Desorption of Implanted Deuterium in Heavy Ion-Irradiated Zry-2
Abstract
1. Introduction
2. Experimental Procedures
3. Results and Discussions
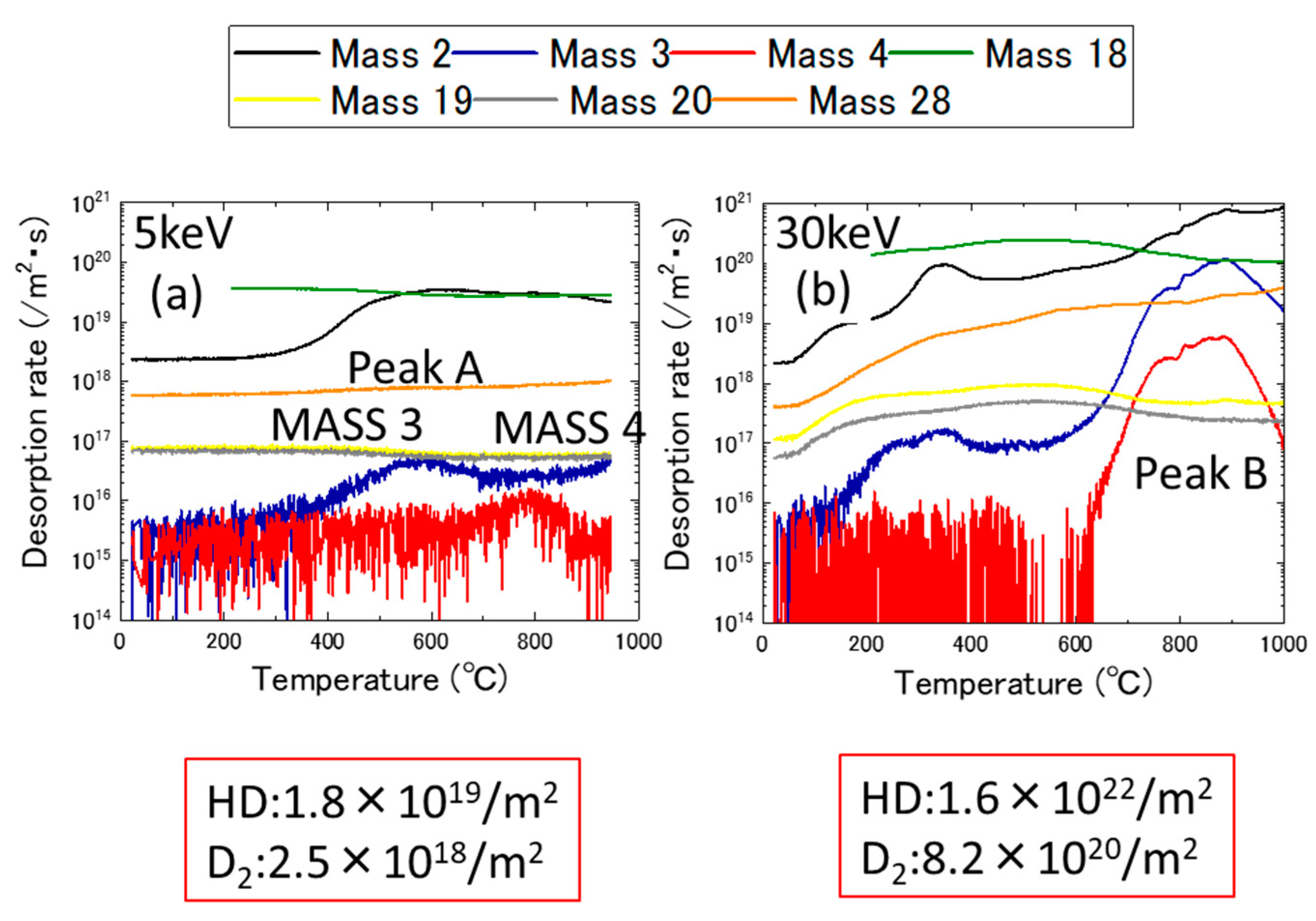
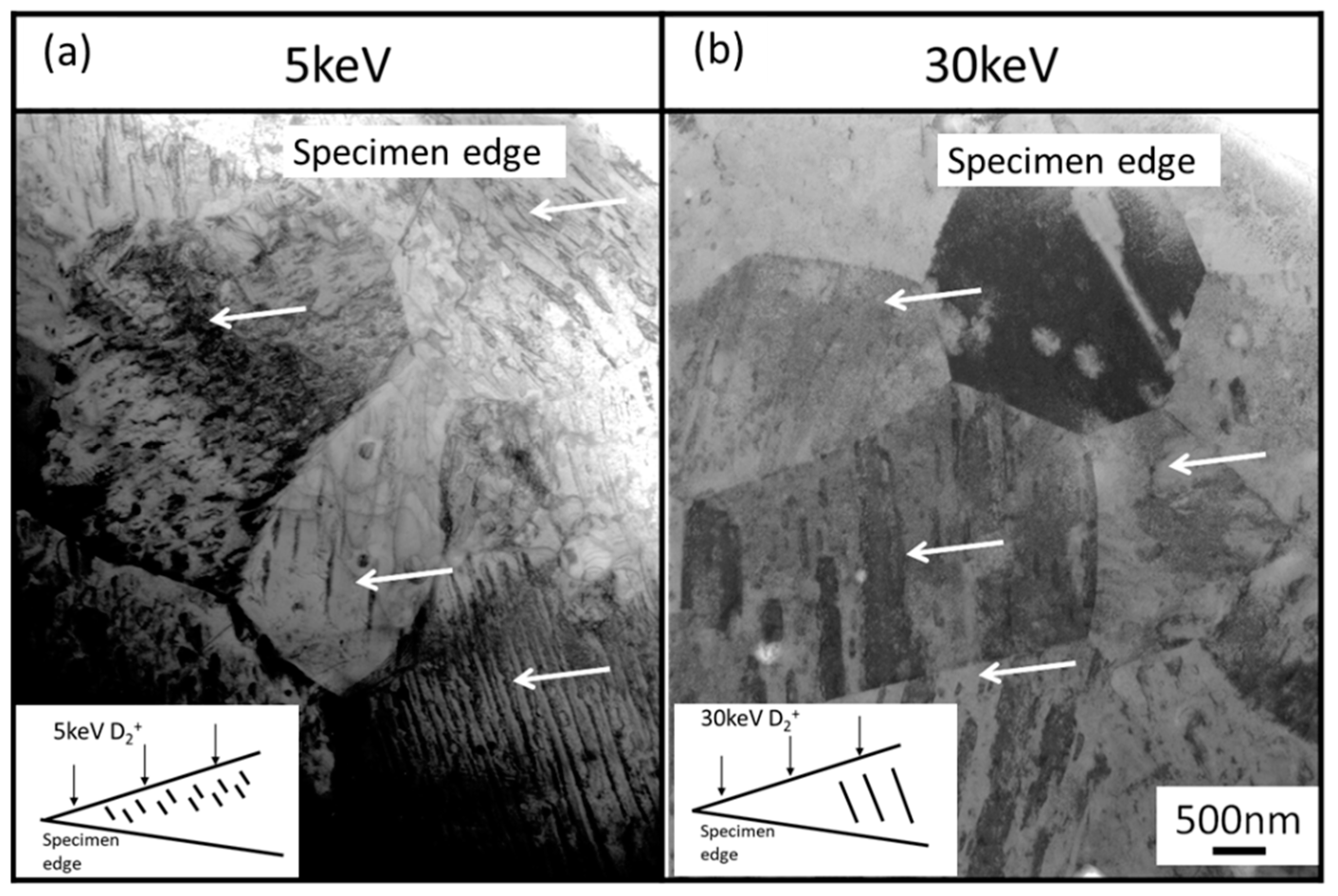
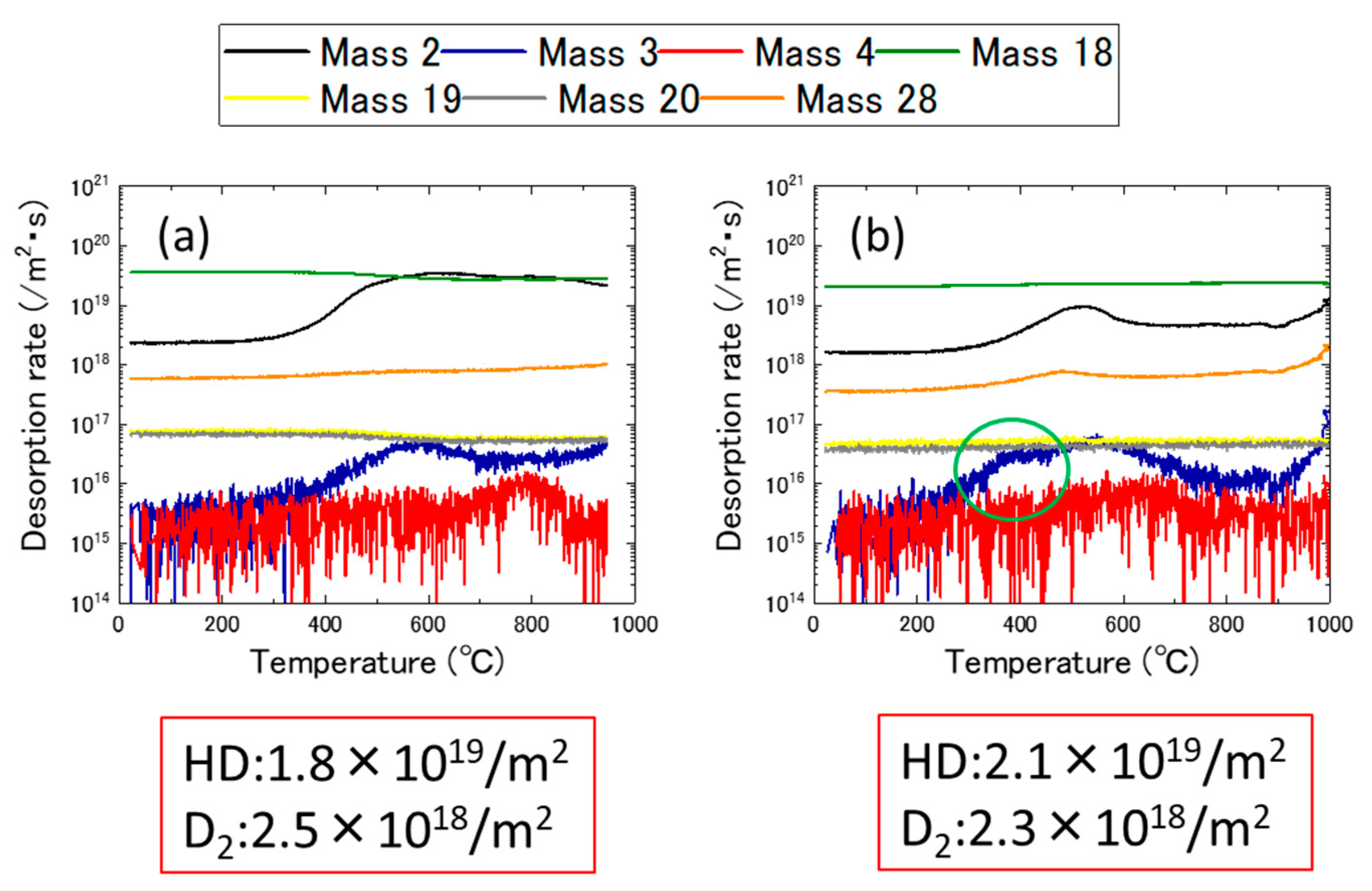
3.1. Ion Dose and Energy Dependance of Thermal Desorption Behavior
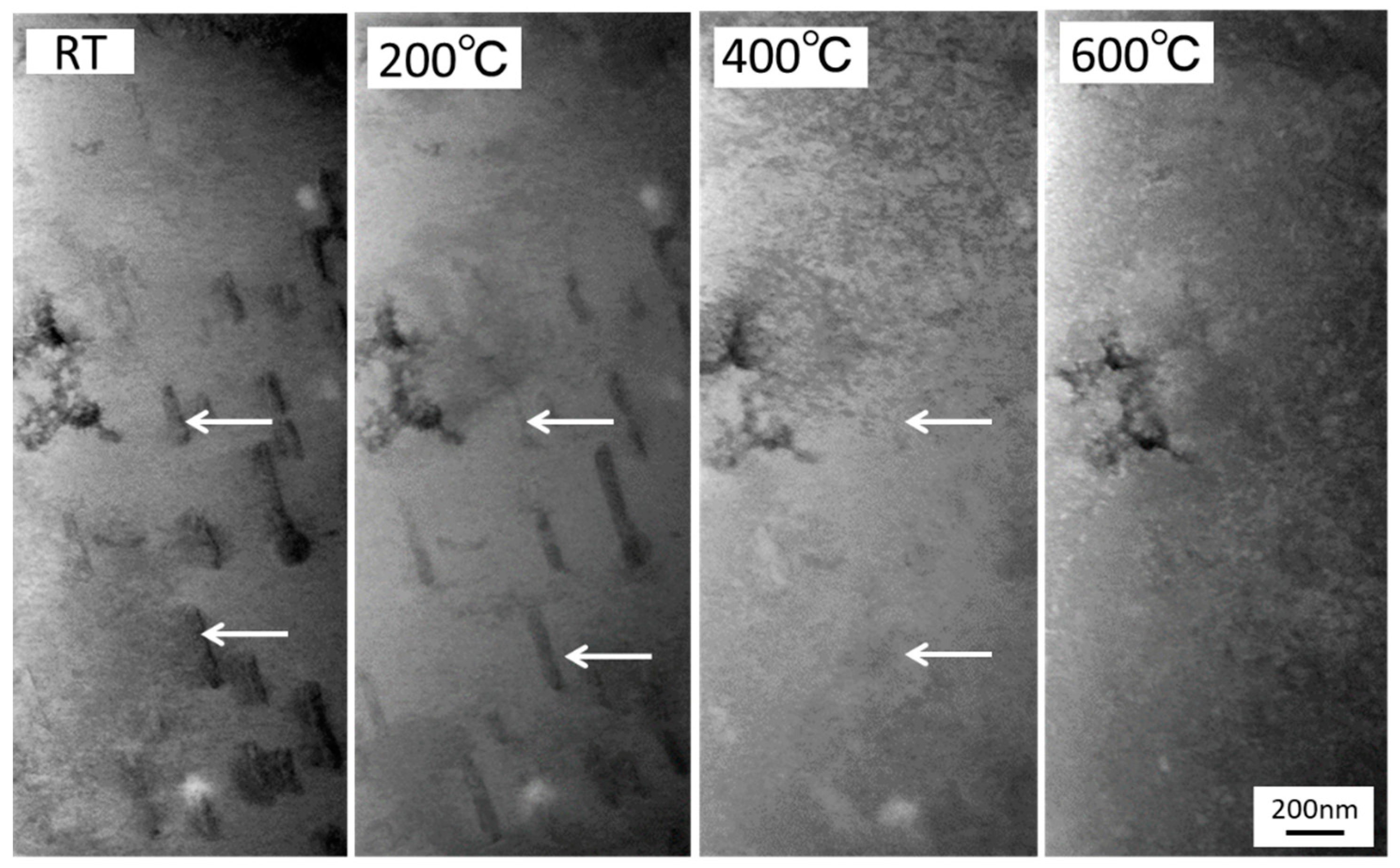
3.2. Effects of Nickel Ion Irradiation
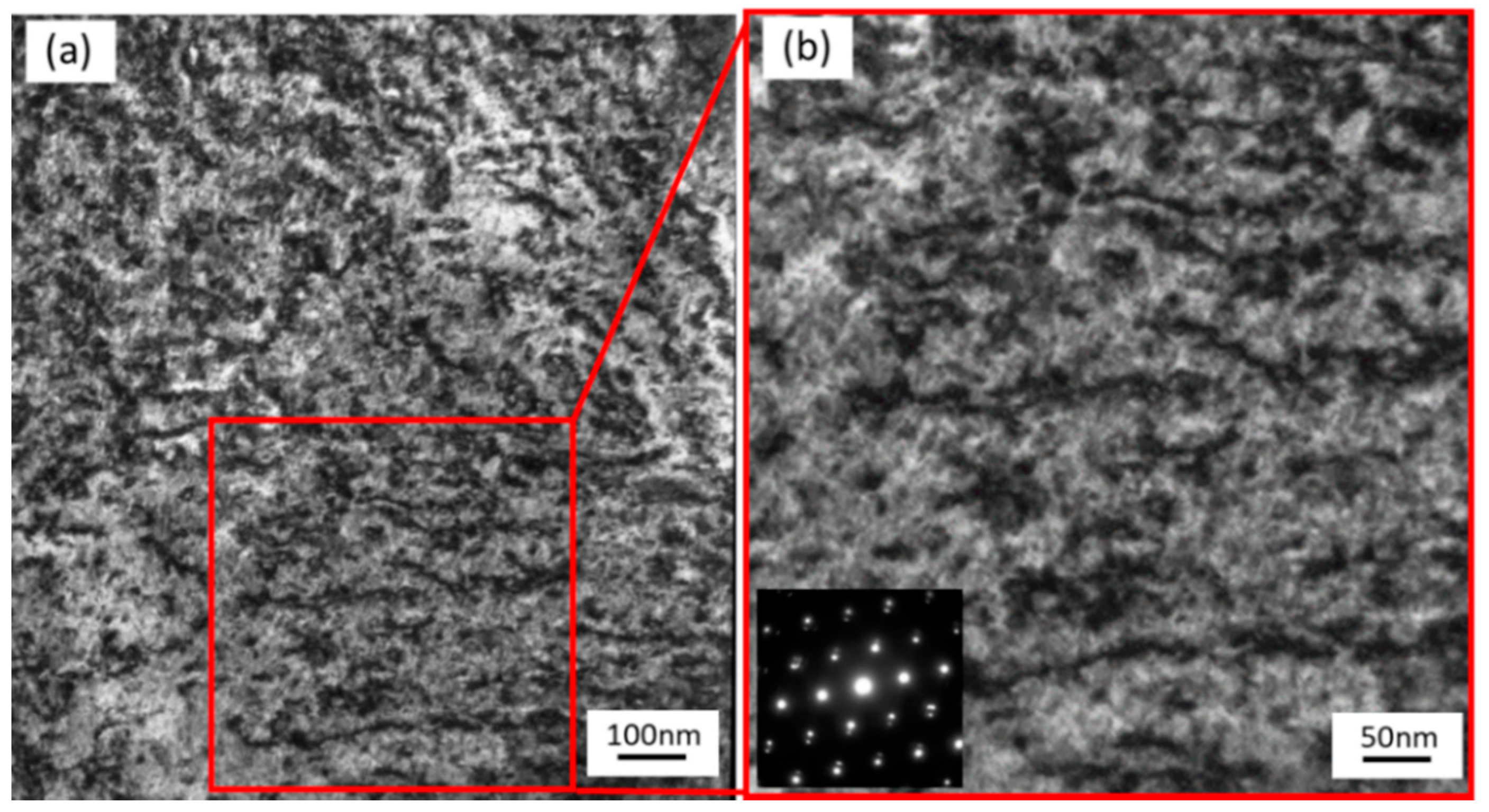
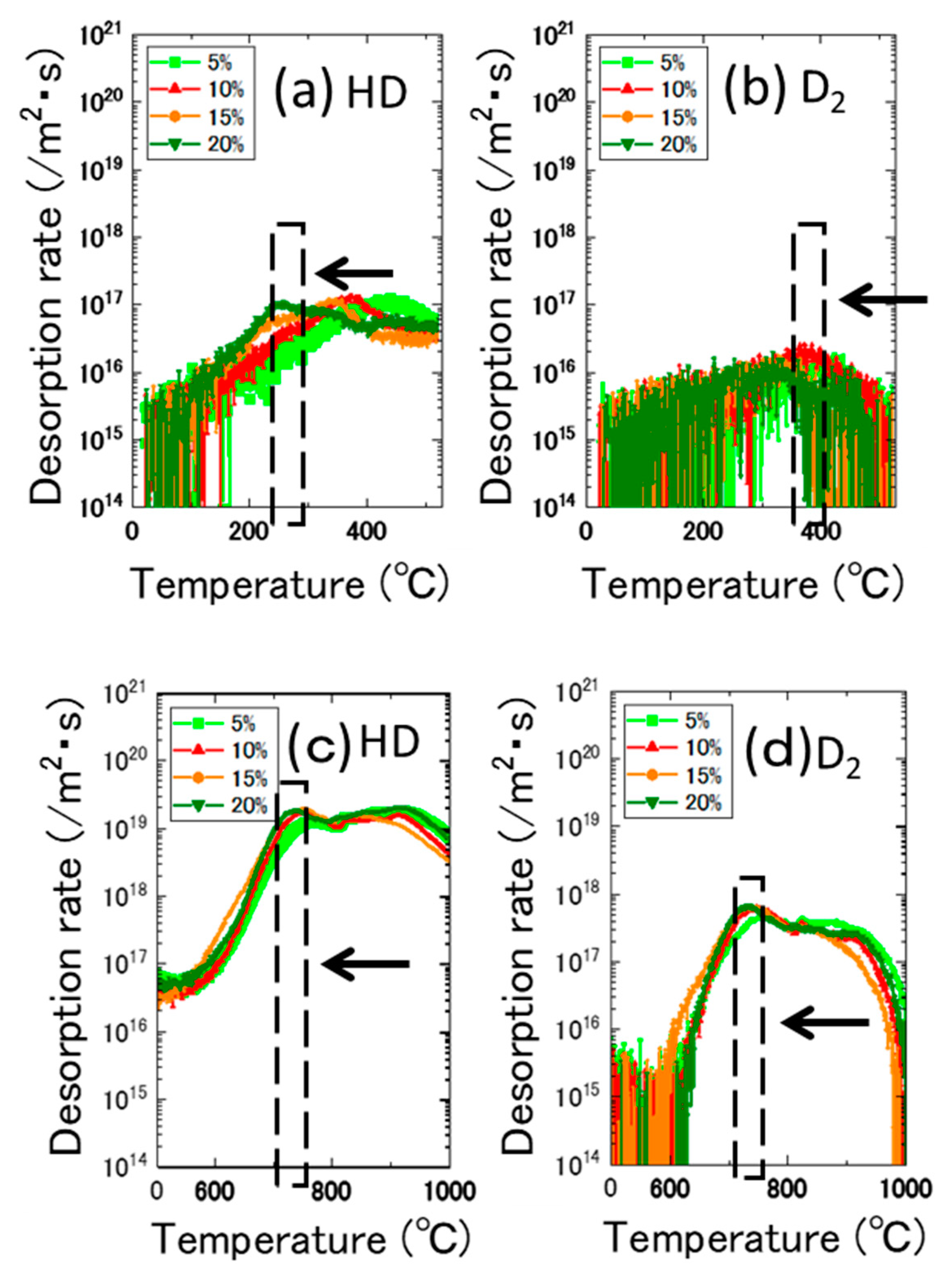
3.3. Effects of Dislocations Formed by Cold Work
4. Summary
- (1)
- The desorption spectrum shows two major desorption stages (named Peak A and Peak B) in the temperature ranges of 400–600 °C and 700–900 °C, respectively.
- (2)
- As the thermal annealing experiments of ion-irradiated samples and cold-worked samples demonstrated, stage A corresponded to the recovery stage of weak trapping sites (i.e., the dislocation loops formed by irradiation and the hydrides that formed in the surface region of the specimens).
- (3)
- Stage B corresponds to the strong trapping sites explained by the tangled dislocations and hydrides that formed in the thick region of the specimens.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jonstsons, A.; Kelly, P.M.; Blake, R.G.; Farrell, K. Neutron Irradiation-Induced Defect Structure in Zirconium. ASTM STP 1979, 683, 46–61. [Google Scholar]
- Griffiths, M. A Review of Microstructure Evolution in Zirconium Alloys During Irradiation. J. Nucl. Mater. 1988, 159, 190–218. [Google Scholar] [CrossRef]
- Griffiths, M.; Loretto, M.H.; Smallman, R.E. Electron Damage in Zirconium. J. Nucl. Mater. 1983, 115, 313–332. [Google Scholar] [CrossRef]
- Griffiths, M.; Styles, R.C.; Woo, C.H.; Pillipp, F.; Frank, W. Study of Point Defect Mobilities in Zirconium During Electron Irradiation in a High-Voltage Electron Microscope. J. Nucl. Mater. 1944, 208, 324–334. [Google Scholar] [CrossRef]
- Lee, D.; Koch, E.F. Irradiation Damage in Zircaloy-2 Produced by High Dose Ion Bombardment. J. Nucl. Mater. 1974, 50, 162–174. [Google Scholar] [CrossRef]
- Tournadre, L.; Onimus, F.; Bechade, J.-L.; Gilbon, D.; Cloue, J.-M.; Mardon, J.-P.; Feaugas, X.; Toader, O.; Bachelet, C. Experimental Study of the Nucleation and Growth of C-Component Loops Under Charge Particle Irradiation of Recrystallized Zircaloy-4. J. Nucl. Mater. 2012, 425, 76–82. [Google Scholar] [CrossRef]
- Tournadre, L.; Onimus, F.; Bechade, J.-L.; Gilbon, D.; Cloue, J.-M.; Mardon, J.-P.; Feaugas, X. Toward a Better Understanding of the Hydrogen Impact on the Radiation Induced Growth of Zirconium Alloys. J. Nucl. Mater. 2013, 441, 222–231. [Google Scholar] [CrossRef]
- Yamada, S.; Kameyama, T. Observation of C-Component Dislocation Structures Formed in Pure Zr and Zr-base Alloy by Self-Ion Accelerator Irradiation. J. Nucl. Mater. 2012, 422, 167–172. [Google Scholar] [CrossRef]
- Shinohara, Y.; Abe, H.; Iwai, T.; Sekimura, N.; Kido, T.; Yamamoto, H.; Taguchi, T. In situ TEM Observation of Growth Process of Zirconium Hydride in Zircaloy-4 During Hydrogen Ion Implantation. J. Nucl. Sci. Technol. 2009, 46, 564–571. [Google Scholar] [CrossRef]
- Idress, Y.; Yao, Z.; Kirk, M.A.; Daymond, M.R. In situ Study of Defect Accumulation in Zirconium Under Heavy Ion Irradiation. J. Nucl. Mater. 2013, 433, 95–107. [Google Scholar] [CrossRef]
- Chemelle, P.; Knorr, D.B.; Van der Sand, J.B.; Pelloux, R.M. Morphology and Composition of Second Phase Precipitates in Zircaloy-2. J. Nucl Mater. 1983, 113, 58–64. [Google Scholar] [CrossRef]
- Taegtstrom, P.; LimBaeck, M.; Dahlbaeck, M.; Andersson, T.; Pettersson, H. Effects of Hydrogen Pickup and Second Phase Particle Dissolution on the in-Reactor Corrosion Performance of BWR Cladding. ASTM STP 2002, 1423, 96–118. [Google Scholar]
- Valizadeh, S.; Ledergerber, G.; Abolhassani, S.; Jaedernaes, D.; Dahlbaeck, M.; Mader, E.V.; Zhou, G.; Wright, J.; Hallstadius, L. Effects of Secondary Phase Particle Dissolution on the In-Reactor Performance of BWR Cladding. STM STP 2011, 1529, 729–753. [Google Scholar]
- Sundell, G.; Thuvander, M.; Tejland, P.; Dahlbaeck, M.; Hallstadius, L.; Andren, H.-O. Redistribution of Alloying Elements in Zircaloy-2 After In-Reactor Exposure. J. Nucl. Mater. 2014, 454, 178–185. [Google Scholar] [CrossRef]
- Griffiths, M.; Gilbert, R.W.; Carpenter, G.J.C. Phase Instability Decomposition and Redistribution on Intermetallic precipitates Zircaloy-2 and -4 During Neutron Irradiation. J. Nucl. Mater. 1987, 150, 53–66. [Google Scholar] [CrossRef]
- Griffiths, M.; Gilbert, R.W.; Fidleris, V.; Tucker, R.P.; Adamson, R.B. Neutron Damage in Zirconium Alloys Irradiated at 644 to 710K. J. Nucl. Mater. 1987, 150, 159–168. [Google Scholar] [CrossRef]
- Griffiths, M.; Gilbert, R.W. The Formation of c-Component defects in Zirconium Alloys During Neutron Irradiation. J. Nucl. Mater. 1987, 150, 169–181. [Google Scholar] [CrossRef]
- Ziegler, Z.F. SRIM–The Stopping and Ranges of Ions in Matter. Available online: http://www.strim.org (accessed on 13 November 2019).
- Watanabe, H.; Takahashi, K.; Yasunaga, K.; Wan, Y.; Aono, Y.; Maruno, Y.; Hashizume, K. Effects of an Alloying Element on a C-Component Loop Formation and Precipitate Resolution in Zr Alloys During Ion Irradiation. J. Nucl. Sci. Technol. 2018, 55, 1212–1224. [Google Scholar] [CrossRef]
- Nakamichi, M.; Kinoshita, C.; Yasuda, K.; Fukada, S. Formation and Growth Process of Dislocation Loops in Zircaloys under Electron Irradiation. J. Nucl. Sci. Technol. 1987, 34, 1079–1086. [Google Scholar] [CrossRef]
- Sonoda, T.; Sawabe, T.; Kitajima, S.; Nishikawa, N. Radiation Damage Accumulation and Dissolution of Second Phase Particles in Zircaloy-2 by means of Ion Accelerator. In Proceedings of the WRFPM 2014, Sendai, Japan, 14–17 September 2014; p. 100091. [Google Scholar]
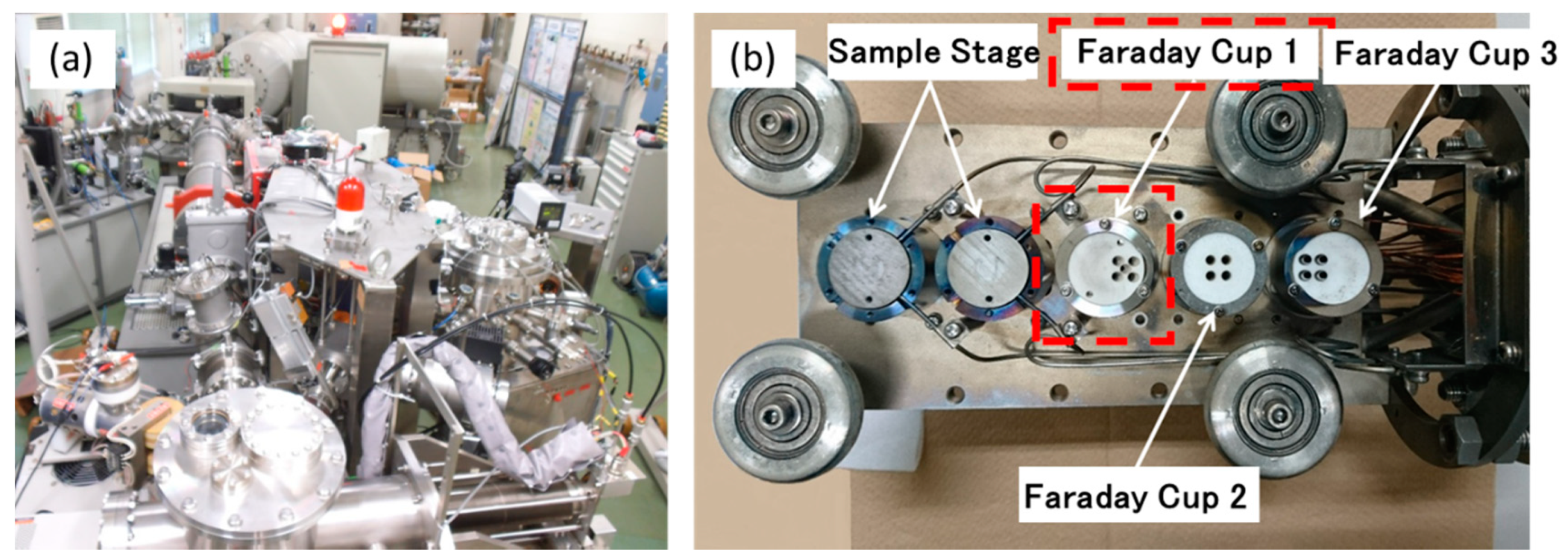
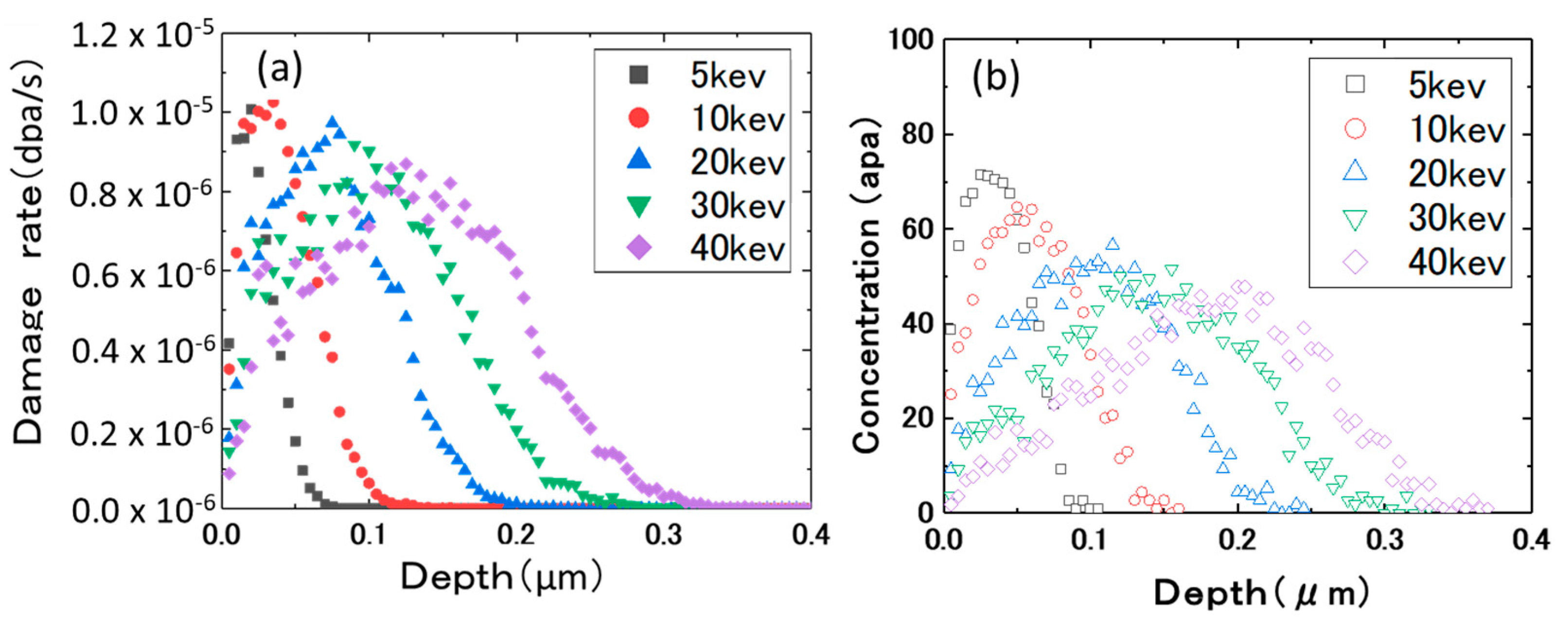
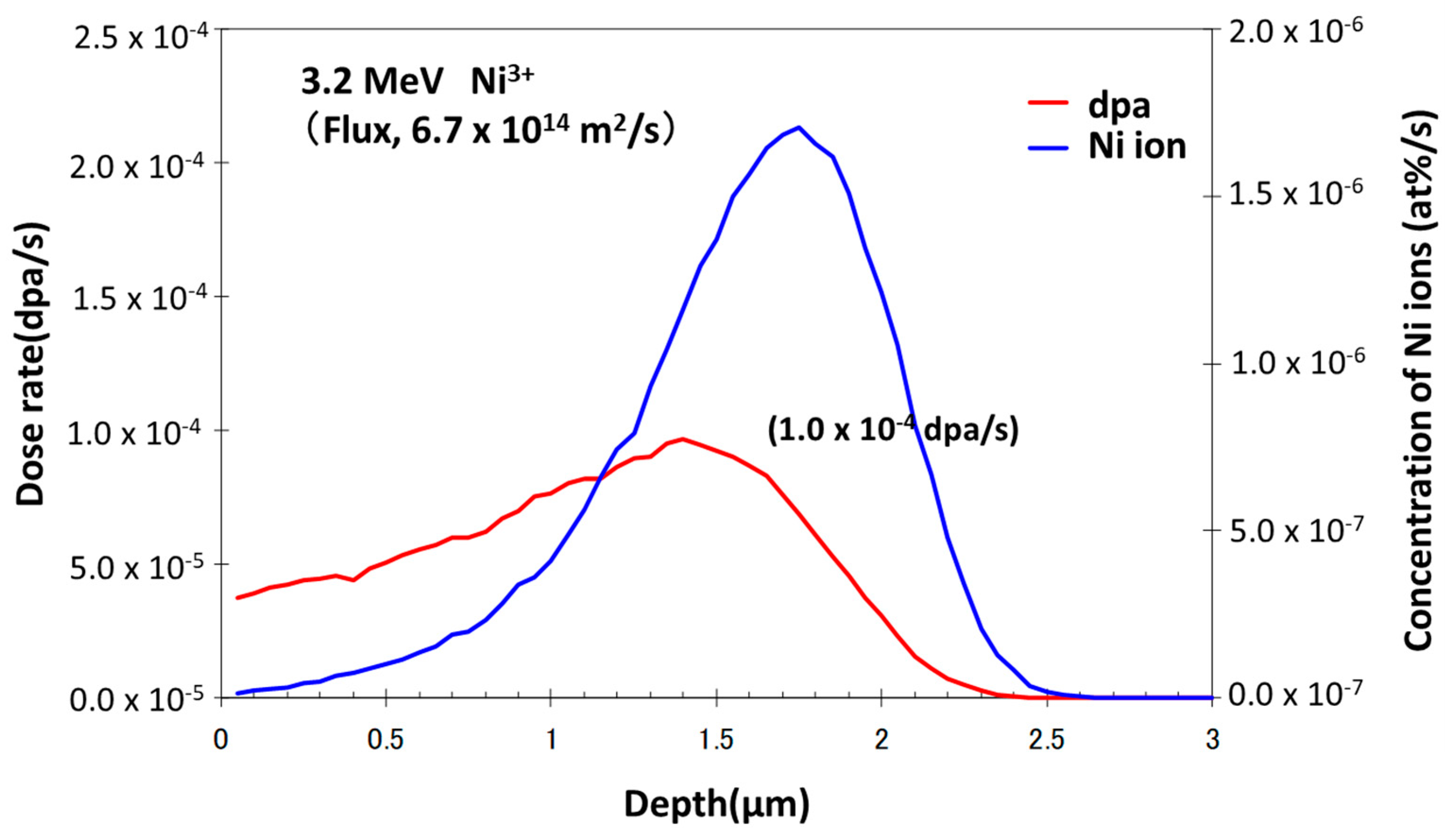
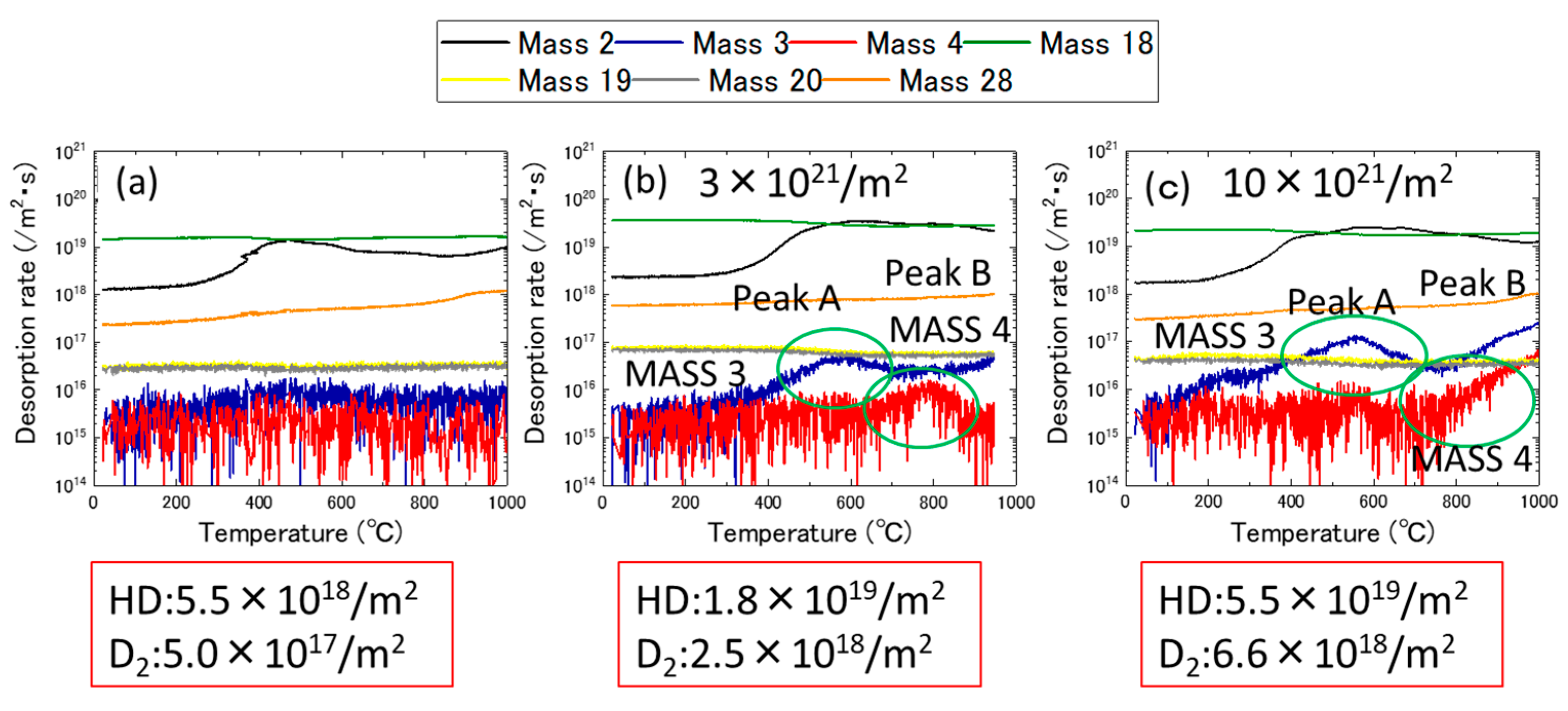
| Sn | Fe | Cr | Ni | H (wtppm) | Zr | |
|---|---|---|---|---|---|---|
| Zircaloy-2 | 1.38 | 0.15 | 0.09 | 0.05 | 46 | Bal. |
| Cold Work (%) | HD (m−2) | D2(m−2) |
|---|---|---|
| 0 | 1.8 × 1019 | 2.5 × 1018 |
| 5 | 4.0 × 1021 | 1.0 × 1020 |
| 10 | 3.8 × 1021 | 9.7 × 1019 |
| 15 | 4.2 × 1021 | 9.5 × 1019 |
| 20 | 4.8 × 1021 | 9.9 × 1019 |
| 25 | 4.0 × 1021 | 1.3 × 1020 |
| Stage | Temp. (°C) | Type of Trapping |
|---|---|---|
| Stage A | 400–600 | Weak Trap (dislocation loop, hydrides formed in surface region) |
| Stage B | 700–900 | Strong Trap (tangled dislocation, hydrides formed in thick region) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, H.; Saita, Y.; Takahashi, K.; Yasunaga, K. Desorption of Implanted Deuterium in Heavy Ion-Irradiated Zry-2. Quantum Beam Sci. 2021, 5, 9. https://doi.org/10.3390/qubs5020009
Watanabe H, Saita Y, Takahashi K, Yasunaga K. Desorption of Implanted Deuterium in Heavy Ion-Irradiated Zry-2. Quantum Beam Science. 2021; 5(2):9. https://doi.org/10.3390/qubs5020009
Chicago/Turabian StyleWatanabe, Hideo, Yoshiki Saita, Katsuhito Takahashi, and Kazufumi Yasunaga. 2021. "Desorption of Implanted Deuterium in Heavy Ion-Irradiated Zry-2" Quantum Beam Science 5, no. 2: 9. https://doi.org/10.3390/qubs5020009
APA StyleWatanabe, H., Saita, Y., Takahashi, K., & Yasunaga, K. (2021). Desorption of Implanted Deuterium in Heavy Ion-Irradiated Zry-2. Quantum Beam Science, 5(2), 9. https://doi.org/10.3390/qubs5020009





