Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
Diagnosis of MS Disorder
2. Innate Immunity in MS
2.1. Role of TLRs in MS
2.2. NOD-Like Receptors in MS
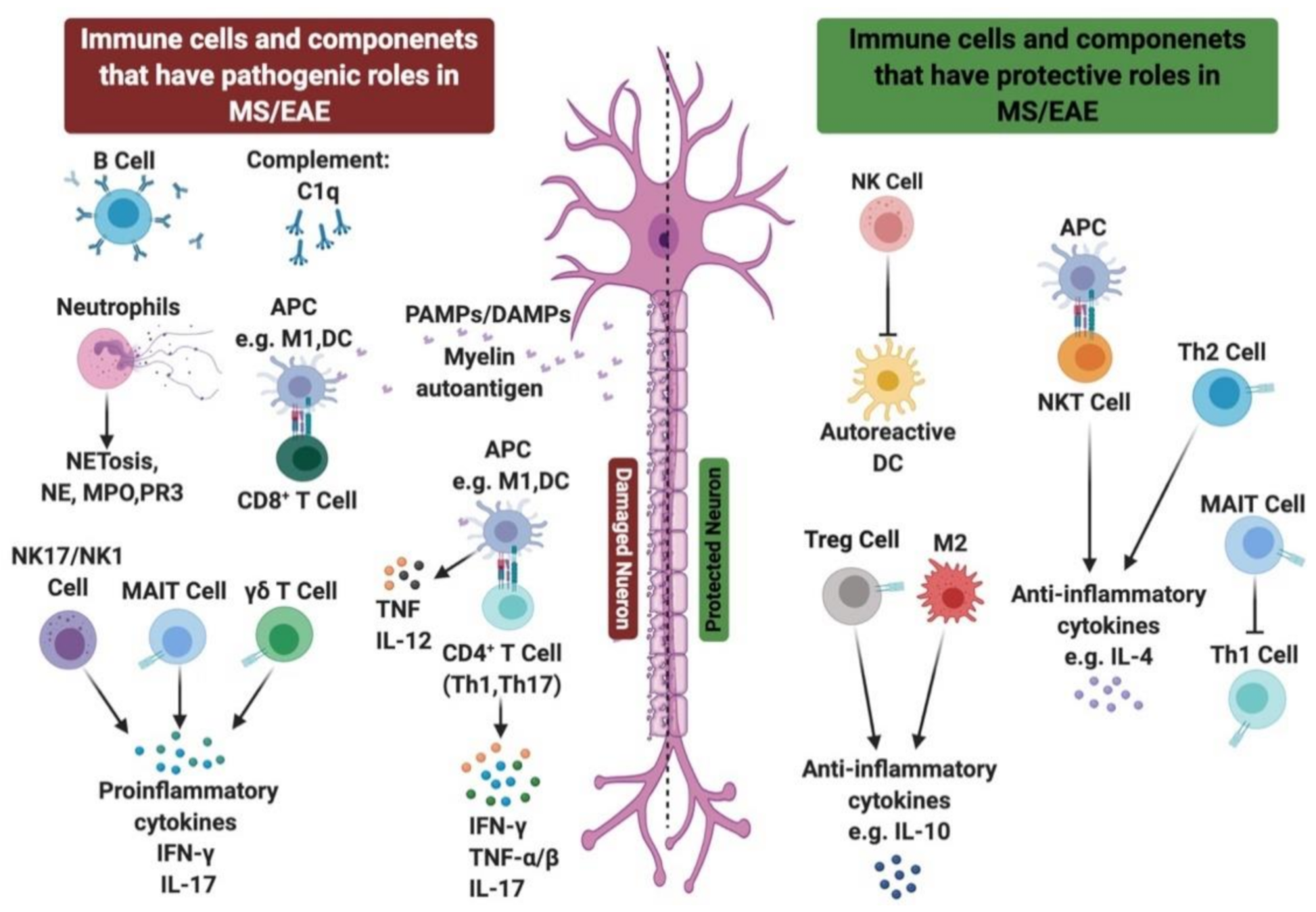
2.3. Role of Innate Immune Cells in MS Disease
2.3.1. Role of Neutrophils in MS/EAE
2.3.2. Role of NK Cells in MS/EAE
2.3.3. Role of NKT Cells in MS/EAE
2.3.4. Role of γδ T Cells in MS/EAE
2.3.5. Role of MAIT Cells in MS/EAE
2.4. Role of Complement System in MS
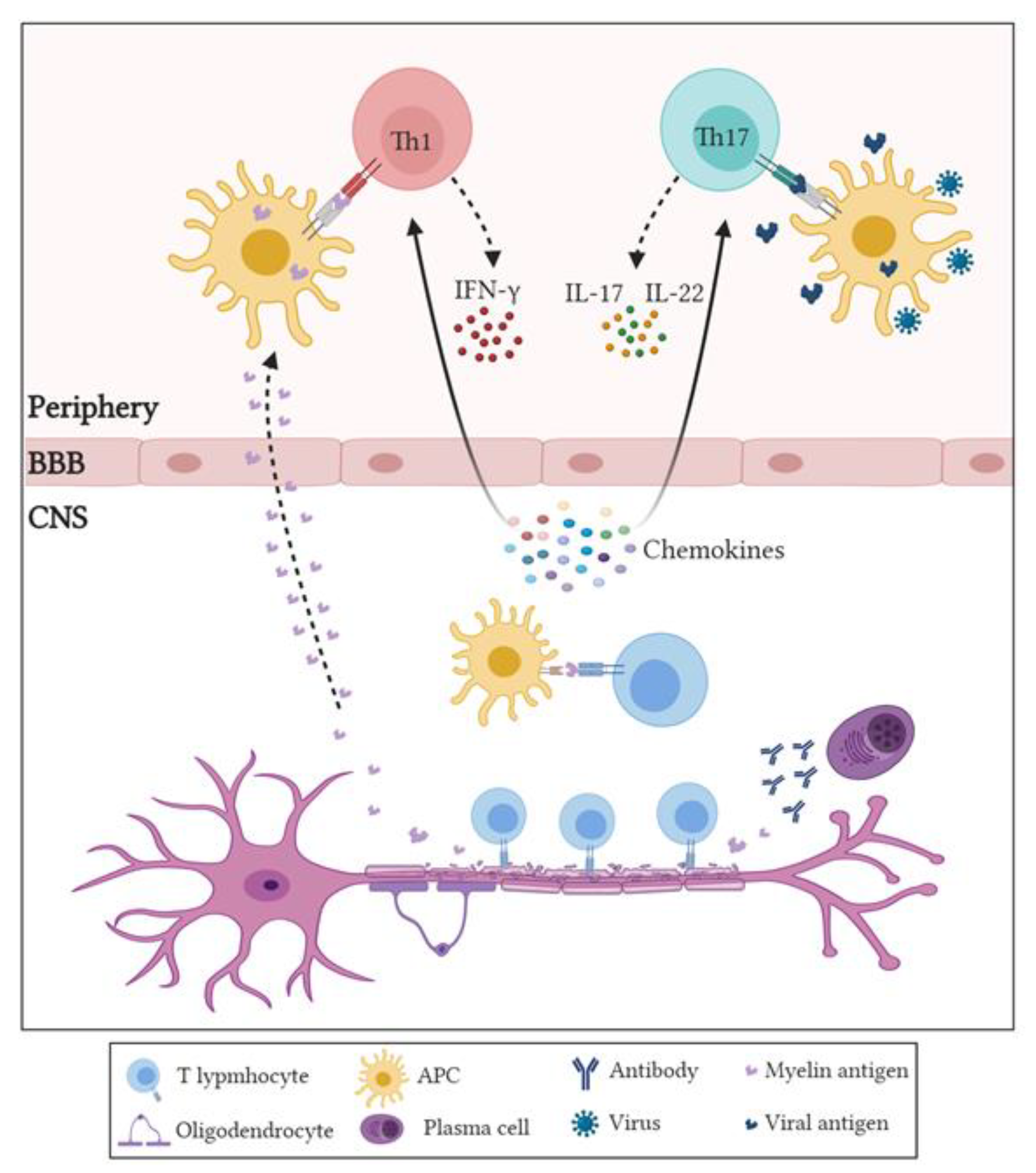
3. Role of Adaptive Immune Cells in MS
3.1. Role of CD4+ T Cells in MS and EAE
3.2. Role of CD8+ Cells in MS
3.3. Role of Regulatory T Cells (Tregs) in MS or EAE
3.4. Role of B Cells in MS/EAE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AIM2 | Absent in melanoma 2 |
| APCs | Antigen-presenting cells |
| ALLO | Allopregnanolone |
| ASC | Apoptosis-associated speck-like protein |
| BBB | Blood–brain barrier |
| CIS | Clinical isolated syndrome |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DAMPs | Danger-associated molecular patterns |
| DCs | Dendritic cells |
| DMF | Dimethyl fumarate |
| EAE | Experimental autoimmune encephalitis |
| G-CSF | Granulocyte-colony stimulating factor |
| GA | Glatiramer acetate |
| GABA-A | Gamma aminobutyric acid-A |
| GalCer | Galactosylceramide |
| GWAS | Genome-wide association studies |
| HLA | Human leukocyte antigen |
| IFN | Interferon |
| IL- | Interleukin |
| Ig | Immunoglobulin |
| LFA-1 | Lymphocyte function-associated antigen 1 |
| MAIT | Mucosal-associated invariant T |
| MBP | Myelin basic protein |
| MHC | Major histocompatibility complex |
| MMF | Monomethyl fumarate |
| MOG | Myelin oligodendrocyte glycoprotein |
| MPO | Myeloperoxidase |
| MR1 | MHC-related molecule 1 |
| MRI | Magnetic resonance imaging |
| MS | Multiple sclerosis |
| NETs | Neutrophils’ extracellular traps |
| NK | Natural killer |
| NKT | Natural killer T |
| NLRs | NOD-like receptors |
| NOD | Nucleotide binding oligomerization domain |
| PAMPs | Pathogen-associated molecular patterns |
| PPMS | Primary progressive multiple sclerosis |
| PRMS | Progressive relapsing multiple sclerosis |
| PRRs | Pattern recognition receptors |
| ROS | Reactive oxygen species |
| RRMS | Relapsing remitting multiple sclerosis |
| SPMS | Secondary progressive multiple sclerosis |
| TCR | T cell receptor |
| Th | T helper |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| Tregs | Regulatory T cells |
| VLA-4 | Very late antigen-4. |
References
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Popescu, B.F.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis. Continuum 2013, 19, 901–921. [Google Scholar] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Chard, D.T.; Ciccarelli, O. Clinically isolated syndromes. Lancet Neurol. 2012, 11, 157–169. [Google Scholar] [CrossRef]
- Gourraud, P.; Harbo, H.F.; Hauser, S.L.; Baranzini, S.E. The genetics of multiple sclerosis: An up-to-date review. Immunol. Rev. 2012, 248, 87–103. [Google Scholar] [CrossRef]
- Holmøy, T.; Hestvik, A.L. Multiple sclerosis: Immunopathogenesis and controversies in defining the cause. Curr. Opin. Infect. Dis. 2008, 21, 271–278. [Google Scholar] [CrossRef]
- Egg, R.; Reindl, M.; Deisenhammer, F.; Linington, C.; Berger, T. Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult. Scler. 2001, 7, 285–289. [Google Scholar] [CrossRef]
- Robinson, A.P.; Harp, C.T.; Noronha, A.; Miller, S.D. The experimental autoimmune encephalomyelitis (EAE) model of MS: Utility for understanding disease pathophysiology and treatment. Handb. Clin. Neurol. 2014, 122, 173–189. [Google Scholar]
- Giralt, M.; Molinero, A.; Hidalgo, J. Active induction of experimental autoimmune encephalomyelitis (EAE) with MOG35-55 in the mouse. Methods Mol. Biol. 2018, 1791, 227–232. [Google Scholar]
- Farooqi, N.; Gran, B.; Constantinescu, C.S. Are current disease-modifying therapeutics in multiple sclerosis justified on the basis of studies in experimental autoimmune encephalomyelitis? J. Neurochem. 2010, 115, 829–844. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Sandrock, A.W.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Yednock, T.A.; Cannon, C.; Fritz, L.C.; Sanchez-Madrid, F.; Steinman, L.; Karin, N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992, 356, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.C.; Sloboda, A.E.; McReynolds, R.A.; Levine, S.; Oronsky, A.L.; Kerwar, S.S. Suppression of experimental allergic encephalomyelitis by mitoxantrone. J. Clin. Immunol. Immunopathol. Res. 1985, 35, 35–42. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple sclerosis: Pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2016, 19, 1–10. [Google Scholar]
- Calabresi, P.A. Diagnosis and management of multiple sclerosis. Am. Fam. Physician 2004, 70, 1935–1944. [Google Scholar]
- Olson, J.K.; Miller, S.D. The innate immune response affects the development of the autoimmune response in Theiler’s virus-induced demyelinating disease. J. Immunol. 2009, 182, 5712–5722. [Google Scholar] [CrossRef]
- Tosi, M.F. Innate immune responses to infection. J. Allergy Clin. Immunol. 2005, 116, 241–250. [Google Scholar] [CrossRef]
- Deerhake, M.E.; Biswas, D.D.; Barclay, W.E.; Shinohara, M.L. Pattern recognition receptors in multiple sclerosis and its animal models. Front. Immunol. 2019, 10, 2644. [Google Scholar] [CrossRef]
- Pone, E.J.; Zan, H.; Zhang, J.; Al-Qahtani, A.; Xu, Z.; Casali, P. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: Relevance to microbial antibody responses. Crit. Rev. Immunol. 2010, 30, 1–29. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Z.; Jiang, M.; Zhang, E.; Trippler, M.; Broering, R.; Bucchi, A.; Krux, F.; Dittmer, U.; Yang, D.; et al. Toll-like receptor-induced innate immune responses in non-parenchymal liver cells are cell type-specific. Immunology 2010, 129, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Mosser, D.M. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013, 37, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Lai, W.; Rivest, S.; Hart, R.P.; Satoskar, A.R.; Popovich, P.G. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007, 102, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sloane, J.A.; Batt, C.; Ma, Y.; Harris, Z.; Trapp, B.; Vartanian, T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl. Acad. Sci. USA 2010, 107, 11555–11560. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, K.H.; Sloane, J.A. Regulation of remyelination in multiple sclerosis. FEBS Lett. 2011, 585, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Tuohy, T.M.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Chu, F.; Zhu, J.; Jin, T. Inflammatory role of TLR-Myd88 signaling in multiple sclerosis. Front. Mol. Neurosci. 2020, 12, 314. [Google Scholar] [CrossRef]
- Marta, M. Toll-like receptors in multiple sclerosis mouse experimental models. Ann. N. Y. Acad. Sci. 2009, 1173, 458–462. [Google Scholar] [CrossRef]
- Touil, T.; Fitzgerald, D.; Zhang, G.; Rostami, A.; Gran, B. Cutting Edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β. J. Immunol. 2006, 177, 7505–7509. [Google Scholar] [CrossRef]
- Giacomini, E.; Severa, M.; Rizzo, F.; Mechelli, R.; Annibali, V.; Ristori, G.; Riccieri, V.; Salvetti, M.; Coccia, E.M. IFN-β therapy modulates B-cell and monocyte crosstalk via TLR7 in multiple sclerosis patients. Eur. J. Immunol. 2013, 43, 1963–1972. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, J.; Tang, Y.; Speer, D.; Sujkowska, D.; Markovic-Plese, S. IFN-β1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J. Immunol. 2009, 182, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shin, J.; Nahm, M.H. NOD-like receptors in infection, immunity, and diseases. Yonsei Med. J. 2016, 57, 5. [Google Scholar] [CrossRef] [PubMed]
- Hachim, M.Y.; Khalil, B.A.; Elemam, N.M.; Maghazachi, A.A. Pyroptosis: The missing puzzle among innate and adaptive immunity crosstalk. J. Leukoc. Biol. 2020, 108, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Hernández-Pedro, N.Y.; Espinosa-Ramirez, G.; de la Cruz, V.; Pineda, B.; Sotelo, J. Initial immunopathogenesis of multiple sclerosis: Innate immune response. Clin. Dev. Immunol. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Muhammad, J.S.; Jayakumar, M.N.; Elemam, N.M.; Venkatachalam, T.; Raju, T.K.; Hamoudi, R.A.; Maghazachi, A.A. Gasdermin D hypermethylation inhibits pyroptosis and LPS-induced IL-1β release from NK92 cells. ImmunoTargets Ther. 2019, 8, 29–41. [Google Scholar] [CrossRef]
- Yap, J.K.; Pickard, B.S.; Chan, E.W.; Gan, S. The role of neuronal NLRP1 inflammasome in Alzheimer’s disease: Bringing neurons into the neuroinflammation game. Mol. Neurobiol. 2019, 56, 7741–7753. [Google Scholar] [CrossRef]
- Tan, M.; Yu, J.; Jiang, T.; Zhu, X.; Tan, L. The NLRP3 inflammasome in alzheimer’s disease. Mol. Neurobiol. 2013, 48, 875–882. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; He, X.; Yu, H.; Feng, J. Ghrelin attenuates neuroinflammation and demyelination in experimental autoimmune encephalomyelitis involving NLRP3 inflammasome signaling pathway and pyroptosis. Front. Pharmacol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.; Quintana, F.J.; Weiner, H.L. The innate immune system in demyelinating disease. Immunol. Rev. 2012, 248, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Vereyken, E.J.; Glim, J.E.; Heijnen, P.D.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, C.E.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 809. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Vinet, J.; Brouwer, N.; Brendecke, S.; Biagini, G.; Biber, K.; Boddeke, H.W.; Eggen, B.J. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia 2014, 62, 1724–1735. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Shi, M.; Zheng, C.; Shen, D.; Zhu, J.; Zheng, X.; Cui, L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018, 318, 1–7. [Google Scholar] [CrossRef]
- Karni, A.; Abraham, M.; Monsonego, A.; Cai, G.; Freeman, G.J.; Hafler, D.; Khoury, S.J.; Weiner, H.L. Innate immunity in multiple sclerosis: Myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 2006, 177, 4196–4202. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Baker, G.B.; Power, C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front. Cell Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef]
- Reyes-García, M.G.; Hernández-Hernández, F.; Hernández-Téllez, B.; García-Tamayo, F. GABA receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J. Neuroimmunol. 2007, 188, 64–68. [Google Scholar] [CrossRef]
- Bhat, R.; Axtell, R.; Mitra, A.; Miranda, M.; Lock, C.; Tsien, R.W.; Steinman, L. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 2580–2585. [Google Scholar] [CrossRef]
- Legroux, L.; Arbour, N. Multiple sclerosis and T lymphocytes: An entangled story. J. Neuroimmune Pharmacol. 2015, 10, 528–546. [Google Scholar] [CrossRef]
- Rumble, J.M.; Huber, A.K.; Krishnamoorthy, G.; Srinivasan, A.; Giles, D.A.; Zhang, X.; Wang, L.; Segal, B.M. Neutrophil-related factors as biomarkers in EAE and MS. J. Exp. Med. 2015, 212, 23–35. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Sun, E. Neutrophil extracellular traps in autoimmune diseases. Chin. Med. J. 2018, 131, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Dittel, B.N. Inflammatory disease severity is ameliorated by inhibition of neutrophil-derived MPO that supports endothelial/epithelial integrity. J. Immunol. 2017, 198, 127. [Google Scholar]
- Yu, G.; Zheng, S.; Zhang, H. Inhibition of myeloperoxidase by N-acetyl lysyltyrosylcysteine amide reduces experimental autoimmune encephalomyelitis-induced injury and promotes oligodendrocyte regeneration and neurogenesis in a murine model of progressive multiple sclerosis. NeuroReport 2018, 29, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ray, A.; Miller, N.M.; Hartwig, D.; Pritchard, K.A.; Dittel, B.N. Inhibition of myeloperoxidase at the peak of experimental autoimmune encephalomyelitis restores blood-brain barrier integrity and ameliorates disease severity. J. Neurochem. 2015, 136, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Herges, K.; de Jong, B.A.; Kolkowitz, I.; Dunn, C.; Mandelbaum, G.; Ko, R.M.; Maini, A.; Han, M.H.; Killestein, J.; Polman, C.; et al. Protective effect of an elastase inhibitor in a neuromyelitis optica-like disease driven by a peptide of myelin oligodendroglial glycoprotein. Mult. Scler. 2012, 18, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Minohara, M.; Matsuoka, T.; Li, W.; Osoegawa, M.; Ishizu, T.; Ohyagi, Y.; Kira, J. Upregulation of myeloperoxidase in patients with opticospinal multiple sclerosis: Positive correlation with disease severity. J. Neuroimmunol. 2006, 178, 156–160. [Google Scholar] [CrossRef]
- Maghazachi, A.A. Compartmentalization of human natural killer cells. Mol. Immunol. 2005, 42, 523–529. [Google Scholar] [CrossRef]
- Glimcher, L.; Shen, F.W.; Cantor, H. Identification of a cell-surface antigen selectively expressed on the natural killer cell. J. Exp. Med. 1977, 145, 1–9. [Google Scholar] [CrossRef]
- Maghazachi, A.A. Role of natural killer cells in multiple sclerosis. ISRN Immunol. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Høglund, R.A.; Maghazachi, A.A. Multiple sclerosis and the role of immune cells. World J. Exp. Med. 2014, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Al-Jaderi, Z.; Høglund, R.A.; Holmøy, T.; Harbo, H.F.; Norgauer, J.; Maghazachi, A.A. Identification of human NK17/NK1 cells. PLoS ONE 2011, 6, e26780. [Google Scholar] [CrossRef] [PubMed]
- Sand, K.L.; Knudsen, E.; Rolin, J.; Al-Falahi, Y.; Maghazachi, A.A. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell. Mol. Life Sci. 2009, 66, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaderi, Z.; Maghazachi, A.A. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins 2013, 5, 1932–1947. [Google Scholar] [CrossRef] [PubMed]
- Maghazachi, A.A.; Sand, K.L.; Al-Jaderi, Z. Glatiramer acetate, dimethyl fumarate, and monomethyl fumarate upregulate the expression of CCR10 on the surface of natural killer cells and enhance their chemotaxis and cytotoxicity. Front. Immunol. 2016, 7, 437. [Google Scholar] [CrossRef] [PubMed]
- Vego, H.; Sand, K.L.; Høglund, R.A.; Fallang, L.; Gundersen, G.; Holmøy, T.; Maghazachi, A.A. Monomethyl fumarate augments NK cell lysis of tumor cells through degranulation and the upregulation of Nkp46 and CD107a. Cell. Mol. Immunol. 2016, 13, 57–64. [Google Scholar] [CrossRef]
- Al-Jaderi, Z.; Maghazachi, A.A. Vitamin D3 and monomethyl fumarate enhance natural killer cell lysis of dendritic cells and ameliorate the clinical score in mice suffering from experimental autoimmune encephalomyelitis. Toxins 2015, 7, 4730–4744. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Høglund, R.A.; Holmøy, T.; Harbo, H.F.; Maghazachi, A.A. A one year follow-up study of natural killer and dendritic cells activities in multiple sclerosis patients receiving glatiramer acetate (GA). PLoS ONE 2013, 8, e62237. [Google Scholar]
- Balato, A.; Unutmaz, D.; Gaspari, A.A. Natural killer T cells: An unconventional T-cell subset with diverse effector and regulatory functions. J. Investig. Dermatol. 2009, 129, 1628–1642. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wan, Q. NKT cells in neurological diseases. Front. Cell. Neurosci. 2019, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Latha, T.S.; Reddy, M.C.; Durbaka, P.V.; Rachamallu, A.; Pallu, R.; Lomada, D. γδ T cell-mediated immune responses in disease and therapy. Front. Immunol. 2014, 5, 571. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.J.; Gao, Y.L.; Raine, C.S.; Brosnan, C.F. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J. Immunol. 1996, 157, 941–949. [Google Scholar] [PubMed]
- Spahn, T.W.; Issazadah, S.; Salvin, A.J.; Weiner, H.L. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33–35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR δ chain gene. Eur. J. Immunol. 1999, 29, 4060–4071. [Google Scholar] [CrossRef]
- Treiner, E.; Liblau, R.S. Mucosal-associated invariant T cells in multiple sclerosis: The jury is still out. Front. Immunol. 2015, 6, 503. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Guerri, L.; Dusseaux, M.; Martin, E.; Soudais, C.; Lantz, O. Mucosal-associated invariant T cells: Unconventional development and function. Trends Immunol. 2011, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Miyake, S.; Chiba, A.; Lantz, O.; Yamamura, T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int. Immunol. 2011, 23, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Barnum, S.R. Complement biosynthesis in the central nervous system. Crit. Rev. Oral Biol. Med. 1995, 6, 132–146. [Google Scholar] [CrossRef]
- Wren, D.R.; Noble, M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in absence of antibody. Proc. Natl. Acad. Sci. USA 1989, 86, 9025–9029. [Google Scholar] [CrossRef]
- Johns, T.G.; Bernard, C.C. Binding of complement component Clq to myelin oligodendrocyte glycoprotein: A novel mechanism for regulating CNS inflammation. Mol. Immunol. 1997, 34, 33–38. [Google Scholar] [CrossRef]
- Bhat, R.; Steinman, L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron 2009, 64, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; Oldstone, M.B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: Mechanism for autoimmunity. Science 1985, 230, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Chastain, E.M.; Terry, R.L.; Miller, S.D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev. 2013, 255, 197–209. [Google Scholar] [CrossRef]
- Keller, C.W.; Sina, C.; Kotur, M.B.; Ramelli, G.; Mundt, S.; Quast, I.; Ligeon, L.; Weber, P.; Becher, B.; Münz, C.; et al. ATG-dependent phagocytosis in dendritic cells drives myelin-specific CD4+ T cell pathogenicity during CNS inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, e11228–e11237. [Google Scholar] [CrossRef]
- Lovett-Racke, A.E.; Yang, Y.; Racke, M.K. Th1 versus Th17: Are T cell cytokines relevant in multiple sclerosis? Biochim. Biophys. Acta 2011, 1812, 246–251. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef]
- Panitch, H.S.; Hirsch, R.L.; Haley, A.S.; Johnson, K.P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1987, 1, 893–895. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Segal, B.M. The diversity of encephalitogenic CD4+ T cells in multiple sclerosis and its animal models. J. Clin. Med. 2019, 8, 120. [Google Scholar] [CrossRef]
- Salou, M.; Nicol, B.; Garcia, A.; Laplaud, D.A. Involvement of CD8+ T cells in multiple sclerosis. Front. Immunol. 2015, 6, 604. [Google Scholar] [CrossRef]
- Babbe, H.; Roers, A.; Waisman, A.; Lassmann, H.; Goebels, N.; Hohlfeld, R.; Friese, M.; Schröder, R.; Deckert, M.; Schmidt, S.; et al. Clonal expansions of Cd8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 2000, 192, 393–404. [Google Scholar] [CrossRef]
- Chevalier, G.; Suberbielle, E.; Monnet, C.; Duplan, V.; Martin-Blondel, G.; Farrugia, F.; Le Masson, G.; Liblau, R.; Gonzalez-Dunia, D. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog 2011, 7, e1002393. [Google Scholar] [CrossRef]
- Booss, J.; Esiri, M.M.; Tourtellotte, W.W.; Mason, D.Y. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J. Neurol Sci. 1983, 62, 219–232. [Google Scholar] [CrossRef]
- Lucchinetti, C.F.; Popescu, B.F.; Bunyan, R.F.; Moll, N.M.; Roemer, S.F.; Lassmann, H.; Brück, W.; Parisi, J.E.; Scheithauer, B.W.; Weigand, S.D.; et al. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 2011, 365, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Battistini, L.; Piccio, L.; Rossi, B.; Bach, S.; Galgani, S.; Gasperini, C.; Ottoboni, L.; Ciabini, D.; Caramia, M.D.; Bernardi, G.; et al. CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: A critical role for P-selectin glycoprotein ligand-1. Blood 2003, 101, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Whitaker, J.N.; Huang, Z.; Liu, D.; Coleclough, C.; Wekerle, H.; Raine, C.S. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J. Immunol. 2001, 166, 7579–7587. [Google Scholar]
- Najafian, N.; Chitnis, T.; Salama, A.D.; Zhu, B.; Benou, C.; Yuan, X.; Clarkson, M.R.; Sayegh, M.H.; Khoury, S.J. Regulatory functions of CD8+CD28-T cells in an autoimmune disease model. J. Clin. Investig. 2003, 112, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- York, N.R.; Mendoza, J.P.; Ortega, S.B.; Benagh, A.; Tyler, A.F.; Firan, M.; Karandikar, N.J. Immune regulatory CNS-reactive CD8+T cells in experimental autoimmune encephalomyelitis. J. Autoimmun. 2010, 35, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Boyden, A.W.; Itani, F.R.; Crawford, M.P.; Karandikar, N.J. CD8(+) T-cells as immune regulators of multiple sclerosis. Front. Immunol. 2015, 6, 619. [Google Scholar] [CrossRef]
- He, F.; Balling, R. The role of regulatory T cells in neurodegenerative diseases. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 153–180. [Google Scholar] [CrossRef]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J. Neuroinflamm. 2017, 14, 117. [Google Scholar] [CrossRef]
- Kipnis, J.; Avidan, H.; Caspi, R.R.; Schwartz, M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: A dialogue with microglia. Proc. Natl. Acad. Sci. USA 2004, 101, 14663–14669. [Google Scholar] [CrossRef]
- Walsh, J.T.; Kipnis, J. Regulatory T cells in CNS injury: The simple, the complex and the confused. Trends Mol. Med. 2011, 17, 541–547. [Google Scholar] [CrossRef]
- Calvo-Barreiro, L.; Eixarch, H.; Montalban, X.; Espejo, C. Combined therapies to treat complex diseases: The role of the gut microbiota in multiple sclerosis. Autoimmun. Rev. 2018, 17, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R. Multiple sclerosis, melatonin, and neurobehavioral diseases. Front. Endocrinol. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.R.; Carmack, C.E.; Shinton, S.A.; Kemp, J.D.; Hayakawa, K. Resolution and characterization of pro-B and pre–pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 1991, 173, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Touil, H.; Pikor, N.B.; Gommerman, J.L.; Prat, A.; Bar-Or, A. B cells in the multiple sclerosis central nervous system: Trafficking and contribution to CNS-compartmentalized inflammation. Front. Immunol. 2015, 6, 636. [Google Scholar] [CrossRef]
- Al-ani, M.R.; Raju, T.K.; Hachim, M.Y.; Hachim, I.Y.; Elemam, N.M.; Guimei, M.; Bendardaf, R.; Maghazachi, A.A. Rituximab prevents the development of experimental autoimmune encephalomyelitis (EAE): Comparison with prophylactic, therapeutic or combinational regimens. J. Inflamm. Res. 2020, 13, 151–164. [Google Scholar] [CrossRef]
- Lin, M.; Wang, Z.; Han, X. B cells with regulatory function in animal models of autoimmune and non-autoimmune diseases. Open J. Immunol. 2015, 5, 9–17. [Google Scholar] [CrossRef][Green Version]
- Kowarik, M.C.; Cepok, S.; Sellner, J.; Grummel, V.; Weber, M.S.; Korn, T.; Berthele, A.; Hemmer, B. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflamm. 2012, 9, 93. [Google Scholar] [CrossRef]
- Häusser-Kinzel, S.; Weber, M.S. The role of B Cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front. Immunol. 2019, 10, 201. [Google Scholar] [CrossRef]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef]
- Pikor, N.B.; Prat, A.; Bar-Or, A.; Gommerman, J.L. Meningeal tertiary lymphoid tissues and multiple sclerosis: A gathering place for diverse types of immune cells during CNS autoimmunity. Front. Immunol. 2016, 6, 657. [Google Scholar] [CrossRef] [PubMed]
- Negron, A.; Robinson, R.R.; Stüve, O.; Forsthuber, T.G. The role of B cells in multiple sclerosis: Current and future therapies. Cell Immunol. 2019, 339, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.D.; Dittel, B.N.; Hardardottir, F.; Janeway, C.A., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996, 184, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaiban, S.; Al-Ani, M.; Elemam, N.M.; Al-Aawad, M.H.; Al-Rawi, Z.; Maghazachi, A.A. Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci 2021, 3, 12. https://doi.org/10.3390/sci3010012
Dhaiban S, Al-Ani M, Elemam NM, Al-Aawad MH, Al-Rawi Z, Maghazachi AA. Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci. 2021; 3(1):12. https://doi.org/10.3390/sci3010012
Chicago/Turabian StyleDhaiban, Sarah, Mena Al-Ani, Noha Mousaad Elemam, Mahmood H. Al-Aawad, Zeinab Al-Rawi, and Azzam A. Maghazachi. 2021. "Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis" Sci 3, no. 1: 12. https://doi.org/10.3390/sci3010012
APA StyleDhaiban, S., Al-Ani, M., Elemam, N. M., Al-Aawad, M. H., Al-Rawi, Z., & Maghazachi, A. A. (2021). Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci, 3(1), 12. https://doi.org/10.3390/sci3010012






