Process Controls of the Live Root Zone and Carbon Sequestration Capacity of the Sundarbans Mangrove Forest, Bangladesh
Abstract
1. Introduction
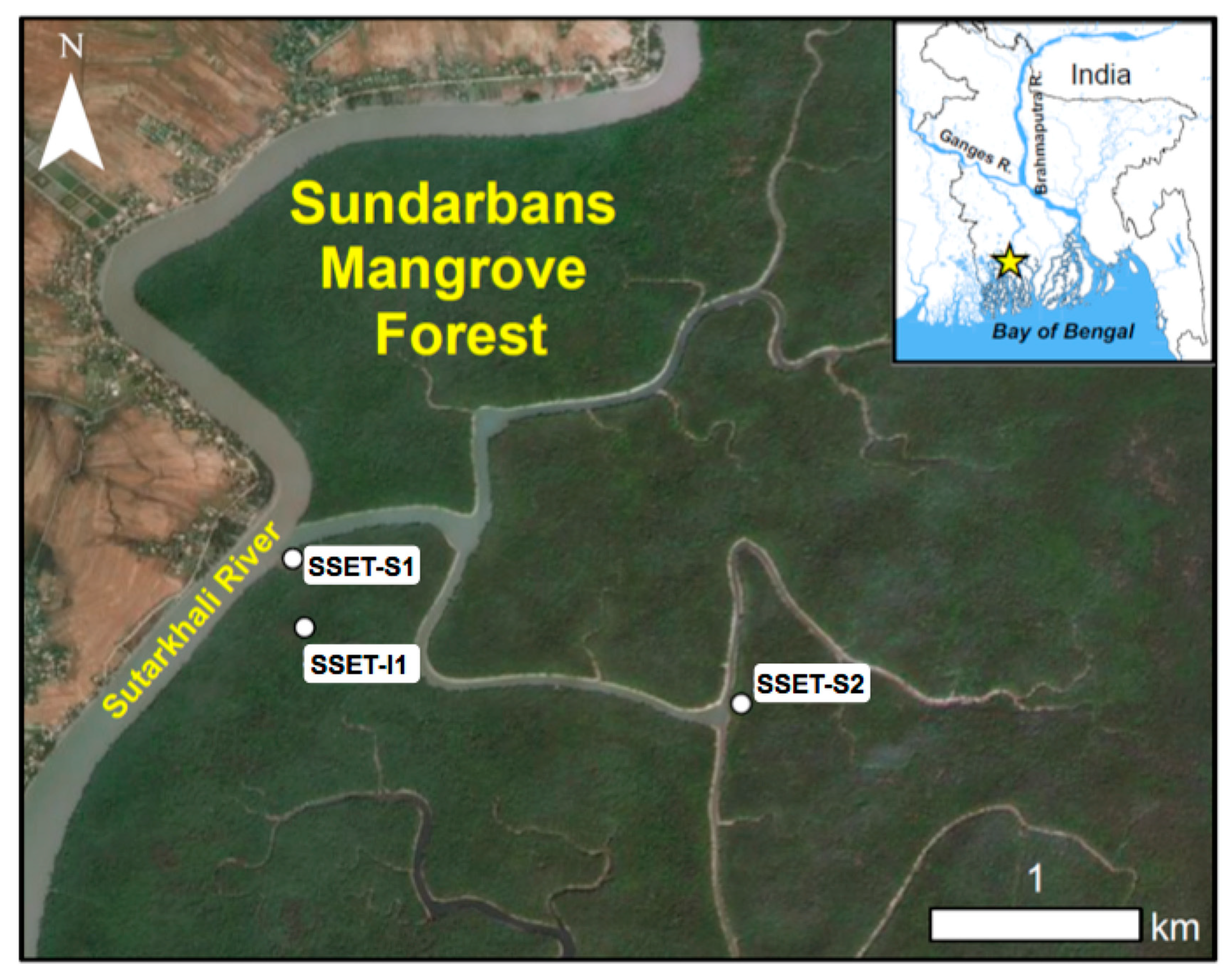
2. Study Area
3. Materials and Methods
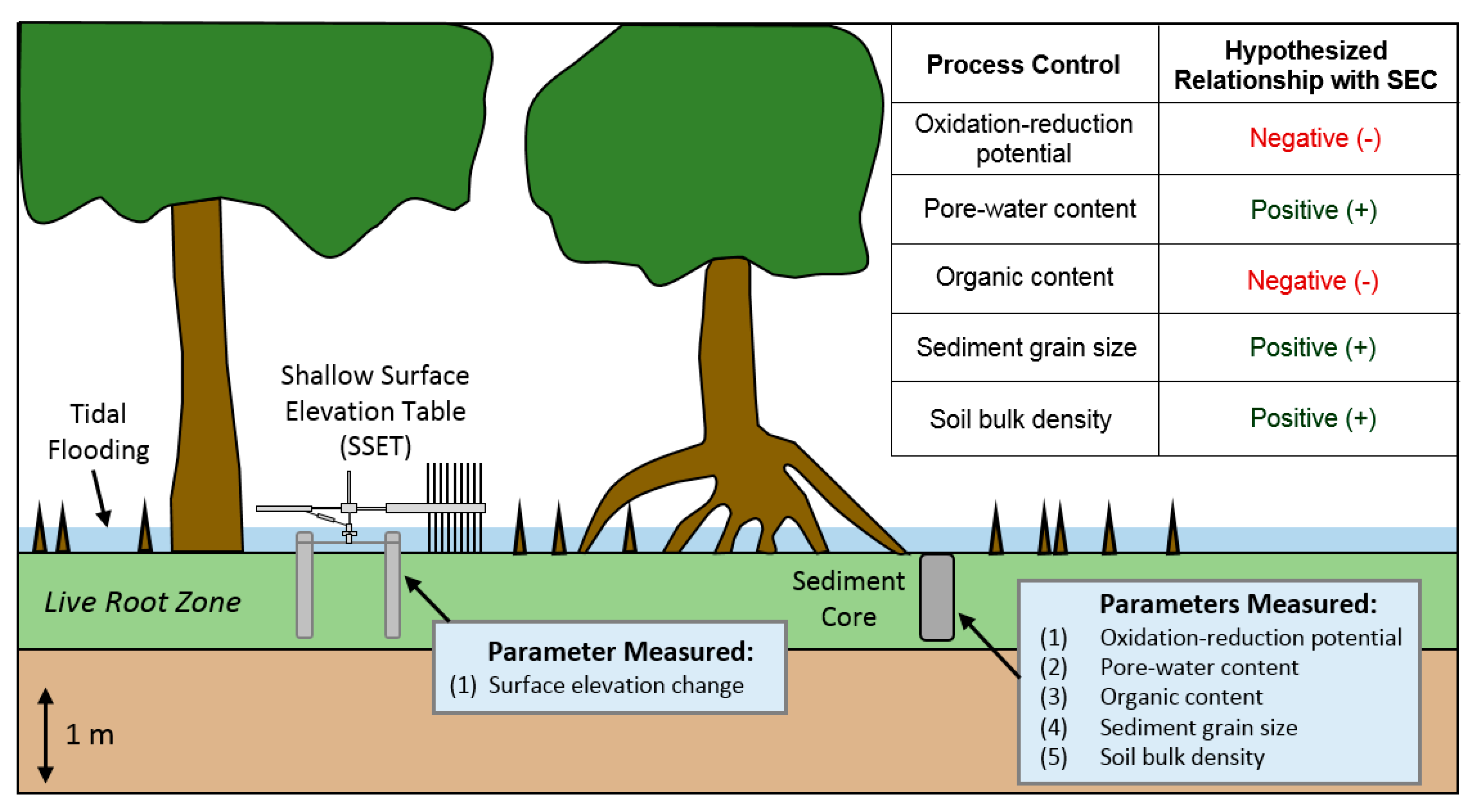
3.1. Site Selection and Near-Surface Elevation Dynamics
3.2. Oxidation-Reduction Potential
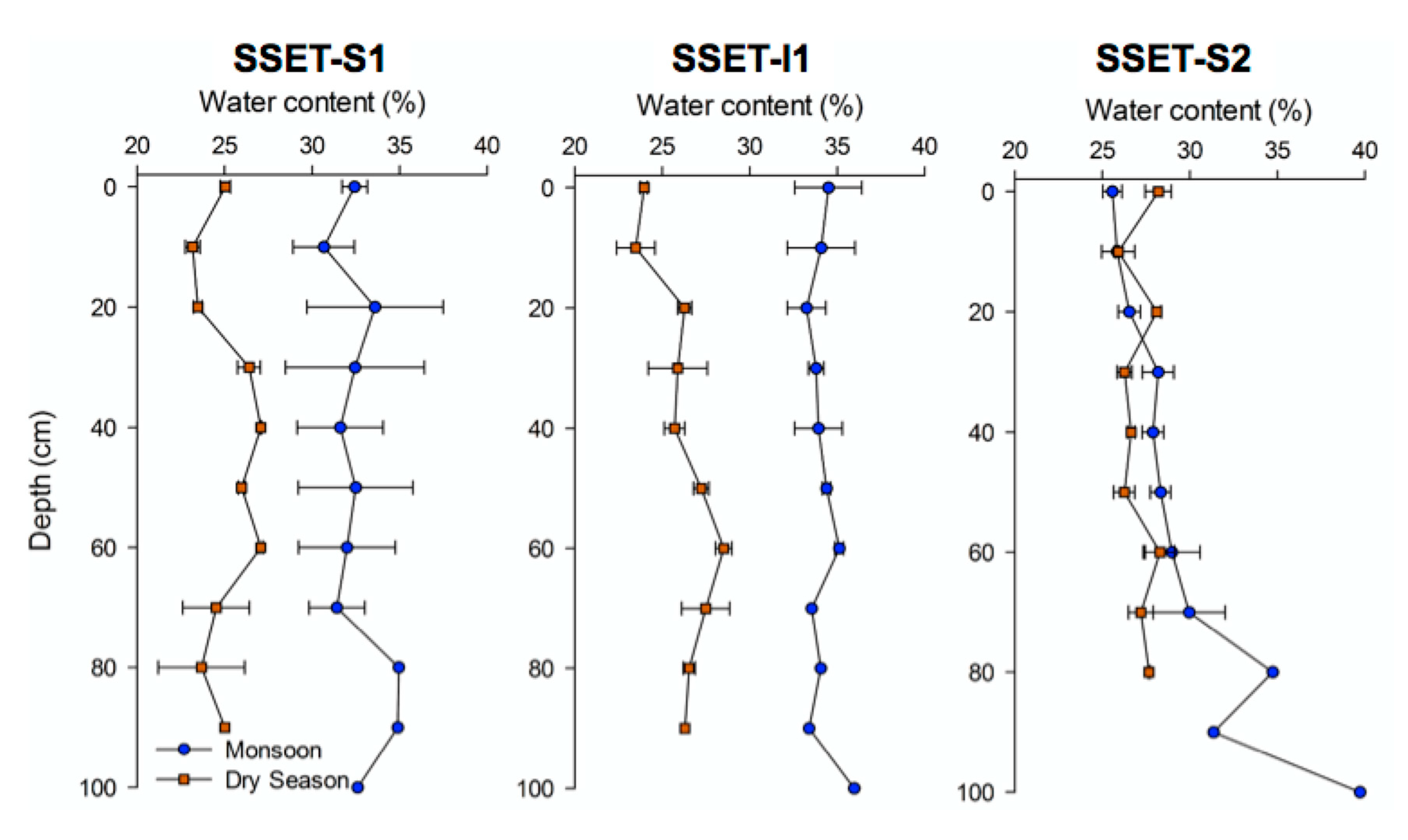
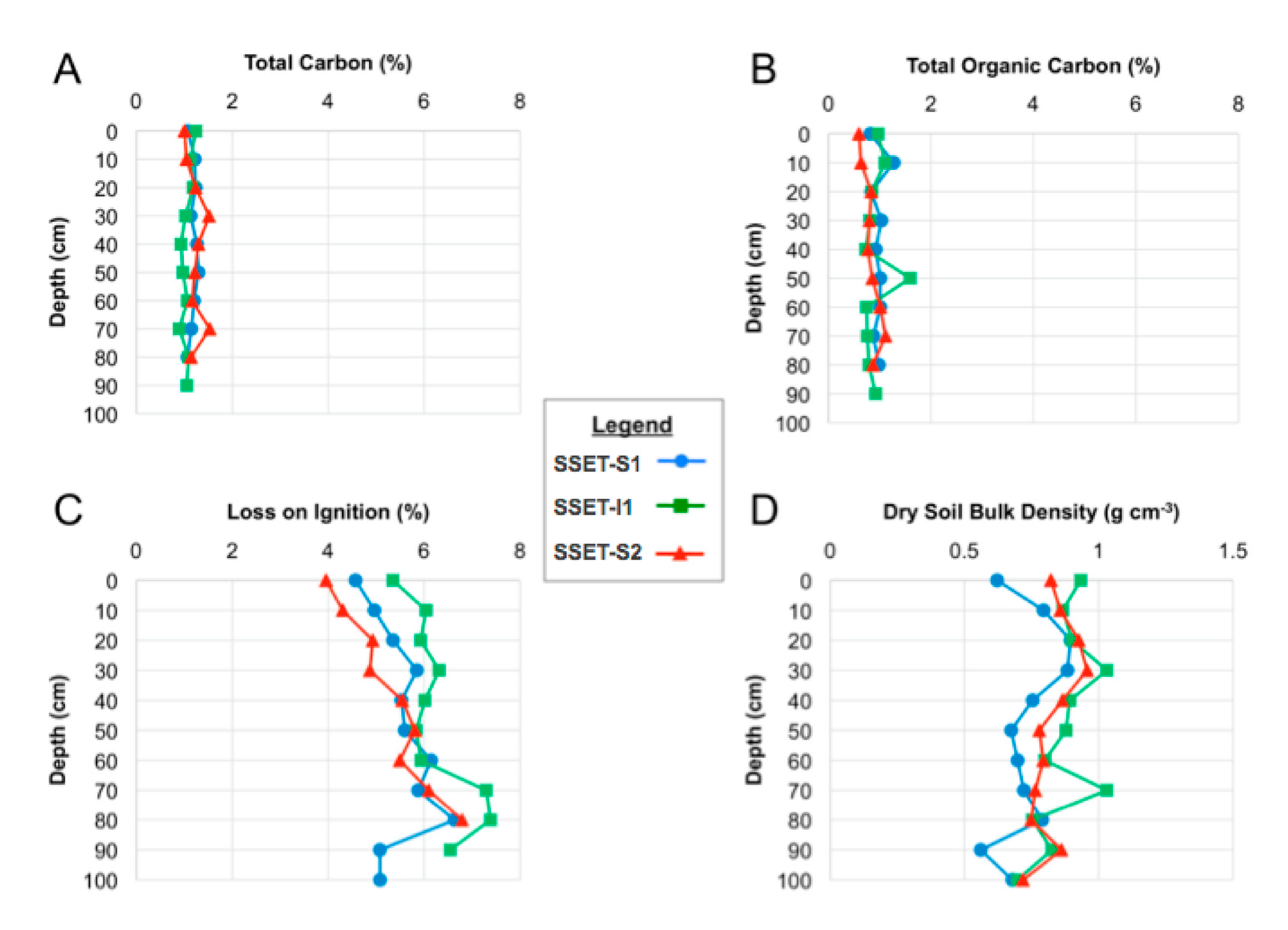
3.3. Soil Pore-Water Content, Organic Content, and Bulk Density
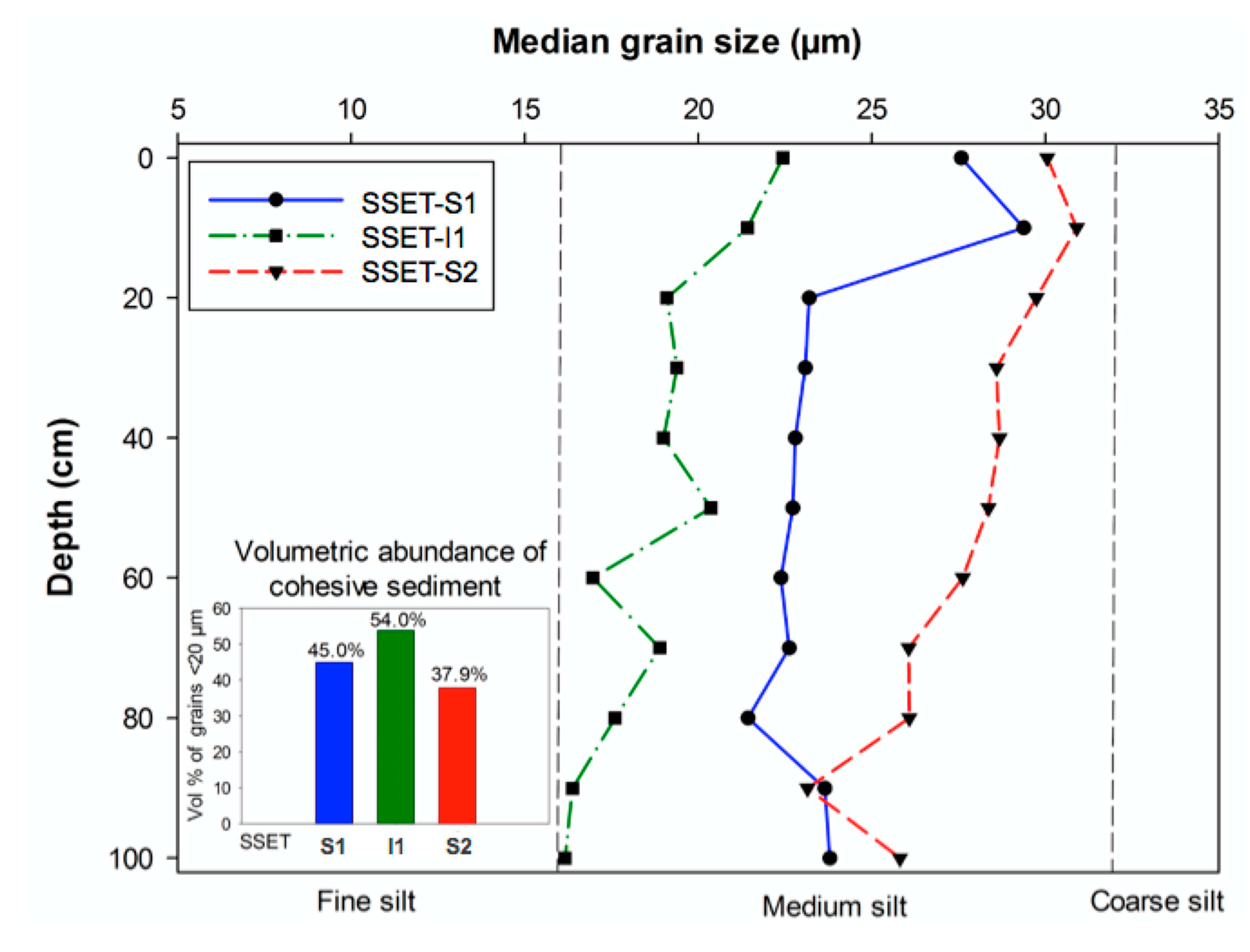
3.4. Granulometry
3.5. Statistical Analyses
4. Results
4.1. Near-Surface Elevation Dynamics
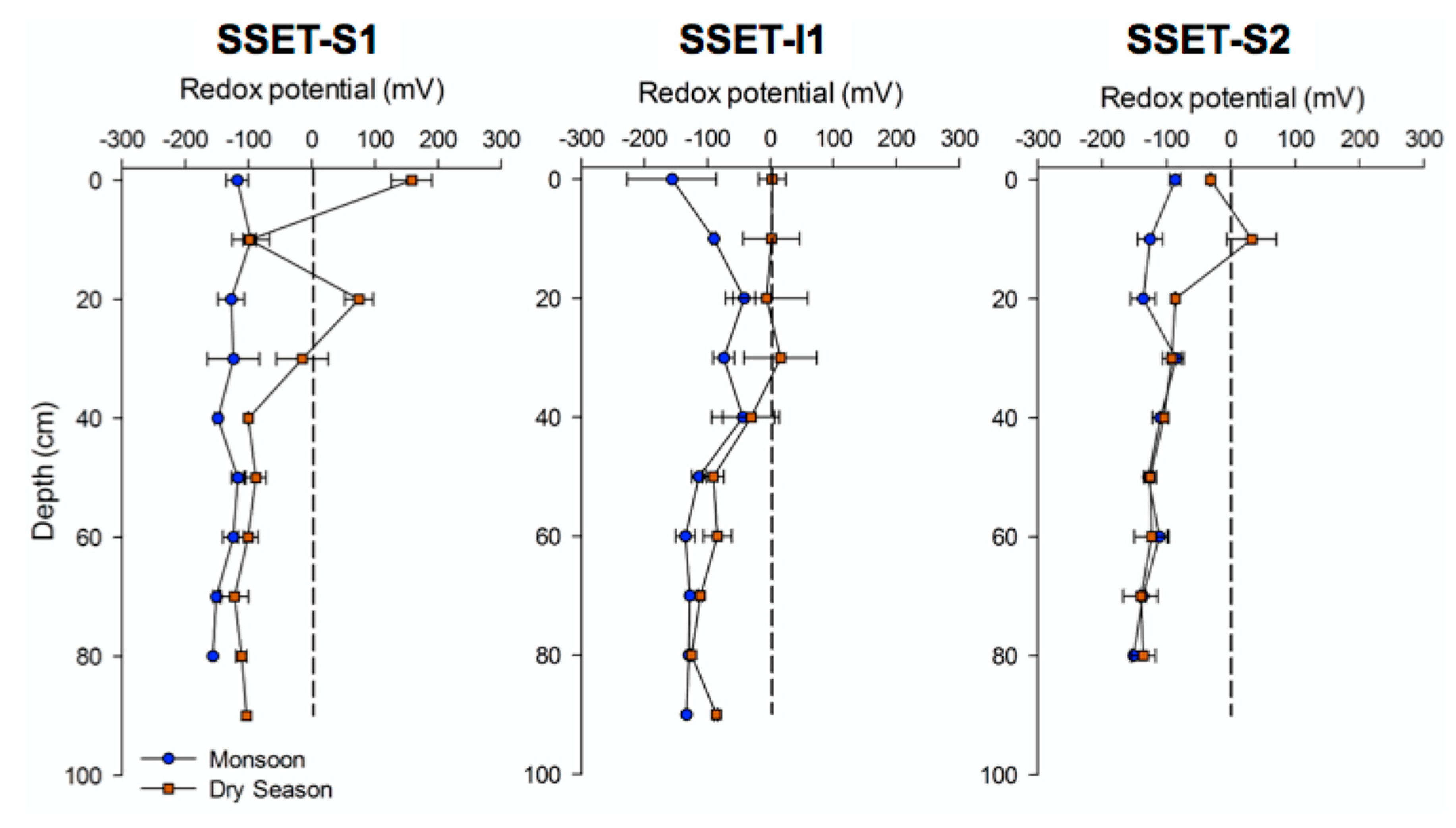
4.2. Oxidation-Reduction Potential
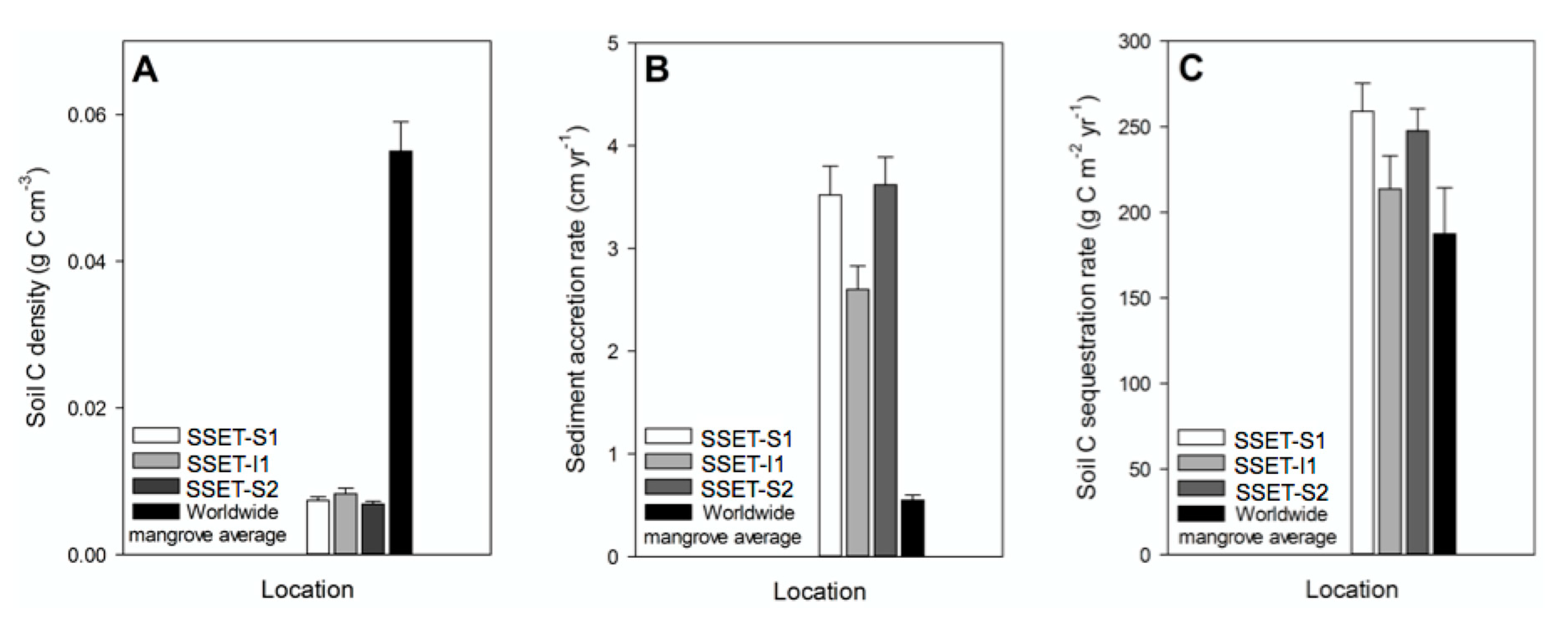
4.3. Soil Pore-Water Content, Organic Content, Bulk Density, and Sequestration Rates
4.4. Granulometry
5. Discussion
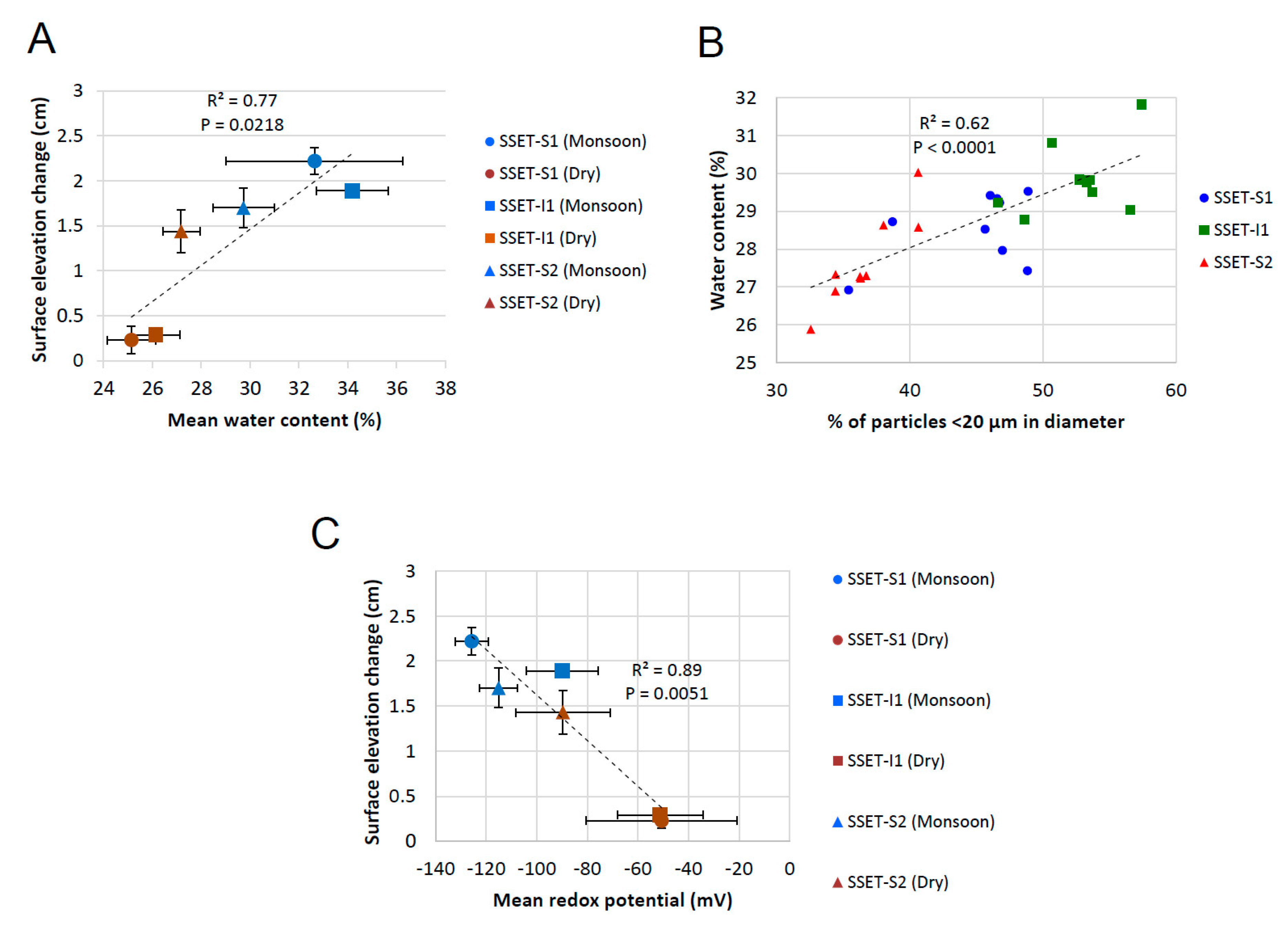
5.1. Effects of Below-Ground Process Controls on Surface Elevation Change
5.2. Carbon Sequestration Capacity of the SMF
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Danielsen, F. The Asian Tsunami: A Protective Role for Coastal Vegetation. Science 2005, 310, 643. [Google Scholar] [CrossRef]
- Das, S.; Vincent, J.R. Mangroves protected villages and reduced death toll during Indian super cyclone. Proc. Natl. Acad. Sci. USA 2009, 106, 7357–7360. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and Sediment Transport in Mangrove Forests. Estuar. Coast. Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Victor, S.; Golbuu, Y.; Wolanski, E.; Richmond, R.H. Fine sediment trapping in two mangrove-fringed estuaries exposed to contrasting land-use intensity, Palau, Micronesia. Wetl. Ecol. Manag. 2004, 12, 277–283. [Google Scholar] [CrossRef]
- Krauss, K.W.; McKee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef]
- McKee, K.L. Biophysical controls on accretion and elevation change in Caribbean mangrove ecosystems. Estuar. Coast. Shelf Sci. 2011, 91, 475–483. [Google Scholar] [CrossRef]
- McKee, K.L.; Cahoon, D.R.; Feller, I.C. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob. Ecol. Biogeogr. 2007, 16, 545–556. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: Projections of Sea Level Rise; IPCC: Geneva, Switzerland, 2013.
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J.-F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K.; et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–241. [Google Scholar] [CrossRef]
- FAO. The world’s Mangroves 1980–2005; FAO: Roma, Italy, 2007.
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Ellison, J.C.; Stoddart, D.R. Mangrove Ecosystem Collapse during Predicted Sea-Level Rise: Holocene Analogues and Implications. J. Coast. Res. 1991, 7, 151–165. [Google Scholar]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Ellison, J.C. Mangrove Retreat with Rising Sea-level, Bermuda. Estuar. Coast. Shelf Sci. 1993, 37, 75–87. [Google Scholar] [CrossRef]
- Krauss, K.W.; Cahoon, D.R.; Allen, J.A.; Ewel, K.C.; Lynch, J.C.; Cormier, N. Surface Elevation Change and Susceptibility of Different Mangrove Zones to Sea-Level Rise on Pacific High Islands of Micronesia. Ecosystems 2010, 13, 129–143. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Bennion, V.; Grinham, A.; Cahoon, D.R. The Role of Surface and Subsurface Processes in Keeping Pace with Sea Level Rise in Intertidal Wetlands of Moreton Bay, Queensland, Australia. Ecosystems 2011, 14, 745–757. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Cahoon, D.R.; Friess, D.A.; Guntenspergen, G.R.; Krauss, K.W.; Reef, R.; Rogers, K.; Saunders, M.L.; Sidik, F.; Swales, A.; et al. The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 2015, 526, 559–563. [Google Scholar] [CrossRef]
- Bomer, E.J.; Wilson, C.A.; Hale, R.P.; Hossain, A.N.M.; Rahman, F.M.A. Surface elevation and sedimentation dynamics in the Ganges-Brahmaputra tidal delta plain, Bangladesh: Evidence for mangrove adaptation to human-induced tidal amplification. Catena 2020, 187, 104312. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Lynch, J.C. Vertical accretion and shallow subsidence in a mangrove forest of southwestern Florida, USA. Mangroves Salt Marshes 1997, 1, 173–186. [Google Scholar] [CrossRef]
- Hopkins, D.W. Carbon mineralization. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; Canadian Society of Soil Science: Pinawa, MB, Canada; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Rogers, K.; Saintilan, N.; Cahoon, D. Surface Elevation Dynamics in a Regenerating Mangrove Forest at Homebush Bay, Australia. Wetl. Ecol. Manag. 2005, 13, 587–598. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Hensel, P.; Rybczyk, J.; McKee, K.L.; Proffitt, C.E.; Perez, B.C. Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurricane Mitch. J. Ecol. 2003, 91, 1093–1105. [Google Scholar] [CrossRef]
- Hale, R.P.; Wilson, C.A.; Bomer, E.J. Seasonal Variability of Forces Controlling Sedimentation in the Sundarbans National Forest, Bangladesh. Front. Earth Sci. 2019, 7, 211. [Google Scholar] [CrossRef]
- Marion, C.; Anthony, E.J.; Trentesaux, A. Short-term (≤2 yrs) estuarine mudflat and saltmarsh sedimentation: High-resolution data from ultrasonic altimetery, rod surface-elevation table, and filter traps. Estuar. Coast. Shelf Sci. 2009, 83, 475–484. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior; Wiley: Hoboken, NJ, USA, 2005; p. 592. [Google Scholar]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major Role of Marine Begetation on the Oceanic Carbon Cycle; Vliz: Oostende, Belgium, 2004; p. 22. [Google Scholar]
- Twilley, R.R.; Chen, R.H.; Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut. 1992, 64, 265–288. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Riul, P.; Cifuentes-Jara, M.; Manrow-Villalobos, M.; Horta, P.A.; Simonassi, J.C.; Fonseca, A.L.; Pagliosa, P.R. Global controls on carbon storage in mangrove soils. Nat. Clim. Chang. 2018, 8, 534–538. [Google Scholar] [CrossRef]
- Cameron, C.C.; Palmer, C.A. The Mangrove Peat of the Tobacco Range Islands, Belize Barrier Reef, Central America; Smithsonian: Washington, DC, USA, 1995. [Google Scholar]
- Vegas-Vilarrúbia, T.; Baritto, F.; López, P.; Meleán, G.; Ponce, M.E.; Mora, L.; Gómez, O. Tropical Histosols of the lower Orinoco Delta, features and preliminary quantification of their carbon storage. Geoderma 2010, 155, 280–288. [Google Scholar] [CrossRef]
- Whelan, K.R.T.; Smith, T.J.; Cahoon, D.R.; Lynch, J.C.; Anderson, G.H. Groundwater control of mangrove surface elevation: Shrink and swell varies with soil depth. Estuaries 2005, 28, 833–843. [Google Scholar] [CrossRef]
- Rogers, K.G.; Goodbred, S.L.; Mondal, D.R. Monsoon sedimentation on the ‘abandoned’ tide-influenced Ganges–Brahmaputra delta plain. Estuar. Coast. Shelf Sci. 2013, 131, 297–309. [Google Scholar] [CrossRef]
- Swales, A.; Bentley, S.J.; Lovelock, C.E. Mangrove-forest evolution in a sediment-rich estuarine system: Opportunists or agents of geomorphic change? Earth Surf. Process. Landf. 2015, 40, 1672–1687. [Google Scholar] [CrossRef]
- Iftekhar, M.S.; Saenger, P. Vegetation dynamics in the Bangladesh Sundarbans mangroves: A review of forest inventories. Wetl. Ecol. Manag. 2008, 16, 291–312. [Google Scholar] [CrossRef]
- Iftekhar, M.S.; Islam, M.R. Managing mangroves in Bangladesh: A strategy analysis. J. Coast. Conserv. 2004, 10, 139–146. [Google Scholar] [CrossRef]
- Islam, M.J.; Khan, F.A. Timber Volume Inventory. UNDP/UNESCO Mangrove Ecosystems Occasional Papers, No.2.; United Nations Development Program: New Delhi, India, 1988; p. 31. [Google Scholar]
- Ahmad, N. Economic Geography of East Pakistan; Oxford University Press: Oxford, UK, 1968. [Google Scholar]
- Allison, M.A. Geologic Framework and Environmental Status of the Ganges-Brahmaputra Delta. J. Coast. Res. 1998, 14, 827–836. [Google Scholar]
- Giri, C.; Pengra, B.; Zhu, Z.; Singh, A.; Tieszen, L.L. Monitoring mangrove forest dynamics of the Sundarbans in Bangladesh and India using multi-temporal satellite data from 1973 to 2000. Estuar. Coast. Shelf Sci. 2007, 73, 91–100. [Google Scholar] [CrossRef]
- Wilson, C.; Goodbred, S.; Small, C.; Gilligan, J.; Sams, S.; Mallick, B.; Hale, R. Widespread infilling of tidal channels and navigable waterways in human-modified tidal deltaplain of southwest Bangladesh. Elem. Sci. Anthr. 2017, 5, 78. [Google Scholar] [CrossRef]
- Shaha, D.C.; Cho, Y.-K. Salt Plug Formation Caused by Decreased River Discharge in a Multi-channel Estuary. Sci. Rep. 2016, 6, 27176. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Lynch, J.C.; Perez, B.C.; Segura, B.; Holland, R.D.; Stelly, C.; Stephenson, G.; Hensel, P. High-Precision Measurements of Wetland Sediment Elevation: II. The Rod Surface Elevation Table. J. Sediment. Res. 2002, 72, 734–739. [Google Scholar] [CrossRef]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; United States Environmental Protection Agency, Environmental Sciences Division National Exposure Research Laboratory NCEA-C-1282: Las Vegas, NV, USA, 2002; pp. 1–23. [Google Scholar]
- Harris, D.; Horwáth, W.R.; van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or CARBON-13 isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Allison, M.; Kepple, E. Modern sediment supply to the lower delta plain of the Ganges-Brahmaputra River in Bangladesh. Geo-Mar. Lett. 2001, 21, 66–74. [Google Scholar] [CrossRef]
- Mehta, A.J. On estuarine cohesive sediment suspension behavior. J. Geophys. Res. Oceans 2012, 14303–14314. [Google Scholar] [CrossRef]
- Pethick, J.; Orford, J.D. Rapid rise in effective sea-level in southwest Bangladesh: Its causes and contemporary rates. Glob. Planet. Chang. 2013, 111, 237–245. [Google Scholar] [CrossRef]
- Bangladesh Meteorology Department Normal monthly rainfall. Available online: http://live.bmd.gov.bd/p/Normal-Monthly-Rainfall/ (accessed on 13 March 2019).
- Cahoon, D.R.; Perez, B.C.; Segura, B.D.; Lynch, J.C. Elevation trends and shrink–swell response of wetland soils to flooding and drying. Estuar. Coast. Shelf Sci. 2011, 91, 463–474. [Google Scholar] [CrossRef]
- Nuttle, W.K.; Hemond, H.F.; Stolzenbach, K.D. Mechanisms of water storage in salt marsh sediments: The importance of dilation. Hydrol. Process. 1990, 4, 1–13. [Google Scholar] [CrossRef]
- Brammer, H. Can Bangladesh Be Protected From Floods? Dhaka University Press: Dhaka, Bangladesh, 2004. [Google Scholar]
- Rogers, K.; Saintilan, N. Relationships between Surface Elevation and Groundwater in Mangrove Forests of Southeast Australia. J. Coast. Res. 2008, 1, 63–69. [Google Scholar] [CrossRef]
- Boivin, P.; Garnier, P.; Tessier, D. Relationship between Clay Content, Clay Type, and Shrinkage Properties of Soil Samples. Soil Sci. Soc. Am. J. 2004, 68, 1145–1153. [Google Scholar] [CrossRef]
- Fredlund, M.D.; Fredlund, D.G.; Wilson, G.W. Prediction of the Soil-Water Characteristic Curve from Grain-Size Distribution and Volume-Mass Properties; Department of Civil Engineering, University of Saskatoon: Saskatoon, SK, Canada, 1997; p. 12. [Google Scholar]
- Gupta, S.C.; Larson, W.E. Estimating soil water retention characteristics from particle size distribution, organic matter percent, and bulk density. Water Resour. Res. 1979, 15, 1633–1635. [Google Scholar] [CrossRef]
- Allison, M.A.; Khan, S.R.; Goodbred, S.L.; Kuehl, S.A. Stratigraphic evolution of the late Holocene Ganges–Brahmaputra lower delta plain. Sediment. Geol. 2003, 155, 317–342. [Google Scholar] [CrossRef]
- Brinson, M.M.; Lugo, A.E.; Brown, S. Primary Productivity, Decomposition and Consumer Activity in Freshwater Wetlands. Annu. Rev. Ecol. Syst. 1981, 12, 123–161. [Google Scholar] [CrossRef]
- Middleton, B.A.; McKee, K.L. Degradation of mangrove tissues and implications for peat formation in Belizean island forests. J. Ecol. 2001, 89, 818–828. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Cahoon, D.R.; Hensel, P.F.; Spencer, T.; Reed, D.J.; McKee, K.L.; Saintilan, N. Coastal Wetland Vulnerability to Relative Sea-Level Rise: Wetland Elevation Trends and Process Controls. In Wetlands and Natural Resource Management; Verhoeven, J.T.A., Beltman, B., Bobbink, R., Whigham, D.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 190, pp. 271–292. ISBN 978-3-540-33186-5. [Google Scholar]
- McIvor, A.; Spencer, T.; Moller, I.; Spalding, M. The Response of Mangrove Soil Surface Elevation to Sea Level Rise; Natural Coastal Protection Series: Report 3; Cambridge Coastal Research Unit Working Paper 42; University of Cambridge: Cambridge, UK, 2013; p. 59. [Google Scholar]
- Bird, M.I.; Fifield, L.K.; Chua, S.; Goh, B. Calculating Sediment Compaction for Radiocarbon Dating of Intertidal Sediments. Radiocarbon 2004, 46, 421–435. [Google Scholar] [CrossRef]
- Van Asselen, S.; Stouthamer, E.; van Asch, T.W.J. Effects of peat compaction on delta evolution: A review on processes, responses, measuring and modeling. Earth Sci. Rev. 2009, 92, 35–51. [Google Scholar] [CrossRef]
- Meckel, T.A.; ten Brink, U.S.; Williams, S.J. Current subsidence rates due to compaction of Holocene sediments in southern Louisiana. Geophys. Res. Lett. 2006, 33, L11403. [Google Scholar] [CrossRef]
- Törnqvist, T.E.; Wallace, D.J.; Storms, J.E.A.; Wallinga, J.; van Dam, R.L.; Blaauw, M.; Derksen, M.S.; Klerks, C.J.W.; Meijneken, C.; Snijders, E.M.A. Mississippi Delta subsidence primarily caused by compaction of Holocene strata. Nat. Geosci. 2008, 1, 173–176. [Google Scholar] [CrossRef]
- Meckel, T.A.; Ten Brink, U.S.; Williams, S.J. Sediment compaction rates and subsidence in deltaic plains: Numerical constraints and stratigraphic influences. Basin Res. 2007, 19, 19–31. [Google Scholar] [CrossRef]
- Victor, S.; Neth, L.; Golbuu, Y.; Wolanski, E.; Richmond, R.H. Sedimentation in mangroves and coral reefs in a wet tropical island, Pohnpei, Micronesia. Estuar. Coast. Shelf Sci. 2006, 66, 409–416. [Google Scholar] [CrossRef]
- Lutzow, M.V.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Goodbred, S.L.; Kuehl, S.A. The significance of large sediment supply, active tectonism, and eustasy on margin sequence development: Late Quaternary stratigraphy and evolution of the Ganges–Brahmaputra delta. Sediment. Geol. 2000, 133, 227–248. [Google Scholar] [CrossRef]
- Hale, R.; Bain, R.; Goodbred Jr., S.; Best, J. Observations and Scaling of Tidal Mass Transport Acrossthe Lower Ganges-Brahmaputra Delta Plain: Implications for Delta Management and Sustainability; Research Gate: Berlin, Germany, 2018. [Google Scholar]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Kuehl, S.A.; Allison, M.A.; Goodbred, S.L.; Kudrass, H. The Ganges-Brahmaputra Delta. In River Deltas-Concepts, Models, and Examples; Giosan, L., Bhattacharya, J.P., Eds.; Society for Sedimentary Geology (SEPM): Tulsa, OK, USA, 2005; ISBN 978-1-56576-113-1. [Google Scholar]
- Sasmito, S.D.; Murdiyarso, D.; Friess, D.A.; Kurnianto, S. Can mangroves keep pace with contemporary sea level rise? A global data review. Wetl. Ecol. Manag. 2016, 24, 263–278. [Google Scholar] [CrossRef]
- Aucour, A.-M.; France-Lanord, C.; Pedoja, K.; Pierson-Wickmann, A.-C.; Sheppard, S.M.F. Fluxes and sources of particulate organic carbon in the Ganga-Brahmaputra river system. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Galy, V.; France-Lanord, C.; Beyssac, O.; Faure, P.; Kudrass, H.; Palhol, F. Efficient organic carbon burial in the Bengal fan sustained by the Himalayan erosional system. Nature 2007, 450, 407–410. [Google Scholar] [CrossRef]
- Rogers, K.G.; Goodbred, S.L. The Sundarbans and Bengal Delta: The World’s Largest Tidal Mangrove and Delta System. In Landscapes and Landforms of India; Kale, V.S., Ed.; Springe: Dordrecht, The Netherlands, 2014; pp. 181–187. ISBN 978-94-017-8028-5. [Google Scholar]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-history, movement, and habitat use of Scylla serrata (Decapoda, Portunidae): Current knowledge and future challenges. Hydrobiologia 2016, 763, 5–21. [Google Scholar] [CrossRef]
- Webster, J.R.; Benfield, E.F. Vascular Plant Breakdown in Freshwater Ecosystems. Annu. Rev. Ecol. Syst. 1986, 17, 567–594. [Google Scholar] [CrossRef]
- Hossain, M.; Siddique, M.R.H.; Abdullah, S.M.R.; Saha, S.; Ghosh, D.C.; Rahman, M.S.; Limon, S.H. Nutrient Dynamics Associated with Leaching and Microbial Decomposition of Four Abundant Mangrove Species Leaf Litter of the Sundarbans, Bangladesh. Wetlands 2014, 34, 439–448. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Adame, M.F.; Bennion, V.; Hayes, M.; O’Mara, J.; Reef, R.; Santini, N.S. Contemporary Rates of Carbon Sequestration Through Vertical Accretion of Sediments in Mangrove Forests and Saltmarshes of South East Queensland, Australia. Estuaries Coasts 2014, 37, 763–771. [Google Scholar] [CrossRef]
- Higgins, S.A.; Overeem, I.; Rogers, K.G.; Kalina, E.A. River linking in India: Downstream impacts on water discharge and suspended sediment transport to deltas. Elem. Sci. Anthr. 2018, 6, 20. [Google Scholar] [CrossRef]
- Hutchison, J.; Manica, A.; Swetnam, R.; Balmford, A.; Spalding, M. Predicting Global Patterns in Mangrove Forest Biomass: Global patterns in mangrove biomass. Conserv. Lett. 2014, 7, 233–240. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Heider, C.; Cole, T.G.; Dwire, K.A.; Donato, D.C. Ecosystem Carbon Stocks of Micronesian Mangrove Forests. Wetlands 2011, 31, 343–352. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Donato, D.; Kauffman, J.B.; Kurnianto, S.; Stidham, M.; Kanninen, M. Carbon Storage in Mangrove and Peatland Ecosystems: A Preliminary Account from Plots in Indonesia; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2010. [Google Scholar]
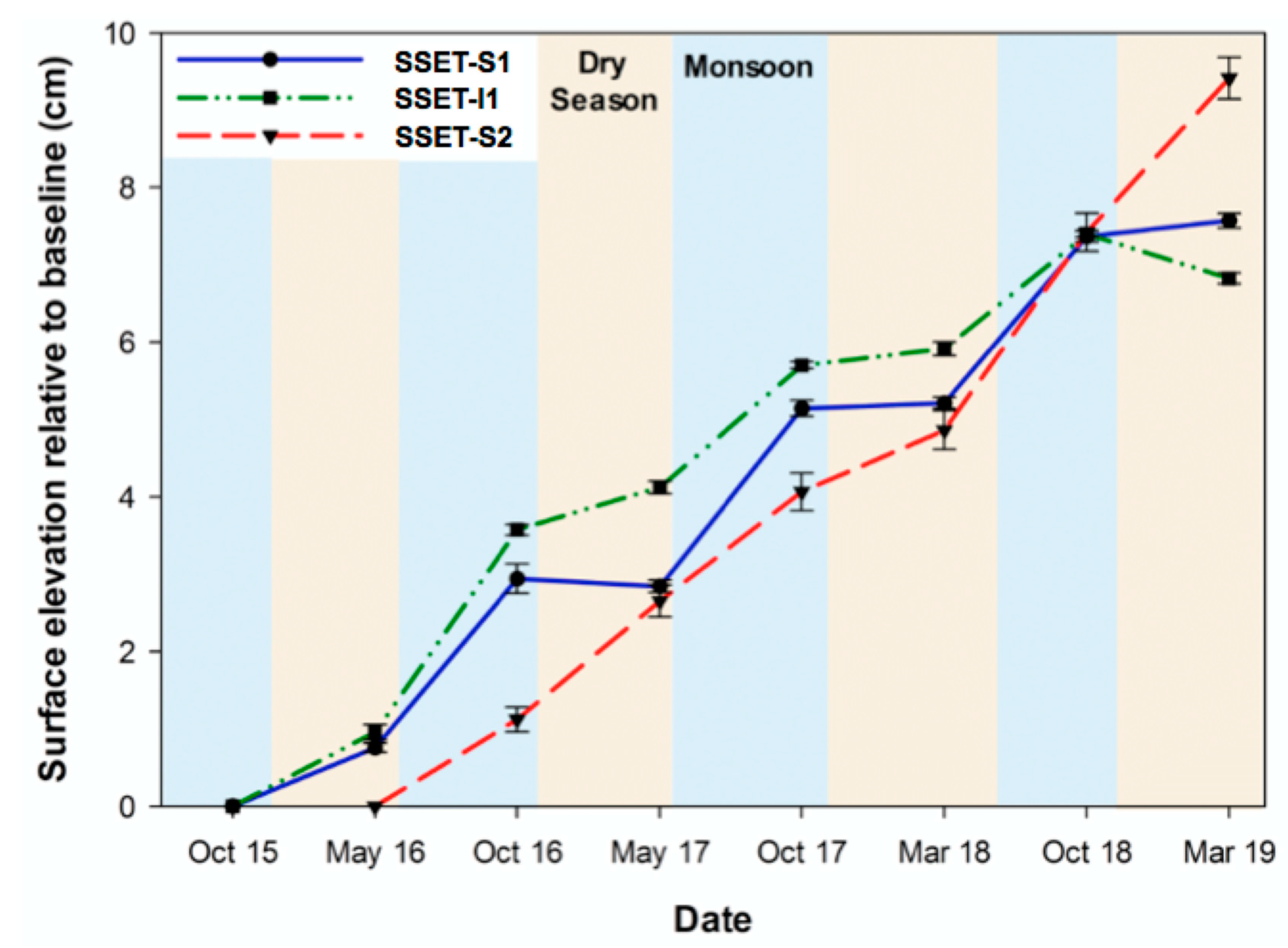

| Station | Record (year) | Mean Annual SEC (cm year−1) | Mean Monsoon SEC (cm year−1) | Mean Dry Season SEC (cm year−1) |
|---|---|---|---|---|
| SSET-S1 | 3.5 | 2.16 ± 0.19 | 2.22 ± 0.15 | 0.23 ± 0.08 |
| SSET-I1 | 3.5 | 1.95 ± 0.14 | 1.89 ± 0.05 | 0.29 ± 0.08 |
| SSET-S2 | 3.0 | 3.14 ± 0.46 | 1.70 ± 0.22 | 1.44 ± 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bomer, E.J.; Wilson, C.A.; Elsey-Quirk, T. Process Controls of the Live Root Zone and Carbon Sequestration Capacity of the Sundarbans Mangrove Forest, Bangladesh. Sci 2020, 2, 54. https://doi.org/10.3390/sci2030054
Bomer EJ, Wilson CA, Elsey-Quirk T. Process Controls of the Live Root Zone and Carbon Sequestration Capacity of the Sundarbans Mangrove Forest, Bangladesh. Sci. 2020; 2(3):54. https://doi.org/10.3390/sci2030054
Chicago/Turabian StyleBomer, Edwin J., Carol A. Wilson, and Tracy Elsey-Quirk. 2020. "Process Controls of the Live Root Zone and Carbon Sequestration Capacity of the Sundarbans Mangrove Forest, Bangladesh" Sci 2, no. 3: 54. https://doi.org/10.3390/sci2030054
APA StyleBomer, E. J., Wilson, C. A., & Elsey-Quirk, T. (2020). Process Controls of the Live Root Zone and Carbon Sequestration Capacity of the Sundarbans Mangrove Forest, Bangladesh. Sci, 2(3), 54. https://doi.org/10.3390/sci2030054





