Abstract
Antibiotic-sparing treatments are required to prevent the further emergence of antimicrobial resistance in Neisseria gonorrhoeae. Commensal Neisseria species have previously been found to inhibit the growth of pathogenic Neisseria species. For example, a previous study found that three out of five historical isolates of Neisseria mucosa could inhibit the growth of N. gonorrhoeae. In this study, we used agar overlay assays to assess if 24 circulating and historical isolates of Neisseria mucosa could inhibit the growth of 28 circulating and historical isolates of N. gonorrhoeae. Although pitting around each colony of N. mucosa created an optical illusion of decreased growth of N. gonorrhoeae, we found no evidence of inhibition (n = 24). In contrast, positive controls of Streptococcus pneumoniae and Escherichia coli demonstrated a strong inhibitory effect against the growth of N. gonorrhoeae.
1. Introduction
A number of countries worldwide are reporting an increasing incidence of sexually transmitted infections due to Neisseria gonorrhoeae [1]. This, combined with increasing antimicrobial resistance in this organism, has led to efforts to find novel therapies to treat and prevent this infection [2,3,4,5]. One of these strategies has been to use antiseptics to prevent the acquisition and transmission of N. gonorrhoeae to and from the oropharynx [2,6]. The prevalence of N. gonorrhoeae in the pharynx may reach 10% in high-risk populations and N. gonorrhoeae has been shown to be highly susceptible in vitro to antiseptics such as those based on essential oils [6,7,8,9]. A pilot clinical study found that an essential oil-based mouthwash reduced the prevalence of pharyngeal N. gonorrhoeae, as assessed by culture [10]. These findings provided the motivation for two randomized controlled trials that assessed if essential oil-based mouthwashes could reduce the incidence of N. gonorrhoeae and other STIs in men who have sex with men [2,6].
One of these was the preventing resistance in gonorrhoea (PReGo) study conducted in our centre [2]. This placebo-controlled trial randomized high-risk men who have sex with men to intensive use of an essential oil-based mouthwash and gargle, or placebo, to try to reduce the incidence of bacterial STIs in this population. The study found that mouthwash increased rather than decreased the incidence of oropharyngeal N. gonorrhoeae. The same essential oil-based mouthwash had a similar though statistically non-significant effect in the other study that used a slightly different study design (the OMEGA study) [6]. One of the possible explanations for these surprising results is that essential oil-based mouthwashes could reduce the abundance of commensal bacteria that have an inhibitory effect on N. gonorrhoeae. One such commensal bacteria is Neisseria mucosa, which has recently been shown to inhibit the growth of N. gonorrhoeae by Aho et al. [11].
N. mucosa is a healthy core component of the oropharyngeal microbiome and even low concentrations of an essential oil-based mouthwash have been shown to be bactericidal to Neisseria spp. [7]. If the use of the essential oil-based mouthwashes reduce the prevalence/abundance of N. mucosa, and N. mucosa inhibits the growth of N. gonorrhoeae, then the essential oil-based mouthwash could increase the susceptibility for N. gonorrhoeae infection [2]. In a similar vein, a randomized controlled trial established that nasal inoculation with N. lactamica reduced the incidence of colonization with N. meningitidis [12]. If the in-vitro anti-gonococcal effect of N. mucosa could be confirmed, N. mucosa might be evaluated as a probiotic to prevent gonococcal infection.
This provided the motivation for the current study where we tested the hypothesis that N. mucosa could inhibit the growth of N. gonorrhoeae. Our central objective was to assess if our locally circulating isolates of N. mucosa and other commensal Neisseria, including those circulating in the PReGo participants, were able to inhibit the growth of N. gonorrhoeae.
2. Results
Agar overlay assays were used to assess if 21 circulating and historical isolates of Neisseria mucosa, and 16 isolates from other Neisseria species, could inhibit the growth of 26 circulating and historical isolates of N. gonorrhoeae.
None of the commensal Neisseria or N. meningitidis exhibited any activity against N. gonorrhoeae (Table S1). The isolate of S. pneumoniae demonstrated clear evidence of inhibition against all nine strains of N. gonorrhoeae (median diameter of inhibition = 21 mm) (Figure 1a,b; Table S1). The inhibitory effect of E. coli was less pronounced (Figure 1a,b; Table S1). Inhibition was evident in three out of nine N. gonorrhoeae strains tested—the median diameter of inhibition was 11 mm (Table S1).
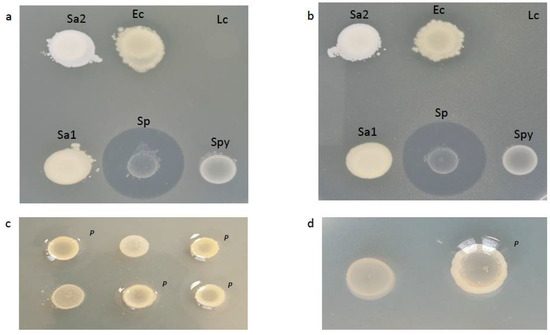
Figure 1.
Agar overlay assay testing the ability of various bacterial species to inhibit the growth of a lawn of N. gonorrhoeae strain RL1 (a) and strain 21.189 (b). Only the colonies of Escherichia coli (Ec) and Streptococcus pneumoniae (Sp) inhibited the growth. The colonies of N. mucosa in (c,d) did not exhibit any inhibitory effect on the growth of N. gonorrhoeae strain 21.163 (c) and strain WHO-W (d). A close-up of one of the N. mucosa colonies in (d) demonstrates the pitting (p) of the upper layer of agar around the right-hand colony of N. mucosa. Ec—Escherichia coli (ATCC 25922); Lc—Lactobacillus crispatus (LMG 9479); p—pitting; Sa—Staphylococcus aureus (1:ATCC 29213, 2:ATCC 25913); Sp—Streptococcus pneumoniae (ATCC 49619); Spy—Streptococcus pyogenes (LMG 14238).
A proportion of the colonies of N. mucosa exhibited a repellant effect, whereby they repelled the layer of agar poured over them (Figure 1c,d; Table S1). This created ‘pitting’ colonies or a convex slope between the top of the second layer of agar and the edge of each N. mucosa colony, which created an illusion of reduced N. gonorrhoeae growth around each N. mucosa colony [13] (Figure 2). Closer visual inspection, however, confirmed that N. gonorrhoeae growth over this convex slope around the N. mucosa colonies was not macroscopically distinguishable from that elsewhere (Figure 1c,d).

Figure 2.
A schematic illustration of the difference between growth-inhibition and pitting in the agar overlay assay. An agar plate in cross section is depicted, on which 3 bacterial colonies (labeled ‘inhibitory’, ‘non-inhibitory’ and ‘pitting’) were spotted and incubated for 24 h before a layer of GCB agar containing 106 CFU/mL of N. gonorrhoeae was poured over the plate (grey layer). Whilst the ‘non-inhibitory’ colony had no effect on the growth of N. gonorrhoeae (red line), and the ‘inhibitory’ colony had a clear inhibitory effect, the major effect of the ‘pitting’ colony was to repel the second layer of agar, thus creating an area around it which appears more translucent from above. Close visual inspection, including from the lateral aspect, of the depressed sections of the second layer of agar around the ‘pitting’ colony revealed the uninhibited growth of N. gonorrhoeae.
3. Discussion
Unlike Aho et al., we could find no evidence that N. mucosa or any other commensal Neisseria was able to inhibit the growth of N. gonorrhoeae [11]. This was despite using a large number of clinical and reference strains of N. gonorrhoeae as target strains, and the largest collection of commensal Neisseria tested to date as inhibitory bacteria.
How can these discordant findings be explained? Aho found this inhibitory effect in three out of five N. mucosa isolates. The isolates were all obtained from ATCC collections and did not include any recent clinical isolates. No photos were provided of the agar overlay assays showing that N. mucosa inhibited the growth of N. gonorrhoeae. However, one image of N. mucosa inhibiting the growth of N. flavescens was provided.
In our study, we followed an identical agar overlay protocol utilizing a larger panel of isolates of N. mucosa and N. gonorrhoeae. The experiments were performed by a laboratory technician with over 25 years of experience culturing Neisseria species (SA). The plates were examined by this person and two others with extensive experience in culturing Neisseria species (CK and JL). All three concurred that pitting around each colony of N. mucosa created an optical illusion of decreased growth around the colony. Close visual inspection confirmed that there was no inhibition of growth. We did not ascertain what the molecular determinants of this repellant effect were, as this was not an objective of this study.
We consider this a parsimonious explanation for the different findings between the two studies. It could be possible that only certain strains of N. mucosa are able to inhibit specific strains of N. gonorrhoeae, and that we did not include any of these combinations in our experiments. We did, however, test one of the three isolates of N. mucosa shown to have an inhibitory effect by Aho et al. This isolate (ATCC 25996) had no effect on the growth of 23 contemporarily circulating strains of N. gonorrhoeae in our laboratory. We did not have access to, and therefore did not include any of the same strains of N. gonorrhoeae used by Aho et al. As a result, we cannot exclude the possibility that our N. mucosa strains would have had an inhibitory effect on the N. gonorrhoeae strains used by Aho et al. Furthermore, our experiments were not conducted in duplicate. In pilot studies, we found that N. mucosae did not inhibit the growth of N. gonorrhoeae, and our experiment was thus designed to maximize the chances of detecting any inhibitory effect on N. gonorrhoeae. We thus evaluated if any strains of N. mucosa we could access (n = 24) could inhibit the growth of a large panel of circulating and type strains of N. gonorrhoeae (n = 28). This constitutes the largest experiment to have assessed this effect. The previous largest experiment was conducted with four isolates of N. mucosa tested against one isolate of N. gonorrhoeae, and a further one isolate of N. mucosa tested against seven isolates of N. gonorrhoeae [11]. Because we found no evidence of inhibition in any of the pair-wise comparisons in our experiments, we consider it unlikely that repeating the experiments in triplicate would change our findings.
We mainly included pharyngeal N. gonorrhoeae target strains. Since these were isolated from asymptomatic individuals, they may have adapted to live with oral commensals. Therefore, a greater number of strains from anatomical sites other than the pharynx should be included in future studies. We also cannot completely exclude the possibility that an unevaluated different experimental condition, such as storage of the isolates or the source of the agar used, was responsible for the differences in the results between the two studies. It could be argued that a further weakness of the study is that inhibition was only assessed via visual inspection. This is, however, the standard method of assessing growth inhibition in the agar overlay assay [11]. Our study, unlike that of Aho et al., did include positive controls. These showed clear and consistent evidence of inhibition. Taken together, these findings suggest that N. mucosa is unlikely to have a significant inhibitory effect on the growth of N. gonorrhoeae—at least in the agar overlay assays evaluated here. More importantly for our current research, we consider it unlikely that a broad range of N. mucosa isolates contained a sufficiently potent compound against our currently circulating strains of N. gonorrhoeae to be able to explain the findings of the PReGo and OMEGA studies. A further strength of this study is that we assessed if currently circulating isolates of commensal Neisseria were able to inhibit the growth of N. gonorrhoeae. The previous largest study by Aho et al. was limited to testing historical isolates from collections [11].
More recent studies by Kim et al. have found that Neisseria elongata is able to kill N. gonorrhoeae in vivo and in a mouse infection model [14,15]. The toxic compound was found to be differentially methylated DNA that was taken up by N. gonorrhoeae via transformation [15]. N. elongata is one of the less prevalent commensal Neisseria spp. In the PReGo and ComCom studies, for example, we only isolated N. elongata from one individual [16]. This isolate showed no evidence of inhibiting the growth of N. gonorrhoeae in the agar-overlay assay (Table S1). We cannot exclude the possibility that this isolate could exhibit an inhibitory effect on the growth of N. gonorrhoeae if assessed in more sensitive assays, such as those used by Kim et al. [15]. Commensal Neisseria may also inhibit the growth of N. gonorrhoeae via type 6 secretory systems. In a series of elegant experiments, Custodia et al. have established that a type 6 secretory system, in certain strains of N. cinerea, is able to reduce the survival of the gonococcus by five-fold [17,18]. Another recent study has found that N. cinerea forms microcolonies of epithelial cells in a way that impairs the colonization of the epithelium by N. meningitidis [19]. These effects can only be detected in experiments using cell models, which we did not do. Other studies of meningococcal colonization have illustrated how complex the interactions between pathogenic Neisseria and other bacterial species may be. Audry et al., for example, have established that the initial meningococcal colonization of the nasopharynx may not result in inflammation in the short term, as the bacteria may be trapped within the mucus lining [20]. During this entrapped state, the interaction with Streptococcus mitis but not Moraxella catarralis, triggered invasive disease via degradation of the encasing mucins. These findings illustrate that whilst the results of in vitro assessments of growth inhibition are important, great caution should be exercised in extrapolating these findings to what happens in vivo [21].
In conclusion, we concur with Aho et al., that commensal microbes represent a possible source of antimicrobial compounds that could play an important role in reducing the emergence of AMR in N. gonorrhoeae and other bacteria [11,22,23,24]. Based on our findings, we consider it more likely that such anti-gonococcal compounds will be discovered from organisms such as S. pneumoniae, rather than N. mucosa [23,25,26,27,28,29,30,31,32].
4. Materials and Methods
4.1. Origin of Bacterial Isolates
4.1.1. Inhibitory/Producer Bacterial Isolates
Most Neisseria isolates were obtained from two clinical studies conducted at our centre:
- (i)
- The Preventing Resistance in Gonorrhoea Study (PReGo), a single-centre randomized controlled trial conducted at the Institute of Tropical Medicine in Antwerp, Belgium, between 2019 and 2020, that assessed the efficacy of an antiseptic mouthwash to prevent STIs among 343 MSM using PrEP [2].
- (ii)
- The Commensals in the Community Study (ComCom), a survey of the oropharyngeal microbiomes of Institute of Tropical Medicine (ITM) employees conducted in June 2020 [16]. In both studies, oropharyngeal swabs (ESwabTM COPAN Diagnostics Inc., Brescia, Italy) were taken and inoculated onto blood and modified Thayer–Martin agar plates using the streak plate technique, and incubated at 35–37 °C and 5% CO2. Plates were examined after 48 h, and Neisseria-like colonies were selected based on a positive oxidase test and a Gram stain. Neisseria-like colonies were enriched on blood agar plates and stored in skim milk at −80 °C. Cultures of Neisseria-like colonies were shipped to Laboratoire des Hôpitaux Universitaires de Bruxelles-Universitair Laboratorium Brussel (LHUB-ULB), where species were identified using matrix-assisted laser desorption/ionization time-of-flight mass Spectrometry (MALDI-TOF MS), on a MALDI Biotyper® Sirius IVD system using the MBT Compass IVD software and library (Bruker Daltonics, Bremen, Germany) consisting of 9607 spectra.
All N. mucosa isolates
obtained from the PReGo and ComCom studies (n = 14), as well as a random selection
of N. meningitidis (n = 3) and other commensal Neisseria obtained from these two studies—N. subflava (n = 4), N. cinerea (n = 2), N. lactamica (n = 1), N. oralis (n = 3), N. longate (n = 1), and Neisseria spp. (n = 1; Table S1)—were included in the present work.
In addition, we also included 6 N. mucosa isolates from our ITM historical collection. Five of these were ATCC strains and one was a historical clinical specimen obtained from a patient in 1977, and the DSM4631/ATCC 25996 isolate used by Aho et al. was obtained from the DSMZ (https://www.dsmz.de/collection/catalogue/details/culture/DSM-46, accessed 2 October 2021).
4.1.2. N. gonorrhoeae Target Strains
Three strains of N. gonorrhoeae were used as target strains for all experiments (WHO-F, WHO-X and MoNg003—a clinical isolate obtained from an individual with asymptomatic pharyngeal N. gonorrhoeae infection attending our STI clinic in 2020). In addition, one ATCC strain of N. gonorrhoeae, WHO-W and 23 other circulating strains of N. gonorrhoeae were tested against some of the putative inhibitory bacteria (Table S1).
4.1.3. Non-Neisseria Isolates
Six ATCC strains of species previously shown to inhibit the growth of N. gonorrhoeae were included to serve as potential positive control for the agar overlay inhibition tests: Streptococcus pneumoniae (n = 1; ATCC 49619), Escherichia coli (n = 1; ATCC 25922), Staphylococcus aureus (n = 2; ATCC 29213, ATCC 25913), Streptococcus pyogenes (n = 1; LMG 14238) and Lactobacillus crispatus (n = 1; LMG 9479) [23,25,26,27,28,29,30,31,32] (available at https://www.atcc.org accessed 1 November 2021).
4.2. Agar Overlay Assay
The details of the agar overlay assay have been described elsewhere [11]. Briefly, all strains used in the experiment were propagated on Columbian blood agar plates for 18–24 h. The cultures were suspended in 10 µL of phosphate-buffered saline (PBS) containing 109 CFU/mL of inhibitory strains. These were spotted onto GC agar and incubated in 5% CO2 at 35–37 °C for 24 h. Then, 10 mL of melted GCB agar containing 106 CFU/mL of a target strain was added to each spotted plate. The plates were then re-incubated for 24 to 48 h. The diameter of the zone of inhibition surrounding each producer strain was assessed at 24 h.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sci4010008/s1, Table S1: Inhibitory activity of various commensal Neisseria and other species in agar overlay assay; Table S2: Clinical study, anatomical site, year of isolation and antimicrobial susceptibilities of isolates used in the study.
Author Contributions
Conceptualization, C.K., S.A., S.S.M.-B., I.D.B. and J.L.; methodology, S.A., N.G.; software, C.K.; validation, S.A., J.L.; formal analysis, S.A., C.K.; investigation, S.A., N.G., D.M., T.d.B.; data curation, C.V.D.; writing—original draft preparation, C.K.; writing—review and editing, C.K.; visualization, S.A., C.K.; supervision, C.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics approval was obtained from ITM’s Institutional Review Board (1276/18 and 1351/20) and from the Ethics Committee of the University of Antwerp (19/06/058 and AB/ac/003).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the relevant data generated during this study is provided in Table S1.
Acknowledgments
We would like to thank the PReGo and ComCom study participants for providing the samples used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Van Dijck, C.; Tsoumanis, A.; Rotsaert, A.; Vuylsteke, B.; Van den Bossche, D.; Paeleman, E.; De Baetselier, I.; Brosius, I.; Laumen, J.; Buyze, J. Antibacterial mouthwash to prevent sexually transmitted infections in men who have sex with men taking HIV pre-exposure prophylaxis (PReGo): A randomised, placebo-controlled, crossover trial. Lancet Infect. Dis. 2021, 21, 657–667. [Google Scholar] [CrossRef]
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouttier, S.; Yeo, A.; Any-Grah, A.A.S.A.; Geiger, S.; Huang, N.; Nicolas, V.; Villebrun, S.; Faye-Kette, H.; Ponchel, G.; Koffi, A.A.; et al. Characterization and in vitro evaluation of a vaginal gel containing Lactobacillus crispatus for the prevention of gonorrhea. Int. J. Pharm. 2020, 588, 119733. [Google Scholar] [CrossRef]
- Kenyon, C.; Laumen, J.; Manoharan-Basil, S. Choosing New Therapies for Gonorrhoea: We Need to Consider the Impact on the Pan-Neisseria Genome. A Viewpoint. Antibiotics 2021, 10, 515. [Google Scholar] [CrossRef]
- Chow, E.P.; Williamson, D.A.; Hocking, J.S.; Law, M.G.; Maddaford, K.; Bradshaw, C.S.; McNulty, A.; Templeton, D.J.; Moore, R.; Murray, G.L. Antiseptic mouthwash for gonorrhoea prevention (OMEGA): A randomised, double-blind, parallel-group, multicentre trial. Lancet Infect. Dis. 2021, 21, 647–656. [Google Scholar] [CrossRef]
- Van Dijck, C.; Cuylaerts, V.; Sollie, P.; Spychala, A.; De Baetselier, I.; Laumen, J.; Crucitti, T.; Kenyon, C. The development of mouthwashes without anti-gonococcal activity for controlled clinical trials: An in vitro study. F1000Research 2019, 8, 1620. [Google Scholar] [CrossRef]
- Tsoumanis, A.; Hens, N.; Kenyon, C.R. Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies. Sex. Transm. Dis. 2018, 45, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Barbee, L.A.; Khosropour, C.M.; Dombrowski, J.C.; Manhart, L.E.; Golden, M.R. An estimate of the proportion of symptomatic gonococcal, chlamydial and non-gonococcal non-chlamydial urethritis attributable to oral sex among men who have sex with men: A case-control study. Sex. Transm. Infect. 2016, 92, 155–160. [Google Scholar] [CrossRef]
- Chow, E.P.; Howden, B.P.; Walker, S.; Lee, D.; Bradshaw, C.S.; Chen, M.Y.; Snow, A.; Cook, S.; Fehler, G.; Fairley, C.K. Antiseptic mouthwash against pharyngeal Neisseria gonorrhoeae: A randomised controlled trial and an in vitro study. Sex. Transm. Infect. 2017, 93, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Aho, E.L.; Ogle, J.M.; Finck, A.M. The Human Microbiome as a Focus of Antibiotic Discovery: Neisseria mucosa Displays Activity Against Neisseria gonorrhoeae. Front. Microbiol. 2020, 11, 577762. [Google Scholar] [CrossRef] [PubMed]
- Deasy, A.M.; Guccione, E.; Dale, A.P.; Andrews, N.; Evans, C.M.; Bennett, J.S.; Bratcher, H.B.; Maiden, M.C.; Gorringe, A.R.; Read, R.C. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin. Infect. Dis. 2015, 60, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Kandi, V. Bacterial Colony Characters: Pitting Colonies. J. Med. Microbiol. Diagn. 2015, 4, 1000I102. [Google Scholar] [CrossRef] [Green Version]
- So, M.; Rendon, M.A. Tribal warfare: Commensal Neisseria kill pathogen Neisseria gonorrhoeae using its DNA. Microb. Cell 2019, 6, 544–546. [Google Scholar] [CrossRef]
- Kim, W.J.; Higashi, D.; Goytia, M.; Rendon, M.A.; Pilligua-Lucas, M.; Bronnimann, M.; McLean, J.A.; Duncan, J.; Trees, D.; Jerse, A.E.; et al. Commensal Neisseria Kill Neisseria gonorrhoeae through a DNA-Dependent Mechanism. Cell Host Microbe 2019, 26, 228–239.e8. [Google Scholar] [CrossRef]
- Laumen, J.G.E.; Manoharan-Basil, S.S.; Abdellati, S.; De Baetselier, I.; Van Dijck, C.; Martiny, D.; Serrano, G.; Bottieau, E.; Kenyon, C. Antimicrobial susceptibility of commensal Neisseria in the general population and men who have sex with men in Belgium. Sci. Rep. 2022, 12, 9. [Google Scholar] [CrossRef]
- Custodio, R.; Ford, R.M.; Ellison, C.J.; Liu, G.; Mickute, G.; Tang, C.M.; Exley, R.M. Type VI secretion system killing by commensal Neisseria is influenced by expression of type four pili. eLife 2021, 10, e63755. [Google Scholar] [CrossRef]
- Custodio, R.; Ford, R.M.; Ellison, C.J.; Liu, G.; Mickute, G.; Tang, C.M.; Exley, R.M. Type VI secretion system killing by commensal Neisseria is influenced by the spatial dynamics of bacteria. bioRxiv 2020. [Google Scholar] [CrossRef]
- Custodio, R.; Johnson, E.; Liu, G.; Tang, C.M.; Exley, R.M. Commensal Neisseria cinerea impairs Neisseria meningitidis microcolony development and reduces pathogen colonisation of epithelial cells. PLoS Pathog. 2020, 16, e1008372. [Google Scholar] [CrossRef]
- Audry, M.; Robbe-Masselot, C.; Barnier, J.P.; Gachet, B.; Saubamea, B.; Schmitt, A.; Schonherr-Hellec, S.; Leonard, R.; Nassif, X.; Coureuil, M. Airway Mucus Restricts Neisseria meningitidis Away from Nasopharyngeal Epithelial Cells and Protects the Mucosa from Inflammation. mSphere 2019, 4, e00494-19. [Google Scholar] [CrossRef] [Green Version]
- Kahler, C.M. Neisseria species and their complicated relationships with human health. Microbiol. Aust. 2021, 42, 79–83. [Google Scholar] [CrossRef]
- De Block, T.; Laumen, J.G.E.; Van Dijck, C.; Abdellati, S.; De Baetselier, I.; Manoharan-Basil, S.S.; Van den Bossche, D.; Kenyon, C. WGS of Commensal Neisseria Reveals Acquisition of a New Ribosomal Protection Protein (MsrD) as a Possible Explanation for High Level Azithromycin Resistance in Belgium. Pathogens 2021, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-Y.; Alcorn, T.M.; Cohen, M.S. Effects of H2O2-Producing Lactobacilli on Neisseria gonorrhoeae Growth and Activity. J. Infect. Dis. 1994, 170, 1209–1215. [Google Scholar] [CrossRef]
- Lin, E.Y.; Adamson, P.C.; Klausner, J.D. Epidemiology, Treatments, and Vaccine Development for Antimicrobial-Resistant Neisseria gonorrhoeae: Current Strategies and Future Directions. Drugs 2021, 81, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Lux, T.; Nuhn, M.; Hakenbeck, R.; Reichmann, P. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J. Bacteriol. 2007, 189, 7741–7751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, M.E.; Duncan, W.C.; Knox, J.M. Bacterial interference of Neisseria gonorrhoeae by alpha-haemolytic streptococci. Sex. Transm. Infect. 1980, 56, 235–238. [Google Scholar] [CrossRef] [Green Version]
- Kraus, S.J.; Geller, R.C.; Perkins, G.H.; Rhoden, D.L. Interference by Neisseria gonorrhoeae growth by other bacterial species. J. Clin. Microbiol. 1976, 4, 288–295. [Google Scholar] [CrossRef]
- Bisaillon, J.G.; Beaudet, R.; Saheb, S.A.; Morisset, R. Interference of Neisseria gonorrhoeae growth by aerobic bacterial representatives of the urogenital flora. Rev. Can. Biol. 1980, 39, 201–208. [Google Scholar]
- Bisaillon, J.G.; Beaudet, R.; Lafond, L.; Saheb, S.A.; Sylvestre, M. Antigonococcal and antibacterial spectra of some bacterial isolates of the urogenital flora. Rev. Can. Biol. 1981, 40, 215–227. [Google Scholar]
- Kaye, D.; Levison, M. In vitro inhibition of growth of Neisseria gonorrhoeae by genital microorganisms. Sex. Transm. Dis. 1977, 4, 1–3. [Google Scholar] [CrossRef]
- Saigh, J.H.; Sanders, C.C.; Sanders, W.E., Jr. Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: Evidence for a protective effect against infection. Infect. Immun. 1978, 19, 704–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, A.; Saheb, S.; Bisaillon, J.; Beaudet, R.; Sylvestre, M. In vitro inhibition of Neisseria gonorrhoeae growth by strict anaerobes. Infect. Immun. 1980, 28, 766–770. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).