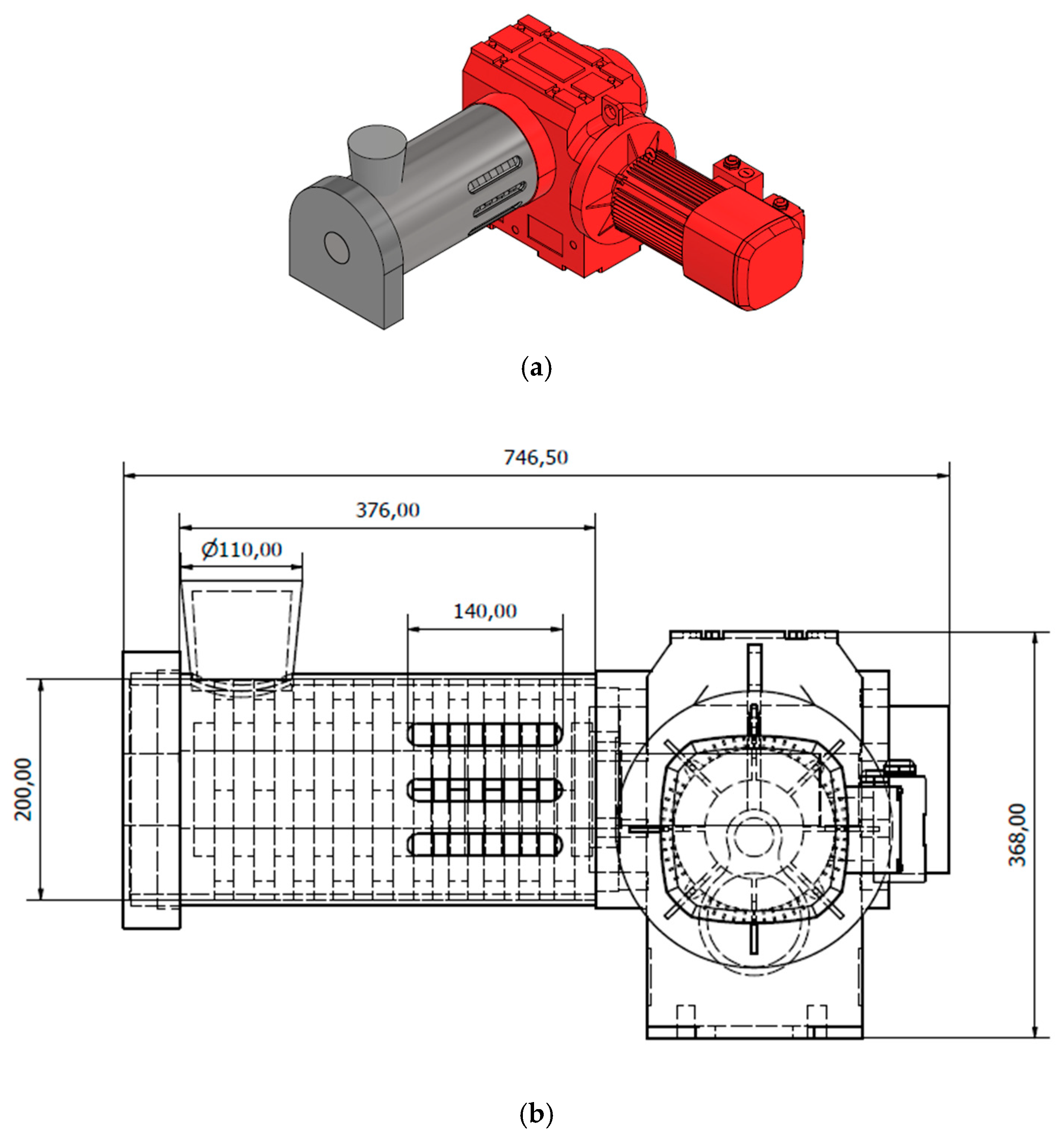
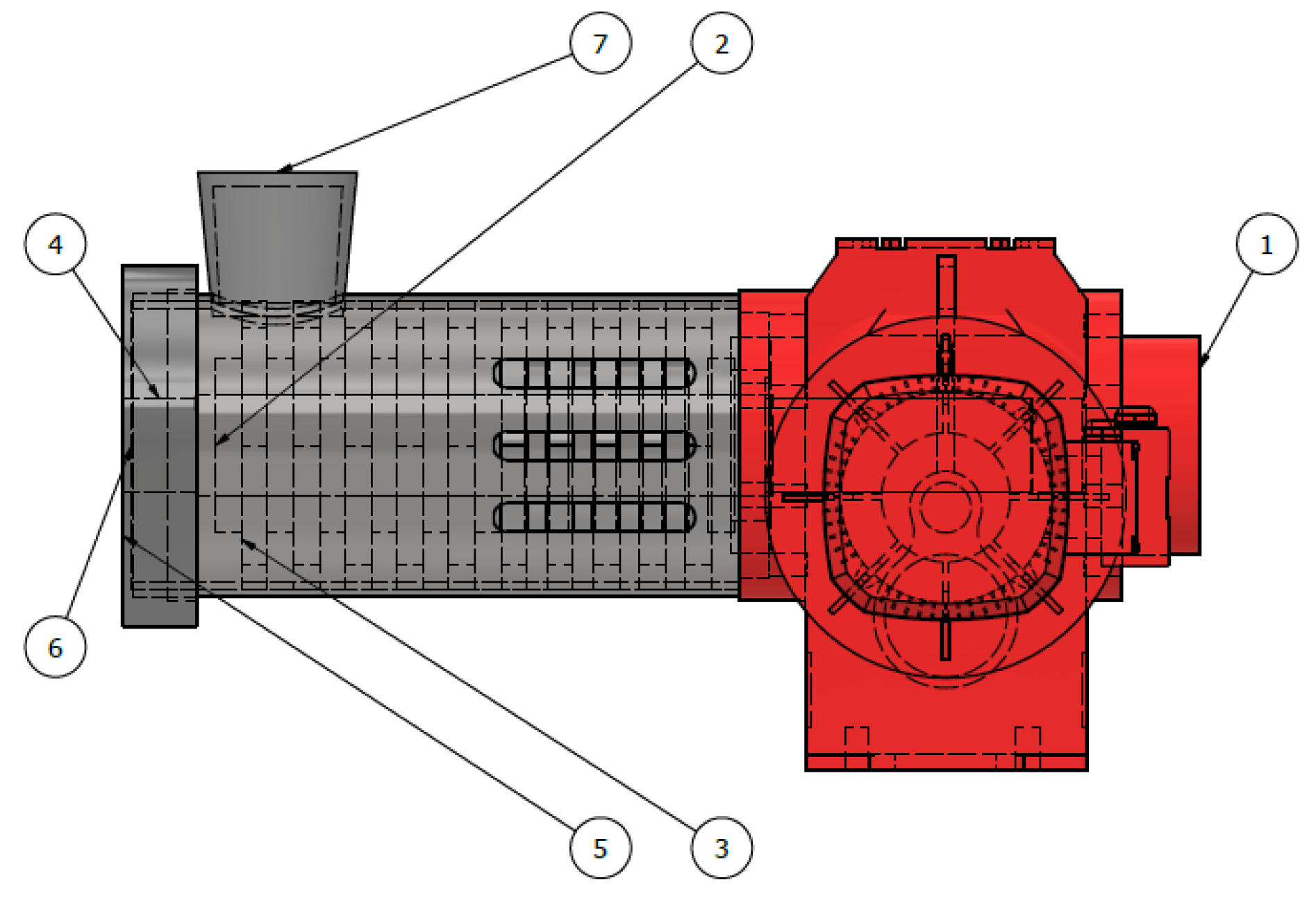
3.1. Simulation of the Main Part of the Mill
Finally, for the simulation of the blade, a fixed support was defined in the center corresponding to the axis on which it is anchored. We also defined the shear force on each side of the blade with the value of 51.3 N. We also defined a mesh predetermined by the program.
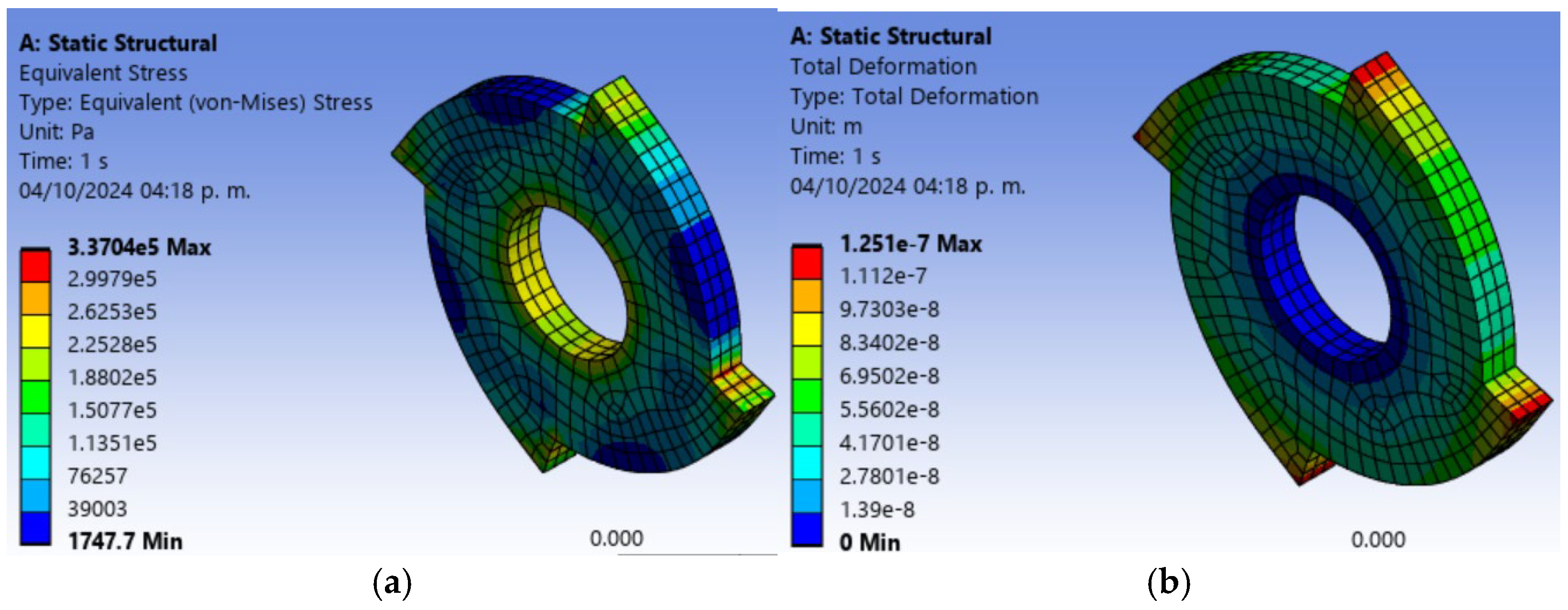
Figure 4 shows a finite element analysis of the most important part of the design, which corresponds to the blade. For the design of the blades, it is necessary to have a material with good mechanical strength and good machinability for this cutting tool. That is why SAE 1045 steel was selected for the design of the blades because it is a carbon steel that has excellent mechanical properties and versatility; it also has a high mechanical strength that varies and has a high impact resistance [
22], not to mention that it is a material that can be easily machined [
23,
24]. To select the steel, the properties mentioned above are taken into account, and the values based on this company are taken because they provide us with a commercial value and also general values because this steel can improve its properties with hot or cold thermal treatments. An SAE 1045 steel with a density of 10.85 g/cm
3 and Young’s modulus of 206 GPa [
25,
26] was selected for the design. In the analysis of each tip or knife, the shear force mentioned above is possible, and thus it was obtained that the maximum effort is presented in the four tips and is 3.3 × 10
5 Pa and a minimum effort of 1747.7 Pa, which is a minimum percentage. For this reason, it can be concluded that the material selected for the knife is adequate because it withstands the stress to which it is subjected. It can also be observed that the maximum deformation that is presented is 1.25 × 10
−7 m in the corners of the knife and the minimum deformation is zero in the center that is supported on the shaft.
The prospects of this work would be to reach a detailed design for the model to achieve the construction of a prototype and to find an experimental efficiency by checking if the processing time is reduced. In addition, a grinding comparison between a conventional knife mill with a drying pre-processing and the knife mill with free convection drying.
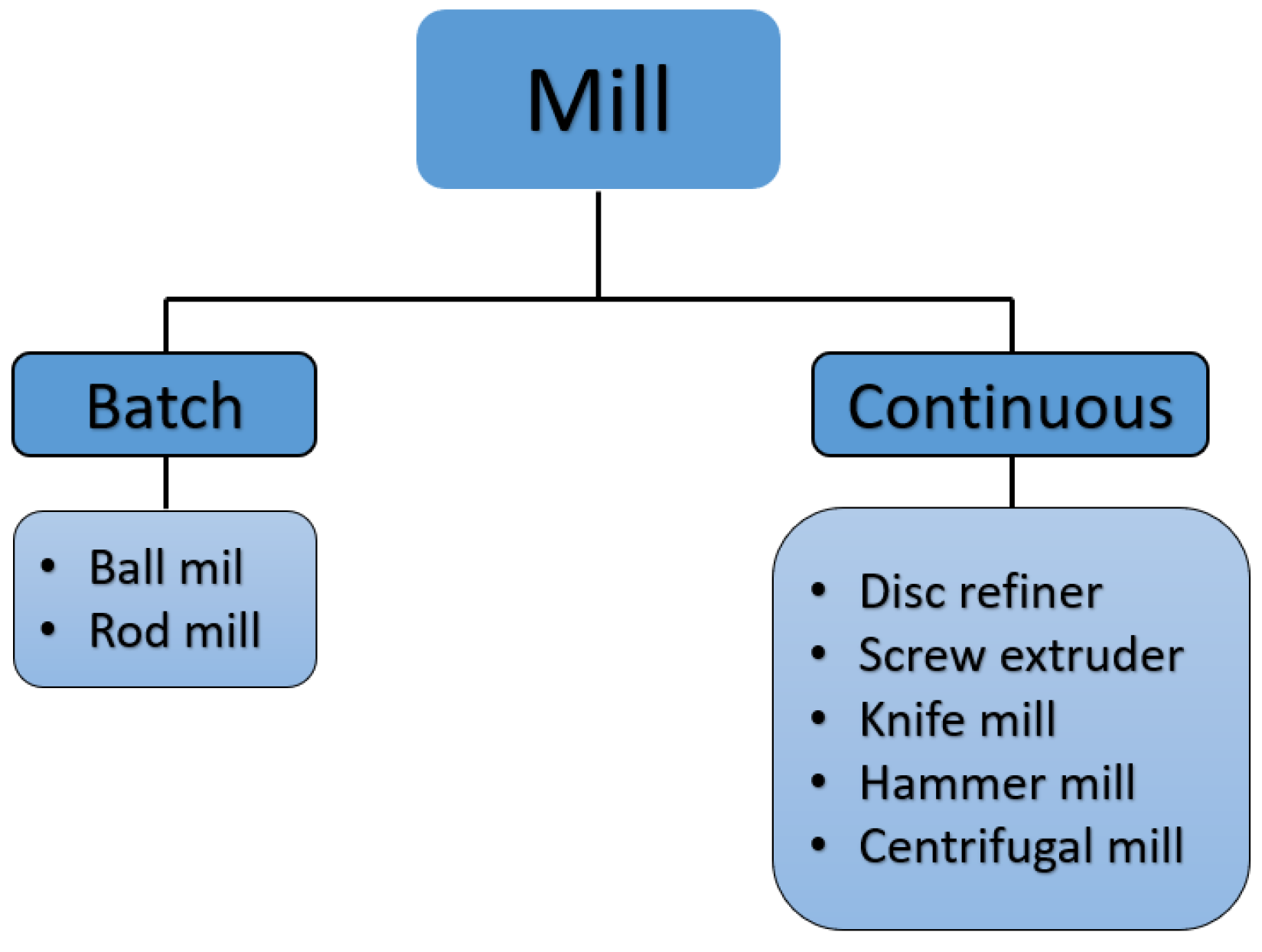
The type of nucleated material can affect the grinding process and the material of the blades, due to the humidity that is trapped in the material. For this reason, it is convenient to establish a maximum of 10% moisture as a control parameter to enter the grinding process and avoid further wear of the material. Another factor to take into account is the rotation speed. Its rotation speed can vary from 2000 to 25,000 rpm or more, depending on the model because some knife mills can grind hard materials and other knife mills can grind slightly softer materials.
3.2. Products Obtained from Milling of Biomass
In the quest to optimize yields from the milling process, a series of 24 meticulously designed experiments were conducted, varying both temperature, biomass and particle size. The temperatures selected for this study are 180 °C, 220 °C, and 260 °C, while the particle sizes tested include 0.5 mm, 1 mm, 2 mm, and 5 mm. By systematically altering these parameters, we aim to uncover the intricate relationships between temperature, particle size, and milling efficiency. This exploration not only seeks to enhance yield outcomes but also to contribute valuable insights into the milling process, ultimately paving the way for improved industrial applications and resource management. The platform chemicals were obtained experimentally through hydrothermal reactions in batch reactors equipped with electronic temperature control systems. Furthermore, the measurements of sugars and organic platform molecules were obtained through instrumental chemical analysis with high-resolution liquid chromatography (HPLC) with samples weighed on four-digit analytical balances, generating absolute errors of 0.0001 ten-thousandths of a gram.
The temperature values studied were selected due to the operating conditions in hydrothermal processes that require subcritical or liquid water to attack the complex chemical structures in cellulose and hemicellulose polymers present in biomass, which from 180 °C begin to hydrolyze to obtain simpler chemical molecules such as sugars in the first stage and subsequently, with the increase in temperature, the structural changes begin towards becoming platform molecules such as levulinic acid, furfural and hydroxymethylfurfural with very defined reaction mechanisms that have been presented in our scientific articles related to this work.
3.2.1. Coffee Cherry Waste
Table 3 shows the optimization results for the different particle sizes obtained from the designed mill. Particle sizes were established using sieve systems for granulometry.
The data show that particle size has a significant effect on the yields of various platform chemicals obtained from coffee cherry waste at 180 °C. Smaller particle sizes, especially 0.5 mm, result in higher total yields compared to larger sizes. This is primarily driven by the trend in sugar yield, which decreases from 25.58% at 0.5 mm down to 20.98% at 5 mm particle size. The yield of formic acid remains relatively constant around 6.6–7.0% regardless of particle size. Levulinic acid yield peaks at 1.74% for 2 mm particles, suggesting an optimal size in that range, but more data are needed to confirm this. The data for HMF and furfural yields are incomplete, with values missing for the 2 mm particle size. This makes it difficult to draw conclusions about the effect of particle size on these compounds. Overall, the total yields of all platform chemicals follow the same trend as sugar, with the highest total at 36.28% for 0.5 mm particles and decreasing to 29.88% and 30.58% for 2 mm and 5 mm, respectively. In summary, these data indicate that optimizing the particle size, especially reducing it below 1 mm, can significantly improve the overall yield of platform chemicals from coffee cherry waste at 180 °C, with formic acid and levulinic acid yields also impacted to some degree.
The data collected at 220 °C reveal interesting trends in the yields of platform chemicals derived from coffee cherry waste, particularly in relation to particle size, even though the yields decrease compared to those obtained at 180 °C, due to the beginning of the carbonization processes and decrease in the extraction of the lignocellulosic material. At the smallest particle size of 0.5 mm, the total yield is the lowest at 17.16%, with sugar yield at 10.16%, formic acid at 5.68%, and levulinic acid at 1.33%. As the particle size increases to 1 mm, the total yield rises to 23.25%, driven by a notable increase in sugar yield to 13.99% and formic acid yield to 7.34%. However, the total yield drops significantly at the 2 mm size to 17.09%, despite a higher levulinic acid yield of 3.20%, indicating that while larger particle sizes may enhance certain yields, they can detrimentally affect the overall yields of other compounds. At the larger particle sizes of 5 mm, the total yield is 21.87%, which is slightly lower than that of the 1 mm size but still higher than the 0.5 mm and 2 mm sizes. The formic acid yield remains relatively stable across the 1 mm and 5 mm sizes, while the levulinic acid yield is highest at 2 mm. Notably, HMF and furfural yields are absent for the smaller particle sizes, suggesting that these compounds may not be produced effectively under the conditions tested. Overall, the data indicate that a particle size of 1 mm appears to optimize the yield of platform chemicals at 220 °C, balancing the production of sugar and formic acid while maintaining reasonable levels of levulinic acid. Further investigation into the missing data for HMF and furfural would provide a more comprehensive understanding of the conversion process.
The data collected at 260 °C reveal a distinct shift in the yields of platform chemicals derived from coffee cherry waste compared to those at lower temperatures. At the smallest particle size of 0.5 mm, the total yield is 21.02%, with sugar yield at 10.19%, formic acid at 9.32%, levulinic acid at 1.43%, HMF at 0.05%, and furfural at 0.03%. As the particle size increases to 1 mm, the total yield decreases slightly to 20.35%, driven by a small decrease in sugar yield to 9.5% and levulinic acid to 1.38%. However, the formic acid yield increases to 9.46%, indicating that larger particle sizes may enhance the production of certain compounds at higher temperatures. At the 2 mm particle size, the total yield drops significantly to 12.76%, despite a higher levulinic acid yield of 2.28% and formic acid yield of 9.95%. This suggests that while larger particle sizes may enhance the yields of certain compounds, they can also detrimentally affect the overall yields of other compounds. At the larger particle size of 5 mm, the total yield is 19.04%, which is higher than the 2 mm size but lower than the 0.5 mm and 1 mm sizes. The formic acid yield remains relatively stable across the 0.5 mm, 1 mm, and 2 mm sizes, while the levulinic acid yield is highest at 2 mm. HMF yield is present for the 0.5 mm and 5 mm sizes, while furfural yield is only present for the 0.5 mm size. Overall, the data indicate that a particle size of 0.5 mm appears to optimize the yield of platform chemicals at 260 °C, balancing the production of sugar, formic acid, and levulinic acid while maintaining reasonable levels of HMF and furfural.
In conclusion, the data presented demonstrate the significant impact of particle size on the yields of various platform chemicals obtained from coffee cherry waste at different temperatures. At 180 °C, smaller particle sizes, particularly 0.5 mm, result in the highest total yields, driven primarily by increased sugar production. While formic acid yields remain relatively constant, levulinic acid peaks at 2 mm, suggesting an optimal size in that range. At 220 °C, a particle size of 1 mm appears to strike a balance between maximizing sugar and formic acid yields while maintaining reasonable levels of levulinic acid. However, the missing data for HMF and furfural at this temperature limit the conclusions that can be drawn. At the highest temperature of 260 °C, the trends shift, with 0.5 mm particles yielding the best overall results. While larger sizes enhance certain compound yields, such as formic acid, they can also detrimentally impact the total yield. The presence of HMF and furfural at 260 °C, albeit in small amounts, further highlights the differences in the conversion process at higher temperatures. Overall, these data emphasize the importance of optimizing particle size to maximize the production of platform chemicals from coffee cherry waste, with the optimal size varying depending on the target compounds and the operating temperature.
3.2.2. Peapod Waste
Table 4 shows the optimization results for the different particle sizes obtained from the designed mill with peapod waste. The data collected at 180 °C indicate that particle size significantly influences the yields of various platform chemicals derived from peapod waste. At the smallest particle size of 0.5 mm, the total yield is highest at 45.13%, with notable contributions from sugar (15.63%) and formic acid (19.14%). The yields of levulinic acid (3.99%), HMF (3.69%), and furfural (2.67%) also contribute to this total, suggesting that smaller particle sizes enhance the overall extraction of valuable compounds. As the particle size increases to 1 mm, the total yield slightly increases to 45.52%, driven by a higher yield of HMF (4.57%) and furfural (6.50%), indicating that while sugar yield decreases to 14.46%, the increase in these specific compounds compensates for the loss, maintaining a high overall yield. However, as the particle size continues to increase to 2 mm and 5 mm, the total yield declines significantly, dropping to 36.55% and 34.05%, respectively. The decrease in sugar yield to 11.24% at 2 mm and 11.27% at 5 mm, coupled with a reduction in formic acid yield, indicates that larger particle sizes may hinder the extraction efficiency of these compounds. While levulinic acid yields remain relatively stable across the different sizes, the notable drop in HMF and furfural yields at larger particle sizes suggests that the conversion of peapod waste to platform chemicals is less effective at these sizes. Overall, the data indicate that optimizing particle size, particularly at 0.5 mm or 1 mm, is crucial for maximizing the yield of platform chemicals from peapod waste at 180 °C, highlighting the importance of size reduction in enhancing extraction and conversion efficiency.
The data collected at 220 °C reveal notable trends in the yields of platform chemicals derived from peapod waste, particularly as particle size varies. At the smallest particle size of 0.5 mm, the total yield is 25.41%, with the formic acid yield being the most significant at 19.09%. Sugar yield is relatively low at 1.51%, while levulinic acid contributes 3.72%. As the particle size increases to 1 mm, the total yield rises to 28.44%, driven primarily by an increase in formic acid yield to 21.63% and a slight increase in furfural yield to 1.01%. This suggests that smaller particle sizes may enhance the extraction of certain compounds, particularly formic acid, while maintaining reasonable levels of levulinic acid. However, as the particle size continues to increase to 2 mm and 5 mm, the total yields decline to 22.12% and 25.90%, respectively. The yield of sugar remains low across all sizes, with the highest being 1.53% at 1 mm, while formic acid yields decrease at 2 mm to 17.83% and then slightly recover at 5 mm to 19.14%. Levulinic acid yields remain relatively stable, around 3.1 to 3.77%. The absence of HMF and furfural yields at 2 mm indicates a potential loss of these compounds with larger particle sizes. Overall, the data suggest that a particle size of 1 mm optimizes the yield of platform chemicals at 220 °C, particularly enhancing the production of formic acid, while larger sizes may hinder the extraction efficiency of other valuable compounds. This highlights the importance of optimizing particle size to maximize the overall yield from peapod waste.
The data collected at 260 °C reveal a distinct shift in the yields of platform chemicals derived from peapod waste compared to lower temperatures. At the smallest particle size of 0.5 mm, the total yield is 20.89%, with formic acid being the most significant contributor at 20.89%. Sugar yield is relatively low at 0.54%, while levulinic acid contributes 1.65%. As the particle size increases to 1 mm, the total yield rises to 29.28%, driven primarily by a notable increase in formic acid yield to 28.78%. This suggests that larger particle sizes may enhance the production of certain compounds, particularly formic acid, at higher temperatures. However, as the particle size continues to increase to 2 mm and 5 mm, the total yields decline to 19.65% and 20.32%, respectively. The sugar yield remains low across all sizes, with the highest being 0.85% at 5 mm. Formic acid yields decrease at 2 mm to 19.20% and then increase slightly at 5 mm to 19.86%. Levulinic acid yields remain relatively stable, around 1.65 to 1.77%. The absence of HMF and furfural yields across all particle sizes at 260 °C indicates a potential loss or complete conversion of these compounds at higher temperatures. Overall, the data suggest that a particle size of 1 mm optimizes the yield of platform chemicals at 260 °C, particularly enhancing the production of formic acid, while larger sizes may hinder the extraction efficiency of other valuable compounds. This highlights the importance of optimizing particle size to maximize the overall yield from peapod waste at higher temperatures.
In conclusion, the analysis of the data collected at different temperatures highlights the critical role of particle size optimization in maximizing the yield of platform chemicals from peapod waste. At 180 °C, smaller particle sizes, particularly 0.5 mm and 1 mm, result in the highest total yields, with significant contributions from sugar, formic acid, levulinic acid, HMF, and furfural. As particle size increases, the yields of these compounds decrease, indicating that size reduction is essential for enhancing extraction and conversion efficiency at this temperature. At 220 °C, a particle size of 1 mm appears to be the most effective, optimizing the production of formic acid while maintaining reasonable levels of levulinic acid. Larger particle sizes lead to a decline in total yields, likely due to the loss of HMF and furfural. At the highest temperature of 260 °C, the trends shift, with 1 mm particles yielding the best results, primarily driven by an increase in formic acid production. However, the absence of HMF and furfural at this temperature suggests that these compounds may have been completely converted or lost under the high-temperature conditions. Overall, the data emphasize the importance of carefully selecting the appropriate particle size and temperature to maximize the yield of specific platform chemicals from peapod waste. Continued research and optimization of these parameters will be crucial for developing efficient and sustainable conversion processes for this agricultural waste stream.