Experimental Setup for Irradiation of Cell Cultures at L2A2
Abstract
1. Introduction
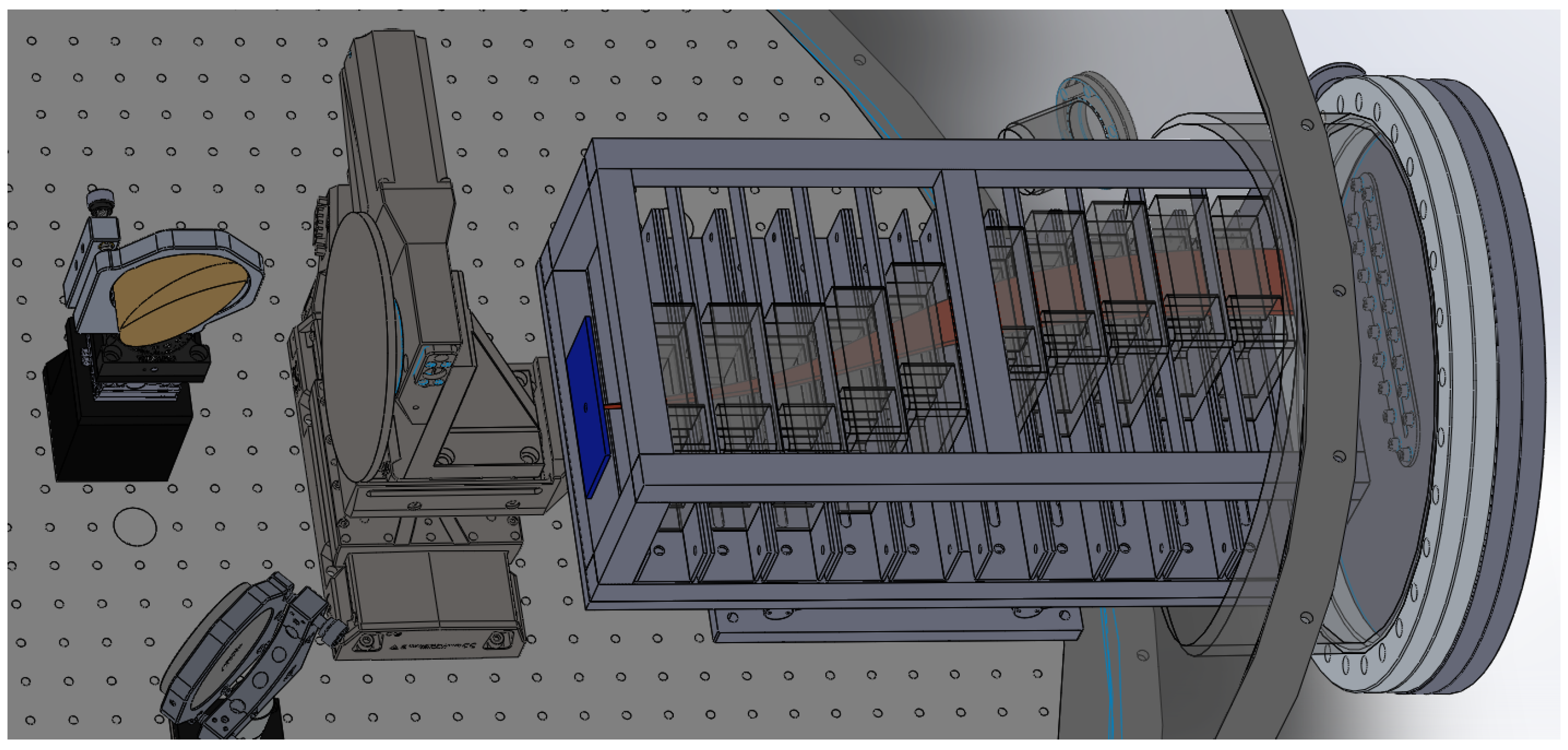
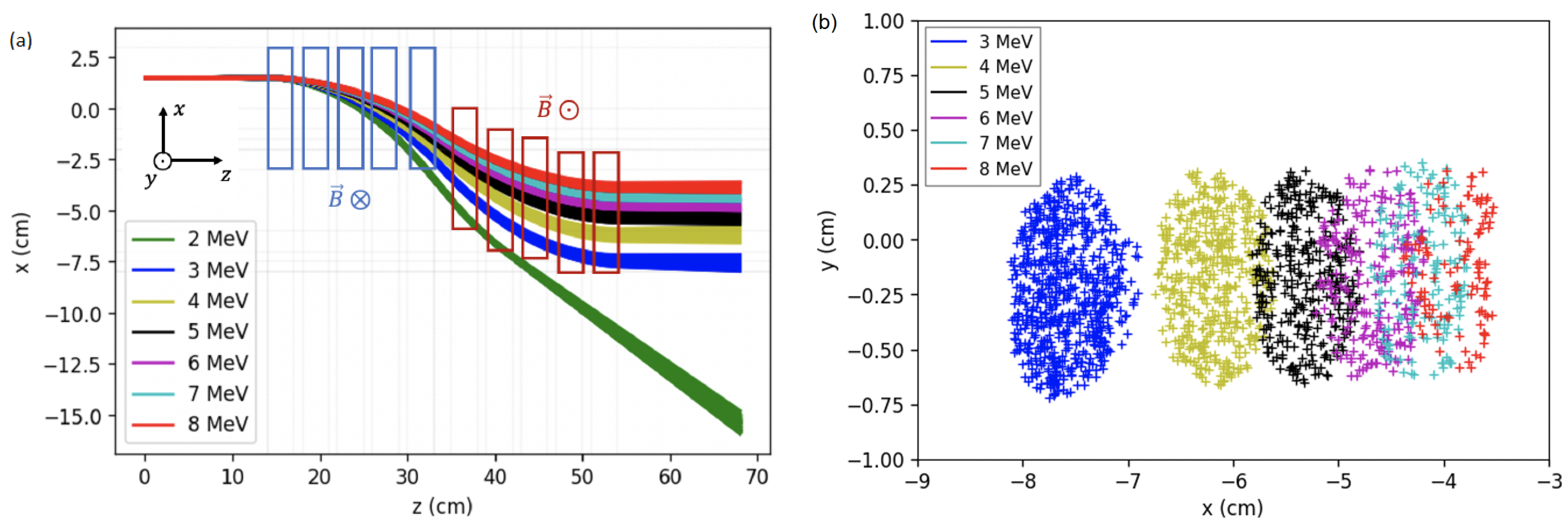
2. Energy Selector
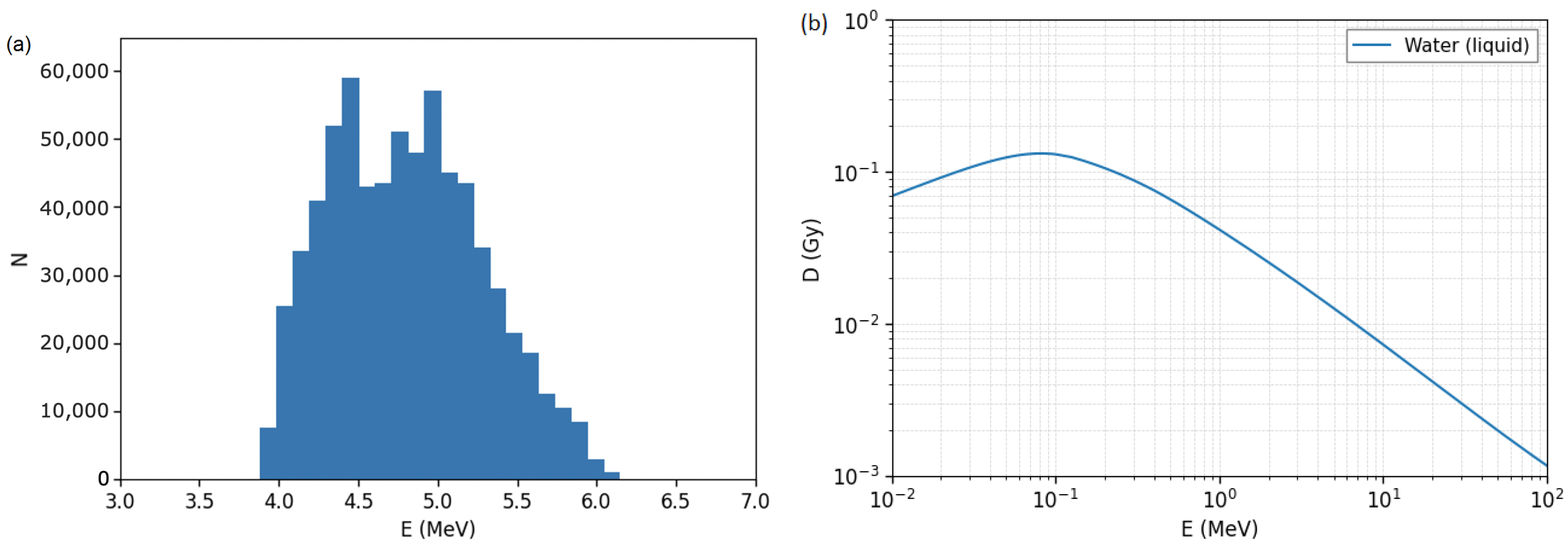
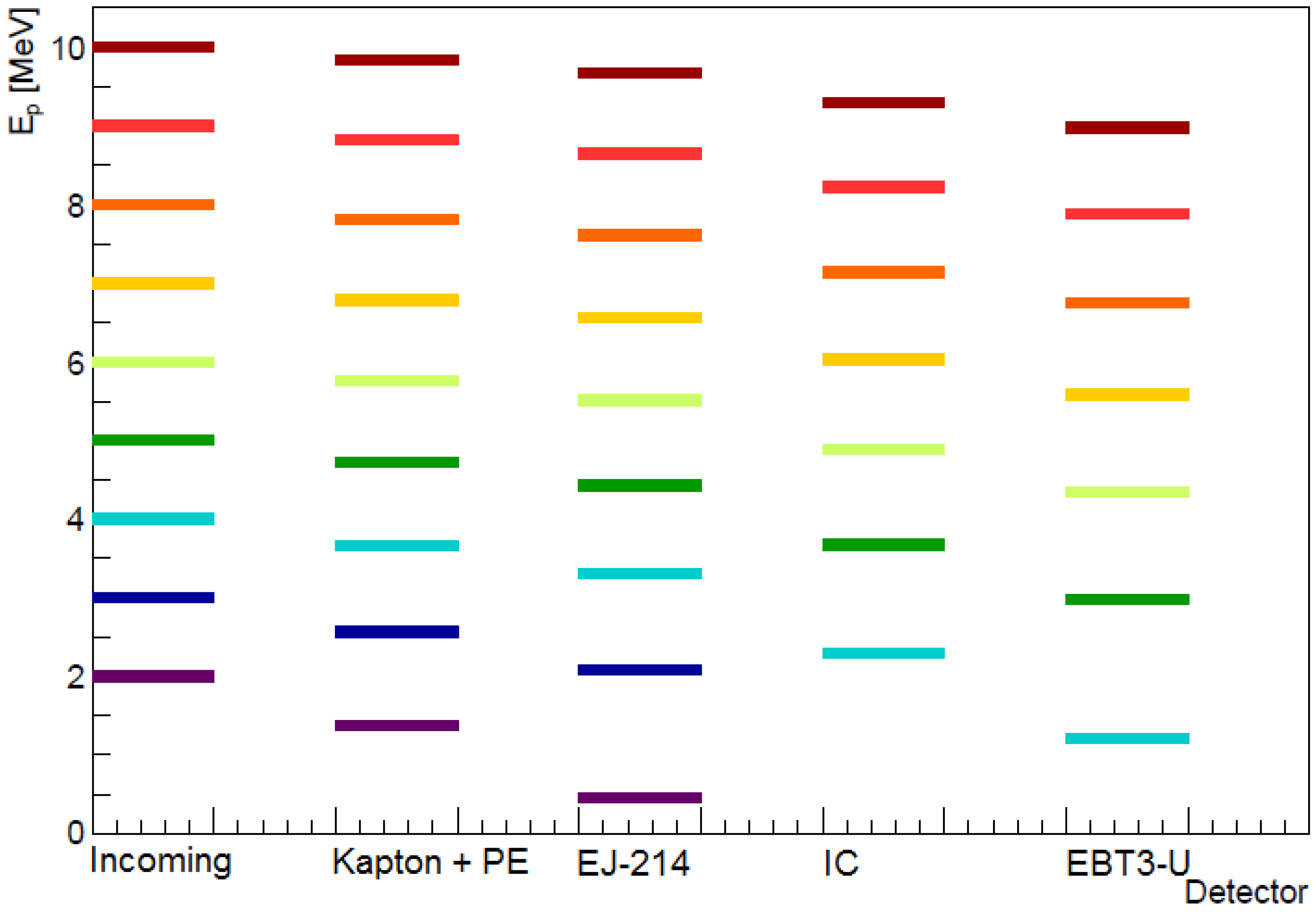
3. Particle Fluence Detectors
4. Cell Cultures
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Friedrich, T.; Scholz, U.; Elsässer, T.; Durante, M.; Scholz, M. Systematic analysis of RBE and related quantities using a database of cell survival experiments with ion beam irradiation. J. Radiat. Res. 2012, 54, 494–514. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Pfuhl, T.; Scholz, M. Update of the particle irradiation data ensemble (PIDE) for cell survival. J. Radiat. Res. 2021, 62, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, N.T.; Sokol, O.; Durante, M.; De Marzi, L.; Pouzoulet, F.; Miszczyk, J.; Olko, P.; Brandenburg, S.; van Goethem, M.J.; Barazzuol, L.; et al. Mapping the Future of Particle Radiobiology in Europe: The INSPIRE Project. Front. Phys. 2020, 8, 565055. [Google Scholar] [CrossRef]
- Patera, V.; Prezado, Y.; Azaiez, F.; Battistoni, G.; Bettoni, D.; Brandenburg, S.; Bugay, A.; Cuttone, G.; Dauvergne, D.; de France, G.; et al. Biomedical Research Programs at Present and Future High-Energy Particle Accelerators. Front. Phys. 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Wéra, A.C.; Riquier, H.; Heuskin, A.C.; Michiels, C.; Lucas, S. In vitro irradiation station for broad beam radiobiological experiments. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 3120–3124. [Google Scholar] [CrossRef]
- Manti, L.; Campajola, L.; Perozziello, F.M.; Kavanagh, J.N.; Schettino, G. Development of a low-energy particle irradiation facility for the study of the biological effectiveness of the ion track end. J. Phys. Conf. Ser. 2012, 373, 012019. [Google Scholar] [CrossRef]
- Baratto-Roldán, A.; Jiménez-Ramos, M.d.C.; Jimeno, S.; Huertas, P.; García-López, J.; Gallardo, M.I.; Cortés-Giraldo, M.A.; Espino, J.M. Preparation of a radiobiology beam line at the 18 MeV proton cyclotron facility at CNA. Phys. Medica Eur. J. Med Phys. 2020, 74, 19–29. [Google Scholar] [CrossRef]
- Viñals, S.; Sánchez-Parcerisa, D.; Fraile, L.M.; España, S.; García, G.; García-Díaz, M.; SánchezTembleque, V.; Udías, J.M. Characterization of the proton pulsed beam at CMAM. EPJ Web Conf. 2021, 253, 04027. [Google Scholar] [CrossRef]
- Auer, S.; Hable, V.; Greubel, C.; Drexler, G.A.; Schmid, T.E.; Belka, C.; Dollinger, G.; Friedl, A.A. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat. Oncol. 2011, 6, 139. [Google Scholar] [CrossRef]
- Hughes, J.R.; Parsons, J.L. FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy. Int. J. Mol. Sci. 2020, 21, 6492. [Google Scholar] [CrossRef]
- Ledingham, K.; Bolton, P.; Shikazono, N.; Ma, C.M. Towards Laser Driven Hadron Cancer Radiotherapy: A Review of Progress. Appl. Sci. 2014, 4, 402–443. [Google Scholar] [CrossRef]
- Yogo, A.; Sato, K.; Nishikino, M.; Mori, M.; Teshima, T.; Numasaki, H.; Murakami, M.; Demizu, Y.; Akagi, S.; Nagayama, S.; et al. Application of laser-accelerated protons to the demonstration of DNA double-strand breaks in human cancer cells. Appl. Phys. Lett. 2009, 94, 181502. [Google Scholar] [CrossRef]
- Chaudhary, P.; Milluzzo, G.; Ahmed, H.; Odlozilik, B.; McMurray, A.; Prise, K.M.; Borghesi, M. Radiobiology Experiments With Ultra-high Dose Rate Laser-Driven Protons: Methodology and State-of-the-Art. Front. Phys. 2021, 9, 75. [Google Scholar] [CrossRef]
- Rösch, T.F.; Szabó, Z.; Haffa, D.; Bin, J.; Brunner, S.; Englbrecht, F.S.; Friedl, A.A.; Gao, Y.; Hartmann, J.; Hilz, P.; et al. A feasibility study of zebrafish embryo irradiation with laser-accelerated protons. Rev. Sci. Instrum. 2020, 91, 063303. [Google Scholar] [CrossRef] [PubMed]
- Brack, F.E.; Kroll, F.; Gaus, L.; Bernert, C.; Beyreuther, E.; Cowan, T.E.; Karsch, L.; Kraft, S.; Kunz-Schughart, L.A.; Lessmann, E.; et al. Spectral and spatial shaping of laser-driven proton beams using a pulsed high-field magnet beamline. Sci. Rep. 2020, 10, 9118. [Google Scholar] [CrossRef] [PubMed]
- Kraft, S.D.; Richter, C.; Zeil, K.; Baumann, M.; Beyreuther, E.; Bock, S.; Bussmann, M.; Cowan, T.E.; Dammene, Y.; Enghardt, W.; et al. Dose-dependent biological damage of tumour cells by laser-accelerated proton beams. New J. Phys. 2010, 12, 085003. [Google Scholar] [CrossRef]
- Bin, J.; Allinger, K.; Assmann, W.; Dollinger, G.; Drexler, G.A.; Friedl, A.A.; Habs, D.; Hilz, P.; Hoerlein, R.; Humble, N.; et al. A laser-driven nanosecond proton source for radiobiological studies. Appl. Phys. Lett. 2012, 101, 243701. [Google Scholar] [CrossRef]
- Zeil, K.; Baumann, M.; Beyreuther, E.; Burris-Mog, T.; Cowan, T.E.; Enghardt, W.; Karsch, L.; Kraft, S.D.; Laschinsky, L.; Metzkes, J.; et al. Dose-controlled irradiation of cancer cells with laser-accelerated proton pulses. Appl. Phys. B 2013, 110, 437–444. [Google Scholar] [CrossRef]
- Raschke, S.; Spickermann, S.; Toncian, T.; Swantusch, M.; Boeker, J.; Giesen, U.; Iliakis, G.; Willi, O.; Boege, F. Ultra-short laser-accelerated proton pulses have similar DNA-damaging effectiveness but produce less immediate nitroxidative stress than conventional proton beams. Sci. Rep. 2016, 6, 32441. [Google Scholar] [CrossRef]
- Pommarel, L.; Vauzour, B.; Mégnin-Chanet, F.; Bayart, E.; Delmas, O.; Goudjil, F.; Nauraye, C.; Letellier, V.; Pouzoulet, F.; Schillaci, F.; et al. Spectral and spatial shaping of a laser-produced ion beam for radiation-biology experiments. Phys. Rev. Accel. Beams 2017, 20, 032801. [Google Scholar] [CrossRef]
- Peñas, J.; Cortina-Gil, D.; Martín, L.; Ruiz, C.; Seimetz, M.; Benlliure, J. A Multi-Shot Wheel-Target Assembly for Laser-Plasma Proton Acceleration. High Power Laser Sci. Eng. 2021, submitted.
- Roth, M.; Schollmeier, M. Ion Acceleration-Target Normal Sheath Acceleration. CERN Yellow Rep. 2016, 1, 231. [Google Scholar]
- Fiorini, F.; Kirby, D.; Borghesi, M.; Doria, D.; Jeynes, J.C.G.; Kakolee, K.F.; Kar, S.; Litt, S.K.; Kirkby, K.J.; Merchant, M.J.; et al. Dosimetry and spectral analysis of a radiobiological experiment using laser-driven proton beams. Phys. Med. Biol. 2011, 56, 6969–6982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doria, D.; Kakolee, K.F.; Kar, S.; Litt, S.K.; Fiorini, F.; Ahmed, H.; Green, S.; Jeynes, J.C.G.; Kavanagh, J.; Kirby, D.; et al. Biological effectiveness on live cells of laser driven protons at dose rates exceeding 109 Gy/s. AIP Adv. 2012, 2, 011209. [Google Scholar] [CrossRef]
- Hanton, F.; Chaudhary, P.; Doria, D.; Gwynne, D.; Maiorino, C.; Scullion, C.; Ahmed, H.; Marshall, T.; Naughton, K.; Romagnani, L.; et al. DNA DSB Repair Dynamics following Irradiation with Laser-Driven Protons at Ultra-High Dose Rates. Sci. Rep. 2019, 9, 4471. [Google Scholar] [CrossRef]
- Yogo, A.; Maeda, T.; Hori, T.; Sakaki, H.; Ogura, K.; Nishiuchi, M.; Sagisaka, A.; Kiriyama, H.; Okada, H.; Kanazawa, S.; et al. Measurement of relative biological effectiveness of protons in human cancer cells using a laser-driven quasimonoenergetic proton beamline. Appl. Phys. Lett. 2011, 98, 053701. [Google Scholar] [CrossRef]
- Schillaci, F.; Pommarel, L.; Romano, F.; Cuttone, G.; Costa, M.; Giove, D.; Maggiore, M.; Russo, A.; Scuderi, V.; Malka, V.; et al. Characterization of the ELIMED Permanent Magnets Quadrupole system prototype with laser-driven proton beams. J. Instrum. 2016, 11, T07005. [Google Scholar] [CrossRef]
- Fritzler, S.; Malka, V.; Grillon, G.; Rousseau, J.P.; Burgy, F.; Lefebvre, E.; d’Humières, E.; McKenna, P.; Ledingham, K.W.D. Proton beams generated with high-intensity lasers: Applications to medical isotope production. Appl. Phys. Lett. 2003, 83, 3039–3041. [Google Scholar] [CrossRef]
- Manti, L.; Perozziello, F.; Borghesi, M.; Candiano, G.; Chaudhary, P.; Cirrone, G.; Doria, D.; Gwynne, D.; Leanza, R.; Prise, K.M.; et al. The radiobiology of laser-driven particle beams: Focus on sub-lethal responses of normal human cells. J. Instrum. 2017, 12, C03084. [Google Scholar] [CrossRef][Green Version]
- Bayart, E.; Flacco, A.; Delmas, O.; Pommarel, L.; Levy, D.; Cavallone, M.; Megnin-Chanet, F.; Deutsch, E.; Malka, V. Fast dose fractionation using ultra-short laser accelerated proton pulses can increase cancer cell mortality, which relies on functional PARP1 protein. Sci. Rep. 2019, 9, 10132. [Google Scholar] [CrossRef]
- Berger, M.J.; Coursey, J.S.; Zucker, M.A.; Chang, J. Stopping-power and range tables for electrons, protons, and helium ions. In NIST Standard Reference Database 124; NIST: Gaithersburg, MD, USA, 1998. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM – The Stopping Range of Ions in Matter (2010). Nucl. Instrum. Meth. Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Seimetz, M.; Peñas, J.; Llerena, J.; Benlliure, J.; García López, J.; Millán-Callado, M.; Benlloch, J. PADC nuclear track detector for ion spectroscopy in laser-plasma acceleration. Phys. Medica Eur. J. Med. Phys. 2020, 76, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Milluzzo, G.; Ahmed, H.; Romagnani, L.; Doria, D.; Chaudhary, P.; Maiorino, C.; McIlvenny, A.; McMurray, A.; Polin, K.; Katzir, Y.; et al. Dosimetry of laser-accelerated carbon ions for cell irradiation at ultra-high dose rate. J. Phys. Conf. Ser. 2020, 1596, 012038. [Google Scholar] [CrossRef]
- Seimetz, M.; Bellido, P.; Soriano, A.; García López, J.; Jiménez-Ramos, M.C.; Fernández, B.; Conde, P.; Crespo, E.; González, A.J.; Hernández, L.; et al. Calibration and Performance Tests of Detectors for Laser-Accelerated Protons. IEEE Trans. Nucl. Sci. 2015, 62, 3216–3224. [Google Scholar] [CrossRef]
- Richter, C.; Karsch, L.; Dammene, Y.; Kraft, S.D.; Metzkes, J.; Schramm, U.; Schürer, M.; Sobiella, M.; Weber, A.; Zeil, K.; et al. A dosimetric system for quantitative cell irradiation experiments with laser-accelerated protons. Phys. Med. Biol. 2011, 56, 1529–1543. [Google Scholar] [CrossRef]
- Di Martino, F.; Giannelli, M.; Traino, A.C.; Lazzeri, M. Ion recombination correction for very high dose-per-pulse high-energy electron beams. Med. Phys. 2005, 32, 2204–2210. [Google Scholar] [CrossRef]
- Suckert, T.; Nexhipi, S.; Dietrich, A.; Koch, R.; Kunz-Schughart, L.A.; Bahn, E.; Beyreuther, E. Models for Translational Proton Radiobiology-From Bench to Bedside and Back. Cancers 2021, 13, 4216. [Google Scholar] [CrossRef]
| (mm) | (cm) | E (MeV) | (mGy) |
|---|---|---|---|
| MeV: | |||
| 50 | 2.9–4.9 | ||
| 70 | 3.1–4.7 | ||
| 100 | 3.3–4.4 | ||
| MeV: | |||
| 50 | 3.7–6.4 | ||
| 70 | 3.8–6.1 | ||
| 100 | 4.0–5.8 | ||
| MeV: | |||
| 50 | 4.3–8.1 | ||
| 70 | 4.7–7.6 | ||
| 100 | 5.0–7.2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torralba, A.; Palenciano, L.; Reija, A.; Rigla, J.P.; Peñas, J.; Llerena, J.J.; Contreras-Martínez, R.; Benlliure, J.; Vega, A.; Aguado-Barrera, M.E.; et al. Experimental Setup for Irradiation of Cell Cultures at L2A2. Quantum Beam Sci. 2022, 6, 10. https://doi.org/10.3390/qubs6010010
Torralba A, Palenciano L, Reija A, Rigla JP, Peñas J, Llerena JJ, Contreras-Martínez R, Benlliure J, Vega A, Aguado-Barrera ME, et al. Experimental Setup for Irradiation of Cell Cultures at L2A2. Quantum Beam Science. 2022; 6(1):10. https://doi.org/10.3390/qubs6010010
Chicago/Turabian StyleTorralba, Alberto, Lidia Palenciano, Alicia Reija, Juan Pablo Rigla, Juan Peñas, Juan José Llerena, Ramiro Contreras-Martínez, José Benlliure, Ana Vega, Miguel Elías Aguado-Barrera, and et al. 2022. "Experimental Setup for Irradiation of Cell Cultures at L2A2" Quantum Beam Science 6, no. 1: 10. https://doi.org/10.3390/qubs6010010
APA StyleTorralba, A., Palenciano, L., Reija, A., Rigla, J. P., Peñas, J., Llerena, J. J., Contreras-Martínez, R., Benlliure, J., Vega, A., Aguado-Barrera, M. E., Ruiz, C., & Seimetz, M. (2022). Experimental Setup for Irradiation of Cell Cultures at L2A2. Quantum Beam Science, 6(1), 10. https://doi.org/10.3390/qubs6010010









