Neisseria mucosa Does Not Inhibit the Growth of Neisseria gonorrhoeae
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Origin of Bacterial Isolates
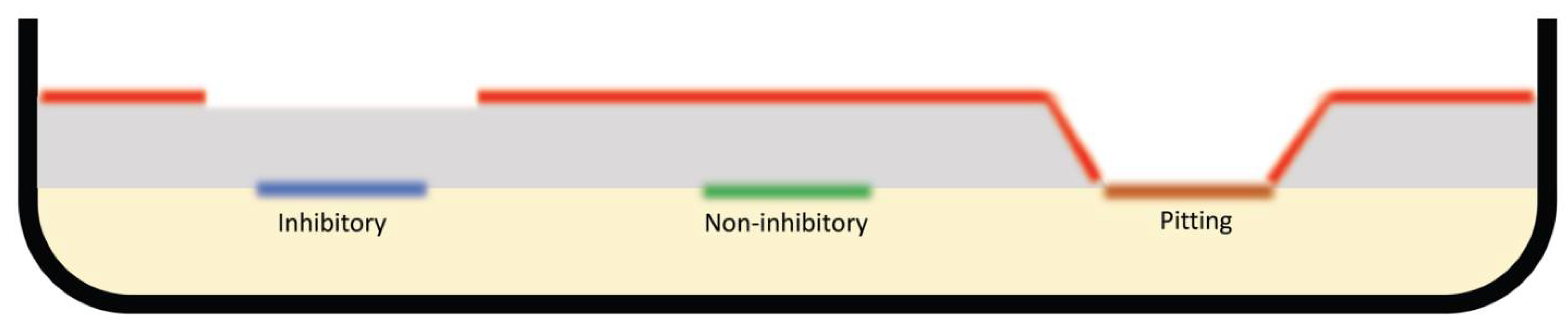
4.1.1. Inhibitory/Producer Bacterial Isolates
- (i)
- The Preventing Resistance in Gonorrhoea Study (PReGo), a single-centre randomized controlled trial conducted at the Institute of Tropical Medicine in Antwerp, Belgium, between 2019 and 2020, that assessed the efficacy of an antiseptic mouthwash to prevent STIs among 343 MSM using PrEP [2].
- (ii)
- The Commensals in the Community Study (ComCom), a survey of the oropharyngeal microbiomes of Institute of Tropical Medicine (ITM) employees conducted in June 2020 [16]. In both studies, oropharyngeal swabs (ESwabTM COPAN Diagnostics Inc., Brescia, Italy) were taken and inoculated onto blood and modified Thayer–Martin agar plates using the streak plate technique, and incubated at 35–37 °C and 5% CO2. Plates were examined after 48 h, and Neisseria-like colonies were selected based on a positive oxidase test and a Gram stain. Neisseria-like colonies were enriched on blood agar plates and stored in skim milk at −80 °C. Cultures of Neisseria-like colonies were shipped to Laboratoire des Hôpitaux Universitaires de Bruxelles-Universitair Laboratorium Brussel (LHUB-ULB), where species were identified using matrix-assisted laser desorption/ionization time-of-flight mass Spectrometry (MALDI-TOF MS), on a MALDI Biotyper® Sirius IVD system using the MBT Compass IVD software and library (Bruker Daltonics, Bremen, Germany) consisting of 9607 spectra.
4.1.2. N. gonorrhoeae Target Strains
4.1.3. Non-Neisseria Isolates
4.2. Agar Overlay Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Van Dijck, C.; Tsoumanis, A.; Rotsaert, A.; Vuylsteke, B.; Van den Bossche, D.; Paeleman, E.; De Baetselier, I.; Brosius, I.; Laumen, J.; Buyze, J. Antibacterial mouthwash to prevent sexually transmitted infections in men who have sex with men taking HIV pre-exposure prophylaxis (PReGo): A randomised, placebo-controlled, crossover trial. Lancet Infect. Dis. 2021, 21, 657–667. [Google Scholar] [CrossRef]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouttier, S.; Yeo, A.; Any-Grah, A.A.S.A.; Geiger, S.; Huang, N.; Nicolas, V.; Villebrun, S.; Faye-Kette, H.; Ponchel, G.; Koffi, A.A.; et al. Characterization and in vitro evaluation of a vaginal gel containing Lactobacillus crispatus for the prevention of gonorrhea. Int. J. Pharm. 2020, 588, 119733. [Google Scholar] [CrossRef]
- Kenyon, C.; Laumen, J.; Manoharan-Basil, S. Choosing New Therapies for Gonorrhoea: We Need to Consider the Impact on the Pan-Neisseria Genome. A Viewpoint. Antibiotics 2021, 10, 515. [Google Scholar] [CrossRef]
- Chow, E.P.; Williamson, D.A.; Hocking, J.S.; Law, M.G.; Maddaford, K.; Bradshaw, C.S.; McNulty, A.; Templeton, D.J.; Moore, R.; Murray, G.L. Antiseptic mouthwash for gonorrhoea prevention (OMEGA): A randomised, double-blind, parallel-group, multicentre trial. Lancet Infect. Dis. 2021, 21, 647–656. [Google Scholar] [CrossRef]
- Van Dijck, C.; Cuylaerts, V.; Sollie, P.; Spychala, A.; De Baetselier, I.; Laumen, J.; Crucitti, T.; Kenyon, C. The development of mouthwashes without anti-gonococcal activity for controlled clinical trials: An in vitro study. F1000Research 2019, 8, 1620. [Google Scholar] [CrossRef]
- Tsoumanis, A.; Hens, N.; Kenyon, C.R. Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies. Sex. Transm. Dis. 2018, 45, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Barbee, L.A.; Khosropour, C.M.; Dombrowski, J.C.; Manhart, L.E.; Golden, M.R. An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: A case-control study. Sex. Transm. Infect. 2016, 92, 155–160. [Google Scholar] [CrossRef]
- Chow, E.P.; Howden, B.P.; Walker, S.; Lee, D.; Bradshaw, C.S.; Chen, M.Y.; Snow, A.; Cook, S.; Fehler, G.; Fairley, C.K. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: A randomised controlled trial and an in vitro study. Sex. Transm. Infect. 2017, 93, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Aho, E.L.; Ogle, J.M.; Finck, A.M. The Human Microbiome as a Focus of Antibiotic Discovery: Neisseria mucosa Displays Activity Against Neisseria gonorrhoeae. Front. Microbiol. 2020, 11, 577762. [Google Scholar] [CrossRef] [PubMed]
- Deasy, A.M.; Guccione, E.; Dale, A.P.; Andrews, N.; Evans, C.M.; Bennett, J.S.; Bratcher, H.B.; Maiden, M.C.; Gorringe, A.R.; Read, R.C. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin. Infect. Dis. 2015, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Kandi, V. Bacterial Colony Characters: Pitting Colonies. J. Med. Microbiol. Diagn. 2015, 4, 1000I102. [Google Scholar] [CrossRef] [Green Version]
- So, M.; Rendon, M.A. Tribal warfare: Commensal Neisseria kill pathogen Neisseria gonorrhoeae using its DNA. Microb. Cell 2019, 6, 544–546. [Google Scholar] [CrossRef]
- Kim, W.J.; Higashi, D.; Goytia, M.; Rendon, M.A.; Pilligua-Lucas, M.; Bronnimann, M.; McLean, J.A.; Duncan, J.; Trees, D.; Jerse, A.E.; et al. Commensal Neisseria Kill Neisseria gonorrhoeae through a DNA-Dependent Mechanism. Cell Host Microbe 2019, 26, 228–239.e8. [Google Scholar] [CrossRef]
- Laumen, J.G.E.; Manoharan-Basil, S.S.; Abdellati, S.; De Baetselier, I.; Van Dijck, C.; Martiny, D.; Serrano, G.; Bottieau, E.; Kenyon, C. Antimicrobial susceptibility of commensal Neisseria in the general population and men who have sex with men in Belgium. Sci. Rep. 2022, 12, 9. [Google Scholar] [CrossRef]
- Custodio, R.; Ford, R.M.; Ellison, C.J.; Liu, G.; Mickute, G.; Tang, C.M.; Exley, R.M. Type VI secretion system killing by commensal Neisseria is influenced by expression of type four pili. eLife 2021, 10, e63755. [Google Scholar] [CrossRef]
- Custodio, R.; Ford, R.M.; Ellison, C.J.; Liu, G.; Mickute, G.; Tang, C.M.; Exley, R.M. Type VI secretion system killing by commensal Neisseria is influenced by the spatial dynamics of bacteria. bioRxiv 2020. [Google Scholar] [CrossRef]
- Custodio, R.; Johnson, E.; Liu, G.; Tang, C.M.; Exley, R.M. Commensal Neisseria cinerea impairs Neisseria meningitidis microcolony development and reduces pathogen colonisation of epithelial cells. PLoS Pathog. 2020, 16, e1008372. [Google Scholar] [CrossRef]
- Audry, M.; Robbe-Masselot, C.; Barnier, J.P.; Gachet, B.; Saubamea, B.; Schmitt, A.; Schonherr-Hellec, S.; Leonard, R.; Nassif, X.; Coureuil, M. Airway Mucus Restricts Neisseria meningitidis Away from Nasopharyngeal Epithelial Cells and Protects the Mucosa from Inflammation. mSphere 2019, 4, e00494-19. [Google Scholar] [CrossRef] [Green Version]
- Kahler, C.M. Neisseria species and their complicated relationships with human health. Microbiol. Aust. 2021, 42, 79–83. [Google Scholar] [CrossRef]
- De Block, T.; Laumen, J.G.E.; Van Dijck, C.; Abdellati, S.; De Baetselier, I.; Manoharan-Basil, S.S.; Van den Bossche, D.; Kenyon, C. WGS of Commensal Neisseria Reveals Acquisition of a New Ribosomal Protection Protein (MsrD) as a Possible Explanation for High Level Azithromycin Resistance in Belgium. Pathogens 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-Y.; Alcorn, T.M.; Cohen, M.S. Effects of H2O2-Producing Lactobacilli on Neisseria gonorrhoeae Growth and Activity. J. Infect. Dis. 1994, 170, 1209–1215. [Google Scholar] [CrossRef]
- Lin, E.Y.; Adamson, P.C.; Klausner, J.D. Epidemiology, Treatments, and Vaccine Development for Antimicrobial-Resistant Neisseria gonorrhoeae: Current Strategies and Future Directions. Drugs 2021, 81, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Lux, T.; Nuhn, M.; Hakenbeck, R.; Reichmann, P. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J. Bacteriol. 2007, 189, 7741–7751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, M.E.; Duncan, W.C.; Knox, J.M. Bacterial interference of Neisseria gonorrhoeae by alpha-haemolytic streptococci. Sex. Transm. Infect. 1980, 56, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Kraus, S.J.; Geller, R.C.; Perkins, G.H.; Rhoden, D.L. Interference by Neisseria gonorrhoeae growth by other bacterial species. J. Clin. Microbiol. 1976, 4, 288–295. [Google Scholar] [CrossRef]
- Bisaillon, J.G.; Beaudet, R.; Saheb, S.A.; Morisset, R. Interference of Neisseria gonorrhoeae growth by aerobic bacterial representatives of the urogenital flora. Rev. Can. Biol. 1980, 39, 201–208. [Google Scholar]
- Bisaillon, J.G.; Beaudet, R.; Lafond, L.; Saheb, S.A.; Sylvestre, M. Antigonococcal and antibacterial spectra of some bacterial isolates of the urogenital flora. Rev. Can. Biol. 1981, 40, 215–227. [Google Scholar]
- Kaye, D.; Levison, M. In vitro inhibition of growth of Neisseria gonorrhoeae by genital microorganisms. Sex. Transm. Dis. 1977, 4, 1–3. [Google Scholar] [CrossRef]
- Saigh, J.H.; Sanders, C.C.; Sanders, W.E., Jr. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: Evidence for a protective effect against infection. Infect. Immun. 1978, 19, 704–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, A.; Saheb, S.; Bisaillon, J.; Beaudet, R.; Sylvestre, M. In vitro inhibition of Neisseria gonorrhoeae growth by strict anaerobes. Infect. Immun. 1980, 28, 766–770. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellati, S.; Laumen, J.; Gonzalez, N.; Manoharan-Basil, S.S.; Van Dijck, C.; De Baetselier, I.; Martiny, D.; de Block, T.; Kenyon, C. Neisseria mucosa Does Not Inhibit the Growth of Neisseria gonorrhoeae. Sci 2022, 4, 8. https://doi.org/10.3390/sci4010008
Abdellati S, Laumen J, Gonzalez N, Manoharan-Basil SS, Van Dijck C, De Baetselier I, Martiny D, de Block T, Kenyon C. Neisseria mucosa Does Not Inhibit the Growth of Neisseria gonorrhoeae. Sci. 2022; 4(1):8. https://doi.org/10.3390/sci4010008
Chicago/Turabian StyleAbdellati, Saïd, Jolein Laumen, Natalia Gonzalez, Sheeba S. Manoharan-Basil, Christophe Van Dijck, Irith De Baetselier, Delphine Martiny, Tessa de Block, and Chris Kenyon. 2022. "Neisseria mucosa Does Not Inhibit the Growth of Neisseria gonorrhoeae" Sci 4, no. 1: 8. https://doi.org/10.3390/sci4010008
APA StyleAbdellati, S., Laumen, J., Gonzalez, N., Manoharan-Basil, S. S., Van Dijck, C., De Baetselier, I., Martiny, D., de Block, T., & Kenyon, C. (2022). Neisseria mucosa Does Not Inhibit the Growth of Neisseria gonorrhoeae. Sci, 4(1), 8. https://doi.org/10.3390/sci4010008





