Abstract
Serial crystallography is a rapidly advancing experimental technology that has seen significant development in recent years. This technique enables the continuous delivery of a series of protein crystal samples to the X-ray beam, allowing for the collection of diffraction data from a large number of crystals at ambient temperature. Despite its advancements, serial crystallography still possesses considerable potential for further development within synchrotron radiation platforms. Currently, several challenges hinder the progress of this technology, including the preparation of numerous microcrystal samples, methods for sample delivery, data acquisition efficiency, and data processing techniques. The device introduced in this paper is designed to facilitate serial crystallographic experiments at the synchrotron radiation station, employing electrospinning in the vacuum cavity to reduce the average flux, mitigate the effects of air ionization on the Taylor cone, and enhance the stability of Taylor cone during the data acquisition process. The diffraction pattern of lysozyme crystals was successfully acquired with this device at the beamlines of the Shanghai Synchrotron Radiation Facility (SSRF).
1. Introduction
The structural determination of biomacromolecules is essential to understand the mechanisms of life activities and rational drug design []. In structural biology, X-ray diffraction is one of the most widely utilized methods. Nonetheless, conventional X-ray crystallography is constrained by the issue of radiation damage. To enhance radiation tolerance by a factor of approximately 30, experiments are typically conducted at low temperatures, such as 100 K [,]. This approach, however, necessitates a sufficiently ordered arrangement of the lattice and a crystal size exceeding 20 μm [], which can pose challenges, as certain proteins do not readily form suitable crystals. At ambient temperature, the susceptibility of protein structures to radiation damage increases, particularly in smaller crystals, which exhibit diminished tolerance to X-ray exposure. Consequently, the radiation tolerance of crystals is significantly enhanced under low-temperature conditions, thereby limiting traditional X-ray crystallography experiments to such environments.
With the advent of X-ray free electron laser (XFEL) technology, the use of femtosecond X-ray pulses facilitates the acquisition of experimental data before the onset of radiation damage, a process known as “diffraction before damage” []. This advancement significantly reduces radiation damage during data acquisition. This technique enables X-ray methods to resolve crystal structures at room temperature [,] with dimensions smaller than one micrometer, effectively overcoming the size and temperature limitations associated with conventional X-ray methods. In 2012, the structure of lysozyme at 1.9 Å was determined [], marking the first high-resolution structure obtained through the serial femtosecond crystallography (SFX) method. Following the success of SFX, the application of serial crystallography in synchrotron facilities began to garner significant attention. Currently, there are only about 10 operational XFEL devices worldwide, and only XFEL devices with high-energy beams can conduct SFX experiments, such as LCLS, SACLA, European XFEL, and PAL-XFEL []. In contrast, a synchrotron facility typically has multiple protein crystallography beamlines; for example, SSRF operates up to six such beamlines simultaneously. If the serial crystallography technology based on synchrotron beamlines can be successfully developed, existing experimental resources can be fully leveraged. In 2014, Stellato et al. demonstrated the feasibility of synchrotron radiation serial crystallography in principle, prompting researchers to explore the application of the loading method used in SFX for synchrotron radiation-based serial crystallography experiments [].
The realization of continuous diffraction in serial crystallography requires efficient and stable sample delivery methods []. The commonly used delivery methods are primarily categorized into two types, namely fixed-target scanning and fluidic phase delivery. Fixed-target methods include microfluidic chips, silicon-based chips, and grid film scanning [,,], while fluidic phase methods encompass aerodynamic virtual nozzles (GDVN) [,], microfluidic electrokinetic sample holders (MESH) [], high-viscosity nozzles (HVE) [,,], and capillaries [], et al. Among the various methodologies, the experimental approach utilizing fluidic phases is particularly advantageous for the investigation of time-resolved serial crystallography []. Therefore, the electrospinning technique has been chosen for sample delivery in this research.
The principle of electrospinning involves utilizing an electrostatic field to exert an electrostatic force on the surface of a polarized charged droplet []. This force overcomes the surface tension, resulting in the formation of a conical structure known as the Taylor cone, from which a micron-scale jet is produced at the droplet’s tip. Neglecting the minimal effects of thermal and magnetic influences, the Taylor cone is the outcome of a balance among the pressure difference at the free surface, the liquid surface tension, and the Maxwell stress. Based on the principle of electrospinning, we can design a serial crystallographic sample delivery device that incorporates a mobile solution phase. The liquid pool used for protein crystallization serves as the spinning solution, from which a micron-scale jet is formed and transported through the X-ray beamline. Subsequently, protein crystals are mixed into the spinning solution. As the spinning solution jet operates continuously, the crystals can be delivered in a steady mode to the optical path for diffraction analysis.
2. Materials and Methods
2.1. Experimental Platform Design
The application of the electrospinning technique in conjunction with a synchrotron radiation apparatus may lead to fluctuations in the Taylor cone, or it may even dissipate upon activation of the light switch. This phenomenon is primarily attributed to the ionization of air. To mitigate this issue, it is crucial to minimize the effects of air ionization throughout the experimental procedure. Consequently, it is advisable to reduce the light flux at a specified vacuum level. Given that the boiling point of an aqueous solution decreases under low pressure, it is essential to consider whether the spinning liquid can remain stable under the experimental conditions []. After testing, we found that adding 20% glycerol and reducing the temperature to 0 °C can effectively maintain the stability of the solution at vacuum status of 10−4 mbar. Therefore, designing a vacuum electrospinning sample delivery device that is compatible with synchrotron radiation facilities is of paramount importance. To facilitate installation at the synchrotron radiation station, a lightweight 3D-printed vacuum chamber was employed. The incident and diffraction windows were sealed with a polyimide film that provides enhanced X-ray transmittance. This vacuum chamber can achieve a vacuum level of 1 × 10−5 mbar under experimental conditions, which meets the requirements of this experiment. It also offers several advantages, including ease of processing, straightforward installation, and low cost. Due to the stringent quality requirements of the X-ray crystallography method for crystal diffraction, directly reducing the flux may compromise the diffraction quality of the crystal. Therefore, a shutter and chopper can be utilized to convert continuous X-ray exposure into a zigzag wave pattern for data collection. This approach reduces the total flux by decreasing pulse width, thereby minimizing the impact of X-rays on the stability of the jet.
The experimental platform primarily consists of a 3D-printed vacuum chamber, a four-way tube, a high-voltage power supply, a vacuum pump, and two microflow injection pumps. The vacuum chamber comprises the main body, an incident window, a diffraction window, positive and negative electrodes, and various connecting components. In the microflow injection system, the mother liquid containing the crystal sample and the protective child liquid are injected by the two microflow pumps. These liquids are then combined in the four-way tube and directed from the positive electrode into the vacuum chamber. Under the strong electric field generated by the high-voltage power supply, electrospinning occurs, enabling the protein crystal to be continuously diffracted along the optical path with the spinning jet.
The main body of the vacuum chamber is a 3D-printed structure, primarily divided into three sections. The central cavity features two windows, one large and one small. The small window serves as the incident window, while the large window functions as the diffraction window. Additionally, there are two small holes, each with a radius of 1 mm, located at the top and bottom, which connect to the positive and negative electrodes. One side of the chamber is linked to the diffractometer, while the opposite side can be directly connected to the vacuum pump via a corrugated tube. When determining the optimal thickness of polyimide film for windows, it is essential to minimize scattering while maintaining an adequate vacuum level. Therefore, the window material should be as thin as possible. A comparison of the vacuum performance of polyimide films with varying thicknesses reveals that, the 50 µm polyimide film exhibits significant deformation and cannot return to its original shape, rendering it unsuitable for repeated use and replacement in experiments. Although the 150 µm film also undergoes deformation, it can recover on its own once normal pressure is restored. To achieve a balance between transparency and stability, the 150 µm thick polyimide film was selected as the diffraction window material, while a thinner 50 µm polyimide film was chosen as the incident window. Calculations indicate that the transmittance of a polyimide film with a total thickness of 200 µm is approximately 95.6%.
The sample chamber is designed to mix crystals with a spinning solution. It has an inner diameter of 8 mm, a length of 2 cm, and a total internal volume of approximately 1 mL. The crystals are stored within the sample chamber, while the mother liquor, injected from the microfluidic pump, flows through the chamber and transports the sample into the vacuum chamber. The flow rate of the spinning solution is denoted as Q, while the crystal number density in the sample chamber is represented by d. As the experiment progresses, the crystals are transported by the rotating solution, described by the equation dN = Qdt · ρ. The total number of samples in the sample room is given by the equation N = ρV. Therefore, we can derive . By integrating, we can obtain . Taking Q = 0.1 µL/min and V = 200 µL, we can obtain . It takes 60 min to collect data for each group in the experiment. Calculations using the formula above indicate that the crystal concentration in the sample room decreases by approximately 2.965%. This essentially means that the concentration remains nearly constant throughout the experiment.
2.2. Off-Beamline Test
The electrospinning parameters were examined using an optical microscope with 10× magnification. A stable Taylor cone was observed, shown in Figure 1, at 0 °C and a vacuum of 10−4 mbar. At this point, the voltage was set to 3800 V, the flow rate was 0.1 µL/min, and the electrode distance was 4 mm. The spinning solution comprised a lysozyme crystallization pool solution, 4 M sodium chloride, 0.1 M citric acid, and 0.1 M sodium acetate. To increase the viscosity of the solution and enhance its stability under low pressure, 20% glycerol was incorporated into the spinning solution. A 20,000-frame high-speed camera was employed to capture the rapid passage of the liquid surface through the optical path following the formation of a liquid droplet from the Taylor cone, shown in Figure 2.
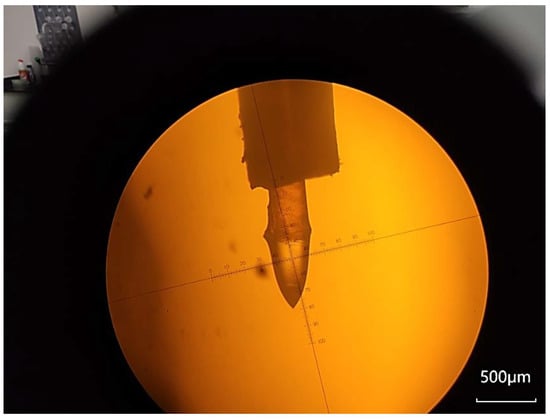
Figure 1.
The microscopic observation of the Taylor cone reveals that the capillary is encased within an electrode formed by the needle tube. It is captured by the SZX16 microscope (Olympus, Tokyo, Japan). The Taylor cone is generated at the liquid level of the solution at the capillary’s outlet. The yellow background depicted in the figure represents polyimide film, while the black frame surrounding the image corresponds to the eyepiece of the microscope.
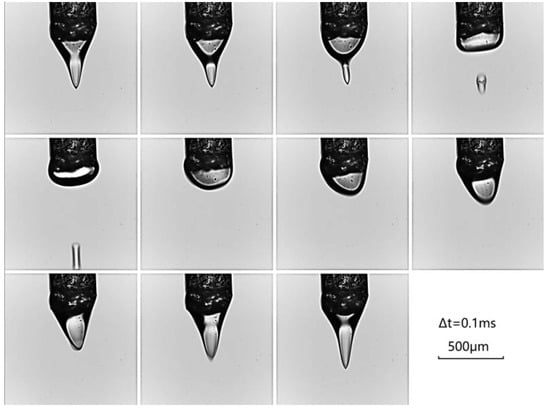
Figure 2.
The solution generates droplets on the micron scale from the apex of the Taylor cone, subsequently traversing the beam. The droplets are captured by the FASTCAM SA-Z high-speed camera (Photron, Tokyo, Japan) equipped with a Aurogon FF 50× high-magnification lens (LAOWA, Heifei, China). The interval between the formation of two droplets is 20 frames, corresponding to a droplet cycle of 1 millisecond. During the droplet formation process at the cone tip, the droplet begins to accelerate after approximately 0.4 ms of quiescence, which can be considered the effective exposure time for data acquisition.
2.3. Installation of the Experiment Platform
The experimental platform, shown in Figure 3, primarily consists of a vacuum chamber, a high-voltage source, a microflow pump, a vacuum pump, and an X-ray chopper. Prior to the experiment, the spinning solution is collected in the injector and connected to the sample chamber via a capillary tube with an outer diameter of 0.35 mm and an inner diameter of 0.25 mm. The coaxial spinning solution is then formed in the four-way tube and enters the vacuum chamber. A polyimide film is used to seal the light entry and exit windows. A 35 mm-long needle is employed for preliminary focusing and alignment. The sealed vacuum chamber is subsequently secured onto the diffractometer, and the vacuum pump is connected to ensure precise focusing. Finally, the chopper is installed along the X-ray path.
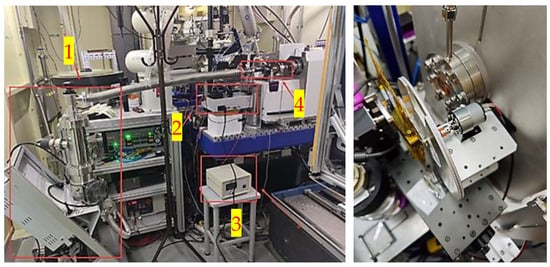
Figure 3.
In the left illustration, the components are designated as follows: 1 represents the vacuum pump, 2 denotes the syringe pump, 3 indicates the high voltage power supply, and 4 refers to the primary structure of the vacuum chamber. The right illustration depicts the chopper utilized for modulating the pulse.
After completing the installation, we start the vacuum pump. Once the vacuum level in the pump reaches 1 × 10−4 mbar, we gradually open the valve to slowly reduce the air pressure in the vacuum chamber. After the pressure in the vacuum chamber stabilizes, we activate the microfluidic pump to eliminate any bubbles in the capillary and set the appropriate flow rate (0.1–0.2 µL/min). We place the sample chamber in an ice-water mixture at 0 °C and gently shake it at 1 Hz frequency to prevent crystal settling. We open the X-ray chopper and adjust it to the appropriate speed. We turn on the high-voltage source and observe the state of the Taylor cone while gradually increasing the voltage to stabilize the Taylor cone. Finally, we open the shutter to obtain the X-ray pulse. After the shutter is opened, the jet immediately disappears, and the original Taylor cone transforms into a circular liquid surface. This phenomenon occurs due to the ionization of air caused by X-rays, which disrupts the electric field distribution between the electrodes, thereby compromising the stability of the Taylor cone.
2.4. Data Collection Strategy
To minimize the impact of X-ray exposure on the stability of the Taylor cone, the shutterless mode commonly employed in the beamline can be modified to a single-pulse exposure mode. This adjustment can be achieved by controlling the X-ray beam switch without altering the beamline hardware. Considering the actual conditions of the beamline, we designed a chopper to generate a 10 ms X-ray pulse, exposing the detector for 25 ms. The chopper features two fan windows of 30°, and the turntable speed was adjusted to 3° per millisecond (120 ms per rotation) to produce the desired 10 ms pulse.
When the shutter is normally open, even with the chopper used to attenuate the X-ray to some extent, significant jitter in the Taylor cone is still observed after the shutter opens. To further enhance the stability of the jet, the shutter and chopper are coordinated to generate pulses, the chopper’s window time is set to 10 ms, the shutter opens for 0.6 s each time, and the intervals are set to 0.6 s, 1.2 s, and 1.8 s, respectively, to further reduce the average flux. Four groups of tests were conducted with the shutter remaining open, and it was found that the stability of the jet during pulse exposure was significantly improved, shown in Figure 4. The results indicate that converting continuous X-ray exposure into pulsed X-ray exposure with specific intervals effectively mitigates the impact of air ionization on jet stability. This approach not only reduces the average X-ray flux but also facilitates the rapid recovery of the jet to a stable state during the vacuum period without X-ray exposure. Experimental results show that even the group with the shortest vacuum period could maintain good stability.

Figure 4.
In the presented figure, State 1 represents the stable condition in the absence of X-ray exposure. State 2 illustrates the significant agitation of the liquid level resulting from X-ray influence. States 3, 4, and 5 depict the Taylor cone configuration induced by X-ray pulses with intervals of 1.8 s, 1.2 s, and 0.6 s, respectively, all of which exhibit a commendable degree of stability. All 5 photos were captured by the OAV B-ZOOM on-axis microscope mounted on an MD3-UP micro-diffractometer (Arinax, Moirans, France). The purple ring with a red crosshair in the figures indicates the position of the beam center, which is calibrated by the on-axis microscope and is the location of the X-ray diffraction spot on the sample.
2.5. Crystallization of Lysozyme
The crystals used in this experiment are lysozyme protein at a concentration of 20 mg/mL, with sizes ranging from 20 to 40 µm in a pool solution containing 0.2 M citric acid, 0.2 M sodium acetate, and 8% NaCl. Due to seeding, the crystal size increases to approximately 50 µm [,,]. After obtaining the crystals, the drops are collected by a pipette from the crystallization plate. Then, the crystal solution is diluted tenfold with spinning solution and transferred into the sample chamber. To protect the crystals, 20% glycerol is added to the pool solution, which is then directly introduced into the sampler of the spinning solution microflow pump.
2.6. Data Collection and Data Processing
Utilizing the on-axis microscope affixed to the diffractometer, along with the motor system, the X-ray beam is aligned with the apex of the Taylor cone. Then, the Python (version 3.10) script is utilized to control the detector for data acquisition, ensuring that the goniometer head remains stationary throughout the data acquisition process. The X-ray diffraction data were collected at beamlines BL17UM and BL18U1. The image containing diffraction points was selected from the diffraction data, which were indexed using CrystFEL (version 0.11.1) software []. A threshold of 150, a signal-to-noise ratio of 3, and a gradient of 5000 were established as the peak-seeking parameters to index the dataset.
3. Results
We acquired several diffraction patterns of lysozyme crystals and subsequently selected them from the dataset. Following preliminary indexing, a total of 112 diffraction data sets were successfully classified as protein crystals, comprising 101 P-lattices and 11 C-lattices. The identified lattice types included 17 monoclinic, 2 orthorhombic, and 93 triclinic structures. The stream file output generated by the CrystFEL software was examined to analyze the obtained cell parameters on an individual basis. The anticipated cell parameters for lysozyme are 7.8 nm, 7.8 nm, and 3.8 nm, with angles measuring 90°, 90°, and 90°, respectively. However, the actual data revealed that only approximately 20% of the entries exhibited α, β, and γ values of 90°. Lysozyme is classified within the tetragonal crystal system, which necessitates the conditions a = b ≠ c and α = β = γ = 90°. In practice, these two conditions are not consistently satisfied. According to prior statistics, there are 16 data points that fulfill the condition a = b ≠ c alone, while 13 data points meet the requirement of α = β = γ = 90°. These erroneous cell parameters typically manifest as 5 parameters being generally consistent with the expected values (deviations < 1%); meanwhile, another parameter frequently exhibits significant deviation (either an axial angle difference exceeding 5° or an axial length discrepancy by several-fold). Nevertheless, based on the results provided by CrystFEL, the majority of these data points are categorized as belonging to the monoclinic and triclinic systems.
The diffraction pattern corresponding to the successfully indexed data was identified. Despite the software yielding indexing results and determining the diffraction pattern as being indicative of protein crystals, the majority of diffraction points in these maps exhibited very low intensities (ranging from 60 to 150), resulting in a relatively poor signal-to-noise ratio (less than 3), shown in Figure 5. The primary factor contributing to this outcome is the inadequate exposure time of the crystal, which led to inaccurate fitting results from the software during the indexing process, ultimately resulting in the failure to obtain correct unit cell parameters. Currently, the principal challenges affecting data quality in experiments conducted at the synchrotron radiation beamlines include short exposure times and insufficient luminous flux available at the station. Results from offline experiments indicate that the effective exposure time for the actual crystal is approximately 0.4 ms, which does not meet the exposure requirements given the luminous flux of conventional synchrotron radiation beamlines. To obtain diffraction data of sufficient quality using this method at synchrotron beamlines, further enhancement of X-ray flux is required, which depends on future technological breakthroughs in synchrotron radiation facilities.
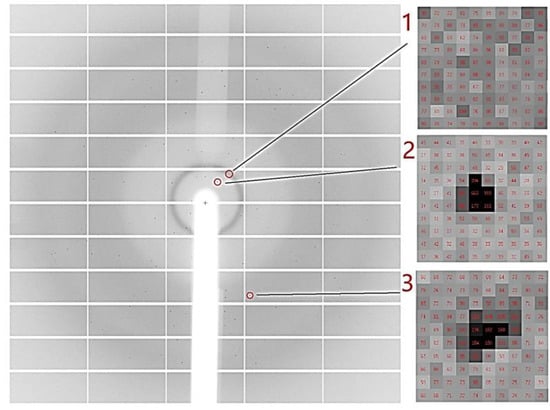
Figure 5.
Diffraction data for lysozyme displayed by software ADXV (version 1.9.14). (1) Illustrates the scattering ring of the solvent, which is classified as background scattering and does not affect data processing. (2) Presents the diffraction points in the low-resolution area (14 Å) of the inner ring, where the diffraction point intensity and signal-to-noise ratio are relatively good. (3) Depicts the diffraction points in the higher resolution area (4 Å), where both the diffraction signal intensity and signal-to-noise ratio are significantly diminished. Unfortunately, in the high-resolution region, most of the diffraction points resemble those shown in (3), resulting in an unsatisfactory final indexing outcome.
4. Conclusions and Future Work
In this study, we developed a vacuum chamber for electrospinning sample loading at a synchrotron radiation beamline. Furthermore, we established a single-pulse acquisition strategy utilizing a chopper to mitigate the effects of X-rays on the electrospinning jet. This device exhibits considerable feasibility for application at synchrotron radiation facilities, effectively addressing the instability in the loading process attributed to air ionization factors. We have successfully achieved the continuous delivery of crystal samples and, for the first time, acquired the diffraction pattern at the synchrotron radiation beamline. It lays a solid experimental foundation and provides a technical framework for the implementation of the electrospinning loading technique in synchrotron radiation environments.
Nonetheless, several challenges remain to be resolved in the application of this method at synchrotron radiation beamlines, particularly regarding inadequate exposure time. This result can primarily be attributed to the relatively low effective X-ray flux of experiments performed on the synchrotron radiation beamline, which leads to suboptimal data quality and significant inaccuracies, thereby hindering the accurate determination of unit cell parameters. Compared to the conventional fixed target method used in synchrotron radiation crystallography, the electrospinning loading technique offers reduced sample preparation costs, enables the continuous acquisition of extensive datasets, and improves data acquisition efficiency. Although the electrospinning process is slower than the GDVN method, it is more compatible with the flux characteristics of synchrotron radiation beamlines, thereby facilitating compliance with exposure time requirements. Additionally, in contrast to low flow rate techniques, such as the HVE nozzle, the electrospinning method generates lower background scattering. With advancements in technology, particularly in the field of high-brightness fourth-generation synchrotron radiation facilities, electrospinning serial crystallography technology is anticipated to have more practical applications, such as structural determination of smaller microcrystals, room-temperature protein structure analysis, and time-resolved studies.
Author Contributions
Conceptualization, J.H.; methodology, L.Y., B.S. and Z.W., software, Z.W. and Q.X. (Qingjie Xiao); validation, Q.X. (Qin Xu) and W.W.; formal analysis, L.Y.; investigation, B.S.; resources, Y.W., Q.W. and Q.X. (Qin Xu); data curation, L.Y.; writing—original draft preparation, L.Y.; writing—review and editing, B.S.; supervision, B.S. and J.H.; project administration, J.H.; funding acquisition, B.S. and Q.X. (Qingjie Xiao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32271248 and 32200988, and the Major Project in Basic and Applied Basic Research of Guangdong Province, grant number 2023B0303000003.
Data Availability Statement
The datasets collected and analyzed during the study were stored at the Data Center of Shanghai Synchrotron Radiation Facility (SSRF) and are available on request.
Acknowledgments
We thank the staff from beamline BL17UM, BL18U, and the User Experiment Assist System of Shanghai Synchrotron Radiation Facility (SSRF) for on-site assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stahlberg, H.; Fotiadis, D.; Scheuring, S.; Rémigy, H.; Braun, T.; Mitsuoka, K.; Fujiyoshi, Y.; Engel, A. Two-dimensional crystals: A powerful approach to assess structure, function and dynamics of membrane proteins. FEBS Lett. 2001, 504, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Stellato, F.; Oberthür, D.; Liang, M.; Bean, R.; Gati, C.; Yefanov, O.; Barty, A.; Burkhardt, A.; Fischer, P.; Galli, L.; et al. Room-temperature macromolecular serial crystallography using synchrotron radiation. IUCrJ 2014, 1 Pt 4, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Zeldin, O.; Gerstel, M.; Garman, E. RADDOSE-3D: Time-and space-resolved modelling of dose in macromolecular crystallography. J. Appl. Crystallogr. 2013, 46, 1225–1230. [Google Scholar] [CrossRef]
- Moukhametzianov, R.; Burghammer, M.; Edwards, P.; Petitdemange, S.; Popov, D.; Fransen, M.; McMullan, G.; Schertler, G.F.; Riekel, C. Protein crystallography with a micrometre-sized synchrotron-radiation beam. Acta Crystallogr. Sect. D Biol. Crystallogr. 2008, 64, 158–166. [Google Scholar] [CrossRef]
- Chapman, H.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef]
- Kern, J.; Alonso-Mori, R.; Hellmich, J.; Tran, R.; Hattne, J.; Laksmono, H.; Glöckner, C.; Echols, N.; Sierra, R.G.; Sellberg, J.; et al. Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. USA 2012, 109, 9721–9726. [Google Scholar] [CrossRef]
- Wright, J. Serial crystallography for the masses? IUCrJ 2015, 2, 3–4. [Google Scholar] [CrossRef]
- Boutet, S.; Lomb, L.; Williams, G.; Barends, T.R.M.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef]
- Huang, N.; Deng, H.; Liu, B.; Wang, D.; Zhao, Z. Features and futures of X-ray free-electron lasers. Innovation 2021, 2, 100097. [Google Scholar] [CrossRef]
- Hattne, J.; Echols, N.; Tran, R.; Kern, J.; Gildea, R.J.; Brewster, A.S.; Alonso-Mori, R.; Glöckner, C.; Hellmich, J.; Laksmono, H.; et al. Accurate macromolecular structures using minimal measurements from X-ray free-electron lasers. Nat. Methods 2014, 11, 545–548. [Google Scholar] [CrossRef]
- Heymann, M.; Opthalage, A.; Wierman, J.; Akella, S.; Szebenyi, D.M.E.; Gruner, S.M.; Fraden, S. Room-temperature serial crystallography using a kinetically optimized microfluidic device for protein crystallization and on-chip X-ray diffraction. IUCrJ 2014, 1, 349–360. [Google Scholar] [CrossRef]
- Roedig, P.; Vartiainen, I.; Duman, R.; Panneerselvam, S.; Stübe, N.; Lorbeer, O.; Warmer, M.; Sutton, G.; Stuart, D.I.; Weckert, E.; et al. A micro-patterned silicon chip as sample holder for macromolecular crystallography experiments with minimal background scattering. Sci. Rep. 2015, 5, 10451. [Google Scholar] [CrossRef] [PubMed]
- Coquelle, N.; Brewster, A.; Kapp, U.; Shilova, A.; Weinhausen, B.; Burghammer, M.; Colletier, J.-P. Raster-scanning serial protein crystallography using micro-and nano-focused synchrotron beams. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- DePonte, D.; Weierstall, U.; Schmidt, K.; Warner, J.; Starodub, D.; Spence, J.C.H.; Doak, R.B. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D Appl. Phys. 2008, 41, 195505. [Google Scholar] [CrossRef]
- Weierstall, U.; Spence, J.C.H.; Doak, R.B. Injector for scattering measurements on fully solvated biospecies. Rev. Sci. Instrum. 2012, 83, 035108. [Google Scholar] [CrossRef]
- Sierra, R.G.; Laksmono, H.; Kern, J.; Tran, R.; Hattne, J.; Alonso-Mori, R.; Lassalle-Kaiser, B.; Glöckner, C.; Hellmich, J.; Schafer, D.W.; et al. Nanoflow electrospinning serial femtosecond crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1584–1587. [Google Scholar] [CrossRef]
- Weierstall, U.; James, D.; Wang, C.; White, T.A.; Wang, D.; Liu, W.; Spence, J.C.H.; Doak, R.B.; Nelson, G.; Fromme, P.; et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014, 5, 3309. [Google Scholar] [CrossRef]
- Conrad, C.; Basu, S.; James, D.; Wang, D.; Schaffer, A.; Roy-Chowdhury, S.; Zatsepin, N.A.; Aquila, A.; Coe, J.; Gati, C.; et al. A novel inert crystal delivery medium for serial femtosecond crystallography. IUCrJ 2015, 2, 421–430. [Google Scholar] [CrossRef]
- Kang, Y.; Zhou, X.; Gao, X.; He, Y.; Liu, W.; Ishchenko, A.; Barty, A.; White, T.A.; Yefanov, O.; Han, G.W.; et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 2015, 523, 561–567. [Google Scholar] [CrossRef]
- Nam, K. Stable sample delivery in viscous media via a capillary for serial crystallography. J. Appl. Crystallogr. 2020, 53, 45–50. [Google Scholar] [CrossRef]
- Panneels, V.; Wu, W.; Tsai, C.; Nogly, P.; Rheinberger, J.; Jaeger, K.; Cicchetti, G.; Gati, C.; Kick, L.M.; Sala, L.; et al. Time-resolved structural studies with serial crystallography: A new light on retinal proteins. Struct. Dyn. 2015, 2, 041718. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Hohman, M.; Brenner, M.; Rutledge, G.C. Experimental characterization of electrospinning: The electrically forced jet and instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
- Ku, B.; Kim, S. Electrohydrodynamic spraying characteristics of glycerol solutions in vacuum. J. Electrost. 2003, 57, 109–128. [Google Scholar] [CrossRef]
- Carpineti, M.; Piazza, R. Metastability and supersaturation limit for lysozyme crystallization. Phys. Chem. Chem. Phys. 2004, 6, 1506–1511. [Google Scholar] [CrossRef]
- Cacioppo, E.; Pusey, M. The solubility of the tetragonal form of hen egg white lysozyme from pH 4.0 to 5.4. J. Cryst. Growth 1991, 114, 286–292. [Google Scholar] [CrossRef]
- Falkner, C.J.; Al-Somali, A.; Jamison, J.; Zhang, J.; Adrianse, S.L.; Simpson, R.L.; Calabretta, M.K.; Radding, W.; Phillips, G.N.; Colvin, V.L. Generation of size-controlled, submicrometer protein crystals. Chem. Mater. 2005, 17, 2679–2686. [Google Scholar] [CrossRef]
- White, T.; Kirian, R.; Martin, A.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).