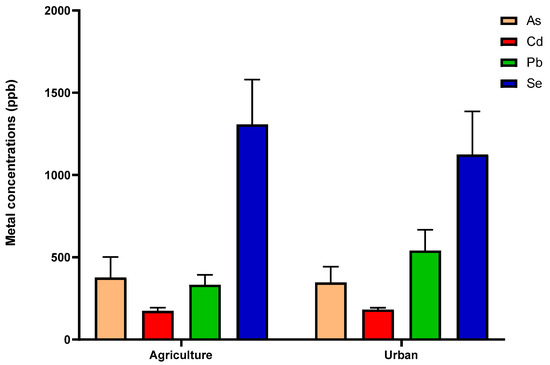
Figure 2.
Mean and SEM values of heavy metal (As, Cd, and Pb) and Se concentrations (ppb) in pollen samples. Pollen samples are identified as originating from either urban or agricultural locations. p-value > 0.05 for all analyses.
Variations were observed in the concentrations of heavy metals (As, Pb, and Cd) and Se detected in honey samples collected from agricultural versus urban areas. The mean concentrations of As, Cd, and Pb detected in honey samples were 1.44, 1.47, and 1.31 times higher in agricultural sites than they were in urban sites, respectively. The mean concentration of Se detected in honey samples was 1.05 times higher in urban sites than it was in agricultural sites. Table 2 summarizes the statistical data of the heavy metals and Se detected in honey samples collected from hives located in agricultural and urban areas. Figure 3 represents boxplots with Tukey whiskers showing concentrations (ppb) of As, Cd, Pb, and Se detected in honey samples identified as originating from urban versus agricultural locations. Overall, no significant variations were observed between urban and agricultural locations for these metals in honey samples (all with p > 0.05).

Table 2.
Statistical data summary of heavy metals and selenium detected in honey samples.
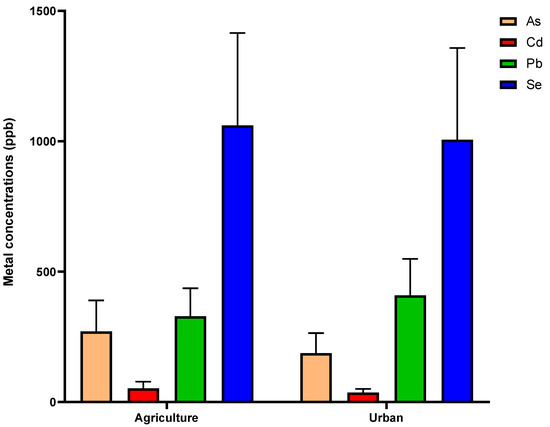
Figure 3.
Mean and SEM values of heavy metal (As, Cd, and Pb) and Se concentrations (ppb) detected in honey samples. Honey samples are identified as originating from either urban or agricultural locations. p-value > 0.05 for all analyses.
3.2. Pesticides
A total of 61 pollen samples were collected and analyzed for the presence of 92 different pesticides. Sixty-four pesticide types were not detected in the pollen samples or were less than the limit of quantitation. Of the 38 chemicals that were detected, 15 were fungicides, 13 were insecticides, 7 were herbicides, 2 were acaricides, and 1 was a pesticide synergist. Chlorpyrifos, Atrazine, Diuron, and Metconazole were observed at the highest levels among pollen samples collected from agricultural areas, with mean concentrations of 17.3, 3.44, 3.21, and 1.74 ppb, respectively. Among the samples collected from urban locations, Triphenylmethyl, Chlorpyrifos, Carbaryl, and Chlorantraniliprole were observed at the highest levels, with mean concentrations of 105.24, 26.01, 16.28, and 11.06 ppb, respectively. Overall, no significant variations were observed for these pesticides in pollen samples from all areas (all with p > 0.05).
Twenty-one honey samples were collected from hives located in urban versus agricultural landscapes and analyzed for 92 different pesticide residues. Seventy-six pesticide types were not detected in honey samples or were less than the limit of quantitation. However, sixteen chemicals were detected in honey samples, nine of which were insecticides, three were herbicides, two were fungicides, one was an acaricide, and one was a pesticide synergist. Coumaphos, Piperonyl butoxide, and Tebuconazole were observed at the highest concentrations in honey samples collected from agricultural areas, with mean concentrations of 0.22, 0.11, and 0.11 ppb, respectively. Coumaphos, Acephate, and 2,4-DMPF were observed at the highest concentrations in honey samples collected from urban areas, with mean concentrations of 1.09, 0.93, and 0.64, respectively. Figure 4 shows the means and standard errors of mean of different pesticide concentrations (ppb) found in honey samples collected from urban and agricultural areas. Overall, no significant variation in pesticide concentrations was observed in honey samples collected from urban and agricultural areas (all with p > 0.05).
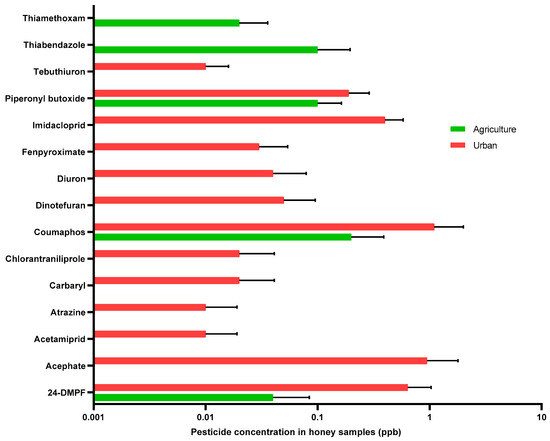
Figure 4.
Mean and SEM concentrations (ppb) of different pesticide residues detected in honey samples collected from urban and agricultural areas. p-value > 0.05 for all analyses.
3.3. Risk Assessment
To determine the risk level that honey bee populations may be exposed to, we compared the data for the concentrations of heavy metals and pesticides detected in the pollen and honey samples in this study to previously reported concentrations for lethal and chronic health impacts of pesticides and heavy metals on honey bees.
3.3.1. Heavy Metals
In Table 3, we compare the results of the heavy metal concentrations in the pollen and honey samples with previously reported concentrations associated with lethal or chronic health impacts of heavy metals.

Table 3.
Effect of different concentrations of heavy metals on honey bees compared with the concentrations we detected in pollen and honey samples.
3.3.2. Pesticides
In Table 4, we compare the results of the pesticide concentrations in the pollen and honey samples with previously reported concentrations associated with lethal or chronic health impacts of pesticides.

Table 4.
Effect of different concentrations of pesticides on honey bees compared with the concentrations we detected in honey and pollen samples.
4. Discussion
Honey bees and their products (pollen, honey, and wax) have been widely used as a bioindicator for environmental pollution, either through the accumulation of different toxins in their products or through the high mortality rates caused by these toxins [55]. Most research has focused on pesticides as major chemical stressors that increase the risk for the phenomenon of colony collapse disorder [56,57]. Studies [18,58,59] investigated a variety of risks associated with honey bees’ exposure to pesticides, either inside hives or during foraging. Although there were confirming results of the effect of heavy metals on honey bees’ survival [46], memory, and foraging behavior [15], few studies examined the contribution of heavy metals in bringing about the colony collapse phenomenon. The presence of pesticides and heavy metals in honey bees and their products simultaneously can alert investigators to the possibility of combinatorial impacts of these toxins, which may exacerbate the risks of harm to bee colonies. The primary objective of this study was to determine the spatial variations in pesticides and heavy metals within pollen and honey samples.
A wide range of heavy metal residues (As, Pb, and Cd) and Se were observed in the pollen and honey samples collected from urban and agricultural landscapes. Based on our data, the lowest and highest (As) concentrations in the honey samples collected from different landscapes were 1 ppb and 1280 ppb, respectively. For all metals, there were no significant differences in the mean concentrations detected in the honey and pollen samples collected from different locations. Thus, there was no spatial variation in the metal concentrations in pollen and honey samples collected from urban and agricultural areas. Similarly, there were no significant differences in the mean pesticide concentrations detected in the pollen and honey samples collected from urban and agricultural locations.
Most bee health research has focused on the effects of insecticides, such as neonicotinoids, by reporting their immediate toxicity and the close connection between these insecticides and the corresponding impacts on bee populations [17,18,52,57]. Fewer studies have reported the effects of other pesticides, such as herbicides, acaricides, and fungicides [60], or different types of adjuvant chemicals that are used along with pesticides, such as piperonyl butoxide (PBO). Piperonyl butoxide is a pesticide synergist used in combination with insecticides to enhance their active properties by inhibiting insect detoxification activity. Although the effect of PBO on different organisms has been reported (for example, reductions in developmental and behavioral orientation in mice [61] and lethality to the cotton whitefly (Bemisia tabaci) [62]), PBO’s effects on honey bees are typically examined for its combinatorial impacts when added to other compounds [58]. For example, the application of PBO with methyl benzoate has been shown to decrease the orientation and flight ability of bees [58].
Synergistic effects of some pesticide mixtures on bee health were reported in which the toxicities of some pesticides were enhanced by the presence of others, such as in Johnson et al. [62], where the toxicity of tau-ßuvalinate increased with the application of Coumaphos. Sometimes, protective beekeeping practices to save bee colonies expose bees to interactive toxins. For example, applying different types of acaricide and fungicide at the same time to control Varroa mite and bacterial infections can interact and produce a higher level of toxicity to bee populations [60]. Pesticide synergism can also reduce bees’ detoxification ability, which in turn increases their sensitivities to environmental toxins [63]. There has been less focus on the synergistic effects of pesticide–heavy metal combination or the synergistic effects of multiple stressors.
Based on the findings of this study, and by comparison with previously published findings, the heavy metal levels observed in northern Colorado in some pollen and honey samples pose severe risks to honey bees, whereas the pesticide levels were observed to be below the established levels of risk for honey bee health. However, future risk-based studies will be necessary to consider the potential for combinatorial effects resulting from the interaction of pesticides with heavy metals. The inclusion of other factors that exacerbate health risks, such as the presence of pathogens, variables of climate change, loss of foraging habitat, etc., may likewise reveal lower thresholds relative to the established concentrations for risk levels.
Most of the pesticide residues detected were below the established levels of risk. However, the detected levels of heavy metals and selenium in some honey and pollen samples pose a lethal or acute risk to honey bees. This investigation sets the stage for future studies to explore the effects of exacerbating combinatorial variables that can impact the health or loss of honey bee populations.
5. Conclusions
The variance in levels of pesticides, selenium, and heavy metals in two distinct landscapes—urban and agricultural areas of northeastern Colorado, USA—was investigated using pollen and honey samples. The quantities of metals and selenium in pollen and honey samples gathered from urban and rural areas did not vary spatially. Additionally, we found no significant spatial variations in the levels of pesticides in pollen and honey samples. According to prior research and a comparison of the levels of heavy metals, selenium, and pesticides in our samples of pollen and honey, we found that some honey and pollen samples include heavy metal and selenium residues that probably constitute a serious health danger to honey bees. Nevertheless, we consider the probability of synergistic chemical effects even when the pesticide residue levels were below known risk criteria. Our findings provide encouragement for further research into honey bee health concerns linked to various contaminant combinations.
Author Contributions
M.M.A. wrote the first draft of the paper. R.B.B. provided critical input and assisted in revising and improving the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All publicly accessible data are in Awad (2023) at https://www.proquest.com/docview/2820206747 (accessed on 31 April 2023).
Acknowledgments
We wish to express immense gratitude to all the beekeepers, and their bees, for volunteering and for their valuable participation in this research. We are also grateful to the Graduate Degree Program of Ecology, Colorado State University for covering the cost of some pollen traps we used in this research.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Types and limits of concentration detections of pesticides surveyed in honey and pollen samples. LOD and LOQ are the limit of detection and limit of quantitation, respectively, which represent the lowest concentrations of pesticides that can be detected.
Table A1.
Types and limits of concentration detections of pesticides surveyed in honey and pollen samples. LOD and LOQ are the limit of detection and limit of quantitation, respectively, which represent the lowest concentrations of pesticides that can be detected.
| Pesticide | Type | LOD-LOQ (ppb) Honey | LOD-LOQ (ppb) Pollen |
|---|---|---|---|
| 2,4-DMPF | Insecticide | 0.29–0.86 | 0.40–1.20 |
| 4-Hydroxy-chlorothalonil | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Acephate | Insecticide | 0.71–2.14 | 1.00–3.00 |
| Acetamiprid | Insecticide | 0.07–0.21 | 0.1–0.30 |
| Ametryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Atrazine | Herbicide | 0.07–0.21 | 0.1–0.30 |
| Avermectin B1a | Acaricide | 0.43–1.29 | 0.60–1.80 |
| Azoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Bendiocarb | Insecticide | 0.09–0.26 | 0.12–0.36 |
| Boscalid | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Bromuconazole | Fungicide | 0.43–1.29 | 0.60–1.80 |
| Carbaryl | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Carbofuran | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Chlorantraniliprole | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Chlorpyrifos | Insecticide | 4.29–12.86 | 6.00–18.00 |
| Clomazone | Herbicide | 0.11–0.34 | 0.16–0.48 |
| Clothianidin | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Coumaphos | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Cyanazine | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Cyantraniliprole | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Cyflufenamid | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Cyprodinil | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Cyromazine | Insecticide | 0.71–2.14 | 1.00–3.00 |
| Difenoconazole | Fungicide | 0.07–0.21 | 0.1–0.30 |
| Diflubenzuron | Acaricide | 2.86–8.57 | 4.00–12.00 |
| Dimoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Dinotefuran | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Diuron | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Fenamidone | Fungicide | 0.07–0.21 | 0.1–0.30 |
| Fenbuconazole | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fenhexamid | Fungicide | 2.86–8.57 | 4.00–12.00 |
| Fenpyroximate | Acaricide | 0.07–0.21 | 0.10–0.30 |
| Fipronil | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Fluazifop | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Fluazinam | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fludioxonil | Fungicide | 0.43–1.29 | 0.60–1.80 |
| Flufenacet | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Flumioxazin | Herbicide | 7.14–21.43 | 10.00–30.00 |
| Fluometuron | Herbicide | 0.29–0.86 | 0.40–1.20 |
| Fluopicolide | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Fluopyram | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Fluoxastrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Flupyradifurone | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Fluxapyroxad | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Fumagillin | Fungicide | 1.43–4.29 | 2.00–6.00 |
| Hexaflumuron | Insecticide | 2.86–8.57 | 4.00–12.00 |
| Imidacloprid | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Indoxacarb | Insecticide | 0.43–1.29 | 0.60–1.80 |
| Malaoxon | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Mandipropamid | Fungicide | 0.06–0.17 | 0.08–0.24 |
| Metalaxyl | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Metazachlor | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Metconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Methiocarb | Insecticide | 0.29–0.86 | 0.40–1.20 |
| Methoprotryne | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Methoxyfenozide | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Metobromuron | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Metolachlor | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Mevinphos | Insecticide | 0.14–0.43 | 0.20–0.60 |
| Myclobutanil | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Napropamide | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Penthiopyrad | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Phenmedipham | Herbicide | 0.14–0.43 | 0.20–0.60 |
| Phosmet | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Picoxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Piperonyl butoxide | pesticide synergist | 0.03–0.09 | 0.04–0.12 |
| Profenophos | Insecticide | 0.57–1.71 | 0.80–2.40 |
| Prometon | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Prometryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Propazine | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Propiconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Pyraclostrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Pyrimethanil | Fungicide | 0.14–0.43 | 0.20–0.60 |
| Spinetoram | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Spinosad | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Spirotetramat | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Sulfentrazone | Herbicide | 2.86–8.57 | 4.00–12.00 |
| Sulfoxaflor | Insecticide | 1.43–4.29 | 2.00–6.00 |
| Tebuconazole | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Tebufenozide | Insecticide | 0.03–0.09 | 0.04–0.12 |
| Tebuthiuron | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Terbutryn | Herbicide | 0.03–0.09 | 0.04–0.12 |
| Tetraconazole | Fungicide | 0.29–0.86 | 0.40- 1.20 |
| Tetramethrin | Insecticide | 0.43–1.29 | 0.60–1.80 |
| Thiabendazole | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Thiacloprid | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Thiamethoxam | Insecticide | 0.07–0.21 | 0.10–0.30 |
| Thiobencarb | Herbicide | 0.43–1.29 | 0.60–1.80 |
| Thiophanate-methyl | Fungicide | 0.07–0.21 | 0.10–0.30 |
| Triadimefon | Fungicide | 0.29–0.86 | 0.40–1.20 |
| Trifloxystrobin | Fungicide | 0.03–0.09 | 0.04–0.12 |
| Triflumizole | Fungicide | 0.07–0.21 | 0.10–0.30 |
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Miller-Struttmann, N. The Complex Causes of Worldwide Bee Declines. Available online: https://phys.org/news/2016-01-complex-worldwide-bee-declines.html (accessed on 20 March 2022).
- The State of Food and Agriculture. 2009. Available online: https://www.fao.org/3/i0680e/i0680e.pdf (accessed on 2 March 2023).
- ARS Honey Bee Health. Available online: https://www.ars.usda.gov/oc/br/ccd/index/ (accessed on 10 February 2022).
- Grozinger, C.M.; Flenniken, M.L. Bee Viruses: Ecology, Pathogenicity, and Impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Feinsinger, P. Habitat Fragmentation, Native Insect Pollinators, and Feral Honey Bees in Argentine. Ecol. Appl. 1994, 4, 378–392. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A Worldwide Survey of Neonicotinoids in Honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Tison, L.; Hahn, M.-L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey Bees’ Behavior Is Impaired by Chronic Exposure to the Neonicotinoid Thiacloprid in the Field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Wernecke, A.; Frommberger, M.; Forster, R.; Pistorius, J. Lethal Effects of Various Tank Mixtures Including Insecticides, Fungicides and Fertilizers on Honey Bees under Laboratory, Semi-Field and Field Conditions. J. Consum. Prot. Food Saf. 2019, 14, 239–249. [Google Scholar] [CrossRef]
- Polykretis, P.; Delfino, G.; Petrocelli, I.; Cervo, R.; Tanteri, G.; Montori, G.; Perito, B.; Branca, J.; Morucci, G.; Gulisano, M. Evidence of Immunocompetence Reduction Induced by Cadmium Exposure in Honey Bees (Apis Mellifera). Environ. Pollut. 2016, 218, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Monchanin, C.; Drujont, E.; Devaud, J.-M.; Lihoreau, M.; Barron, A.B. Metal Pollutants Have Additive Negative Effects on Honey Bee Cognition. J. Exp. Biol. 2021, 24, jeb241869. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet Effects on Honeybee Immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Purdy, J.; Anderson, T.; Fell, R. Risks of Neonicotinoid Insecticides to Honeybees. Environ. Toxicol. Chem. 2014, 33, 719–731. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis Mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Moroń, D.; Grześ, I.M.; Skórka, P.; Szentgyörgyi, H.; Laskowski, R.; Potts, S.G.; Woyciechowski, M. Abundance and Diversity of Wild Bees Along Gradients of Heavy Metal Pollution. J. Appl. Ecol. 2011, 49, 118–125. [Google Scholar] [CrossRef]
- Hladun, K.R.; Di, N.; Liu, T.; Trumble, J.T. Metal Contaminant Accumulation in the Hive: Consequences for whole-colony Health and Brood Production in the Honey Bee (Apis mellifera L.). Environ. Toxicol. Chem. 2016, 35, 322–329. [Google Scholar] [CrossRef]
- Sivakoff, F.S.; Gardiner, M.M. Soil Lead Contamination Decreases Bee Visit Duration at Sunflowers. Urban Ecosyst. 2017, 20, 1221–1228. [Google Scholar] [CrossRef]
- Zhou, X.; Taylor, M.P.; Davies, P.J.; Prasad, S. Identifying Sources of Environmental Contamination in European Honey Bees (Apis mellifera) Using Trace Elements and Lead Isotopic Compositions. Environ. Sci. Technol. 2018, 52, 991–1001. [Google Scholar] [CrossRef]
- Aghamirlou, H.M.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.H.; Akbarzadeh, A.; Nazmara, S. Heavy Metals Determination in Honey Samples Using Inductively Coupled Plasma-Optical Emission Spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Shalaby, T.A.; Prokisch, J.; Fári, M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In CO2 Sequestration, Biofuels and Depollution; Springer: Cham, Switzerland, 2015; pp. 153–232. [Google Scholar]
- Čuvardić, M.S. Selenium in soil. Zb. Matice Srp. Za Prir. Nauk. 2003, 23–37. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Burden, C.M.; Elmore, C.; Hladun, K.R.; Trumble, J.T.; Smith, B.H. Acute exposure to selenium disrupts associative conditioning and long-term memory recall in honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2016, 127, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hladun, K.R.; Smith, B.H.; Mustard, J.A.; Morton, R.R.; Trumble, J.T. Selenium Toxicity to Honey Bee (Apis mellifera L.) Pollinators: Effects on Behaviors and Survival. PLoS ONE 2012, 7, e34137. [Google Scholar] [CrossRef]
- Sabatino, L.; Scordino, M.; Pantò, V.; Chiappara, E.; Traulo, P.; Gagliano, G. Survey of Neonicotinoids and Fipronil in Corn Seeds for Agriculture. Food Addit. Contam. Part B 2013, 6, 11–16. [Google Scholar] [CrossRef]
- Pohorecka, K.; Skubida, P.; Semkiw, P.; Miszczak, A.; Teper, D.; Sikorski, P.; Zagibajło, K.; Skubida, M.; Zdańska, D.; Bober, A. Effects of Exposure of Honey Bee Colonies to Neonicotinoid seed–treated Maize Crops. J. Apic. Sci. 2013, 57, 199–208. [Google Scholar] [CrossRef]
- Castilhos, D.; Dombroski, J.L.D.; Bergamo, G.C.; Gramacho, K.P.; Gonçalves, L.S. Neonicotinoids and Fipronil Concentrations in Honeybees Associated With Pesticide Use in Brazilian Agricultural Areas. Apidologie 2019, 50, 657–668. [Google Scholar] [CrossRef]
- Zawislak, J.; Adamczyk, J.; Johnson, D.R.; Lorenz, G.; Black, J.; Hornsby, Q.; Stewart, S.D.; Joshi, N. Comprehensive Survey of Area-Wide Agricultural Pesticide Use in Southern United States Row Crops and Potential Impact on Honey Bee Colonies. Insects 2019, 10, 280. [Google Scholar] [CrossRef]
- Sheldon, M.; Pinion, C., Jr.; Klyza, J.; Zimeri, A.M. Pesticide Contamination in Central Kentucky Urban Honey: A Pilot Study. J. Environ. Health 2019, 82, 8–13. [Google Scholar]
- Sadowska, M.; Gogolewska, H.; Pawelec, N.; Sentkowska, A.; Krasnodębska-Ostręga, B. Comparison of the Contents of Selected Elements and Pesticides in Honey Bees with Regard to Their Habitat. Environ. Sci. Pollut. Res. 2018, 26, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Naccari, C.; Macaluso, A.; Giangrosso, G.; Naccari, F.; Ferrantelli, V. Risk Assessment of Heavy Metals and Pesticides in Honey From Sicily (Italy). J. Food Res. 2014, 3, 107. [Google Scholar] [CrossRef]
- The Urban and Rural Classifications. Available online: https://www2.census.gov/geo/pdfs/reference/GARM/Ch12GARM.pdf (accessed on 13 May 2022).
- Colorado Revised Statutes 2016 TITLE 25.5. Available online: https://leg.colorado.gov/sites/default/files/images/olls/crs2016-title-25.5.pdf (accessed on 14 September 2022).
- Utaipanon, P.; Holmes, M.J.; Chapman, N.C.; Oldroyd, B.P. Estimating the Density of Honey Bee (Apis mellifera) Colonies Using Trapped Drones: Area Sampled and Drone Mating Flight Distance. Apidologie 2019, 50, 578–592. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Vandame, R.; Castro-Chan, R.; Penilla-Navarro, R.; Gómez, J.; Sánchez, D. Organochlorine Pesticides in Honey and Pollen Samples from Managed Colonies of the Honey Bee Apis mellifera Linnaeus and the Stingless Bee Scaptotrigona Mexicana Guérin from Southern, Mexico. Insects 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of Mineral Content and Heavy Metal of Honey Samples from South and East Region of Turkey by Using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Jones, J.B.; Workman, S.M. Optical Emission Spectrometry. In Agronomy Monographs; ACSESS: Hoboken, NJ USA, 2015; pp. 29–65. [Google Scholar] [CrossRef]
- EN 15662—European Standards. Available online: https://www.en-standard.eu/csn-en-15662-foods-of-plant-origin-multimethod-for-the-determination-of-pesticide-residues-using-gc-and-lc-based-analysis-following-acetonitrile-extraction-partitioning-and-clean-up-by-dispersive-spe-modular-quechers-method/ (accessed on 2 March 2023).
- United States Environmental Protection Agency; Office of Pesticide Programs. Assigning Values to Non-Detected /Non-Quantified Pesticide Residues in Human Health Food Exposure Assessments; Office of Pesticide Programs, US Environmental Protection Agency: Washington, DC, USA, 2000.
- Knowlton, G.F.; Sturtevant, A.P.; Sorenson, C.J. Adult Honey Bee Losses in Utah as Related to Arsenic Poisoning; Bulletin No. 340-; Utah Agricultural Experiment Station: Logan, UT, USA, 1950. [Google Scholar]
- Di, N.; Hladun, K.R.; Zhang, K.; Liu, T.-X.; Trumble, J.T. Laboratory Bioassays on the Impact of Cadmium, Copper and Lead on the Development and Survival of Honeybee (Apis mellifera L.) Larvae and Foragers. Chemosphere 2016, 152, 530–538. [Google Scholar] [CrossRef]
- Pettis, J.S.; Collins, A.M.; Wilbanks, R.; Feldlaufer, M.F. Effects of Coumaphos on Queen Rearing in the Honey Bee, Apis mellifera. Apidologie 2004, 35, 605–610. [Google Scholar] [CrossRef]
- Nakar, R.; Koteswara Rao, S.; Sridevi, D.; Vidyasagar, B. Contact Toxicity of Certain Conventional Insecticides to European Honeybee, Apis mellifera Linnaeus. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3359–3365. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, Y.C.; Adamczyk, J.; Luttrell, R. Influences of Acephate and Mixtures With Other Commonly Used Pesticides on Honey Bee (Apis mellifera) Survival and Detoxification Enzyme Activities. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 209, 9–17. [Google Scholar] [CrossRef]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of Imidacloprid and Varroa Destructor on Survival and Health of European Honey Bees, Apis mellifera. Insect Sci. 2016, 24, 467–477. [Google Scholar] [CrossRef]
- Hatjina, F.; Papaefthimiou, C.; Charistos, L.; Dogaroglu, T.; Bouga, M.; Emmanouil, C.; Arnold, G. Sublethal Doses of Imidacloprid Decreased Size of Hypopharyngeal Glands and Respiratory Rhythm of Honeybees in Vivo. Apidologie 2013, 44, 467–480. [Google Scholar] [CrossRef]
- Sonnet, P.E.; Lye, T.L.; Sackett, R.R. Effects of Selected Herbicides on the Toxicity of Several Insecticides to Honey Bees. Environ. Entomol. 1978, 7, 254–256. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Li, Y.; Zhang, S.; Shi, P.; Li-Byarlay, H.; Luo, S. Pesticide Residues in Beebread and Honey in Apis Cerana Cerana and Their Hazards to Honey Bees and Human. Ecotoxicol. Environ. Saf. 2022, 238, 113574. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.; Maccagnani, B. Honey bees as bioindicators of environmental pollution. Bull. Insectology 2003, 56, 137–139. [Google Scholar]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Frazier, J.; Mullin, C.; Frazier, M.; Ashcraft, S. Pesticides and their involvement in colony collapse disorder. Am. Bee J. 2011, 151, 779–781. [Google Scholar]
- Zhu, Y.-C.; Wang, Y.; Portilla, M.; Parys, K.; Li, W. Risk and Toxicity Assessment of a Potential Natural Insecticide, Methyl Benzoate, in Honey Bees (Apis mellifera L.). Insects 2019, 10, 382. [Google Scholar] [CrossRef]
- Démares, F.; Gibert, L.; Creusot, P.; Lapeyre, B.; Proffit, M. Acute Ozone Exposure Impairs Detection of Floral Odor, Learning, and Memory of Honey Bees, through Olfactory Generalization. Sci. Total. Environ. 2022, 827, 154342. [Google Scholar] [CrossRef]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, O.; Oishi, S. Reproductive and Neurobehavioural Effects in Three-Generation Toxicity Study of Piperonyl Butoxide Administered to Mice. Food Chem. Toxicol. 1992, 30, 1015–1019. [Google Scholar] [CrossRef]
- Devine, G.; Denholm, I. An Unconventional Use of Piperonyl Butoxide for Managing the Cotton Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 1998, 88, 601–610. [Google Scholar] [CrossRef]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic Interactions Between In-Hive Miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic Detoxification Pathways in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).