Assessment of the Residual Life of the Repaired Arousa Bridge
Abstract
1. Introduction
2. Service History of and Repair Interventions on Arousa Bridge
2.1. Description of the Arousa Bridge
2.2. Structural Typology and Materials
2.3. Summary of 2007 Inspection: Observations and Intervention Program
2.4. Design and Implementation of the DuoGuard CPT System
- (1)
- Vertical steel surface: 12 mm rebar diameter, at 250 mm spacing 4 bars/m × 0.012 m × 3.14 × 1 m = 0.15 m2.
- (2)
- Horizontal steel surface: 10 mm rebar diameter, at 200 mm spacing 5 bars/m × 0.010 m × 3.14 × 1 m = 0.16 m2.
- (1)
- Zone 1—500 mm × 400 mm mesh over a surface area of 31 m2, resulting in approximately 150 units per pier, in six rows.
- (2)
- Zone 2—500 mm × 600 mm mesh over 27.2 m2, resulting in approximately 100 units per pier, in four rows.
- (3)
- Zone 3—800 mm × 600 mm mesh over 43.4 m2, resulting in approximately 80 units per pier.

- (1)
- An instant off potential more negative than −720 mV with respect to Ag/AgCl/0.5 M KCl.
- (2)
- A potential decay over 2 h and up to 24 h of at least 100 mV from “Instantaneous OFF”.
- (3)
- A potential decay over a 24 h period or longer of at least 150 mV from the instant off.
2.5. Inspection of 2021
3. Materials and Methods
3.1. Inspections and Protection Criteria
- (1)
- If potentials over an area are more positive than −0.20 V CSE, there is a greater than 90% probability that no reinforcing steel corrosion is occurring in that area at the time of measurement.
- (2)
- If potentials over an area are in the range of −0.20 to −0.35 V CSE, corrosion activity of the reinforcing steel in that area is uncertain.
- (3)
- If potentials over an area are more negative than −0.35 V CSE, there is a greater than 90% probability that reinforcing steel corrosion is occurring in that area at the time of measurement.
3.2. Core and Sacrificial Anode Extraction
4. Results and Discussion
4.1. Structural Health Assessment
4.1.1. Corrosion Risk and Potential Values
4.1.2. Chloride Penetration and Carbonation
4.1.3. Anode Consumption
4.2. Repassivation of the Galvanic System
4.3. Residual Life Estimation of the GCP System
4.3.1. Initial Calculation
- Available charge = charge capacity (Ah) × efficiency (85%) × utilization (85%), according to supplier specification [25].
- Lifetime = available charge capacity (mAh)/current output (mA)
4.3.2. Residual Life of the Repaired Bridge
5. Conclusions
- (1)
- The assessments conducted since the repair of Arousa Bridge in 2011 demonstrate that the applied cathodic protection systems effectively reduced corrosion risks. While some localized corrosion issues emerged in non-repaired areas, no significant deterioration was found in the protected sections.
- (2)
- The main indicator used to assess the corrosion risk was the potential against installed reference electrodes. This offers incomplete information, and it should be complemented with visual inspection and detailed analysis to complete the corrosion risk assessment.
- (3)
- There are indications that the cathodic protection system installed in the bridge could benefit from another period of impressed current to repassivate the steel and reactivate the sacrificial anode. The periodicity of this intervention was not defined previously and should be protocolized.
- (4)
- Reactivating the system with impressed current is likely advantageous in areas with lower humidity levels. Future research should concentrate on these regions, exploring options such as increasing the number of anodes or reducing the anode spacing, analyzing the medium’s electrical transmissivity, examining the impact of humidity on electrical transmissivity, and identifying key factors influencing system performance to recommend impressed current application for repassivation.
- (5)
- A more detailed investigation into the corrosion status of the bridge, including the elaboration of intensity vs. time curves (where available) is needed. Based on the measured differences in corrosion potentials at the installed control electrodes and the increased signs of corrosion, it is possible that a new shot of impressed current for re-alkalinization should be applied to the entire bridge as a preventative measure.
- (6)
- The cathodic protection system was originally designed with a lifespan of 50 years. Our analysis suggests that the actual operational longevity may extend to a real total since repair of 65–83 years. This increase in expected service life highlights the effectiveness of the cathodic protection strategy employed. This follow-up information is very important for decision-making in future interventions on similarly damaged structures.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrne, A.; Norton, B.; Holmes, N. State-of-the-art review of cathodic protection for reinforced concrete structures. Mag. Concr. Res. 2016, 68, 664–677. [Google Scholar] [CrossRef]
- Glass, G.K.; Roberts, A.C.; Davison, N. Hybrid corrosion protection of chloride-contaminated concrete. Proc. Inst. Civ. Eng. Constr. Mater. 2008, 161, 163–172. [Google Scholar] [CrossRef]
- Polder, R.; Pan, Y.; Courage, W.; Peelen, W. Preliminary study of life cycle cost of preventive measures and repair options for corrosion in concrete infrastructure. Heron 2016, 61, 1–13. [Google Scholar]
- Sergi, G. Life extension of existing steel reinforced structures by simple cathodic protection techniques for sustainable durability. In Life-Cycle of Structures and Infrastructure Systems, Proceedings of the 8th International Symposium on Life-Cycle Civil Engineering, Milan, Italy, 2–6 July 2023; CRC Press: Boca Raton, FL, USA, 2023; Volume 2023, pp. 2839–2846. [Google Scholar] [CrossRef]
- Sergi, G. Ten-year results of galvanic sacrificial anodes in steel reinforced concrete. Mater. Corros. 2011, 62, 98–104. [Google Scholar] [CrossRef]
- Stone, C.; Donadio, M.; Christodoulou, C.; Davison, N. Case studies of hybrid and galvanic systems on bridge structures. In MATEC Web of Conferences, Proceedings of the Concrete Solutions 2019—7th International Conference on Concrete Repair, Cluj-Napoca, Romania, 30 September–2 October 2019; EDP Sciences: Jules, France, 2019; Volume 289. [Google Scholar] [CrossRef]
- Astuti, P.; Rafdinal, R.S.; Yamamoto, D.; Andriamisaharimanana, V.; Hamada, H. Effective Use of Sacrificial Zinc Anode as a Suitable Repair Method for Severely Damaged RC Members Due to Chloride Attack. Civ. Eng. J. 2022, 8, 1535–1548. [Google Scholar] [CrossRef]
- Christodoulou, C.; Sharifi, A.; Das, S.; Goodier, C. Cathodic protection on the UK’s Midland Links motorway viaducts. Proc. Inst. Civ. Eng. Bridg. Eng. 2014, 167, 43–53. [Google Scholar] [CrossRef]
- Whitmore, D.; Sergi, G. Long-term Monitoring Provides Data Required to Predict Performance and Perform Intelligent Design of Galvanic Corrosion Control Systems for Reinforced Concrete Structures. In Proceedings of the CORROSION 2021, Virtual, 19–30 April 2021; NACE: Houston, TX, USA, 2021; pp. 1–10. [Google Scholar]
- Mosquera, J.A.B.; Landrove, J.E.P.; Pérez, J.M.M.; Carro-Lopez, D. Structures with chloride attack. Application of cathodic protection in the bridge to Illa de Arousa. Hormigón Acero 2017, 68, 201–207. [Google Scholar] [CrossRef]
- Pérez-Fadón Martínez, S. Puente a la Isla de Arosa. Hormigón Acero 1985, 36, 192–217. Available online: https://www.hormigonyacero.com/index.php/ache/issue/view/197 (accessed on 1 September 2021).
- González, J.L.; Aguilera, F.P.; García, F.R. Proyecto de rehabilitación del puente de la isla de Arosa. Hormigón Acero 2013, 270, 75–89. [Google Scholar]
- ISO 12696:2022; AENOR, Protección Catódica del Acero en el Hormigón. ISO: Geneva, Switzerland, 2022.
- León González, J.; Prieto Aguilera, F. Rehabilitación del Puente de Acceso a la Isla de Arosa; ACHE, Ed.; V Congr. ACHE: Barcelona, Spain, 2011. [Google Scholar]
- Becerra-Mosquera, J.A.; Carro-López, D.; Landrove, J.E.P.; Pérez, J.M.M. Comparison data between hybrid and galvanic anode installation in the Arousa Island Bridge. In Proceedings of the 6th International Conference on Durability of Concrete Structures (ICDCS 2018), Leeds, UK, 18–20 July 2018; pp. 729–735. [Google Scholar]
- Becerra-Mosquera, J.A.; Carro-López, D.; Herrador, M.F. The Arousa Island bridge. Complete refurbishment and 10 years analysis description. Struct. Concr. 2024, 25, 1554–1570. [Google Scholar] [CrossRef]
- ASTM C876-15; Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM Standard: West Conshohocken, PA, USA, 2015; Volume 14, pp. 4–5. [CrossRef]
- UNE-EN 14629:2007; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Chloride Content in Hardened Concrete. CEN: Brussels, Belgium, 2007.
- DNV-RP-B401; Cathodic Protection Design. Det Norske Veritas: Høvik, Norway, 2005. Available online: http://www.dnv.com (accessed on 1 September 2021).
- Lee, D.; Jeong, J.A. Investigation of the Effective Range of Cathodic Protection for Concrete Pile Specimens Utilizing Zinc Mesh Anode. Corros. Sci. Technol. 2024, 23, 195–202. [Google Scholar] [CrossRef]
- AENOR, EHE-08 Regulation of Structural Concrete. Spain, 2008. Available online: https://www.fomento.gob.es/MFOM/LANG_CASTELLANO/ORGANOS_COLEGIADOS/CPH/instrucciones/EHE_es/ (accessed on 1 September 2021). (In Spanish).
- Ahlström, J. Corrosion of Steel in Concrete at Various Moisture and Chloride Levels, 2014. Available online: https://portal.research.lu.se/en/publications/corrosion-of-steel-in-concrete-at-various-moisture-and-chloride-l (accessed on 21 March 2025).
- Bertolini, L.; Bolzoni, F.; Gastaldi, M.; Pastore, T.; Pedeferri, P.; Redaelli, E. Effects of cathodic prevention on the chloride threshold for steel corrosion in concrete. Electrochim. Acta 2009, 54, 1452–1463. [Google Scholar] [CrossRef]
- Page, C.L.; Sergi, G. Developments in Cathodic Protection Applied to Reinforced Concrete. J. Mater. Civ. Eng. 2000, 12, 8–15. [Google Scholar] [CrossRef]
- Davison, N. Duoguard 500 Specification; Specification Numbre CPT523; Concrete Preservation Technologies: Nottingham, UK, 2010. [Google Scholar]




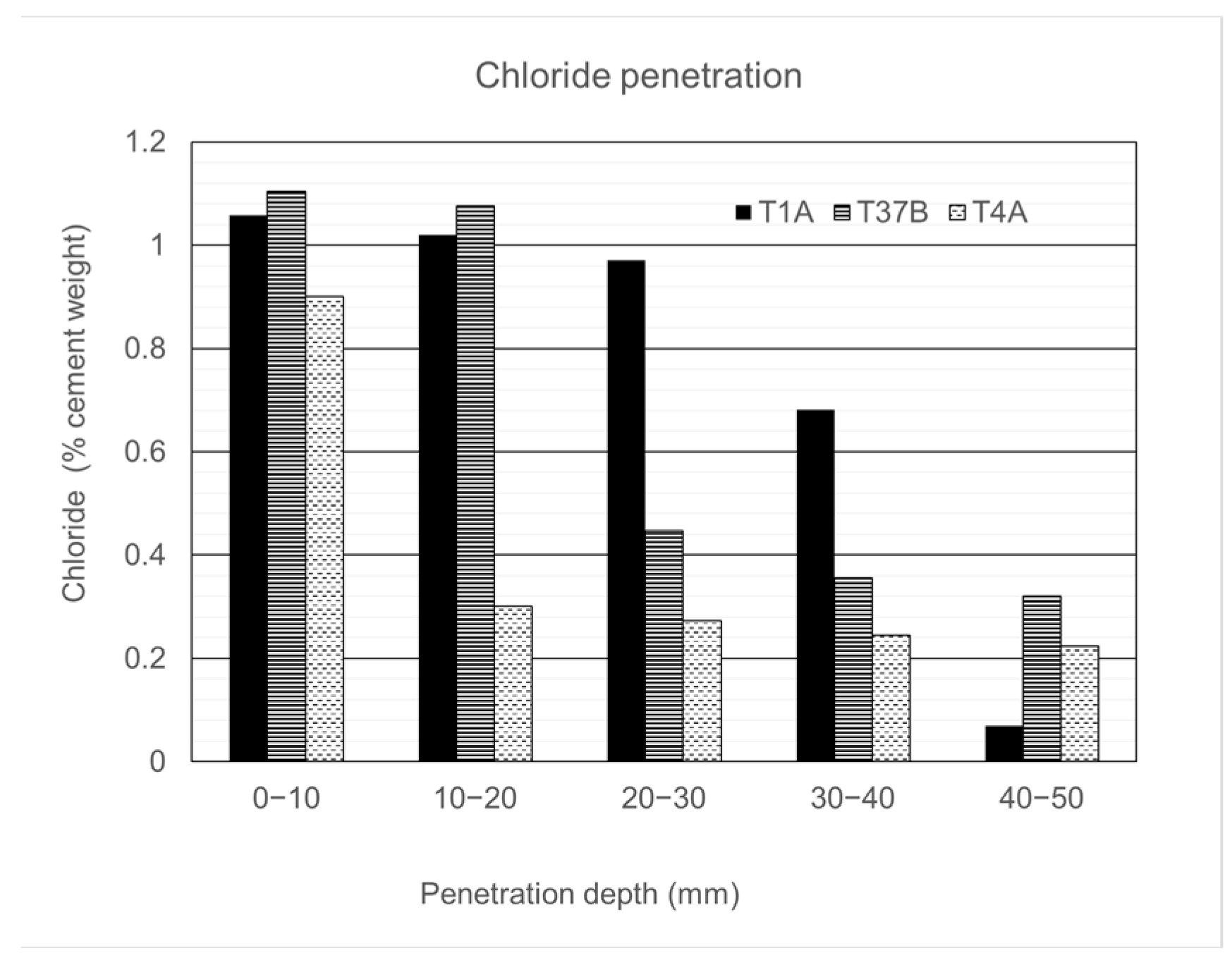
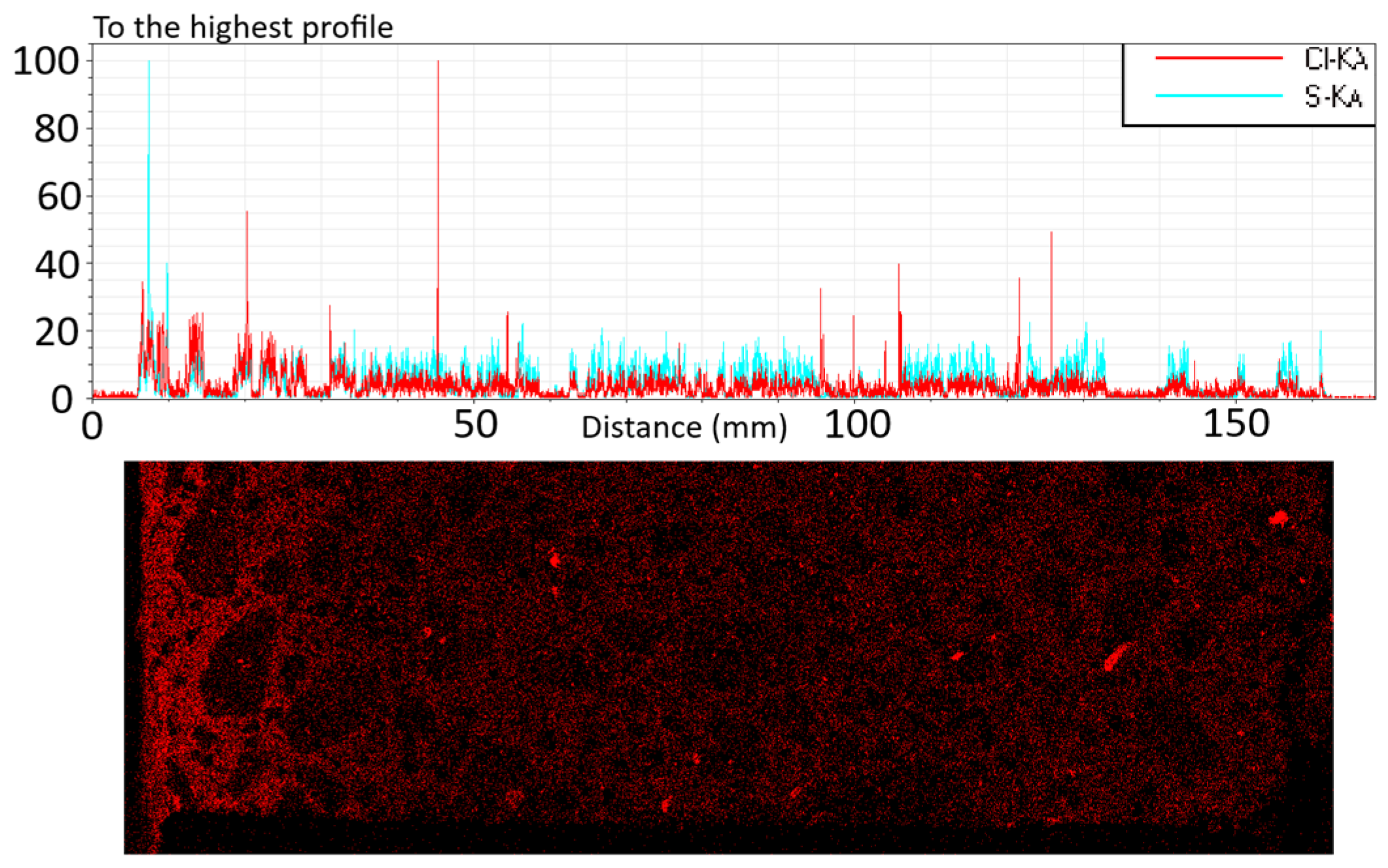





| Sample | Depth (mm) |
|---|---|
| 1A | 8 |
| 1B | 25 |
| 4A | 40 |
| 4B | 15 |
| 37A | 8 |
| 37B | 25 |
| Pier | Remaining Mass of Zinc (%) |
|---|---|
| 4 | 85.8 |
| 9 | 84.2 |
| 37 | 84.3 |
| Zone | Anode Units/m2 Concrete Surface | Ah Capacity/m2 Concrete Surface |
|---|---|---|
| 1 | 5 | 520 |
| 2 | 3.3 | 340 |
| 3 | 2.1 | 215 |
| Zone | Engineering Safety Factor | Anode Current Output (mA/m2) Steel Surface | Anode Lifetime-Years |
|---|---|---|---|
| 1 | 2 | 3.4 | 54 |
| 2 | 2 | 2.2 | 54 |
| 3 | 2 | 1.5 | 54 |
| Pier | Capacity | Estimated Consumption | Estimated Lifetime * | Measured Consumption | Updated Lifetime * |
|---|---|---|---|---|---|
| 4 | 26,510 Ah | 5439.57 Ah | 61 years | 3870.46 Ah | 56–64 years |
| 9 | 27,500 Ah | 6814.82 Ah | 56 years | 4317.50 Ah | 52–59 years |
| 37 | 32,780 Ah | 5468.96 Ah | 66 years | 5179.24 Ah | 61–70 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Mosquera, J.A.; Carro-López, D.; Herrador, M.F.; Eiras-López, J. Assessment of the Residual Life of the Repaired Arousa Bridge. Infrastructures 2025, 10, 141. https://doi.org/10.3390/infrastructures10060141
Becerra-Mosquera JA, Carro-López D, Herrador MF, Eiras-López J. Assessment of the Residual Life of the Repaired Arousa Bridge. Infrastructures. 2025; 10(6):141. https://doi.org/10.3390/infrastructures10060141
Chicago/Turabian StyleBecerra-Mosquera, José Antonio, Diego Carro-López, Manuel F. Herrador, and Javier Eiras-López. 2025. "Assessment of the Residual Life of the Repaired Arousa Bridge" Infrastructures 10, no. 6: 141. https://doi.org/10.3390/infrastructures10060141
APA StyleBecerra-Mosquera, J. A., Carro-López, D., Herrador, M. F., & Eiras-López, J. (2025). Assessment of the Residual Life of the Repaired Arousa Bridge. Infrastructures, 10(6), 141. https://doi.org/10.3390/infrastructures10060141











