Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Characterization
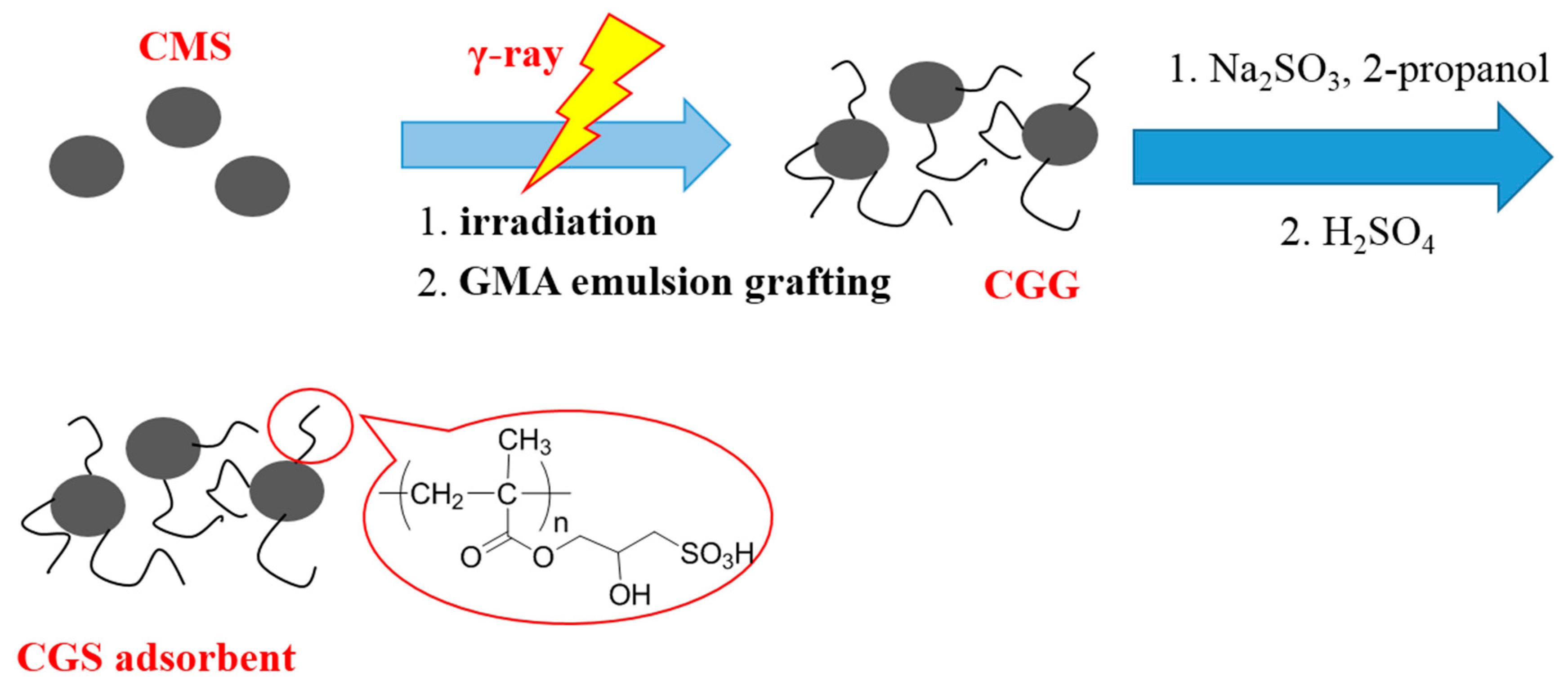
2.3. Preparation of CGS
2.3.1. Radiation-Induced Emulsion Grafting of GMA onto Cellulose Microspheres
2.3.2. Chemical Modification of CGG
2.4. Adsorption of Li+ onto CGS Adsorbents and DIAION SK1B Resin
2.5. Regeneration of the Adsorbent
2.6. Column Adsorption
3. Results and Discussions
3.1. Preparation of the CGS Adsorbents
3.2. Characterizations of the CGS Adsorbents
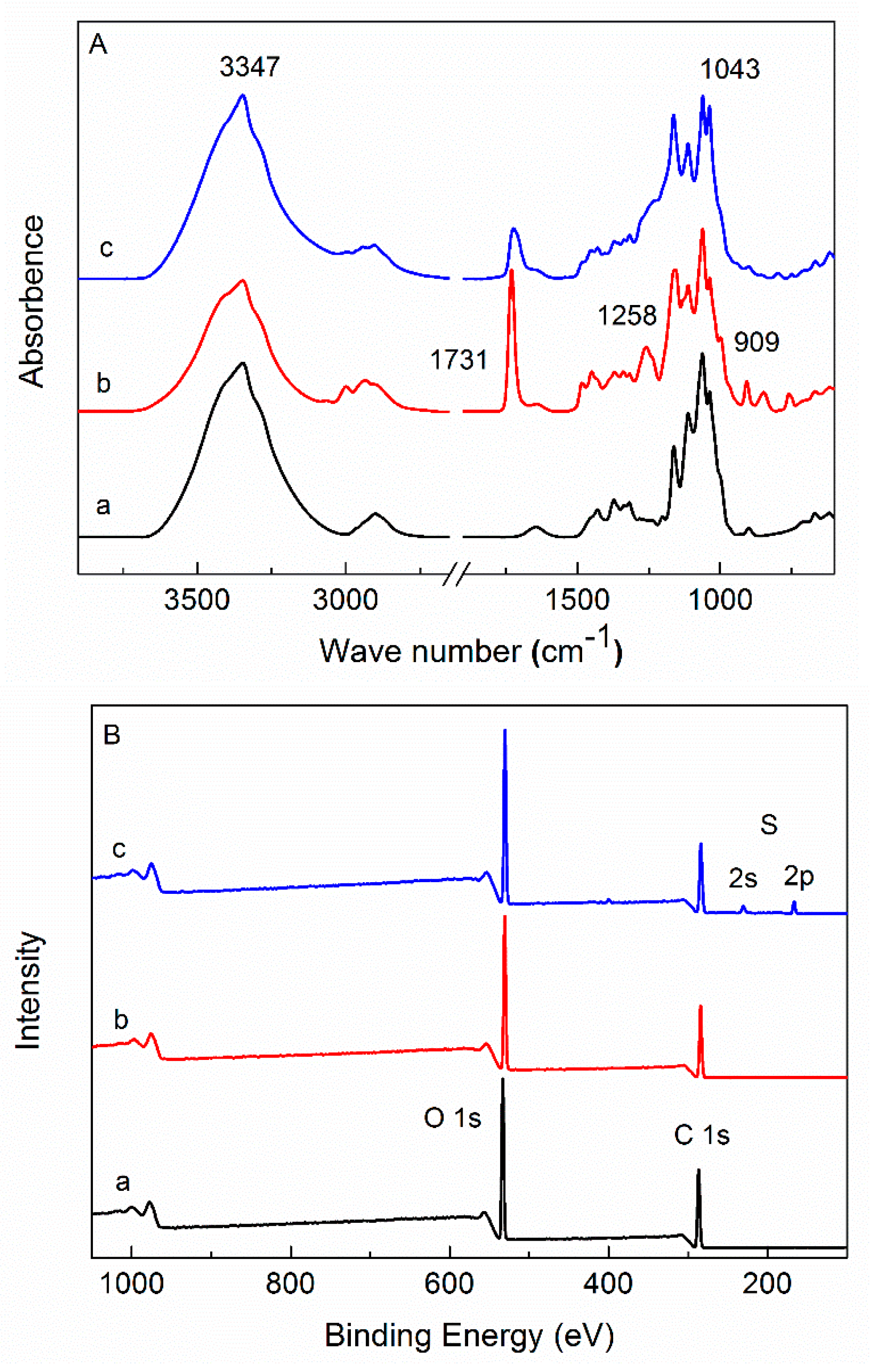
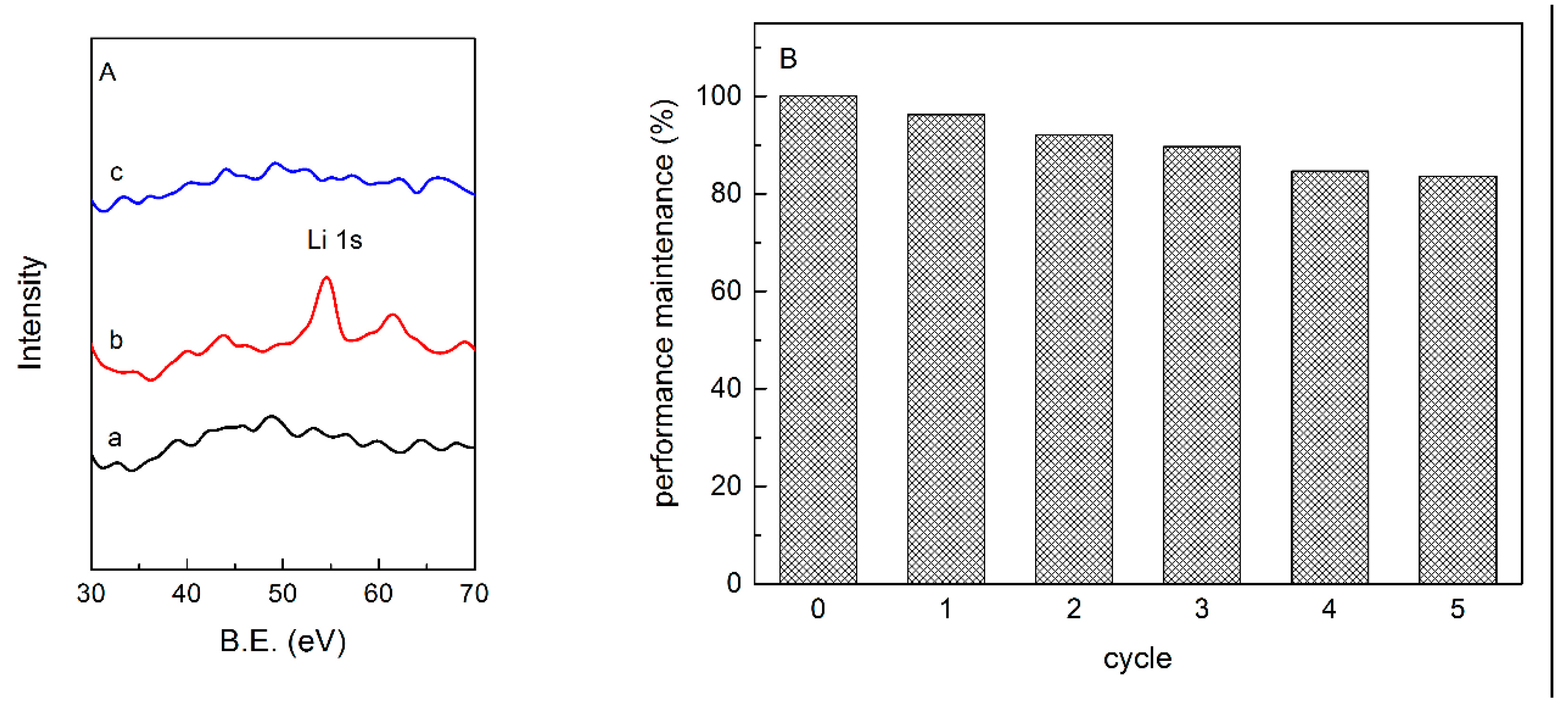
3.2.1. FTIR and XPS Analysis
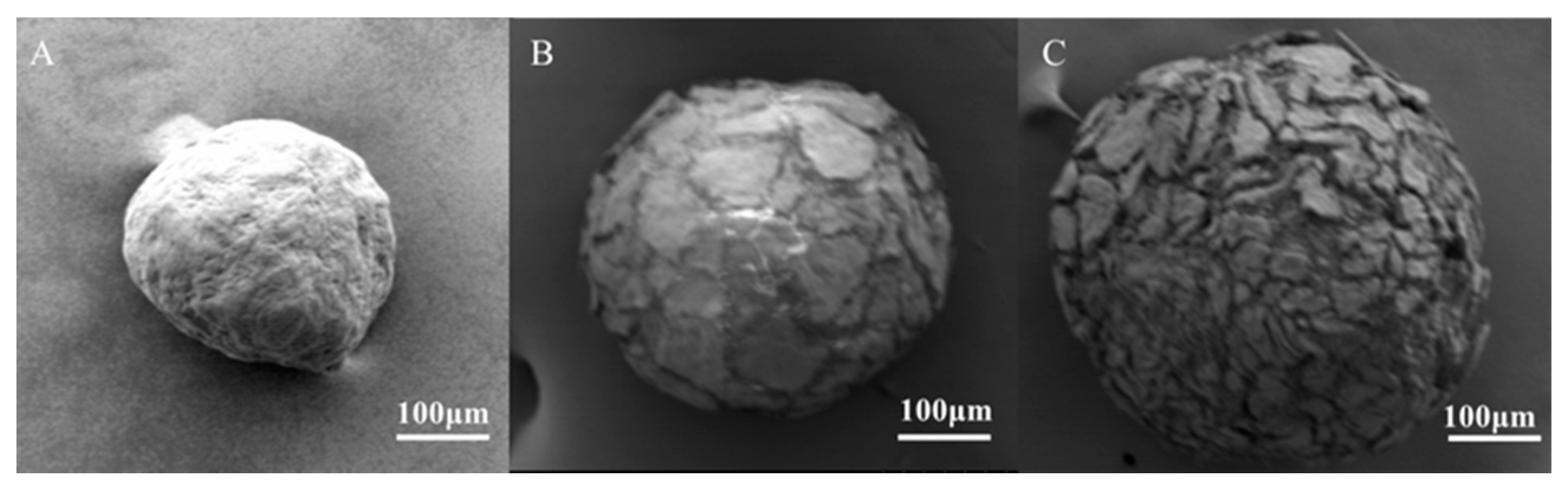
3.2.2. SEM Observation
3.3. Adsorption Performance of Li+ onto CGS Adsorbents
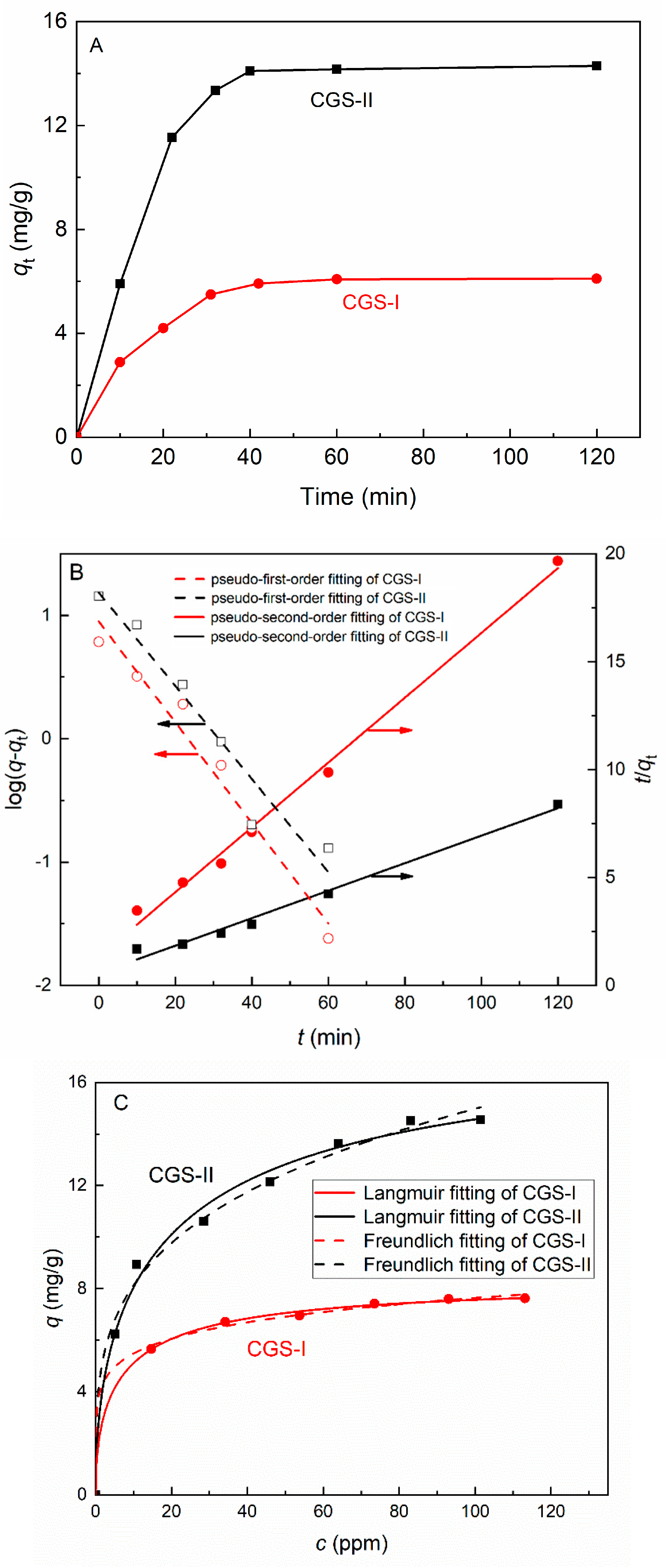
3.3.1. Adsorption Kinetics and Isotherms
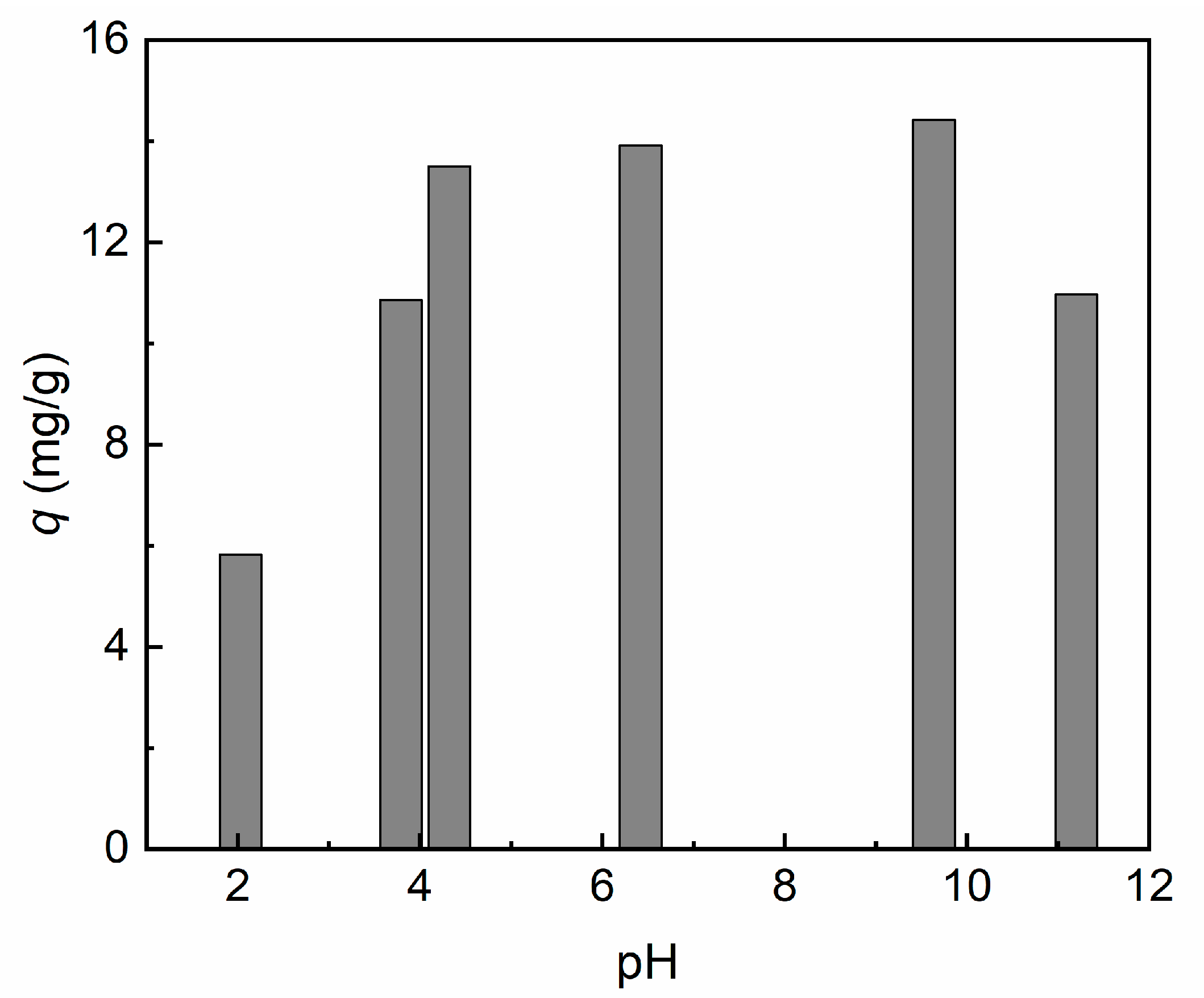
3.3.2. Effect of Solution pH
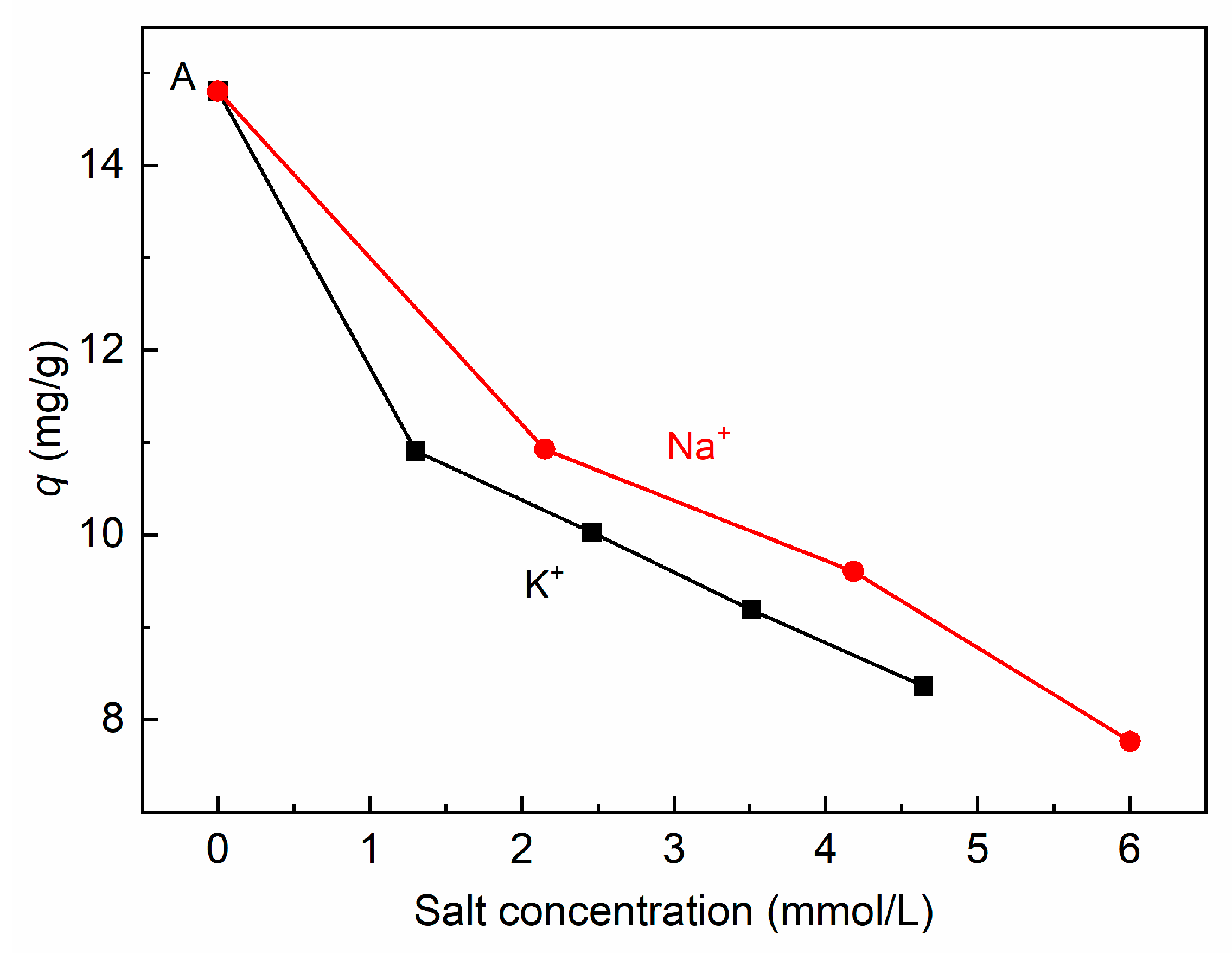
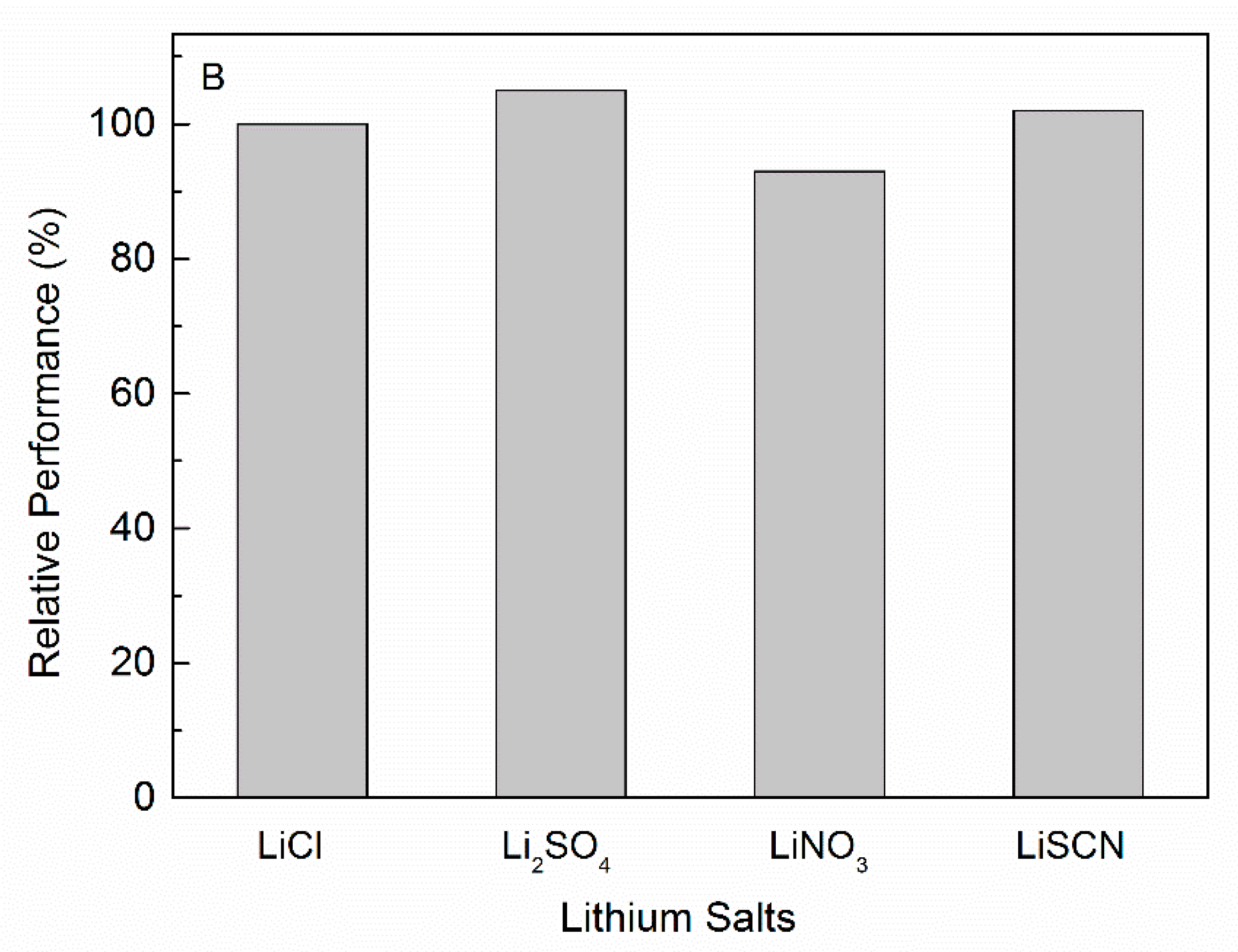
3.3.3. Effect of Different Cations and Anions of Salts
3.3.4. Adsorption Mechanism and the Regeneration of CGS Adsorbents
3.3.5. Column Adsorption Study of CGS Adsorbents for Li+
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebensperger, A.; Maxwell, P.; Moscoso, C. The Lithium Industry: Its Recent Evolution and Future Prospects. Resour. Policy 2005, 30, 218–231. [Google Scholar] [CrossRef]
- Vikstrom, H.; Davidsson, S.; Hook, M. Lithium Availability and Future Production Outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Bradshaw, A.M.; Hamacher, T.; Fischer, U. Is Nuclear Fusion a Sustainable Energy Form? Fusion Eng. Des. 2011, 86, 2770–2773. [Google Scholar] [CrossRef]
- Zhu, G.R.; Wang, P.; Qi, P.F.; Gao, C.J. Adsorption and Desorption Properties of Li+ on PVC-H1.6Mn1.6O4 Lithium Ion-Sieve Membrane. Chem. Eng. J. 2014, 235, 340–348. [Google Scholar] [CrossRef]
- Zhou, Z.; Qin, W.; Fei, W. Extraction Equilibria of Lithium with Tributyl Phosphate in Three Diluents. J. Chem. Eng. Data 2011, 56, 3518–3522. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Qin, W.; Liang, S.K.; Tan, Y.Z.; Fei, W.Y. Recovery of Lithium Using Tributyl Phosphate in Methyl Isobutyl Ketone and FeCl3. Ind. Eng. Chem. Res. 2012, 51, 12926–12932. [Google Scholar] [CrossRef]
- An, J.W.; Kang, D.J.; Tran, K.T.; Kim, M.J.; Lim, T.; Tran, T. Recovery of Lithium from Uyuni Salar Brine. Hydrometallurgy 2012, 117, 64–70. [Google Scholar] [CrossRef]
- Lee, J.; Yu, S.H.; Kim, C.; Sung, Y.E.; Yoon, J. Highly Selective Lithium Recovery from Brine Using a Lambda-MnO2-Ag Battery. Phys. Chem. Chem. Phys. 2013, 15, 7690–7695. [Google Scholar] [CrossRef]
- Ryu, T.; Ryu, J.C.; Shin, J.; Lee, D.H.; Kim, Y.H.; Chung, K.S. Recovery of Lithium by an Electrostatic Field-Assisted Desorption Process. Ind. Eng. Chem. Res. 2013, 52, 13738–13742. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium Recovery from Salt Lake Brine by H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef]
- Luo, X.B.; Guo, B.; Luo, J.M.; Deng, F.; Zhang, S.Y.; Luo, S.L.; Crittenden, J. Recovery of Lithium from Wastewater Using Development of Li Ion-Imprinted Polymers. ACS Sustain. Chem. Eng. 2015, 3, 460–467. [Google Scholar] [CrossRef]
- Recepoglu, Y.K.; Kabay, N.; Yilmaz-Ipek, I.; Arda, M.; Yoshizuka, K.; Nishihama, S.; Yukel, M. Equilibrium and Kinetic Studies on Lithium Adsorption from Geothermal Water by Lambda-MnO2. Solvent Extr. Ion Exch. 2017, 35, 221–231. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.; Kwak, S.Y. Magnetically Separable Magnetite-Lithium Manganese Oxide Nanocomposites as Reusable Lithium Adsorbents in Aqueous Lithium Resources. Chem. Eng. J. 2015, 281, 541–548. [Google Scholar] [CrossRef]
- Ryu, T.; Shin, J.; Lee, D.H.; Ryu, J.; Park, I.; Hong, H.; Kim, B.G.; Lee, J.B.; Huh, Y.S.; Chung, K.S. Improvement of Lithium Adsorption Capacity of Porous Cylinder-Type Lithium Manganese Oxide through Introduction of Additive. Mater. Chem. Phys. 2015, 167, 225–230. [Google Scholar] [CrossRef]
- Chitrakar, R.; Kanoh, H.; Miyai, Y.; Ooi, K. Recovery of Lithium from Seawater using Manganese Oxide Adsorbent (H1.6Mn1.6O4) Derived from Li1.6Mn1.6O4. Ind. Eng. Chem. Res. 2001, 40, 2054–2058. [Google Scholar] [CrossRef]
- Chung, K.S.; Lee, J.C.; Kim, W.K.; Kim, S.B.; Cho, K.Y. Inorganic Adsorbent Containing Polymeric Membrane Reservoir for the Recovery of Lithium from Seawater. J. Membrane Sci. 2008, 325, 503–508. [Google Scholar] [CrossRef]
- Xiao, J.L.; Nie, X.Y.; Sun, S.Y.; Song, X.F.; Li, P.; Yu, J.G. Lithium Ion Adsorption-Desorption Properties on Spinel Li4Mn5O12 and pH-Dependent Ion-Exchange Model. Adv. Powder Technol. 2015, 26, 589–594. [Google Scholar] [CrossRef]
- Ma, L.W.; Chen, B.Z.; Chen, Y.; Shi, X.C. Preparation, Characterization and Adsorptive Properties of Foam-Type Lithium Adsorbent. Mesoporous Mesoporous Mat. 2011, 142, 147–153. [Google Scholar] [CrossRef]
- Xiao, G.P.; Tong, K.F.; Zhou, L.S.; Xiao, J.L.; Sun, S.Y.; Li, P.; Yu, J.G. Adsorption and Desorption Behavior of Lithium Ion in Spherical PVC-MnO2 Ion Sieve. Ind. Eng. Chem. Res. 2012, 51, 10921–10929. [Google Scholar] [CrossRef]
- Kang, H.L.; Liu, R.G.; Huang, Y. Cellulose Derivatives and Graft Copolymers as Blocks for Functional Materials. Polym. Int. 2013, 62, 338–344. [Google Scholar] [CrossRef]
- Kongkaoroptham, P.; Piroonpan, T.; Hemvichian, K.; Suwanmala, P.; Rattanasakulthong, W.; Pasanphan, W. Poly(Ethylene Glycol) Methyl Ether Methacrylate-Graft-Chitosan Nanoparticles as a Biobased Nanofiller for a Poly(Lactic Acid) Blend: Radiation-Induced Grafting and Performance Studies. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Munoz-Munoz, F.; Bucio, E.; Magarinos, B.; Concheiro, A.; Alvarez-Lorenzo, C. Temperature- and pH-Sensitive IPNs Grafted onto Polyurethane by Gamma Radiation for Antimicrobial Drug-Eluting Insertable Devices. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Guin, J.P.; Bhardwaj, Y.K.; Varshney, L. Radiation Grafting: A Voyage from Bio-Waste Corn Husk to an Efficient Thermostable Adsorbent. Carbohydr. Polym. 2018, 183, 151–164. [Google Scholar] [CrossRef]
- Wojnarovits, L.; Foldvary, C.M.; Takacs, E. Radiation-Induced Grafting of Cellulose for Adsorption of Hazardous Water Pollutants: A Review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Yu, T.; Liu, S.; Xu, M.; Peng, J.; Li, J.; Zhai, M. Synthesis of Novel Aminated Cellulose Microsphere Adsorbent for Efficient Cr(VI) Removal. Radiat. Phys. Chem. 2016, 125, 94–101. [Google Scholar] [CrossRef]
- Barsbay, M.; Kavakh, P.A.; Tilki, S.; Kavakh, C.; Guven, O. Porous Cellulosic Adsorbent for the Removal of Cd (II), Pb(II) and Cu(II) Ions from Aqueous Media. Radiat. Phys. Chem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Xu, M.; Ao, Y.; Wang, S.; Peng, J.; Li, J.; Zhai, M. Efficient Adsorption of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquids onto Modified Cellulose Microspheres. Carbohydr. Polym. 2015, 128, 171–178. [Google Scholar] [CrossRef]
- Sokker, H.H.; Badawy, S.M.; Zayed, E.M.; Eldien, F.A.N.; Farag, A.M. Radiation-Induced Grafting of Glycidyl Methacrylate onto Cotton Fabric Waste and its Modification for Anchoring Hazardous Wastes from their Solutions. J. Hazard. Mater. 2009, 168, 137–144. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, J.; Yuan, W.; Yi, Y.; Zhao, L. Recovery of Au(III) by Radiation Synthesized Aminomethyl Pyridine Functionalized Adsorbents Based on Cellulose. Chem. Eng. J. 2016, 283, 504–513. [Google Scholar] [CrossRef]
- Alberti, A.; Bertini, S.; Gastaldi, G.; Iannaccone, N.; Macciantelli, D.; Torri, G.; Vismara, E. Electron beam Irradiated Textile Cellulose Fibres. ESR Studies and Derivatisation with Glycidyl Methacrylate (GMA). Eur. Polym. J. 2005, 41, 1787–1797. [Google Scholar] [CrossRef]
- Chen, I.; Xu, C.; Peng, J.; Han, D.; Liu, S.; Zhai, M. Novel Functionalized Cellulose Microspheres for Efficient Separation of Lithium Ion and Its Isotopes: Synthesis and Adsorption Performance. Molecules 2019, 24, 2762. [Google Scholar] [CrossRef] [PubMed]
- Seko, N.; Bang, L.T.; Tamada, M. Syntheses of Amine-Type Adsorbents with Emulsion Graft Polymerization of Glycidyl Methacrylate. Nucl. Instr. Meth. Phys. Res. B 2007, 265, 146–149. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Synthesis of Iron-Doped Manganese Oxides with an Ion-Sieve Property: Lithium Adsorption from Bolivian Brine. Ind. Eng. Chem. Res. 2014, 53, 3682–3688. [Google Scholar] [CrossRef]
- Huang, Y.; Farooq, M.U.; Lai, S.; Feng, X.; Sampranpiboon, P.; Wang, X.; Huang, W. Model Fitting of Sorption Kinetics Data: Misapplications Overlooked and their Rectifications. AIChE J. 2018, 64, 1793–1805. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Park, M.J.; Nisola, G.M.; Beltran, A.B.; Torrejos, R.E.C.; Seo, J.G.; Lee, S.; Kim, H.; Chung, W. Recyclable Composite Nanofiber Adsorbent for Li+ Recovery from Seawater Desalination Retentate. Chem. Eng. J. 2014, 254, 73–81. [Google Scholar] [CrossRef]
- Arroyo, F.; Morillo, J.; Usero, J.; Rosado, D.; Bakouri, H.E. Lithium Recovery from Desalination Brines using Specific Ion-Exchange Resins. Desalination 2019, 468, 114073. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic Surface Tension and Adsorption Mechanisms of Surfactants at the Air-Water Interface. Adv Colloid Interfac. Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef]
- Knill, C.J.; Kennedy, J.F. Degradation of Cellulose under Alkaline Conditions. Carbohydr. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- Oswald, S. Binding Energy Referencing for XPS in Alkali Metal-Based Battery Materials Research (I): Basic Model Investigations. Appl. Surf. Sci. 2015, 351, 492–503. [Google Scholar] [CrossRef]
- Chu, K.H. Improved Fixed Bed Models for Metal Biosorption. Chem. Eng. J. 2004, 97, 233–239. [Google Scholar] [CrossRef]

| Adsorbents | Equilibrium Time | Maximum Adsorption Capacity (mg/g) | Ref |
|---|---|---|---|
| H2TiO3 | 10 h | 32.6 | [10] |
| Iron-Doped Manganese Oxides | 6 h | 28 | [33] |
| H1.6Mn1.6O4/poly(acrylonitrile) | - | 10.3 | [36] |
| Lewatit K2629/TP 207/TP 208 resin | <30 min | 1.23–2.54 | [37] |
| DIAION SK1B resin | 3 h | 0.23 | This work |
| CGS | 40 min | 16.0 | This work |
| Sample | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | q (mg/g) | R2 | k2 (g mg−1 min−1) | q (mg/g) | R2 | |
| CGS-I | 16.8 | 7.84 | 0.948 | 0.0017 | 6.72 | 0.990 |
| CGS-II | 20.7 | 20.1 | 0.970 | 0.0082 | 15.6 | 0.987 |
| Sample | S Content | Langmuir | Freundlich | ||
|---|---|---|---|---|---|
| % | R2 | qe (mg/g) | R2 | 1/n | |
| CGS-I | 5.7 | 0.999 | 8.02 | 0.938 | 0.140 |
| CGS-II | 8.0 | 0.996 | 16.0 | 0.988 | 0.287 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Yu, T.; Peng, J.; Zhao, L.; Li, J.; Zhai, M. Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups. Quantum Beam Sci. 2020, 4, 6. https://doi.org/10.3390/qubs4010006
Xu C, Yu T, Peng J, Zhao L, Li J, Zhai M. Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups. Quantum Beam Science. 2020; 4(1):6. https://doi.org/10.3390/qubs4010006
Chicago/Turabian StyleXu, Chenxi, Tianlin Yu, Jing Peng, Long Zhao, Jiuqiang Li, and Maolin Zhai. 2020. "Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups" Quantum Beam Science 4, no. 1: 6. https://doi.org/10.3390/qubs4010006
APA StyleXu, C., Yu, T., Peng, J., Zhao, L., Li, J., & Zhai, M. (2020). Efficient Adsorption Performance of Lithium Ion onto Cellulose Microspheres with Sulfonic Acid Groups. Quantum Beam Science, 4(1), 6. https://doi.org/10.3390/qubs4010006





