Thermal Degradation and Microstructural Evolution of Geopolymer-Based UHPC with Silica Fume and Quartz Powder
Abstract
1. Introduction
2. Aim of This Study
3. Materials and Methods
3.1. Materials
3.2. Mixture Proportions and Specimen Preparation
3.3. Test Methods
3.3.1. Testing of Flexural and Compressive Strengths
3.3.2. Thermal Treatment and Specimen Preparation
3.3.3. Microscopic Investigations
4. Results and Discussion
4.1. Compressive Strength
4.1.1. The Impact of Silica Fume
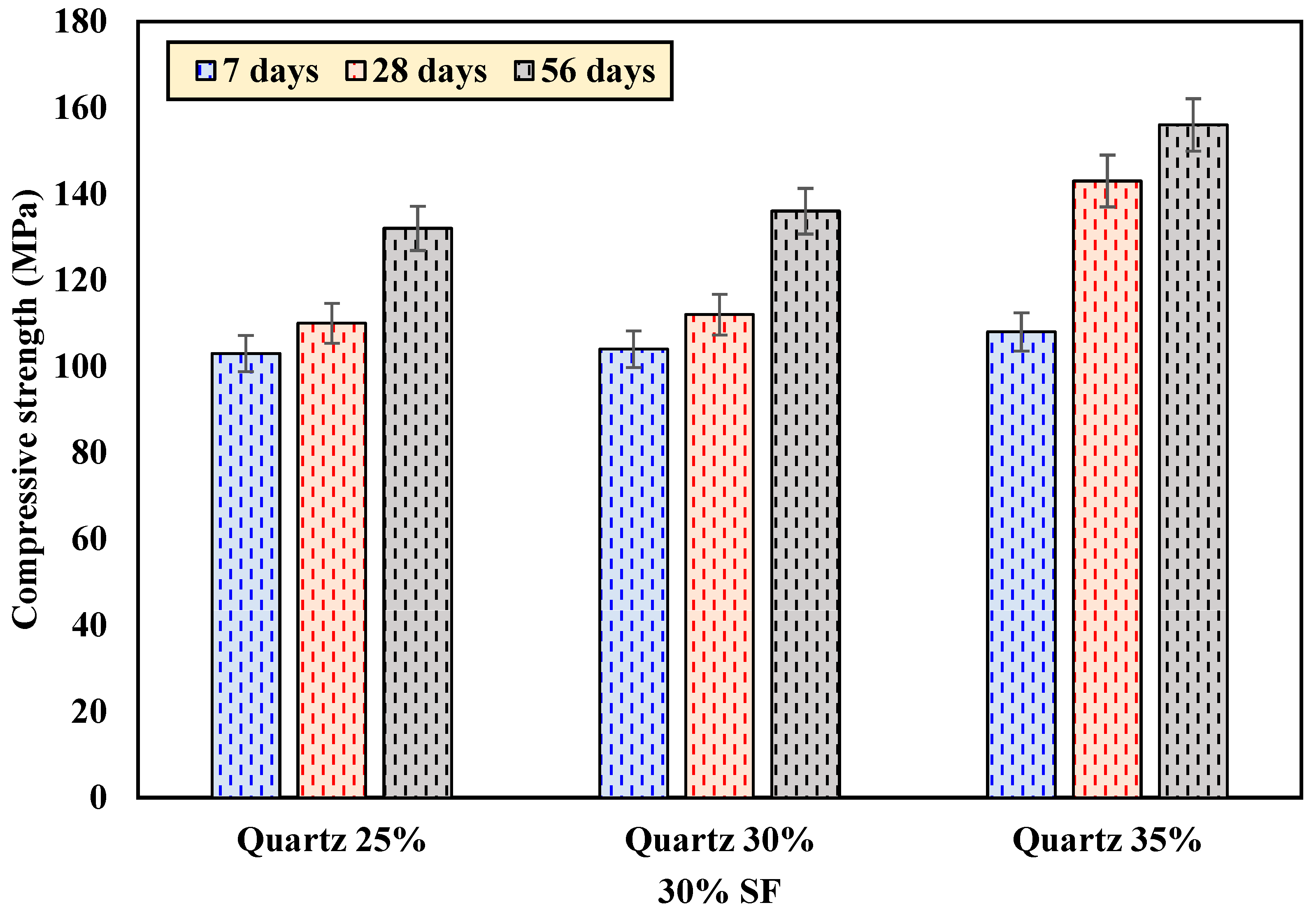
4.1.2. The Effect of Quartz Powder
4.2. Flexural Strength
4.3. Thermal Performance
4.3.1. Physical Appearance
4.3.2. Compressive Strength After High-Temperature Exposure
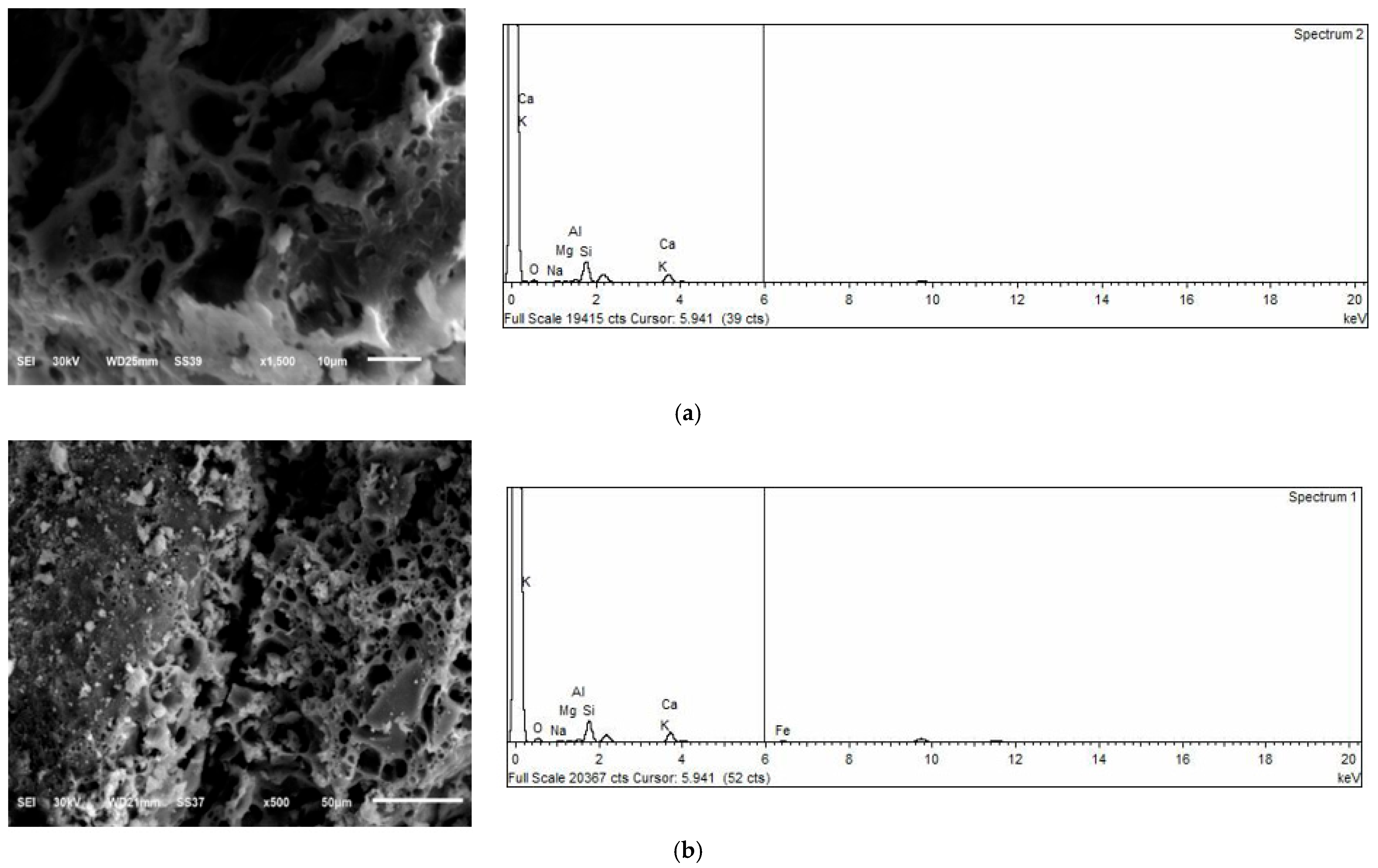
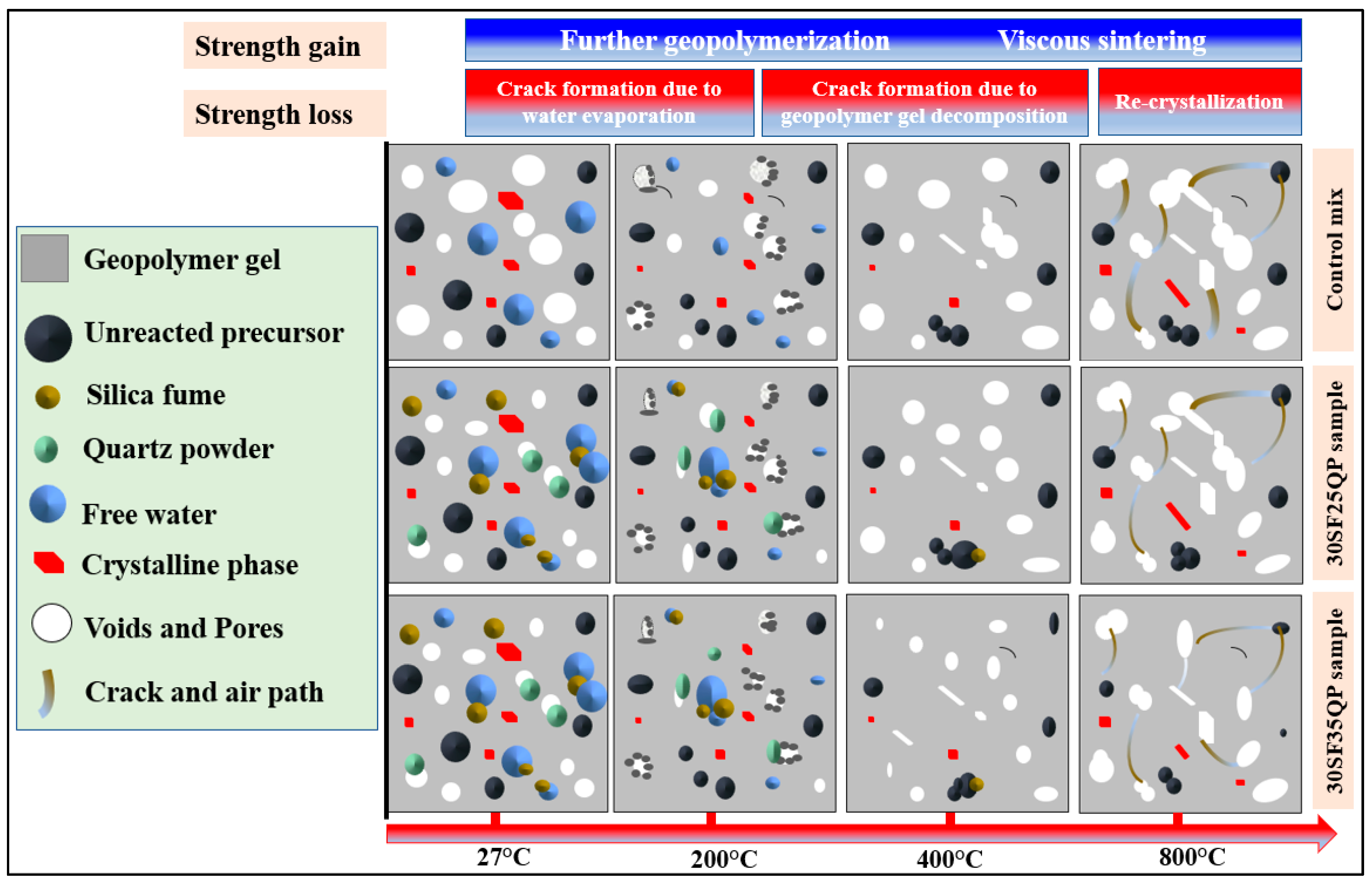
4.4. Microscopic Analysis Before Heating
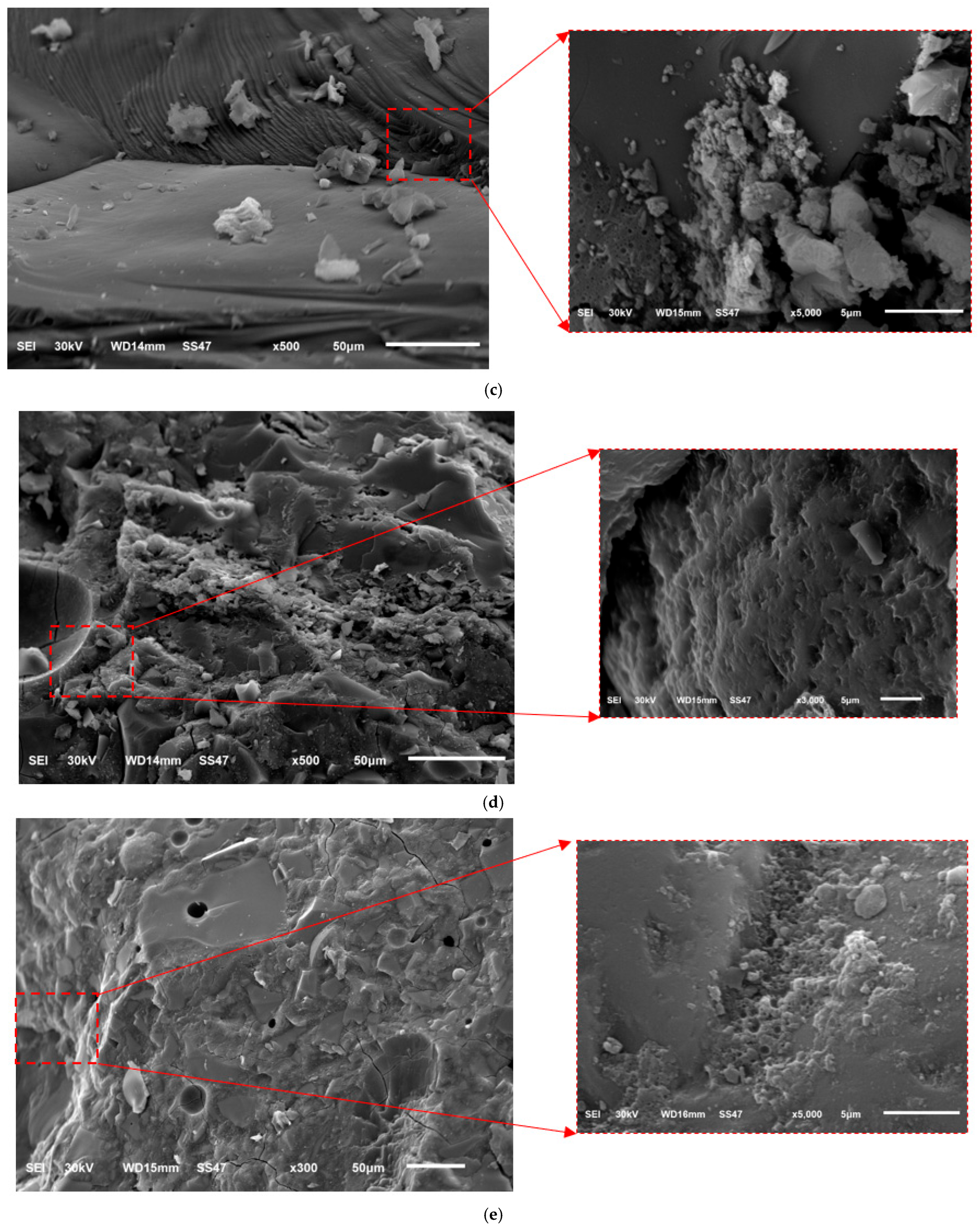
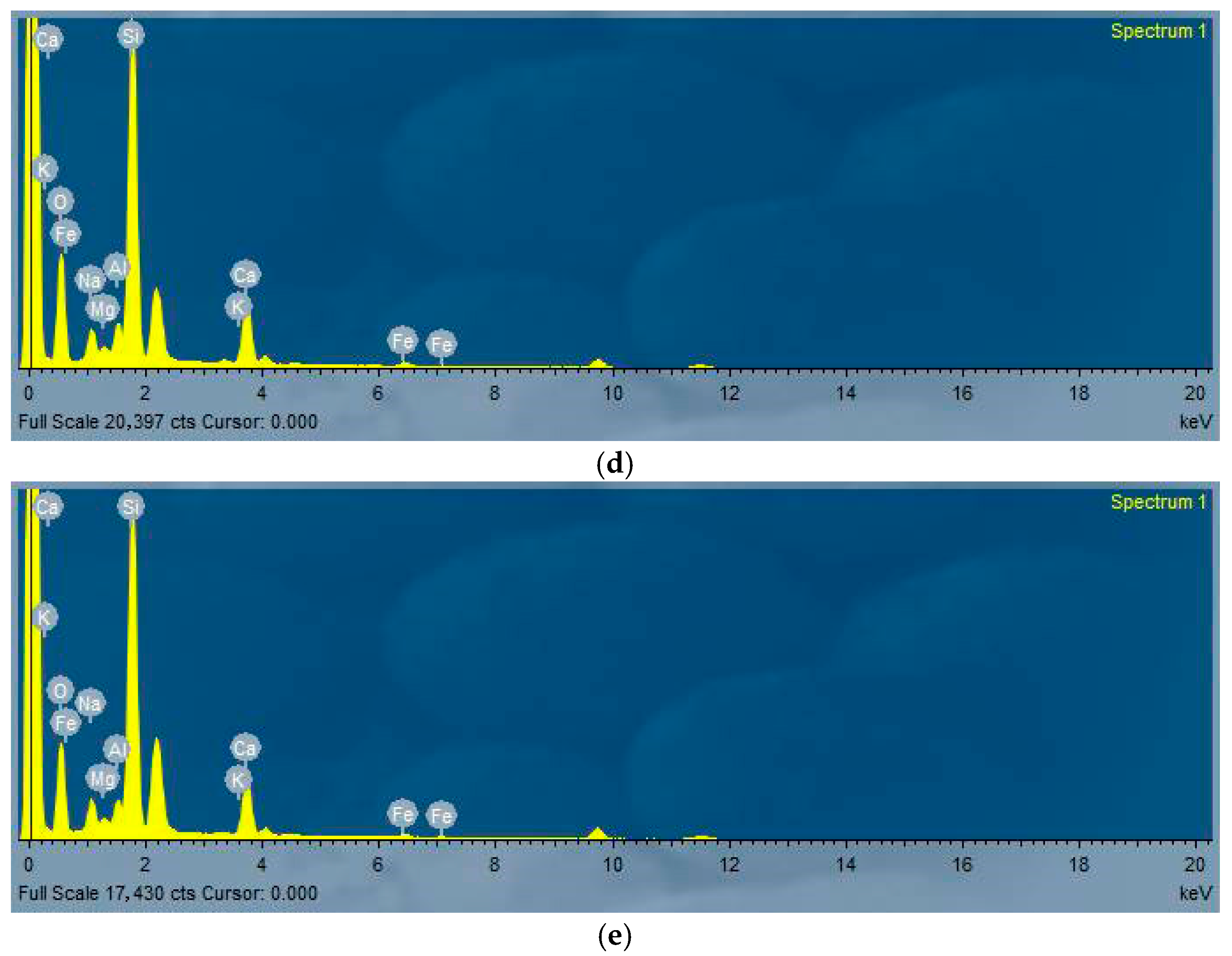
4.5. Microscopic Analysis After Heating
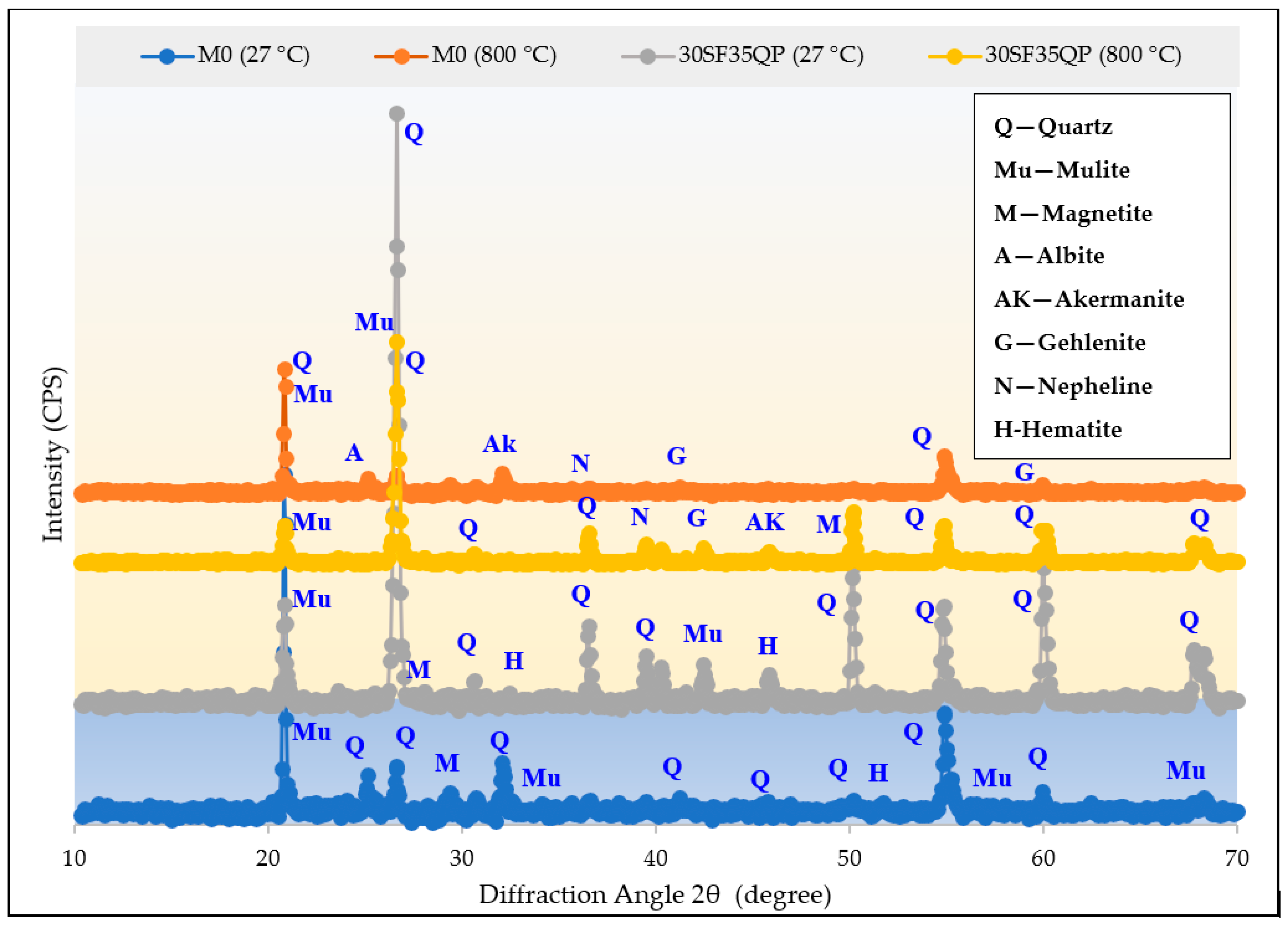
4.6. XRD Analysis Before and After Heating
4.7. Further Discussion
5. Conclusions
- (a)
- The incorporation of SF and QP as partial substitutes for GGBFS and sand, respectively, significantly enhanced the mechanical performance of G-UHPC. SF improved matrix density through pozzolanic reactions, while QP acted as a micro-filler, reducing voids and improving packing. The optimal mix for achieving maximum mechanical performance was determined to be 30% SF and 35% QP, yielding the highest compressive strength among all of the tested mixes.
- (b)
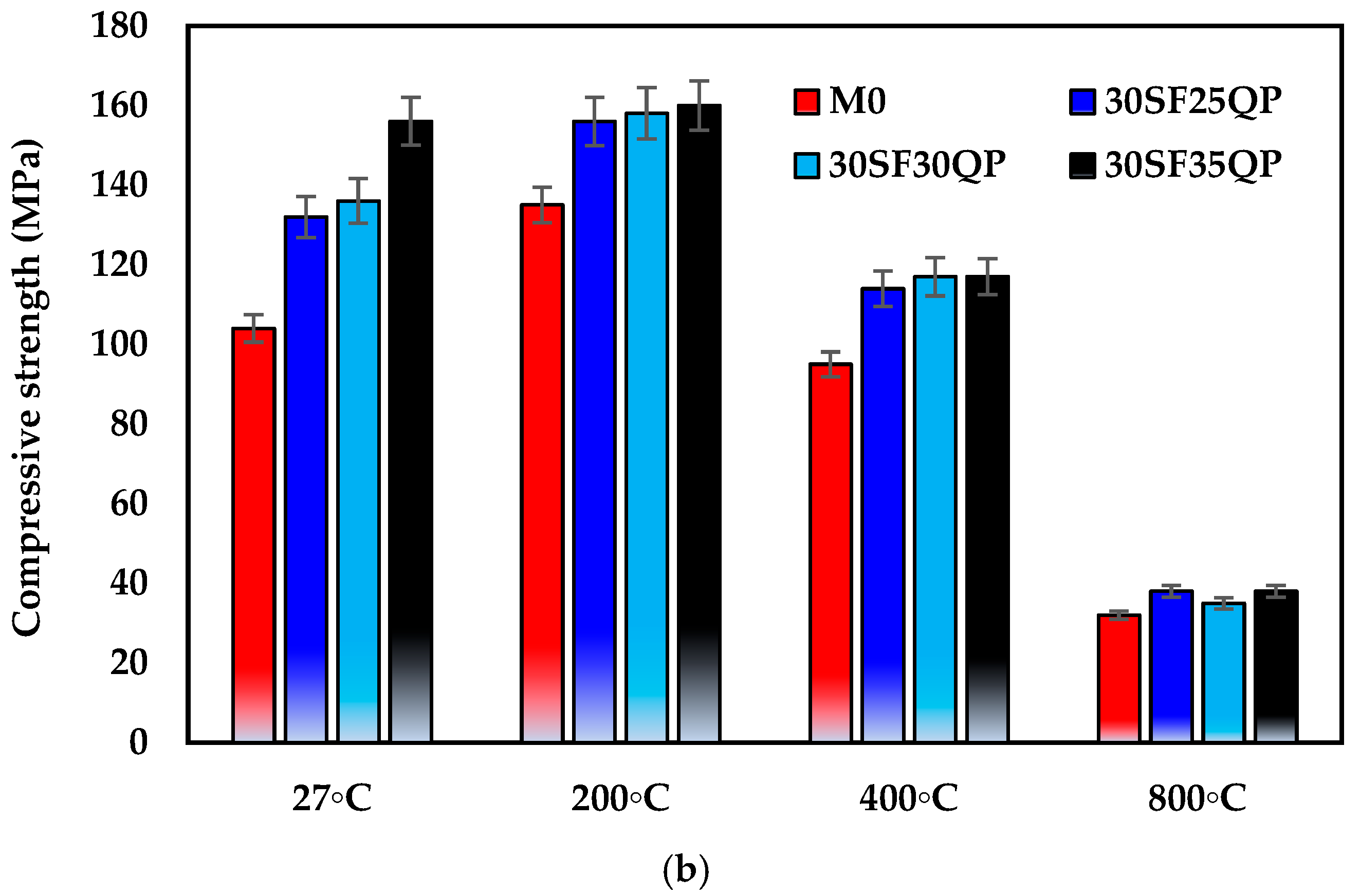
- Under ambient conditions (27 °C), G-UHPC mixtures incorporating 30% SF consistently outperformed those with 15% SF and the control mix (M0). Notably, the 30SF35QP mix achieved a peak compressive strength of 156 MPa, compared to 146.83 MPa for 15SF35QP and 104 MPa for M0, underscoring the synergistic effect of higher SF content and QP addition in enhancing matrix densification and strength development.
- (c)
- G-UHPC with 30% SF and 35% QP attained an impressive compressive strength of 156 MPa at ambient temperature, demonstrating the potential of this formulation for high-strength applications.
- (d)
- At 200 °C, all mixes exhibited enhanced compressive strength due to accelerated geopolymerization, with the control mix gaining 29.8% (135 MPa). This improvement is attributed to the formation of denser C-A-S-H and N-A-S-H gels during thermal treatment.
- (e)
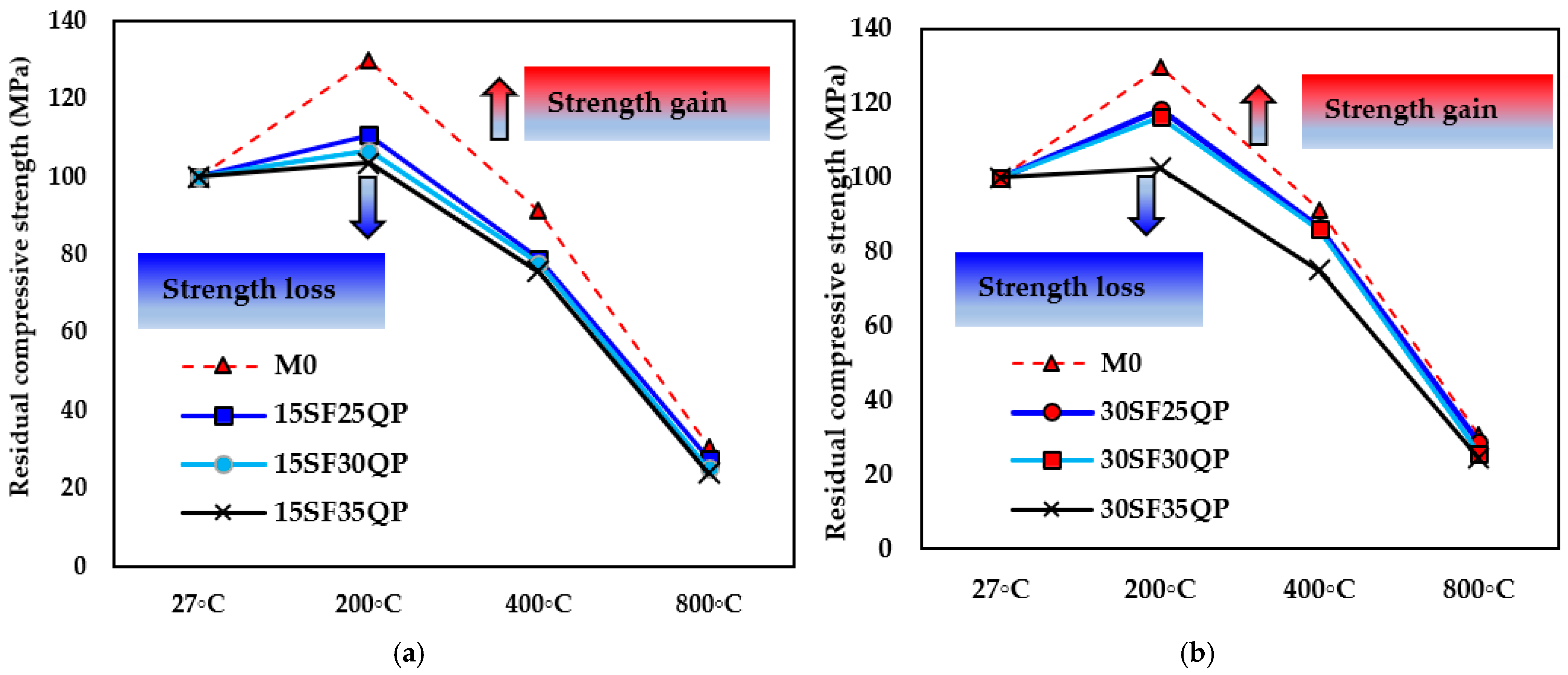
- At 800 °C, the 30SF35QP mix retained 38 MPa of its initial strength, corresponding to 24.4% of its ambient compressive strength—a reduction of 75.6%. Similarly, the 30SF25QP mix also retained 38 MPa, equating to 28% strength retention. Both outperformed the control mix, which retained only 30.8% (32 MPa). These results highlight the superior thermal stability of G-UHPC incorporating 30% SF and 35% QP, demonstrating enhanced resistance to thermal degradation compared to lower SF content mixtures.
- (f)
- Microstructural analysis via SEM revealed that the strength reductions at 400 °C and 800 °C were due to increased porosity and crack formation, driven by the decomposition of C-A-S-H and N-A-S-H gels, water vapor release, and volumetric expansion. However, 30SF35QP exhibited greater compactness and fewer voids, correlating with its superior residual strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shoukry, H.; Perumal, P.; Abadel, A.; Alghamdi, H.; Alamri, M.; Abdel-Gawwad, H.A. Performance of limestone-calcined clay cement mortar incorporating high volume ferrochrome waste slag aggregate. Constr. Build. Mater. 2022, 350, 128928. [Google Scholar] [CrossRef]
- Dong, Y. Performance assessment and design of ultra-high performance concrete (UHPC) structures incorporating life-cycle cost and environmental impacts. Constr. Build. Mater. 2018, 167, 414–425. [Google Scholar] [CrossRef]
- Stengel, T.; Schießl, P. 22—Life cycle assessment (LCA) of ultra high performance concrete (UHPC) structures. In Eco-Efficient Construction and Building Materials; Pacheco-Torgal, F., Cabeza, L.F., Labrincha, J., de Magalhães, A., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 528–564. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Fardis, M.N.; Vayenas, C.G. Effect of composition, environmental factors and cement-lime mortar coating on concrete carbonation. Mater. Struct. 1992, 25, 293–304. [Google Scholar] [CrossRef]
- Elfadaly, E.; Othman, A.M.; Aly, M.H.; Elgarhy, W.A.; Abdellatief, M. Assessing performance and environmental benefits of high-performance geopolymer mortar incorporating pumice and rice straw ash. Sustain. Chem. Pharm. 2025, 44, 101918. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Althoey, F.; Zaid, O.; Martínez-García, R.; de Prado-Gil, J.; Ahmed, M.; Yosri, A.M. Ultra-high-performance fiber-reinforced sustainable concrete modified with silica fume and wheat straw ash. J. Mater. Res. Technol. 2023, 24, 6118–6139. [Google Scholar] [CrossRef]
- Abdellatief, M.; AL-Tam, S.M.; Elemam, W.E.; Alanazi, H.; Elgendy, G.M.; Tahwia, A.M. Development of ultra-high-performance concrete with low environmental impact integrated with metakaolin and industrial wastes. Case Stud. Constr. Mater. 2023, 18, e01724. [Google Scholar] [CrossRef]
- Abdellatief, M.; Murali, G.; Dixit, S. Leveraging machine learning to evaluate the effect of raw materials on the compressive strength of ultra-high-performance concrete. Results Eng. 2025, 25, 104542. [Google Scholar] [CrossRef]
- Plevny, Z. Applications of Ultra-High Performance Concrete; IdeaExchange@UAkron: Akron, OH, USA, 2020. [Google Scholar]
- Abdal, S.; Mansour, W.; Agwa, I.; Nasr, M.; Abadel, A.; Özkılıç, Y.O.; Akeed, M.H. Application of Ultra-High-Performance Concrete in Bridge Engineering: Current Status, Limitations, Challenges, and Future Prospects. Buildings 2023, 13, 185. [Google Scholar] [CrossRef]
- Anunike, G.S.; Tarabin, M.; Hisseine, O.A. Ultra-high-performance concrete for nuclear applications: A review of raw materials and mix design approaches. Constr. Build. Mater. 2024, 438, 136938. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Xiao, J.; Wang, D.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part I. Raw materials and mixture design, Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; He, W. Comparative study on flexural properties of ultra-high performance concrete with supplementary cementitious materials under different curing regimes. Constr. Build. Mater. 2017, 136, 307–313. [Google Scholar] [CrossRef]
- Kioupis, D.; Zisimopoulou, A.; Tsivilis, S.; Kakali, G. Development of porous geopolymers foamed by aluminum and zinc powders. Ceram. Int. 2021, 47, 26280–26292. [Google Scholar] [CrossRef]
- Li, B.; Jiang, G.; Hu, J.; Li, Y.; Wu, F.; Qin, Z.; Wang, S. Specimen size effect on compressive and splitting tensile strengths of sustainable geopolymeric recycled aggregate concrete: Experimental and theoretical analysis. J. Clean. Prod. 2024, 434, 140154. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Abdellatief, M.; Salah, A.; Youssf, O. Valorization of recycled concrete powder, clay brick powder, and volcanic pumice powder in sustainable geopolymer concrete. Sci. Rep. 2025, 15, 11049. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, N.A.M.; Dias, R.L.; Witzke, F.B.; Medeiros-Junior, R.A. Effect of carbonation curing on the physical, mechanical, and microstructural properties of metakaolin-based geopolymer concrete. Constr. Build. Mater. 2023, 406, 133403. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elrahman, M.A.; Abadel, A.A.; Wasim, M.; Tahwia, A. Ultra-high performance concrete versus ultra-high performance geopolymer concrete: Mechanical performance, microstructure, and ecological assessment. J. Build. Eng. 2023, 79, 107835. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Ellatief, M.A.; Bassioni, G.; Heniegal, A.M.; Elrahman, M.A. Influence of high temperature exposure on compressive strength and microstructure of ultra-high performance geopolymer concrete with waste glass and ceramic. J. Mater. Res. Technol. 2023, 23, 5681–5697. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, C.; Guo, Y.; Cui, H.; Xue, J. Improved mechanical and thermal properties of sustainable ultra-high performance geopolymer concrete with cellulose nanofibers. J. Build. Eng. 2025, 102, 112068. [Google Scholar] [CrossRef]
- Ellatief, M.A.; Abadel, A.A.; Federowicz, K.; Elrahman, M.A. Mechanical properties, high temperature resistance and microstructure of eco-friendly ultra-high performance geopolymer concrete: Role of ceramic waste addition. Constr. Build. Mater. 2023, 401, 132677. [Google Scholar] [CrossRef]
- Althoey, F.; Zaid, O.; Alsulamy, S.; Martínez-García, R.; de Prado-Gil, J.; Arbili, M.M. Experimental study on the properties of ultra-high-strength geopolymer concrete with polypropylene fibers and nano-silica. PLoS ONE 2023, 18, e0282435. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wu, M.; Shen, W.; Li, J.; Zhao, D.; Li, P.; Wu, J. Performance and microstructure of ultra-high-performance concrete (UHPC) with silica fume replaced by inert mineral powders. Constr. Build. Mater. 2022, 327, 126996. [Google Scholar] [CrossRef]
- Abdellatief, M.; Elrahman, M.A.; Elgendy, G.; Bassioni, G.; Tahwia, A.M. Response surface methodology-based modelling and optimization of sustainable UHPC containing ultrafine fly ash and metakaolin. Constr. Build. Mater. 2023, 388, 131696. [Google Scholar] [CrossRef]
- Tavares, L.R.C.; Junior, J.F.T.; Costa, L.M.; da Silva Bezerra, A.C.; Cetlin, P.R.; Aguilar, M.T.P. Influence of quartz powder and silica fume on the performance of Portland cement. Sci. Rep. 2020, 10, 21461. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-S.; Wang, X.-Y.; Zhang, G.-Y. Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste. Sustainability 2018, 10, 3369. [Google Scholar] [CrossRef]
- Cai, R.; Wu, T.; Fu, C.; Ye, H. Thermal degradation of potassium-activated ternary slag-fly ash-silica fume binders. Constr. Build. Mater. 2022, 320, 126304. [Google Scholar] [CrossRef]
- Mashri, M.O.M.; Johari, M.A.M.; Ahmad, Z.A.; Mijarsh, M.J.A. Enhancing the properties of UPOFA-based geopolymer mortar via the incorporation of eggshell ash and silica fume. J. Build. Eng. 2023, 65, 105677. [Google Scholar] [CrossRef]
- Wetzel, A.; Middendorf, B. Influence of silica fume on properties of fresh and hardened ultra-high performance concrete based on alkali-activated slag. Cem. Concr. Compos. 2019, 100, 53–59. [Google Scholar] [CrossRef]
- Althoey, F.; Zaid, O.; Alsulamy, S.; Martínez-García, R.; de Prado Gil, J.; Arbili, M.M. Determining engineering properties of ultra-high-performance fiber-reinforced geopolymer concrete modified with different waste materials. PLoS ONE 2023, 18, e0285692. [Google Scholar] [CrossRef] [PubMed]
- ASTM C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 1995; Volume 14. pp. 4–5. [CrossRef]
- E 778-87; Standard Test Methods for Nitrogen in the Analysis Sample of Refuse-Derived Fuel. iTeh Standards: San Francisco, CA, USA, 2004; pp. 3–5.
- ASTM C33/C33M; Standard Specification for Concrete Aggregates, ASTM C33-86. ASTM Standards: West Conshohocken, PA, USA, 2008; Volume 11. p. 11.
- ASTM C109/109M; Standard test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or cube specimens). ASTM Standards: West Conshohocken, PA, USA, 2016; pp. 1–10.
- ASTM C78/C78M-22; Standard Test Method for Flexural Strength of Concrete (Using Simple Beam with Third-Point Loading) 1, Hand The C78-02. ASTM Standards: West Conshohocken, PA, USA, 2010; pp. 1–4.
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N.; Shi, D. Mechanical and fracture properties of ultra-high performance geopolymer concrete: Effects of steel fiber and silica fume. Cem. Concr. Compos. 2020, 112, 103665. [Google Scholar] [CrossRef]
- Murali, G.; Nassar, A.K.; Swaminathan, M.; Kathirvel, P.; Wong, L.S. Effect of silica fume and glass powder for enhanced impact resistance in GGBFS-based ultra high-performance geopolymer fibrous concrete: An experimental and statistical analysis. Def. Technol. 2024, 41, 59–81. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K.H. Influence of silica fume content on microstructure development and bond to steel fiber in ultra-high strength cement-based materials (UHSC). Cem. Concr. Compos. 2016, 71, 97–109. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Dhinakaran, G. Influence of ultrafine TiO2 and silica fume on performance of unreinforced and fiber reinforced concrete. Constr. Build. Mater. 2018, 161, 570–576. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Akeed, M.H.; Qaidi, S.; Bakar, B.H.A. Influence of microsilica and polypropylene fibers on the fresh and mechanical properties of ultra-high performance geopolymer concrete (UHP-GPC). Case Stud. Constr. Mater. 2022, 17, e01367. [Google Scholar] [CrossRef]
- Behnood, A.; Ziari, H. Effects of silica fume addition and water to cement ratio on the properties of high-strength concrete after exposure to high temperatures. Cem. Concr. Compos. 2008, 30, 106–112. [Google Scholar] [CrossRef]
- Li, L.G.; Kwan, A.K.H. Concrete mix design based on water film thickness and paste film thickness. Cem. Concr. Compos. 2013, 39, 33–42. [Google Scholar] [CrossRef]
- Aisheh, Y.I.A.; Atrushi, D.S.; Akeed, M.H.; Qaidi, S.; Tayeh, B.A. Influence of steel fibers and microsilica on the mechanical properties of ultra-high-performance geopolymer concrete (UHP-GPC). Case Stud. Constr. Mater. 2022, 17, e01245. [Google Scholar] [CrossRef]
- Xi, Y.; Siemer, D.D.; Scheetz, B.E. Strength development, hydration reaction and pore structure of autoclaved slag cement with added silica fume. Cem. Concr. Res. 1997, 27, 75–82. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Cruz, A.S.A.; Marvila, M.T.; de Oliveira, L.B.; Monteiro, S.N.; Vieira, C.M.F.; Fediuk, R.; Timokhin, R.; Vatin, N.; Daironas, M. Natural fibers as an alternative to synthetic fibers in reinforcement of geopolymer matrices: A comparative review. Polymers 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, Y.; Guo, M.; Zhang, L.; Ning, H.; Liu, S. Mechanical and fracture properties of ultra-high performance concrete (UHPC) containing waste glass sand as partial replacement material. J. Clean. Prod. 2020, 277, 123501. [Google Scholar] [CrossRef]
- Kathirvel, P.; Sreekumaran, S. Sustainable development of ultra high performance concrete using geopolymer technology. J. Build. Eng. 2021, 39, 102267. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X. Deterioration of ambient-cured and heat-cured fly ash geopolymer concrete by high temperature exposure and prediction of its residual compressive strength. Constr. Build. Mater. 2020, 262, 120924. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, P.; Guo, J.; Wang, J. Effect of Municipal Solid Waste Incineration Fly Ash on the Mechanical Properties and Microstructure of Geopolymer Concrete. Gels 2022, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, J.C.; Cai, R.; Ye, H. Mechanical degradation of ultra-high strength alkali-activated concrete subjected to repeated loading and elevated temperatures. Cem. Concr. Compos. 2021, 121, 104083. [Google Scholar] [CrossRef]
- Ouda, A.S.; Gharieb, M. Behavior of alkali-activated pozzocrete-fly ash paste modified with ceramic tile waste against elevated temperatures and seawater attacks. Constr. Build. Mater. 2021, 285, 122866. [Google Scholar] [CrossRef]
- Cai, R.; Ye, H. Clinkerless ultra-high strength concrete based on alkali-activated slag at high temperatures. Cem. Concr. Res. 2021, 145, 106465. [Google Scholar] [CrossRef]
- Yu, M.; Wang, T.; Chi, Y.; Li, D.; Li, L.Y.; Shi, F. Residual mechanical properties of GGBS-FA-SF blended geopolymer concrete after exposed to elevated temperatures. Constr. Build. Mater. 2024, 411, 134378. [Google Scholar] [CrossRef]
- Swathi, B.; Vidjeapriya, R. Influence of precursor materials and molar ratios on normal, high, and ultra-high performance geopolymer concrete—A state of art review. Constr. Build Mater. 2023, 392, 132006. [Google Scholar] [CrossRef]
- Abdellatief, M.; Adel, B.; Alanazi, H.; Tawfik, T.A. Multiscale optimization analysis of high strength alkali-activated concrete containing waste medical glass under exposure to carbonation and elevated temperatures. Dev. Built Environ. 2024, 19, 100492. [Google Scholar] [CrossRef]
- Wan, X.; Shen, C.; Wang, P.; Zhao, T.; Lu, Y. A study on fracture toughness of ultra-high toughness geopolymer composites based on Double-K Criterion. Constr. Build. Mater. 2020, 251, 118851. [Google Scholar] [CrossRef]
- Gholampour, A.; Ho, V.D.; Ozbakkaloglu, T. Ambient-cured geopolymer mortars prepared with waste-based sands: Mechanical and durability-related properties and microstructure. Compos. B Eng. 2019, 160, 519–534. [Google Scholar] [CrossRef]
- Albidah, A.; Alqarni, A.S.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Behavior of Metakaolin-Based geopolymer concrete at ambient and elevated temperatures. Constr. Build. Mater. 2022, 317, 125910. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Ellatief, M.A.; Heneigel, A.M.; Elrahman, M.A. Characteristics of eco-friendly ultra-high-performance geopolymer concrete incorporating waste materials. Ceram. Int. 2022, 48, 19662–19674. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Shao, R.; Wu, C. Effect of high temperature and cooling method on compression and fracture properties of geopolymer-based ultra-high performance concrete. J. Build. Eng. 2025, 105, 112433. [Google Scholar] [CrossRef]
- Luo, Y.; Li, S.H.; Klima, K.M.; Brouwers, H.J.H.; Yu, Q. Degradation mechanism of hybrid fly ash/slag based geopolymers exposed to elevated temperatures. Cem. Concr. Res. 2022, 151, 106649. [Google Scholar] [CrossRef]













| Oxides (%) | Cao | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 |
|---|---|---|---|---|---|---|
| GGBFS | 38.80 | 32.60 | 15.60 | 6.02 | 1.36 | 1.83 |
| SF | 1.45 | 92.18 | 0.68 | 0.76 | 0.57 | 0.50 |
| QP | 0.04 | 99.65 | 0.085 | 0.003 | 0.01 | - |
| Class | QP | Type I | Type II |
|---|---|---|---|
| A | 25 | 50 | 25 |
| B | 30 | 45 | 25 |
| C | 35 | 40 | 25 |
| Mix ID | GGBFS | SF | Aggregates | NaOH | SS | SP | |
|---|---|---|---|---|---|---|---|
| QP | Sand | ||||||
| M0 | 980 | 0 | 0 | 1100 | 114 | 191 | 19 |
| 15SF25QP | 835 | 116 | 275 | 825 | 114 | 191 | 19 |
| 15SF30QP | 835 | 116 | 330 | 770 | 114 | 191 | 19 |
| 15SF35QP | 835 | 116 | 385 | 715 | 114 | 191 | 19 |
| 30SF25QP | 686 | 233 | 275 | 825 | 114 | 191 | 19 |
| 30SF30QP | 686 | 233 | 330 | 770 | 114 | 191 | 19 |
| 30SF35QP | 686 | 233 | 385 | 715 | 114 | 191 | 19 |
| Mix ID | 7 Days | 28 Days | 56 Days |
|---|---|---|---|
| M0 | 95.0 | 100.0 | 104.0 |
| 15SF25QP | 109.06 | 120.5 | 126.57 |
| 15SF30QP | 104.67 | 127.46 | 139.03 |
| 15SF35QP | 112.67 | 132.8 | 146.83 |
| 30SF25QP | 103.0 | 110.0 | 132.0 |
| 30SF30QP | 104.0 | 112.0 | 136.0 |
| 30SF35QP | 108.0 | 143.0 | 156.0 |
| Temp. | Mass Loss | Residual Compressive Strength | Observable Material Behavior | Ref. |
|---|---|---|---|---|
| Ambient | Negligible | 104–156 MPa | Dense microstructure with minimal unreacted particles; intact matrix with C-A-S-H and N-A-S-H gels. | [24,25,33] |
| 200 °C | Minimal (1–3%) | Increased by 3.5–29.8% | Slight densification due to enhanced geopolymerization; no visible cracking; matrix remains intact. | [22,24,30] |
| 400 °C | Moderate (5–10%) | 75.0–91.3% retention | Onset of microstructural damage; minor voids and cracks form due to bound water evaporation; material remains serviceable. | [24,25,33] |
| 800 °C | Significant (15–20%) | 23.8–30.8% retention | Severe degradation with extensive porosity and cracking; breakdown of C-A-S-H and N-A-S-H gels; compromised structural integrity. | [22,24,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhefny, R.A.; Abdellatief, M.; Elemam, W.E.; Tahwia, A.M. Thermal Degradation and Microstructural Evolution of Geopolymer-Based UHPC with Silica Fume and Quartz Powder. Infrastructures 2025, 10, 192. https://doi.org/10.3390/infrastructures10080192
Elhefny RA, Abdellatief M, Elemam WE, Tahwia AM. Thermal Degradation and Microstructural Evolution of Geopolymer-Based UHPC with Silica Fume and Quartz Powder. Infrastructures. 2025; 10(8):192. https://doi.org/10.3390/infrastructures10080192
Chicago/Turabian StyleElhefny, Raghda A., Mohamed Abdellatief, Walid E. Elemam, and Ahmed M. Tahwia. 2025. "Thermal Degradation and Microstructural Evolution of Geopolymer-Based UHPC with Silica Fume and Quartz Powder" Infrastructures 10, no. 8: 192. https://doi.org/10.3390/infrastructures10080192
APA StyleElhefny, R. A., Abdellatief, M., Elemam, W. E., & Tahwia, A. M. (2025). Thermal Degradation and Microstructural Evolution of Geopolymer-Based UHPC with Silica Fume and Quartz Powder. Infrastructures, 10(8), 192. https://doi.org/10.3390/infrastructures10080192








