Parameters Impacting the 3D Direct Ink Writing (DIW) Silicone Printing Process and Investigating How the Size of 3D-Printed Aortic Heart Valve Models Influences Cardiac Efficiency
Abstract
1. Introduction
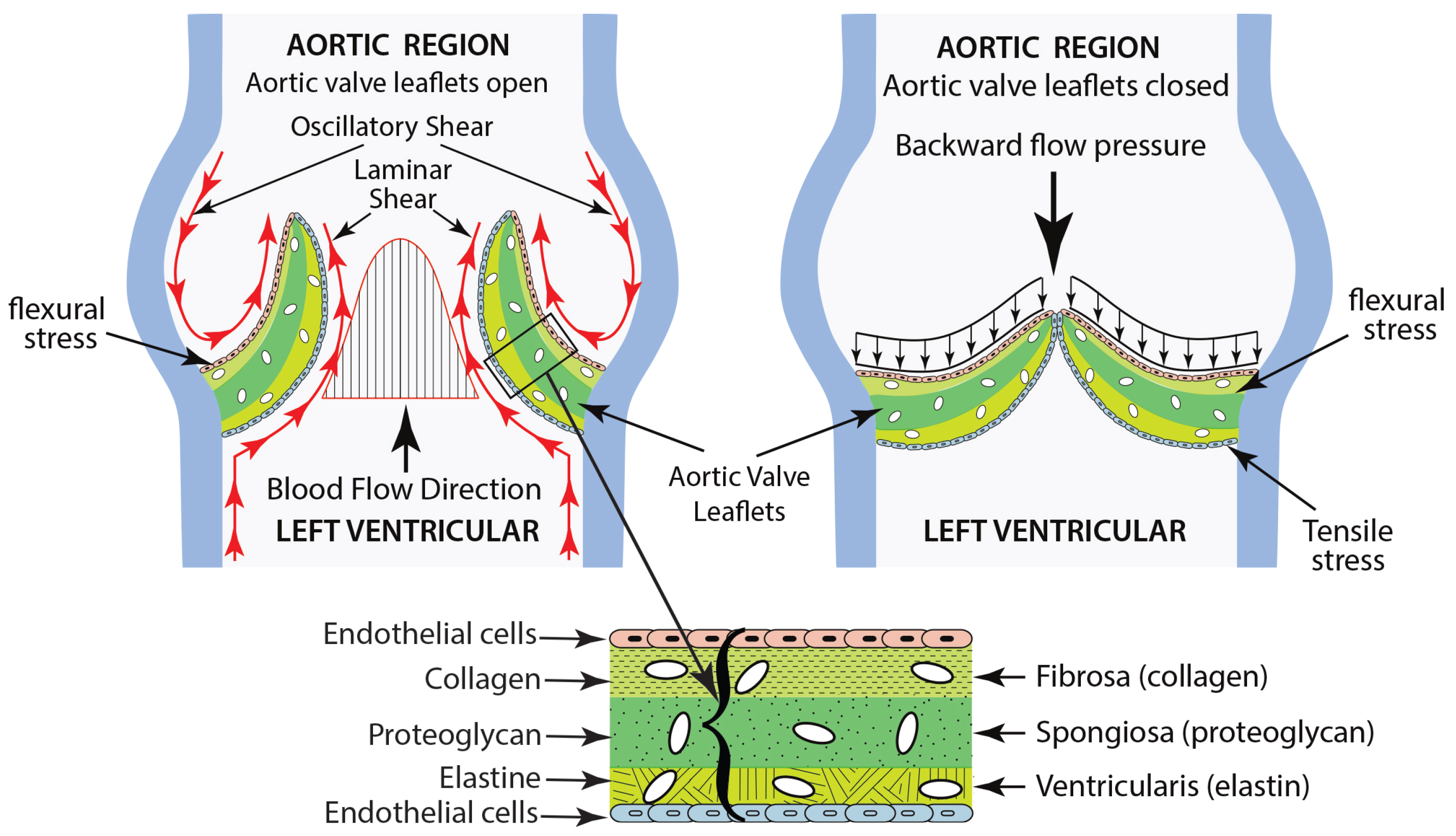
- The fibrosa layer, located on the outflow surface, is composed of collagen fibers that improve its structural integrity.
- The ventricularis layer, situated on the inflow surface, is made up of elastin, which facilitates the expansion and contraction of the valve throughout the cardiac cycle.
- The spongiosa layer is found at the center and is made up of loose connective tissue, permitting the relative movement of the adjacent layers.
- Shear stresses caused by blood flow, which manifest when the valve is in the systolic (open) state.
- Flexural stress, which is generated by transvascular pressure during the opening and closing phases.
- Tensile stresses, which develop when the valve is closed during diastole.
2. Materials and Methods
2.1. Factors Affecting the Direct Ink Writing 3D Silicone Printing Process
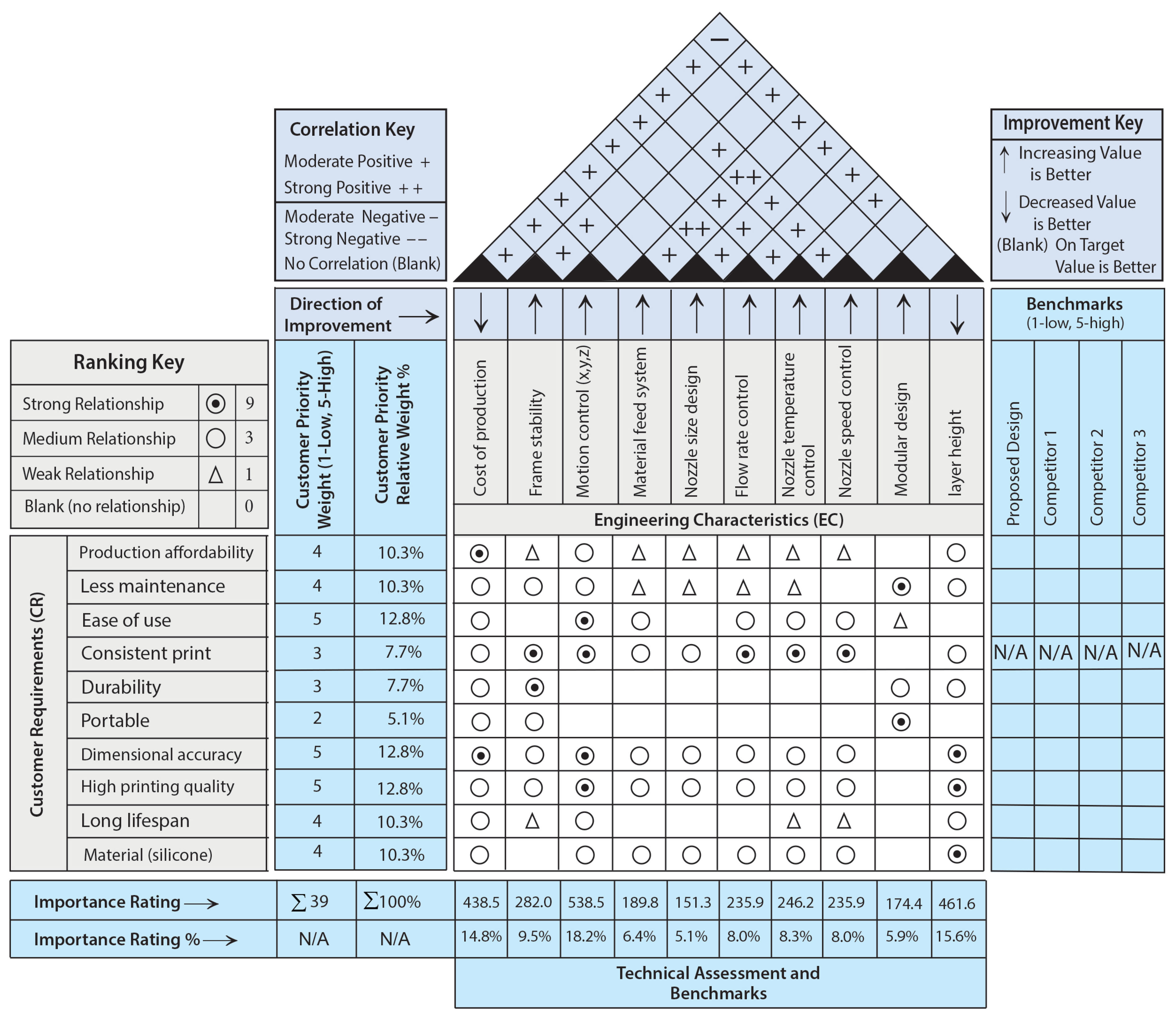
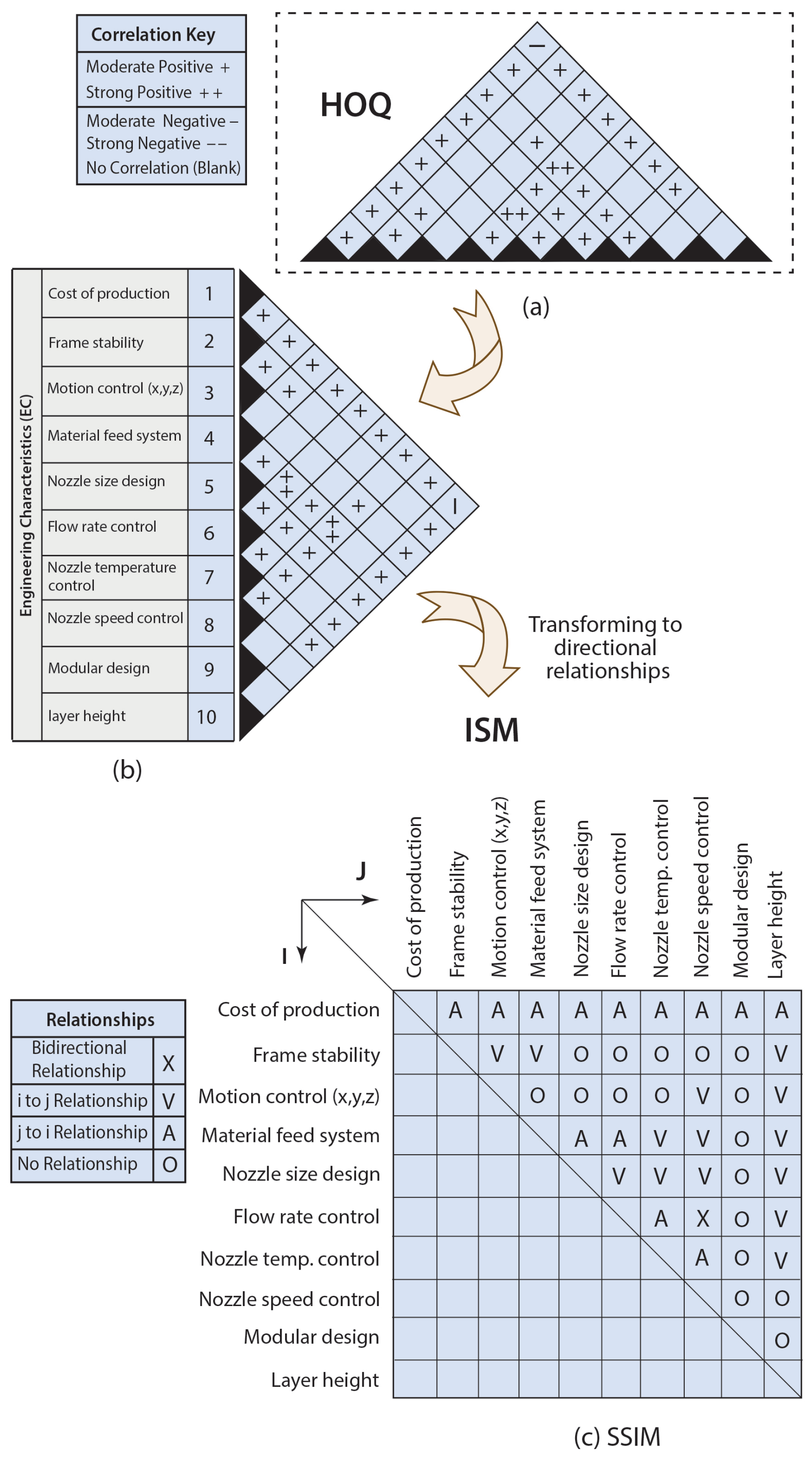
2.1.1. House of Quality
2.1.2. Interpretive Structural Modeling (ISM)
- “V” represents the relationship from column (i) to row (j), but not in both directions;
- “A” denotes the relationship from row (j) to column (i), also not in both directions;
- “X” signifies bidirectional relationships: from column (i) to row (j) and from row (j) to column (i);
- “O” indicates the absence of any relationships between the parameters.
2.2. Silicone Material
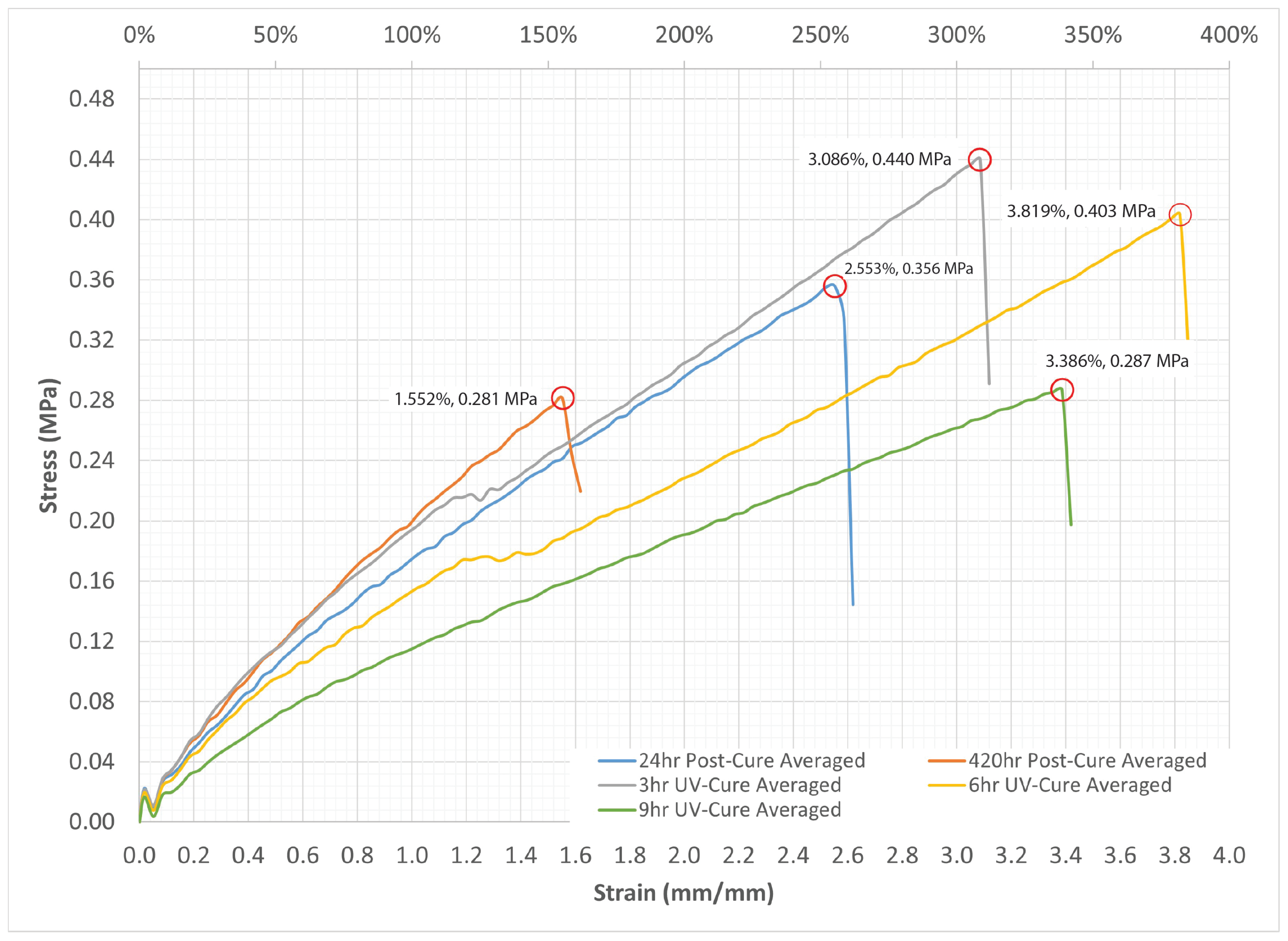
2.3. Tensile Testing of Silicone
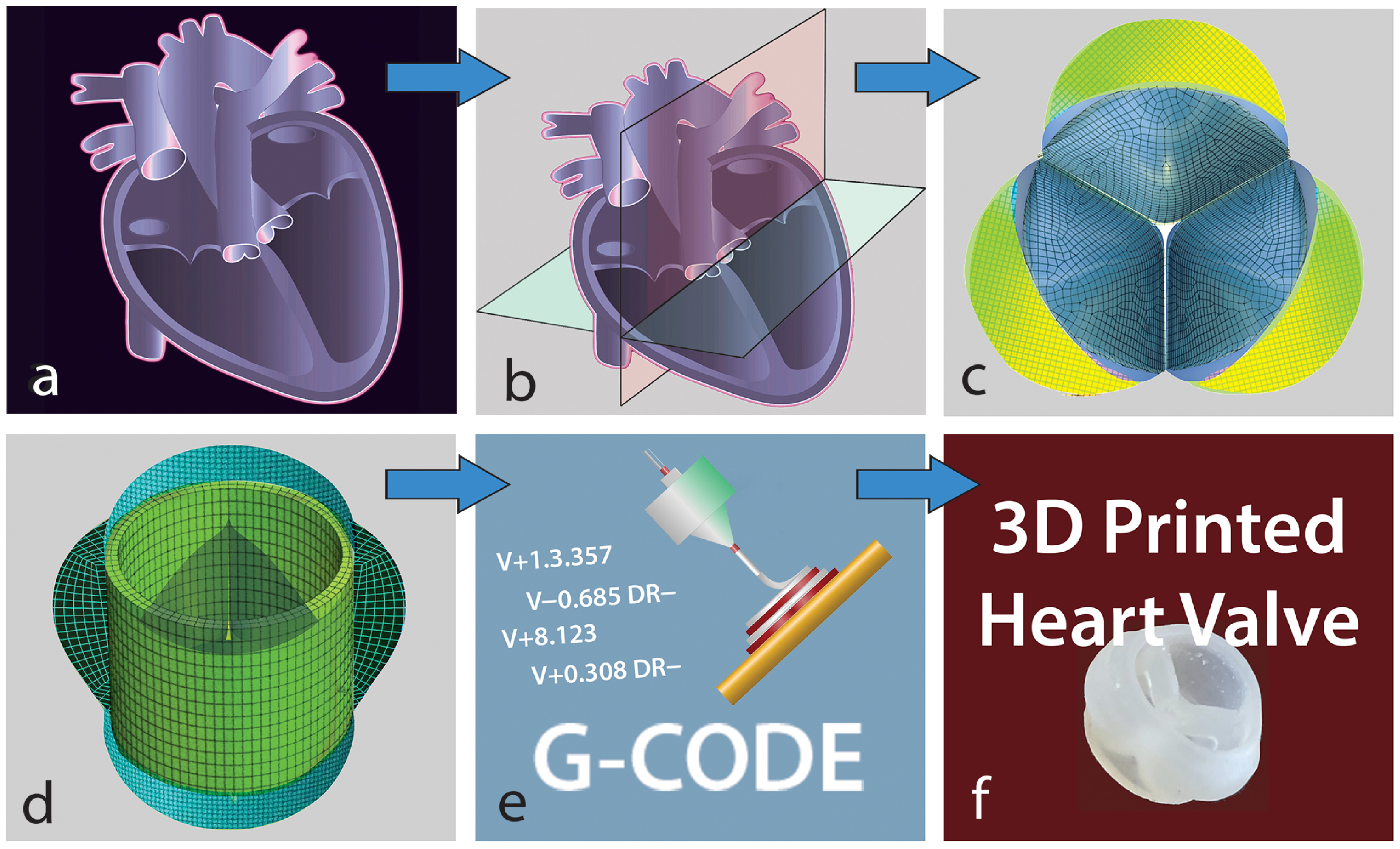
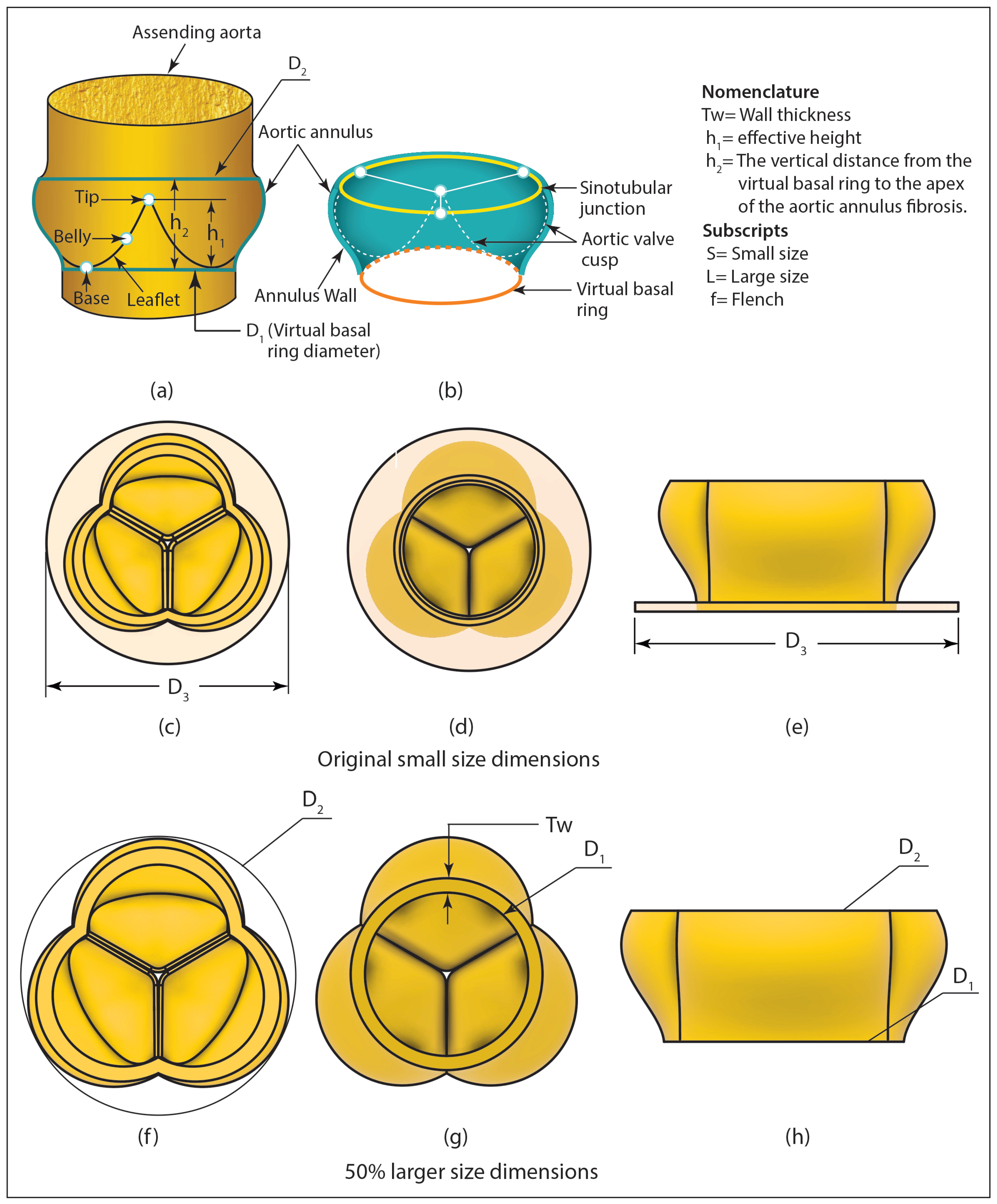
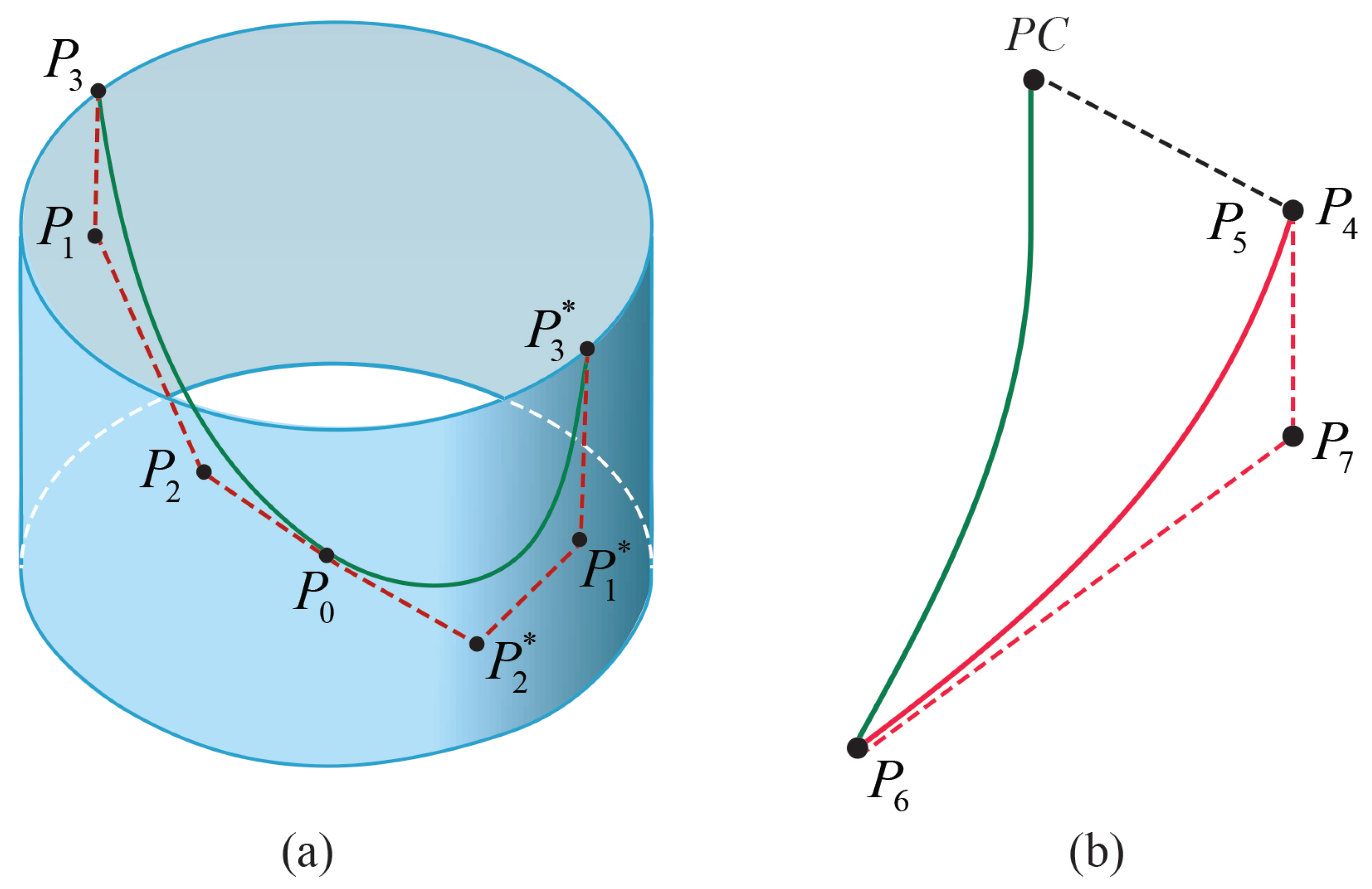
2.4. Geometry Generation for the Heart Valve and the Aorta
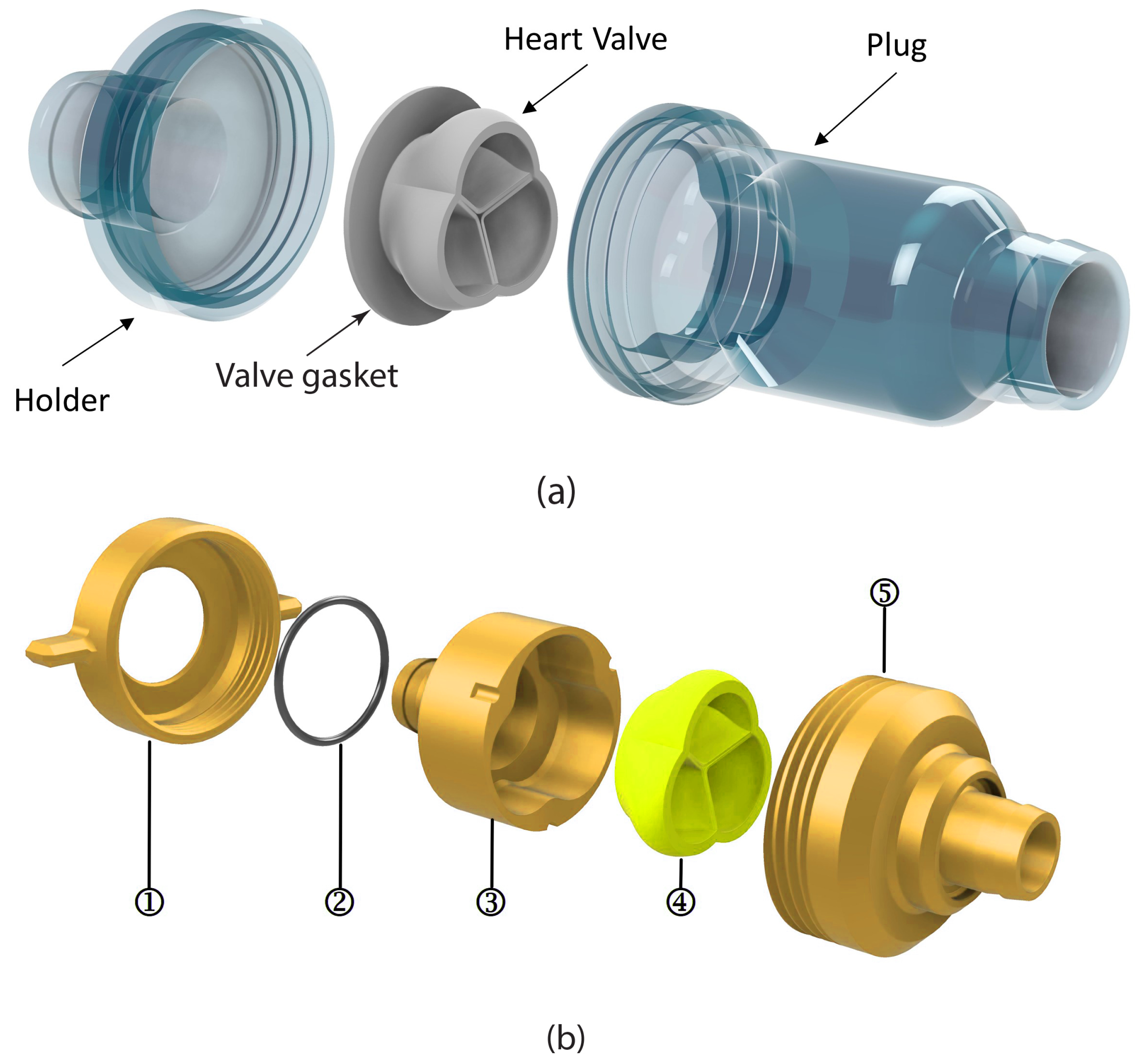
2.5. Experimental Setup
3. Results and Discussions
3.1. Design Analysis of the 3D Printing Machine for Heart Valve Production
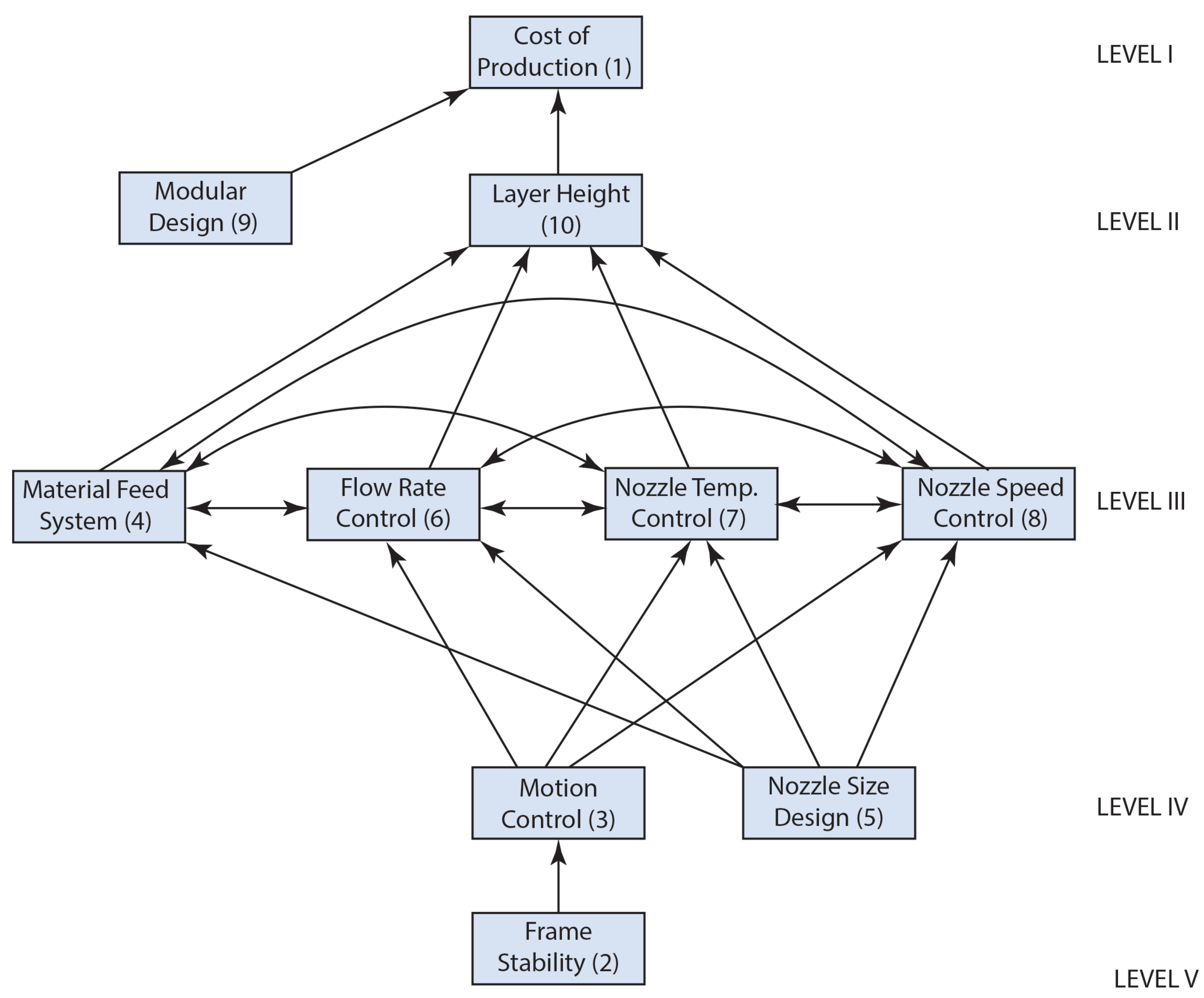
3.1.1. Formation of Digraph
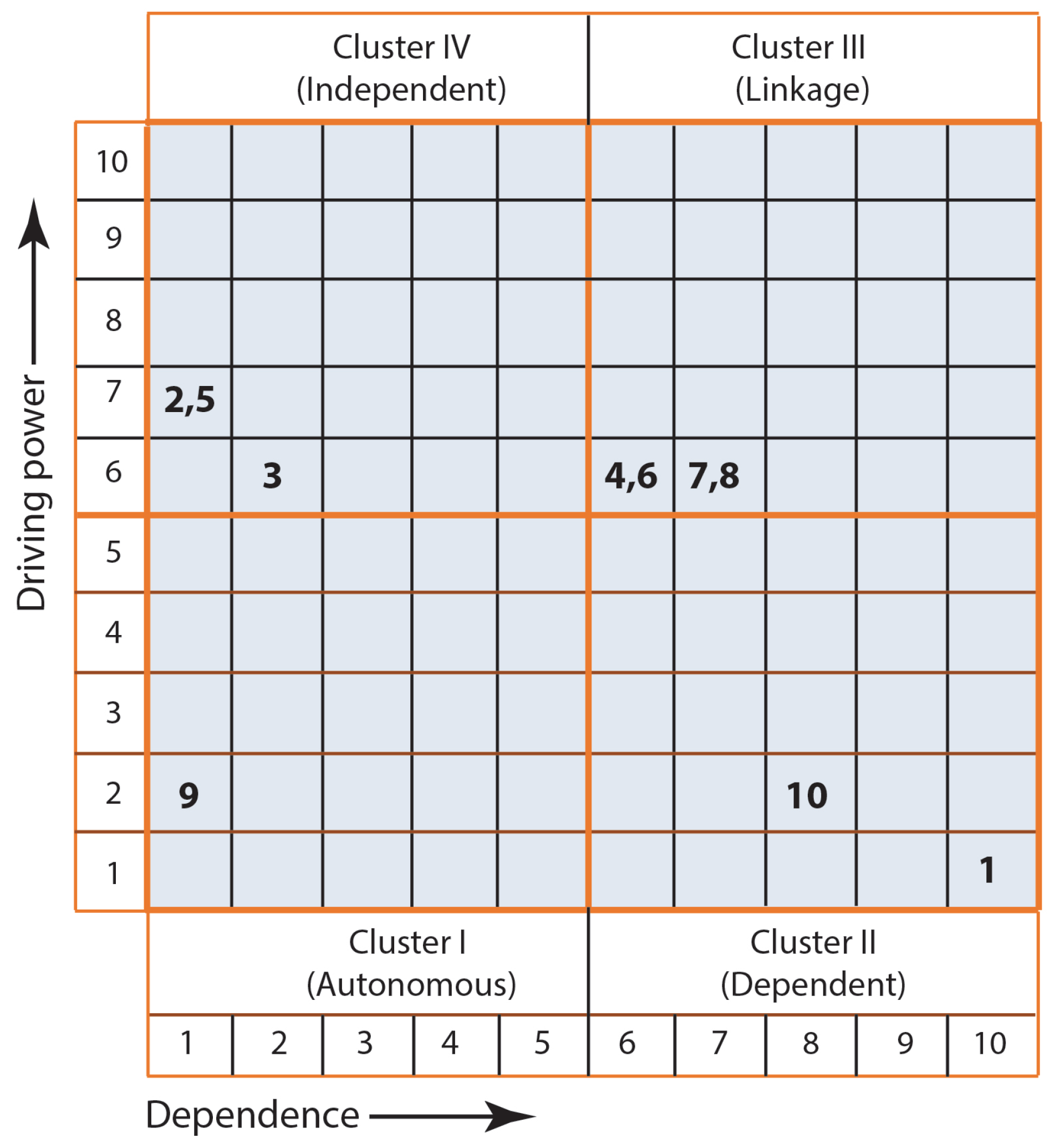
3.1.2. MICMAC Analysis
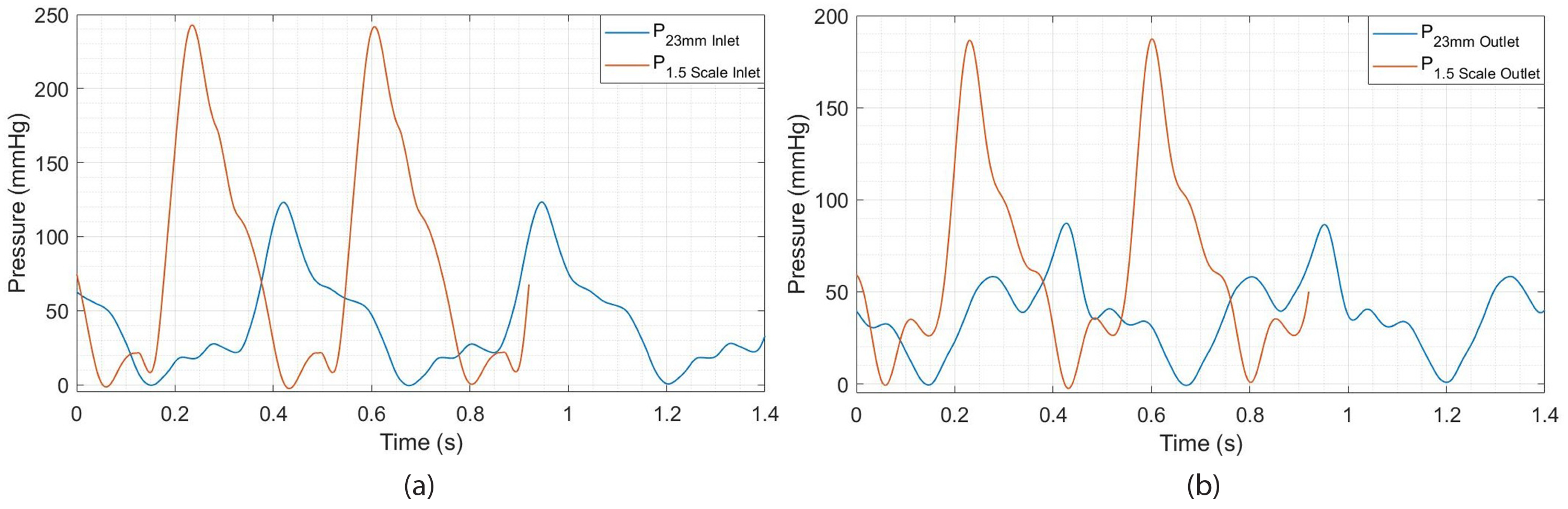
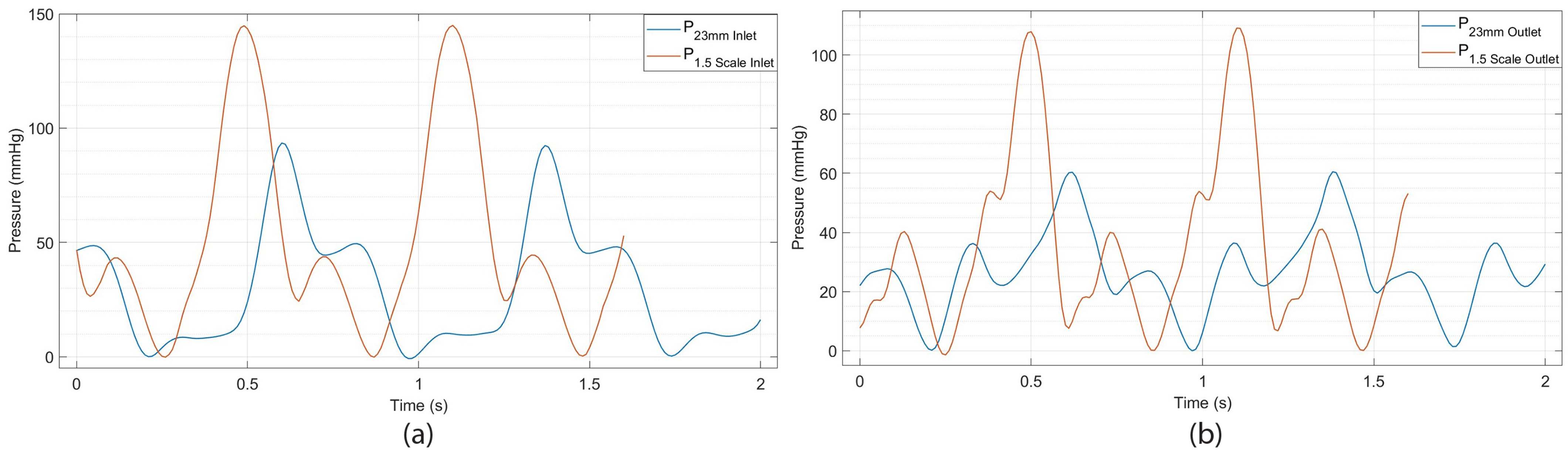
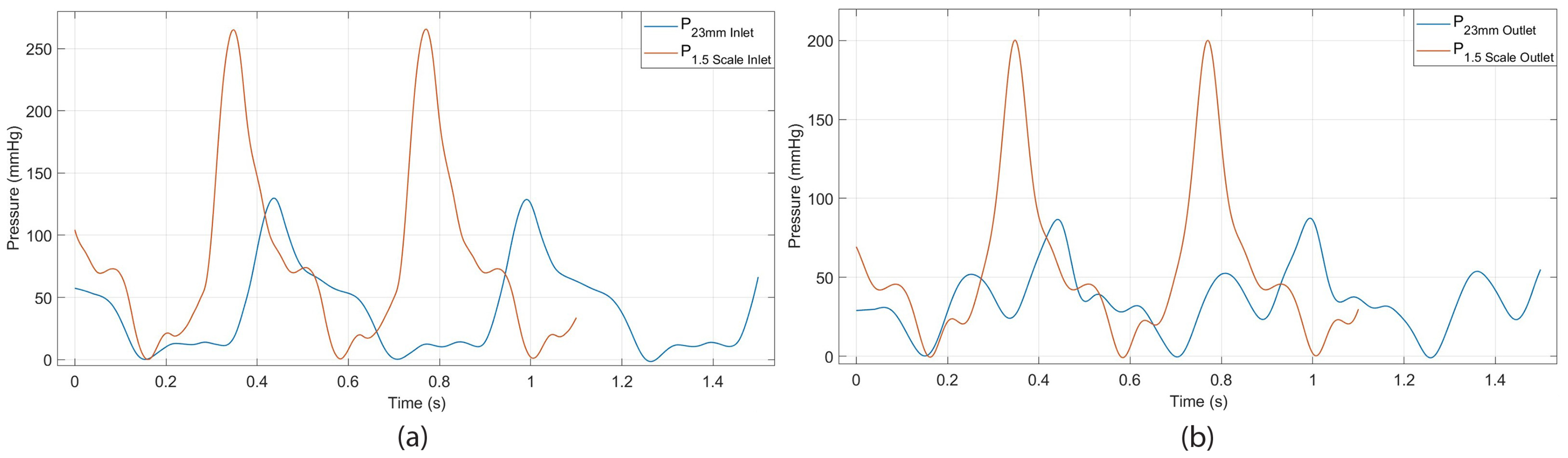
3.2. The Effect of Heart Valve Size on Cardiac Performance
3.2.1. Assessment of 3D-Printed Polymeric Heart Valve Performance
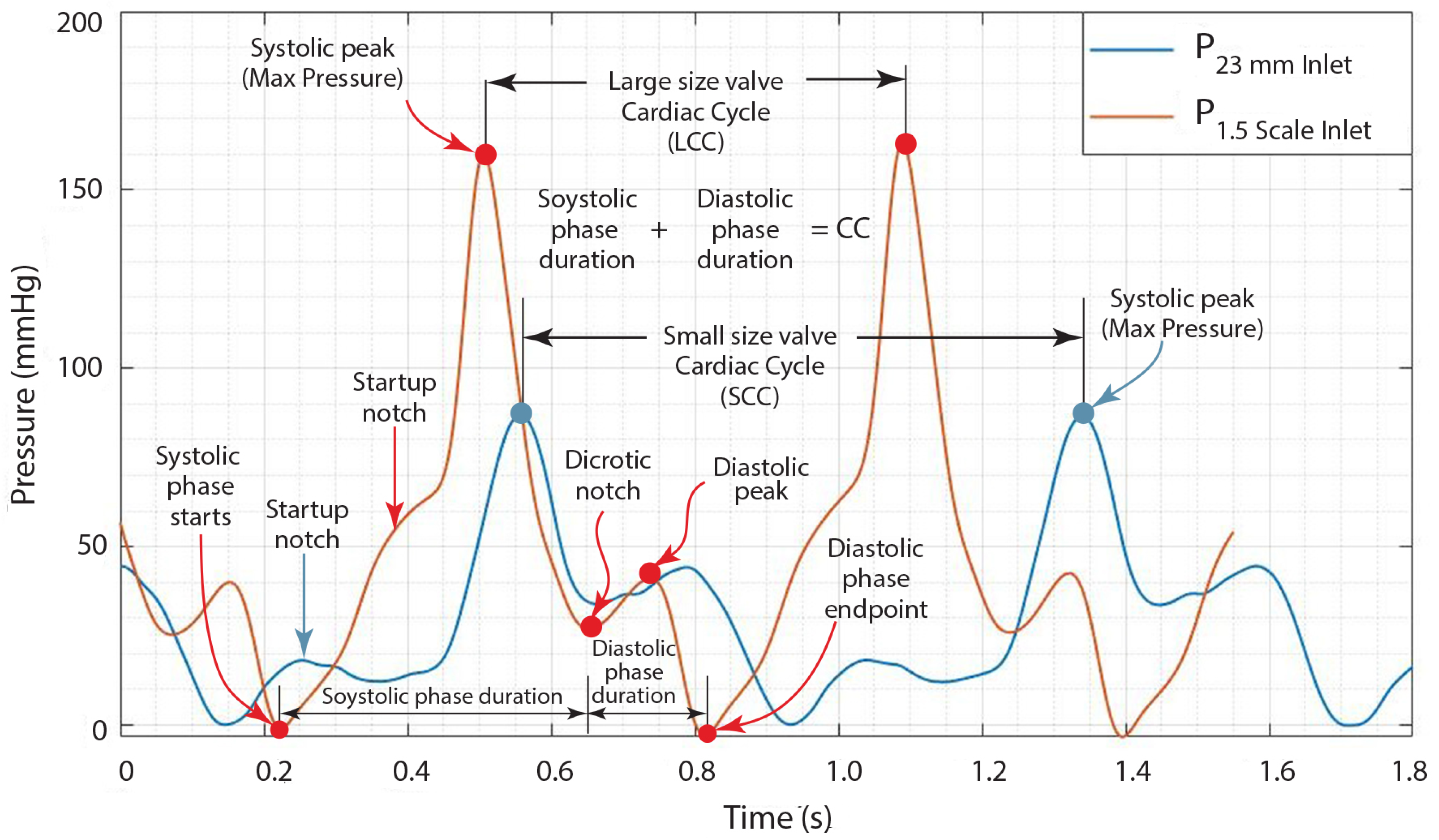
3.2.2. Evaluation of Heart Valve Inlet and Outlet Pressures Plots
- The period of contraction in the ventricles, termed systole;
- The period of relaxation in the ventricles, identified as diastole.
4. Conclusions
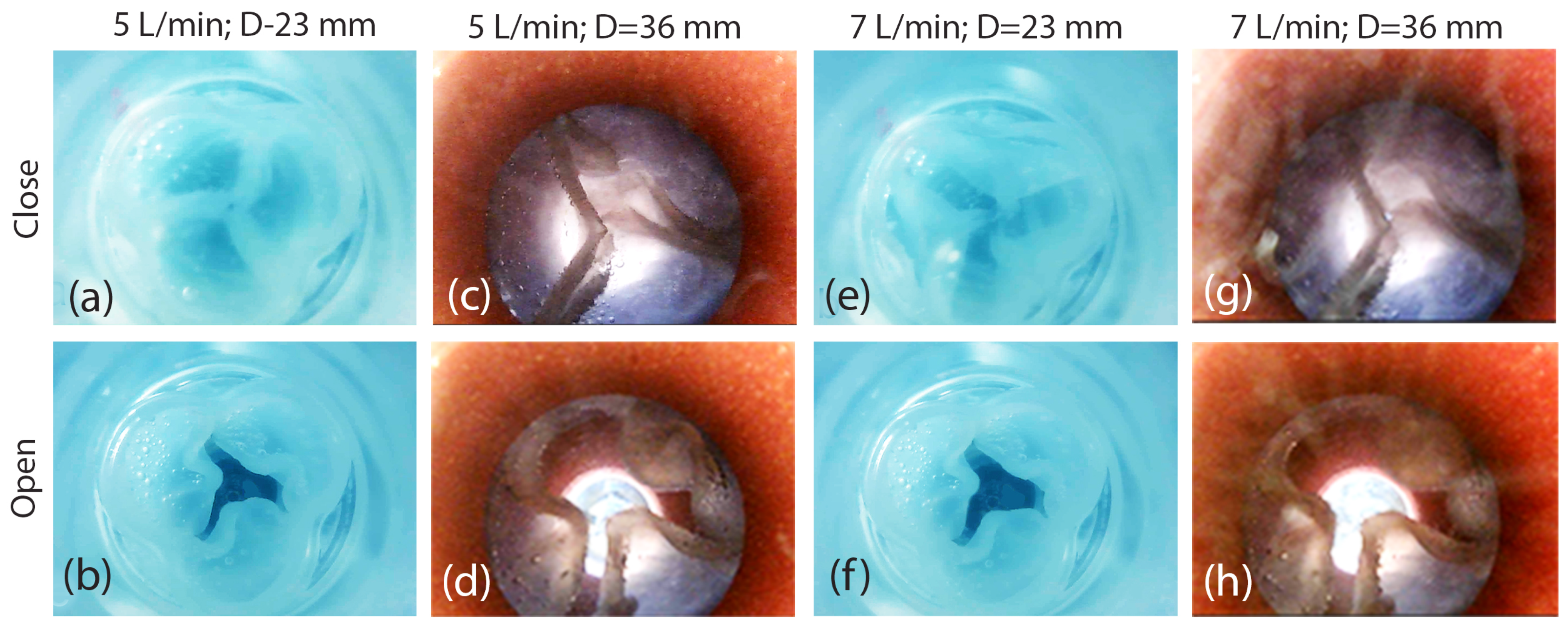
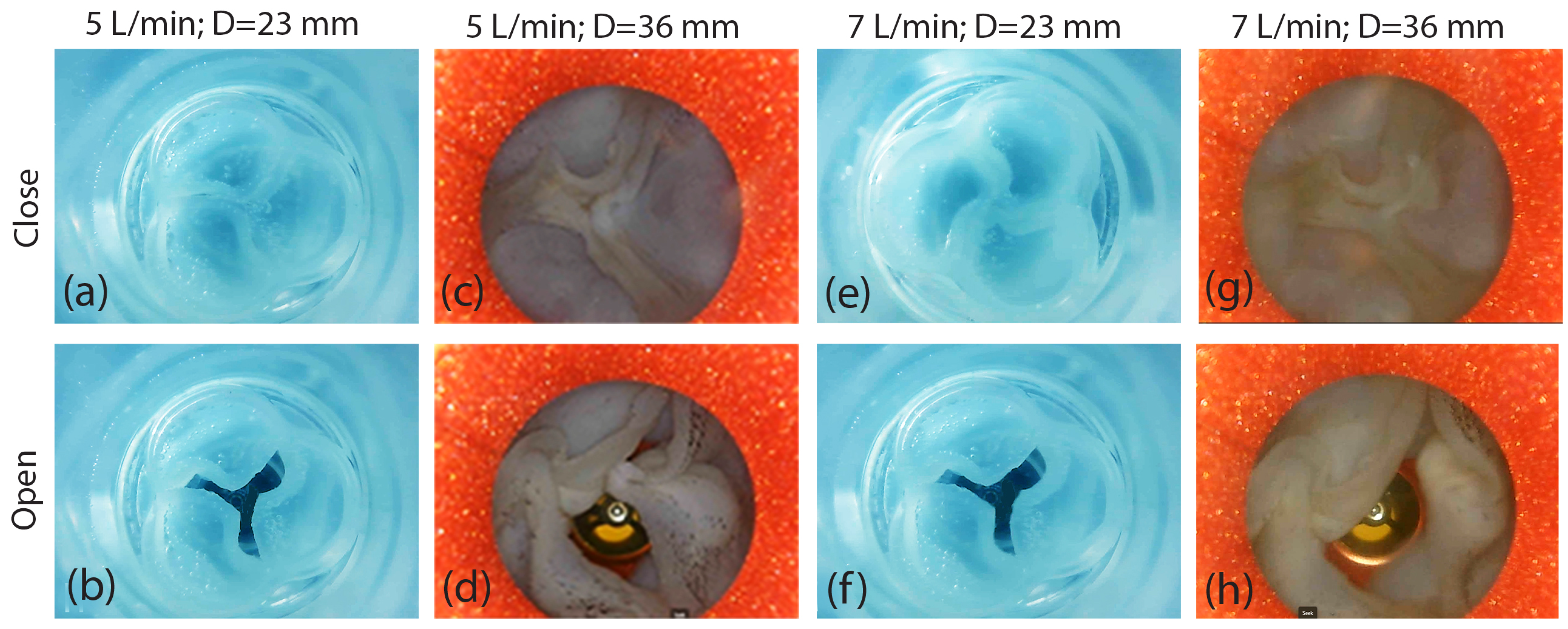
- The small-sized valve successfully closed completely without any leakage.
- The heart valve with a small diameter of 23 mm and leaflet thicknesses of 0.8 mm and 1.6 mm functioned effectively, achieving the desired flow rates of 5 L/min and 7 L/min.
- The heart valve with a small diameter of 23 mm exhibited a symmetrical opening for both flow rates of 5 L/min and 7 L/min, as well as for the two different leaflet thicknesses. Naturally, the valve opening was larger at the flow rate of 7 L/min.
- An increase in valve size leads to a reduction in the duration of the cardiac cycle, signifying a higher heart rate; the heart accelerates its beats as it finishes each cycle more swiftly, achieving its maximum rate.
- A 1.5 times enlargement (a 50% increase in size) of the heart valve results in an almost 100% increase in maximum pressure (systolic peak). This indicates that the pressure in the arteries rises above normal levels during heartbeats, which is a risk factor for heart disease and strokes.
- The heart valve with a diameter measuring 36 mm demonstrated no leakage throughout its operation, and it exhibited symmetrical and similar characteristics at the reduced thickness of the leaflets. A higher flow rate led to a wider valve opening, as expected.
- The enlargement of the aortic valve, characterized by the presence of thicker leaflets, commonly results from the thickening and stiffening of these leaflets, causing a decrease in valve opening and an asymmetrical pattern of valve opening and closing.
Concluding Remarks on Influencing Performance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- The nozzle is the last element of the material feeding system; the stability of the frame in a 3D printing machine plays a crucial role in determining the achievable layer height. A frame that lacks stability may induce vibrations during the printing process, leading to irregular layer deposition and ultimately resulting in poor print quality.
- The motion control system’s performance, and consequently its precision, can be affected by the stability and rigidity of the 3D printer’s frame and its components.
- The motion control of a 3D printer, particularly along the X, Y, and Z axes, has a direct influence on the speed at which the nozzle deposits material, thereby affecting both the overall printing speed and the quality of the finished product.
- The motion control of a 3D printer, specifically along the Z axis, directly affects the layer height. The Z axis controls the vertical movement of the printhead, determining how thickly each layer of material is deposited, essentially defining the layer height.
- A larger nozzle diameter on a 3D printer directly impacts the material feed system, necessitating an increased material flow rate for extrusion. In contrast, a smaller nozzle demands a reduced flow rate.
- In a 3D printer, the regulation of flow rate is crucial as it determines the volume of material extruded from the nozzle. This is achieved by adjusting the speed at which the filament is fed through the extruder, thereby influencing the “thickness” of the material being deposited.
- The material feed system plays a crucial role in regulating the temperature of the 3D printer nozzle. The speed at which material is fed through the nozzle has a direct influence on the amount of heat dissipated from the heating element.
- The material feed system plays a crucial role in regulating the nozzle speed of a 3D printer. The speed at which material is fed into the extruder directly determines the amount of material that can be extruded through the nozzle. This, in turn, affects the required printing speed to ensure consistent material deposition.
- The material feed system in a 3D printer significantly influences the layer height, as it regulates the quantity of material extruded with each motion of the print head. This regulation directly affects the thickness of each layer that is deposited throughout the printing process.
- The size of a 3D printer’s nozzle significantly influences its flow rate. A larger nozzle allows for an increased flow rate, thereby enabling faster printing speeds by extruding a larger volume of material with each pass. In contrast, a smaller nozzle results in a reduced flow rate, which improves the detail and precision of the printed items.
- Additionally, the size of the nozzle affects the nozzle temperature required for optimal operation. Generally, a larger nozzle demands a slightly higher temperature to effectively melt and extrude the material. Furthermore, the nozzle size is a critical factor in determining printing speed. While a larger nozzle can lead to quicker print times, this benefit may come at the expense of print quality and detail. Moreover, the size of the nozzle directly influences the layer height achievable during printing; a larger nozzle permits the use of thicker layers, whereas a smaller nozzle allows for the creation of thinner layers.
- The regulation of nozzle temperature is essential for managing the flow rate in a 3D printer. This is attributed to the direct impact that temperature has on the viscosity of the printing material. Specifically, an elevation in temperature produces a more fluid filament, which can improve the flow rate. In contrast, a reduction in temperature results in a thicker, less fluid filament, consequently diminishing the flow rate.
- The regulation of flow rate has a direct influence on the rate at which material is extruded from the nozzle of a 3D printer, effectively determining the “deposition speed” of the material. This, in turn, affects the overall printing speed while ensuring the quality of the print is preserved. Conversely, the control of nozzle speed has a significant impact on the flow rate of the 3D printer, as the velocity at which the printing material is advanced through the nozzle dictates the volume of material extruded per unit of time. Consequently, an increase in nozzle speed leads to a corresponding rise in flow rate, and the reverse is also true.
- The flow rate control on a 3D printer has a direct impact on the layer height, as it dictates the volume of material extruded for each unit of movement.
- Increased printing speeds necessitate elevated temperatures to enhance heat transfer, as the printing material will have a reduced duration in the melt chamber.
- The temperature of the nozzle on a 3D printer has a direct effect on the layer height, as it regulates the melting and flow of the material, thereby affecting the volume of material deposited in each layer.
References
- Chandrasekhar, J.; Dangas, G.; Mehran, R. Valvular Heart Disease in Women, Differential Remodeling, and Response to New Therapies. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 74. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Prosthetic Heart Valves Selection of the Optimal Prosthesis and Long-Term Management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef]
- Mirsky, N.A.; Ehlen, Q.T.; Greenfield, J.A.; Antonietti, M.; Slavin, B.V.; Nayak, V.V.; Pelaez, D.; Tse, D.T.; Witek, L.; Daunert, S. Three-Dimensional Bioprinting: A comprehensive review for applications in tissue engineering and regenerative medicine. Bioengineering 2024, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Shai, S.-E.; Lai, Y.-L.; Hung, Y.-W.; Hsieh, C.-W.; Su, K.-C.; Wang, C.-H.; Chao, T.-H.; Chiu, Y.-T.; Wu, C.-C.; Hung, S.-C. Long-term survival and regeneration following transplantation of 3d-printed biodegradable pcl tracheal grafts in large-scale porcine models. Bioengineering 2024, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Fergal, B.; Coulter, F.B.; Schaffner, M.; Faber, J.A.; Rafsanjani, A.; Smith, R.; Appa, H.; Zilla, P.; Bezuidenhout, D.; Studart, A.R. Bioinspired heart valve prosthesis made by silicone additive manufacturing. Matter 2019, 1, 266–279. [Google Scholar] [CrossRef]
- Brownell, L. Size a Concern When Replacing Heart Valves. The Harvard Gazete, Wyss Institute Communications. Available online: https://news.harvard.edu/gazette/story/2018/12/cardiologists-can-reduce-leaky-heart-valves-with-3-d-printing/ (accessed on 4 March 2025).
- Eleid, M. Interventional management of paravalvular leak. Heart 2018, 104, 1797–1802. [Google Scholar] [CrossRef]
- Dalby, M.; Panoulas, V. Transcatheter Aortic Valve Implantation: Complications. UpToDae. Available online: https://www.uptodate.com/contents/transcatheter-aortic-valve-implantation-complications (accessed on 24 May 2025).
- Tretter, J.T.; Burbano-Vera, N.H.; Najm, H.K. Multi-modality imaging evaluation and pre-surgical planning for aortic valve-sparing operations in patients with aortic root aneurysm. Ann. Cardiothorac. Surg. 2023, 12, 295–317. [Google Scholar] [CrossRef]
- Myerson, S.G. CMR in Evaluating Valvular Heart Disease: Diagnosis, Severity, and Outcomes. JACC 2021, 14, 2020–2032. [Google Scholar]
- Ertas, A.; Farley-Talamantes, E.; Cuvalci, O.; Gecgel, O. 3D-Printing of Artificial Aortic Heart Valve Using UV-Cured Silicone: Design and Performance Analysis. Bioengineering 2025, 12, 94. [Google Scholar] [CrossRef]
- Amindari, A.; Kirkkopru, K.; Saltik, L.; Sunbuloglu, E. Effect of non-linear leaflet material properties on aortic valve dynamics—A coupled fluid-structure approach. Eng. Solid Mech. 2021, 9, 123–136. [Google Scholar] [CrossRef]
- Alavi, H.; Ruiz, V.; Krasieva, T.; Botvinick, E.L.; Kheradvar, A. Characterizing the collagen fiber orientation in pericardial leaflets under mechanical loading conditions. Ann. Biomed. Eng. 2013, 41, 547–561. [Google Scholar] [CrossRef]
- Stella, J.A.; Liao, J.; Sacks, M.S. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J. Biomech 2007, 40, 3169–3177. [Google Scholar] [CrossRef]
- Moran, D.; Ertas, A.; Gulbulak, U. A Unique Transdisciplinary Engineering-Based Integrated Approach for the Design of Temporary Refugee Housing Using Kano, HOQ/QFD, TRIZ, AD, ISM, and DSM Tools. Designs 2021, 5, 31. [Google Scholar] [CrossRef]
- Warfield, J. Structuring Complex Systems; Battelle Monograph, No. 3; Battelle Memorial Institute: Columbus, OH, USA, 1974. [Google Scholar]
- Mandal, A.; Deshmukh, S.G. Vendor Selection Using Interpretive Structural Modelling (ISM). Int. J. Oper. Prod. Manag. 1994, 14, 52–59. [Google Scholar] [CrossRef]
- Formlabs, C.Y. 3D Print Silicone? Best Silicone 3D Printers and Alternatives. 2024. Available online: https://formlabs.com/global/blog/silicone-3d-printing/ (accessed on 28 April 2024).
- O’Neill, B. Ultimate Guide to Silicone 3D Printing. Aniwaa. 2023. Available online: https://www.aniwaa.com/guide/3d-printers/silicone-3d-printing/ (accessed on 28 April 2024).
- Artificial Heart Valves from Silicone. Available online: https://healthcare-in-europe.com/en/news/artificial-heart-valves-from-silicone.html (accessed on 28 April 2024).
- Zhou, J.; Li, Y.; Li, T.; Tian, X.; Xiong, Y.; Chen, Y. Analysis of the effect of thickness on the performance of polymeric heart valves. J. Funct. Biomater. 2023, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Harary, F.; Norman, R.V.; Cartwright, D. Structural Models: An Introduction to the Theory of Directed Graphs; Willey: New York, NY, USA, 1965. [Google Scholar]
- McCabe, T.J. Describing Cyclomatic Complexity. IEEE Trans. Softw. Eng. 1976, 308, 1976. [Google Scholar]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Gulbulak, U.; Ertas, A.; Baturalp, T.B.; Pavelka, T. The effect of fundamental curves on geometric orifice and coaptation areas of polymeric heart valves. J. Mech. Behav. Biomed. Mater. 2020, 112, 104039. [Google Scholar] [CrossRef]
- Park, K.S. Stents for Transcatheter Aortic Valve Replacement. Master’s Thesis, Division of Biomedical Engineering, Department of Human Biology, University of Cape Town, Cape Town, South Africa, 2017. [Google Scholar]
- Chen, Y.; Haoxiang, L. Pressure distribution over the leaflets and effect of bending stiffness on fluid—Structure interaction of the aortic valve. J. Fluid Mech. 2020, 883, A52. [Google Scholar] [CrossRef]
- Aortic Valve Regurgitation. Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/aortic-valve-regurgitation/symptoms-causes/syc-20353129 (accessed on 21 May 2025).
- Boskovski, M.T.; Gleason, T.G. Current Therapeutic Options in Aortic Stenosis. Circ. Res. 2021, 128, 1398–1417. [Google Scholar] [CrossRef]
- Lee, J.H.; Scotten, L.N.; Hunt, R.; Caranasos, T.G.; Vavalle, J.P.; Griffith, B.E. Bioprosthetic aortic valve diameter and thickness are directly related to leaflet fluttering: Results from a combined experimental and computational modeling study. JTCVS Open 2021, 6, 60–81. [Google Scholar] [CrossRef]
- Hatoum, H.; Moore, B.L.; Dasi, L.P. On the Significance of Systolic Flow Waveform on Aortic Valve Energy Loss. Ann Biomed. Eng. 2018, 46, 2102–2111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Description | Appearance | Viscosity | Specific | Tensile Strength | Elongation | Shore A |
|---|---|---|---|---|---|---|
| Gravity | ASTM D412 | ASTM D412 | ASTM D2240 | |||
| Pa-sec | MPa | % | ||||
| UV Cure | Hazy | 2.5–6.0 | 0.950–1.050 | 0.207–0.621 | 100–400 | 15–25 |
| Viscous Fluid |
| DATASET | Shore A Hardness | Tensile Strength (MPa) | Elongation (%) | |||
|---|---|---|---|---|---|---|
| Avg | Std | Avg | Std | Avr | Std | |
| 6hr UV-Cure | 12.33 | 0.289 | 0.403 | 0.026 | 381.9% | 28.3% |
| Design | Dimensions | Design | Dimensions |
|---|---|---|---|
| Parameters | (mm) | Parameters | (mm) |
| 3.7 | 13.0 | ||
| 2.0 | 17.0 | ||
| 23.0 | 17.5 | ||
| 38.0 | 23.0 | ||
| 36.0 | 42.0.0 | ||
| 56.0 |
| Scale | Flowrate | Heartbeat | Inlet Pressure | Outlet Pressure |
|---|---|---|---|---|
| (L/min) | (RPM) | (mmHg) | (mmHg) | |
| 1 | (LT = 0.8 mm) 5.0 | 75.5 | 86.5874 | 58.8349 |
| 1.5 | (LT = 0.8 mm) 5.0 | 98.2 | 163.3345 | 116.9414 |
| 1 | (LT = 1.6 mm) 5.0 | 72.1 | 92.7598 | 60.6161 |
| 1.5 | (LT = 1.6 mm) 5.0 | 102.5 | 144.9092 | 109.0776 |
| 1 | (LT = 0.8 mm) 7.0 | 112.9 | 123.3549 | 87.2601 |
| 1.5 | (LT = 0.8 mm) 7.0 | 136.7 | 242.8402 | 187.3273 |
| 1 | (LT = 1.6 mm) 7.0 | 108.5 | 126.1436 | 86.5931 |
| 1.5 | (LT = 1.6 mm) 7.0 | 160.1 | 265.6598 | 200.2076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertas, A.; Farley-Talamantes, E.; Cuvalci, O. Parameters Impacting the 3D Direct Ink Writing (DIW) Silicone Printing Process and Investigating How the Size of 3D-Printed Aortic Heart Valve Models Influences Cardiac Efficiency. Designs 2025, 9, 98. https://doi.org/10.3390/designs9040098
Ertas A, Farley-Talamantes E, Cuvalci O. Parameters Impacting the 3D Direct Ink Writing (DIW) Silicone Printing Process and Investigating How the Size of 3D-Printed Aortic Heart Valve Models Influences Cardiac Efficiency. Designs. 2025; 9(4):98. https://doi.org/10.3390/designs9040098
Chicago/Turabian StyleErtas, Atila, Erik Farley-Talamantes, and Olkan Cuvalci. 2025. "Parameters Impacting the 3D Direct Ink Writing (DIW) Silicone Printing Process and Investigating How the Size of 3D-Printed Aortic Heart Valve Models Influences Cardiac Efficiency" Designs 9, no. 4: 98. https://doi.org/10.3390/designs9040098
APA StyleErtas, A., Farley-Talamantes, E., & Cuvalci, O. (2025). Parameters Impacting the 3D Direct Ink Writing (DIW) Silicone Printing Process and Investigating How the Size of 3D-Printed Aortic Heart Valve Models Influences Cardiac Efficiency. Designs, 9(4), 98. https://doi.org/10.3390/designs9040098








