Comparative Performances of Natural Dyes Extracted from Mentha Leaves, Helianthus Annuus Leaves, and Fragaria Fruit for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Natural Dye Sensitizers
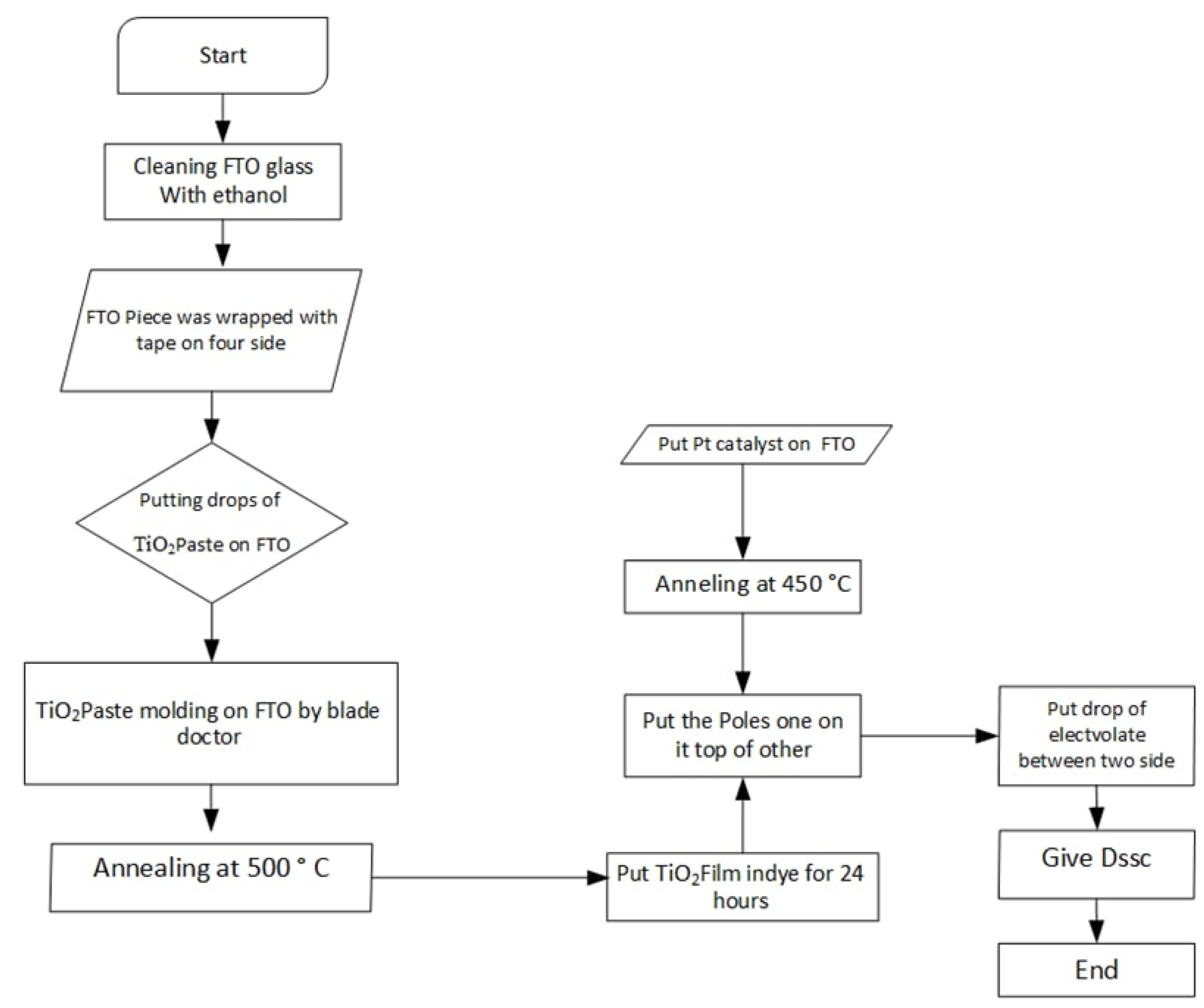
2.3. The TiO2 Paste and Photoelectrode Preparation
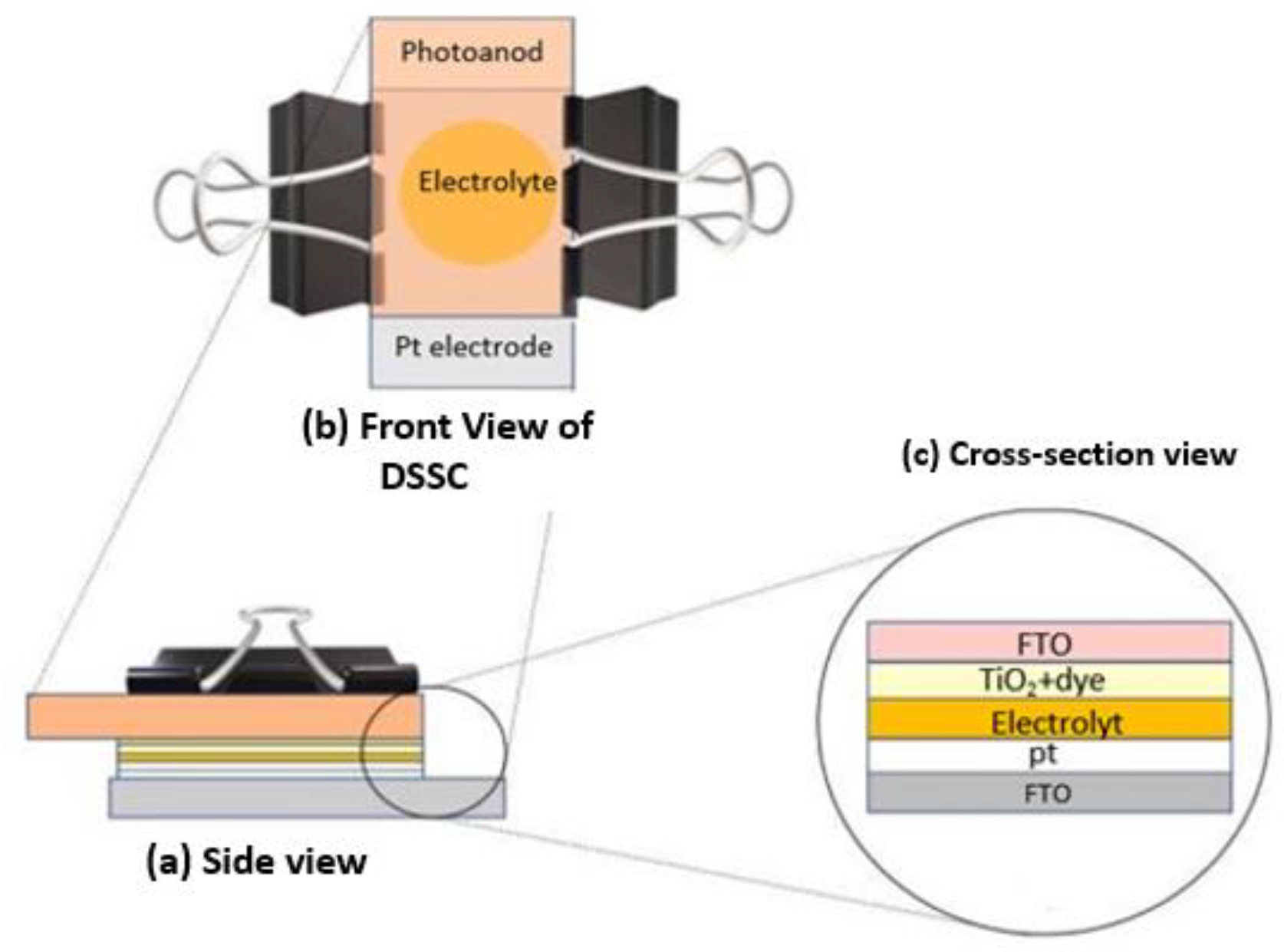
2.4. Fabrication of DSSCs
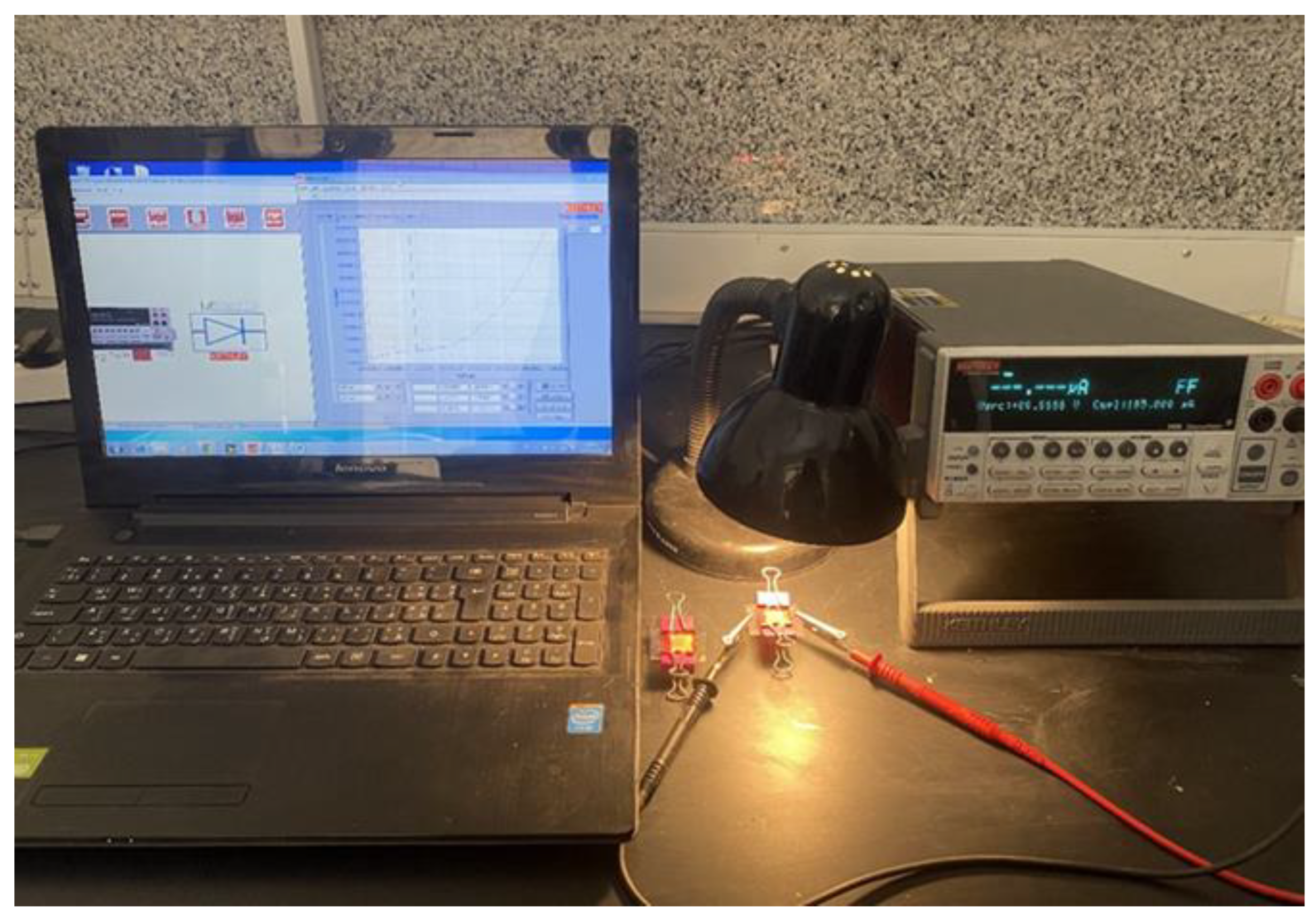
2.5. Measurement of the DSSC’s Photoelectric Conversion Rate
3. Results and Discussion
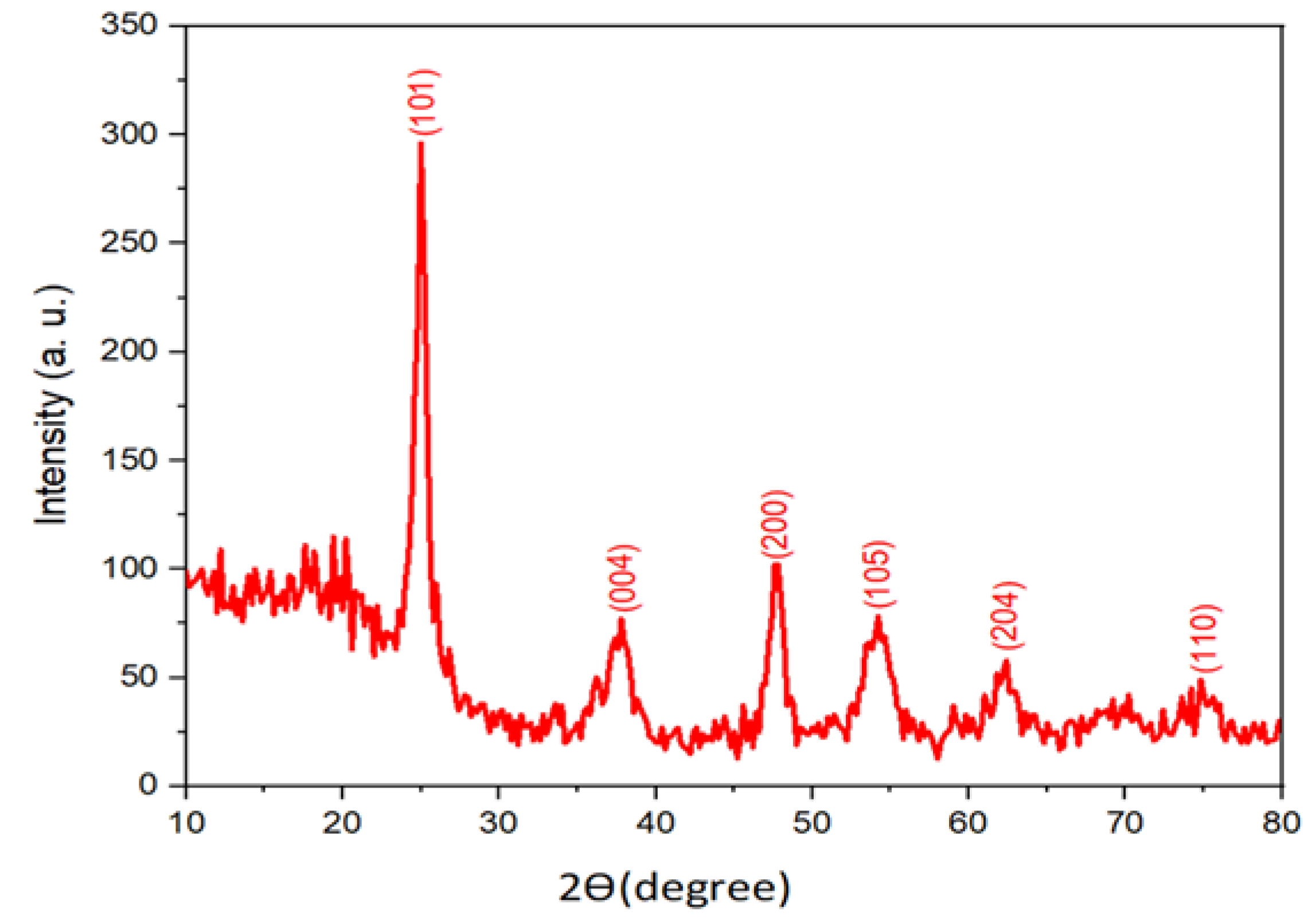
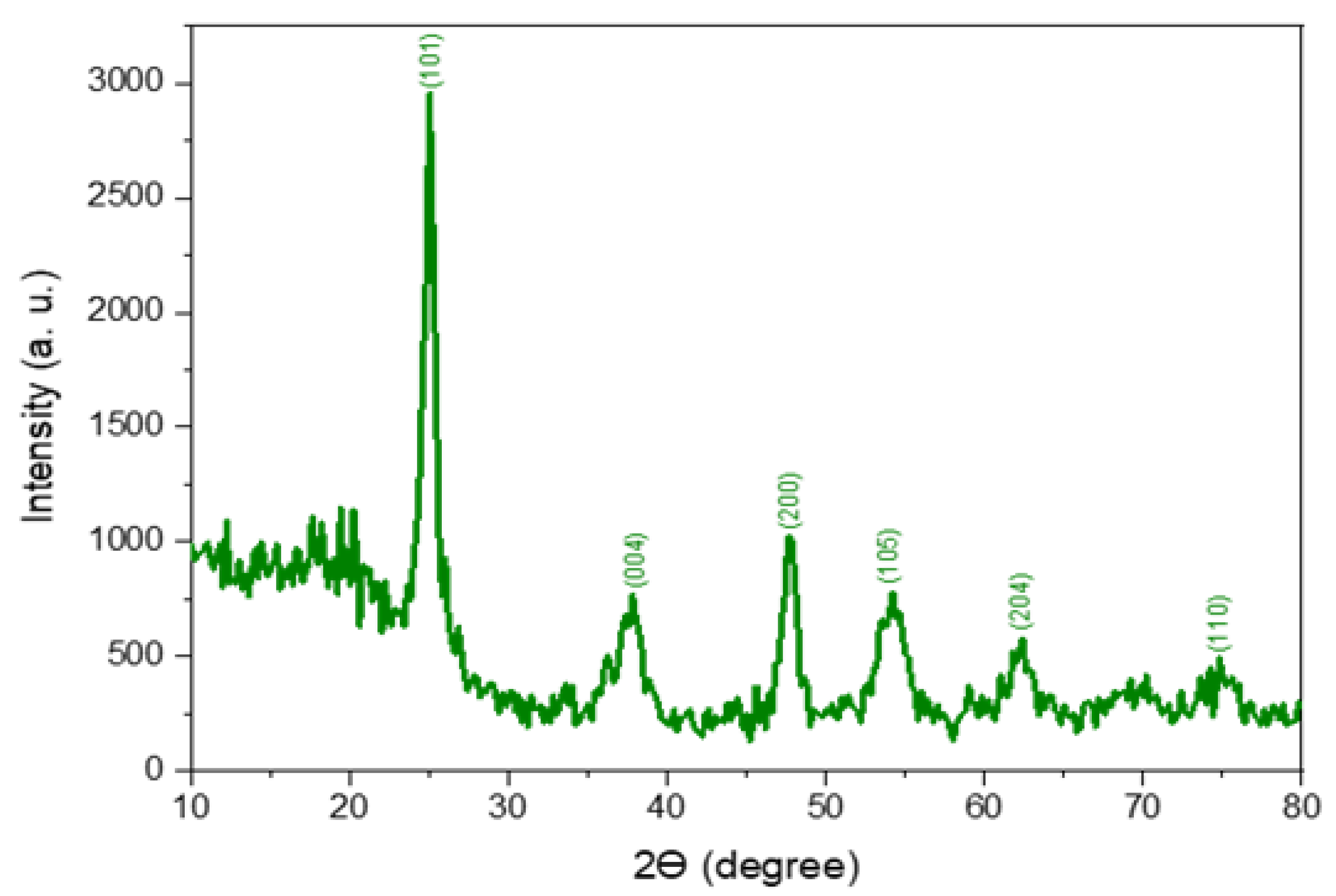
3.1. X-ray Diffraction Analysis
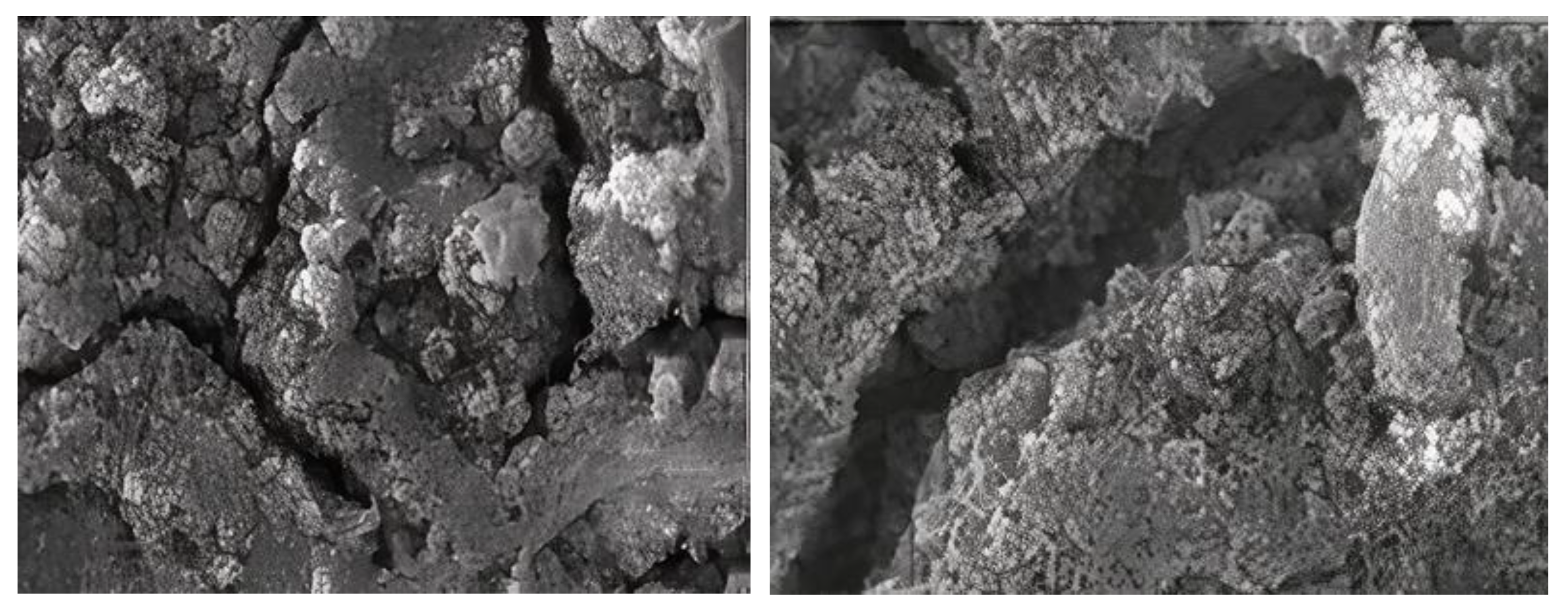
3.2. Field Emission Scanning Electron Microscopy (FESEM)
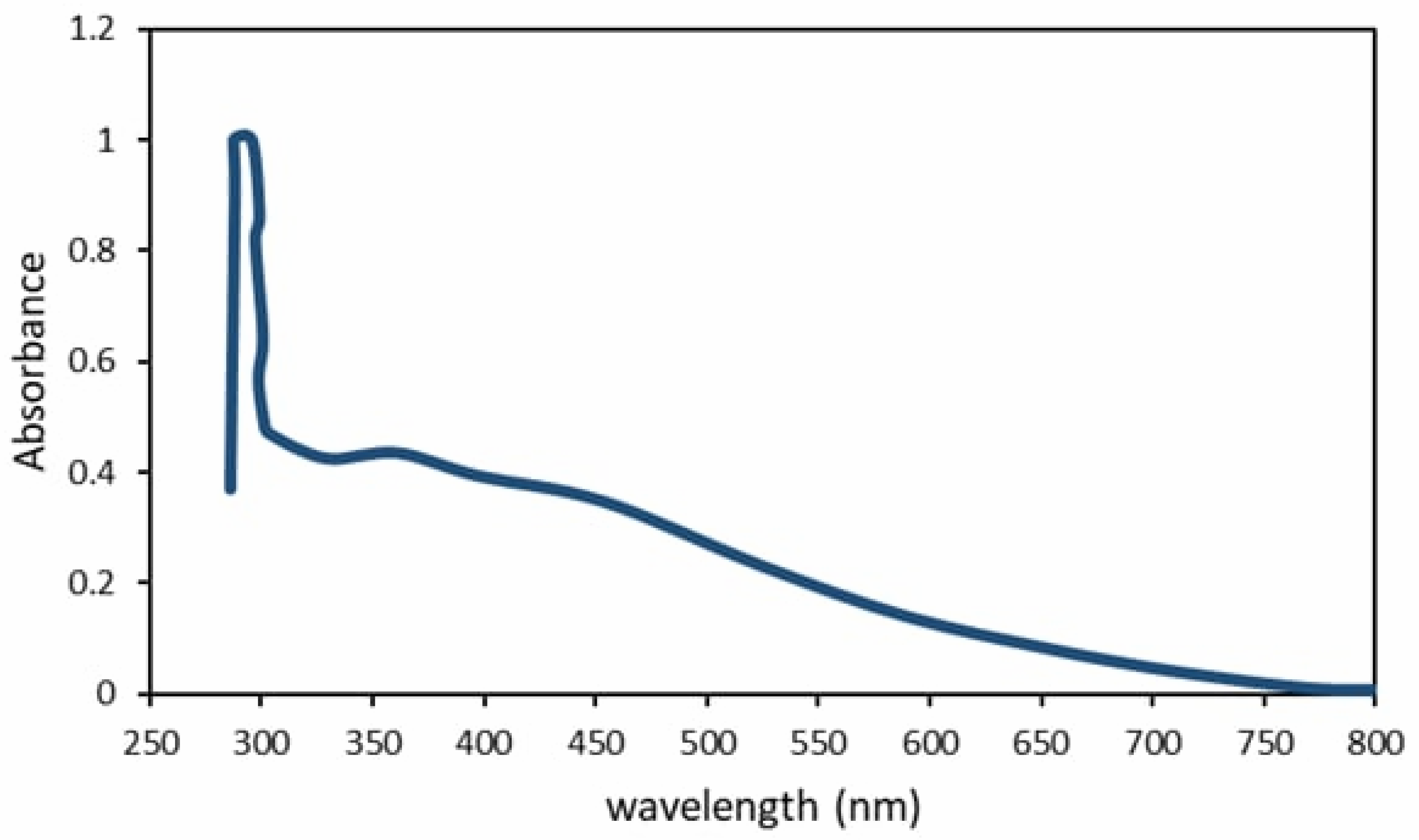
3.3. Absorbance Behavior of the Prepared TiO2 Film
4. UV-Vis Analysis
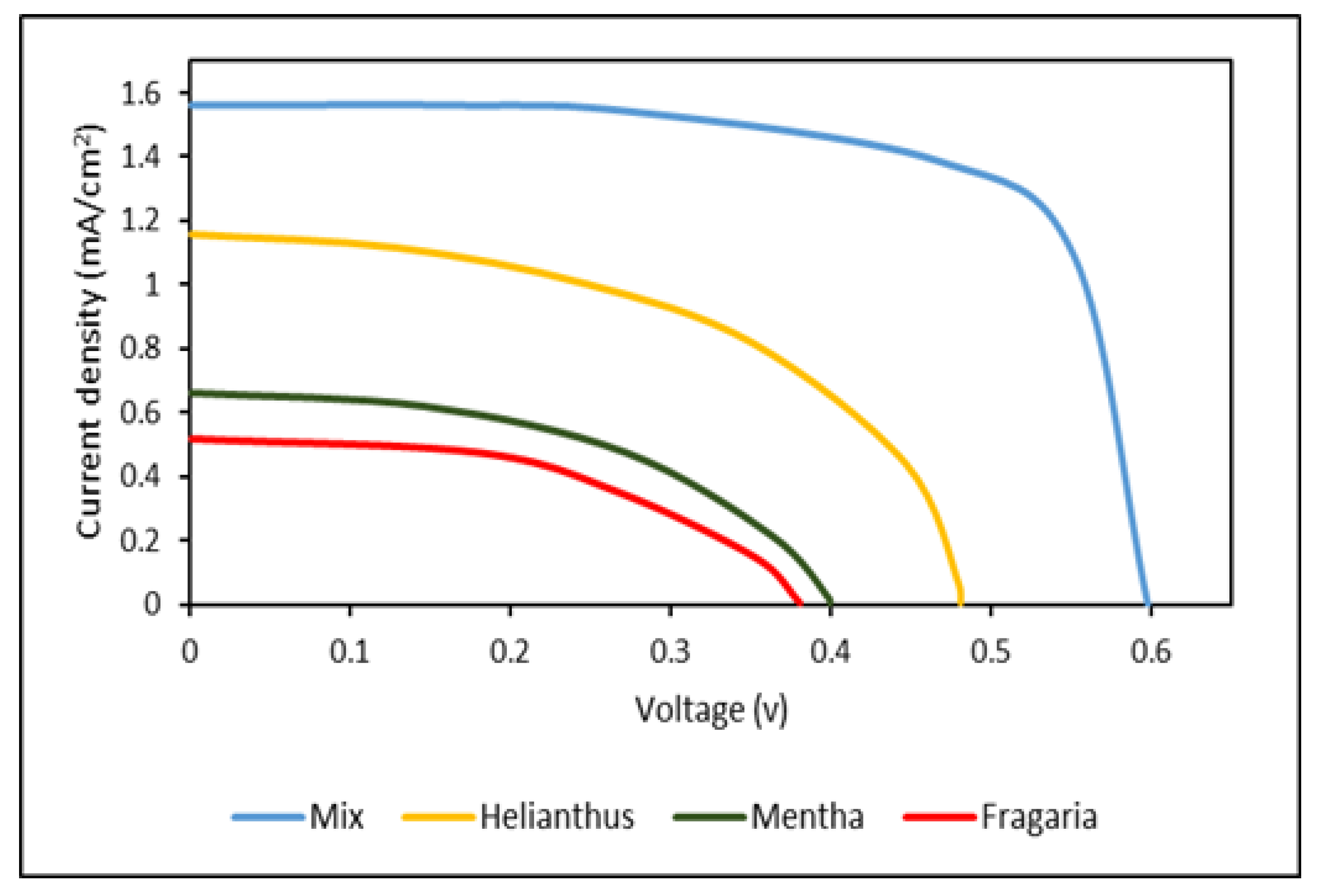
Photovoltaics Performance of DSSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, A.; Khan, J.; Sohail, M.; Hayat, A.; Zhao, T.K.; Ullah, B.; Khan, M.; Uddin, I.; Ullah, S.; Ullah, R.; et al. Fabrication of polymer carbon nitride with organic monomer for effective photocatalytic hydrogen evolution. J. Photochem. Photobiol. A Chem. 2020, 401, 112764. [Google Scholar] [CrossRef]
- Medina-Santana, A.A.; Hewamalage, H.; Cárdenas-Barrón, L.E. Deep Learning Approaches for Long-Term Global Horizontal Irradiance Forecasting for Microgrids Planning. Designs 2022, 6, 83. [Google Scholar] [CrossRef]
- Al-Saeed, Y.W.; Ahmed, A. Evaluating Design Strategies for Nearly Zero Energy Buildings in the Middle East and North Africa Regions. Designs 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Vandewetering, N.; Hayibo, K.S.; Pearce, J.M. Open-Source Design and Economics of Manual Variable-Tilt Angle DIY Wood-Based Solar Photovoltaic Racking System. Designs 2022, 6, 54. [Google Scholar] [CrossRef]
- Bhagavathy, S.M.; Pillai, G. PV Microgrid Design for Rural Electrification. Designs 2018, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Aykapadathu, M.; Nazarinia, M.; Sellami, N. Design and Fabrication of Absorptive/Reflective Crossed CPC PV/T System. Designs 2018, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.U.; Shah, M.Z.; Rasheed, S.; Afzal, W.; Arsalan, M.; Rahman, H.U.; Ullah, M.; Zhao, T.; Ullah, I.; Din, A.U.; et al. Inorganic salt hydrates and zeolites composites studies for thermochemical heat storage. Z. Für Phys. Chem. 2021, 235, 1481–1497. [Google Scholar] [CrossRef]
- Maldon, B.; Thamwattana, N. A Fractional Diffusion Model for Dye-Sensitized Solar Cells. Molecules 2020, 25, 2966. [Google Scholar] [CrossRef]
- Su, R.; Lyu, L.; Elmorsy, M.; El-Shafei, A. Novel metal-free organic dyes constructed with the D-D|A-π-A motif: Sensitization and co-sensitization study. Sol. Energy 2019, 194, 400–414. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Karthika, K.; Dayana, A.M.; Maheswari, G.; Eswaramoorthi, V.; Pavithra, N.; Anandan, S.; Williams, R.V. Reduced graphene oxide embedded titanium dioxide nanocomposite as novel photoanode material in natural dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 13678–13689. [Google Scholar] [CrossRef]
- Cavallo, C.; Di Pascasio, F.; Latini, A.; Bonomo, M.; Dini, D. Nanostructured Semiconductor Materials for Dye-Sensitized Solar Cells. J. Nanomater. 2017, 2017, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.K.; Kříž, J.; Bennett, N.; Chen, B.; Upadhayaya, H.; Reddy, K.R.; Sadhu, V. Functionalized metal oxide nanoparticles for efficient dye-sensitized solar cells (DSSCs): A review. Mater. Sci. Energy Technol. 2020, 3, 472–481. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, H.; Li, Y.; Wang, J.; Zhang, J.; Zhang, M.; Guo, Y.; Wang, P. Dithienopicenocarbazole as the kernel module of low-energy-gap organic dyes for efficient conversion of sunlight to electricity. Energy Environ. Sci. 2015, 8, 3192–3197. [Google Scholar] [CrossRef]
- Bisquert, J.; Cahen, D.; Hodes, G.; Rühle, S.; Zaban, A. Physical Chemical Principles of Photovoltaic Conversion with Nanoparticulate, Mesoporous Dye-Sensitized Solar Cells. J. Phys. Chem. B 2004, 108, 8106–8118. [Google Scholar] [CrossRef]
- Sugathan, V.; John, E.; Sudhakar, K. Recent improvements in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Akinoglu, B.G.; Tuncel, B.; Badescu, V. Beyond 3rd generation solar cells and the full spectrum project. Recent advances and new emerging solar cells. Sustain. Energy Technol. Assess. 2021, 46, 101287. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, R.; Srivastava, P.; Bahadur, L. Natural Pigments from Plants Used as Sensitizers for TiO2 Based Dye-Sensitized Solar Cells. J. Energy 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Nien, Y.-H.; Chen, H.-H.; Hsu, H.-H.; Rangasamy, M.; Hu, G.-M.; Yong, Z.-R.; Kuo, P.-Y.; Chou, J.-C.; Lai, C.-H.; Ko, C.-C.; et al. Study of How Photoelectrodes Modified by TiO2/Ag Nanofibers in Various Structures Enhance the Efficiency of Dye-Sensitized Solar Cells under Low Illumination. Energies 2020, 13, 2248. [Google Scholar] [CrossRef]
- Yu, Z. Liquid Redox Electrolytes for Dye-Sensitized Solar Cells Ze Yu. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2012. Available online: https://www.diva-portal.org/smash/get/diva2:483008/FULLTEXT01.pdf (accessed on 15 September 2022).
- Zeng, K.; Chen, Y.; Zhu, W.-H.; Tian, H.; Xie, Y. Efficient Solar Cells Based on Concerted Companion Dyes Containing Two Complementary Components: An Alternative Approach for Cosensitization. J. Am. Chem. Soc. 2020, 142, 5154–5161. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yan, Q.; Li, C.; Lu, Y.; Tong, Z.; Xie, Y. Light-Absorbing Pyridine Derivative as a New Electrolyte Additive for Developing Efficient Porphyrin Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 57017–57024. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Garud, H.B.; Patil, A.H.; Patil, G.D.; Patil, C.R.; Dongale, T.D.; Patil, P.S. Recent advancements in silica nanoparticles based technologies for removal of dyes from water. Colloids Interface Sci. Commun. 2019, 30, 100181. [Google Scholar] [CrossRef]
- Singh, K.; Arora, S. Removal of Synthetic Textile Dyes From Wastewaters: A Critical Review on Present Treatment Technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Nidheesh, P.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Uribe, C.; Vallejo, W.; Camargo, G.; Muñoz-Acevedo, A.; Quiñones, C.; Schott, E.; Zarate, X. Potential use of an anthocyanin-rich extract from berries of Vaccinium meridionale Swartz as sensitizer for TiO2 thin films—An experimental and theoretical study. J. Photochem. Photobiol. A Chem. 2019, 384, 112050. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, J.; Wang, P.; Wen, H. Developed functionalization of wool fabric with extracts of Lycium ruthenicum Murray and potential application in healthy care textiles. Dye. Pigment. 2018, 163, 308–317. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid-ul-Islam; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Jalali, T.; Arkian, P.; Golshan, M.; Jalali, M.; Osfouri, S. Performance evaluation of natural native dyes as photosensitizer in dye-sensitized solar cells. Opt. Mater. 2020, 110, 110441. [Google Scholar] [CrossRef]
- Golshan, M.; Osfouri, S.; Azin, R.; Jalali, T. Fabrication of optimized eco-friendly dye-sensitized solar cells by extracting pigments from low-cost native wild plants. J. Photochem. Photobiol. A Chem. 2019, 388, 112191. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Ali, S.R.; Khan, S. Progress in dye sensitized solar cell by incorporating natural photosensitizers. Sol. Energy 2019, 181, 490–509. [Google Scholar] [CrossRef]
- Ammar, A.M.; Mohamed, H.; Yousef, M.M.K.; Abdel-Hafez, G.M.; Hassanien, A.S.; Khalil, A.S.G. Dye-Sensitized Solar Cells (DSSCs) Based on Extracted Natural Dyes. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.E.; Lieberwirth, I.; Natalio, F.; Bardeau, J.-F.; Delorme, N.; Emmerling, F.; Barrea, R.; Kappl, M.; Marin, F. Merging Models of Biomineralisation with Concepts of Nonclassical Crystallisation: Is a Liquid Amorphous Precursor Involved in the Formation of the Prismatic Layer of the Mediterranean Fan Mussel Pinna nobilis? Faraday Discuss. 2012, 159, 433–448. [Google Scholar] [CrossRef]
- Jia, H.-L.; Chen, Y.-C.; Ji, L.; Lin, L.-X.; Guan, M.-Y.; Yang, Y. Cosensitization of porphyrin dyes with new X type organic dyes for efficient dye-sensitized solar cells. Dye. Pigment. 2018, 163, 589–593. [Google Scholar] [CrossRef]
- Chandra, R.; Iqbal, H.M.N.; Vishal, G.; Lee, H.-S.; Nagra, S. Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour. Technol. 2019, 278, 346–359. [Google Scholar] [CrossRef]
- Park, N.-G.; van de Lagemaat, J.; Frank, A.J. Comparison of Dye-Sensitized Rutile- and Anatase-Based TiO2 Solar Cells. J. Phys. Chem. B 2000, 104, 8989–8994. [Google Scholar] [CrossRef] [Green Version]
- Zatirostami, A. Increasing the efficiency of TiO2-based DSSC by means of a double layer RF-sputtered thin film blocking layer. Optik 2020, 207, 164419. [Google Scholar] [CrossRef]
- Dhas, V.; Muduli, S.; Agarkar, S.; Rana, A.; Hannoyer, B.; Banerjee, R.; Ogale, S. Enhanced DSSC performance with high surface area thin anatase TiO2 nanoleaves. Sol. Energy 2011, 85, 1213–1219. [Google Scholar] [CrossRef]
- Saadaoui, S.; Ben Youssef, M.A.; Ben Karoui, M.; Gharbi, R.; Smecca, E.; Strano, V.; Mirabella, S.; Alberti, A.; Puglisi, R.A. Performance of natural-dye-sensitized solar cells by ZnO nanorod and nanowall enhanced photoelectrodes. Beilstein J. Nanotechnol. 2017, 8, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Tang, Y.; Yang, K.; Qin, M.; Wang, Y.; Sun, H.; Su, M.; Lu, X.; Zhou, M.; Guo, X. Thiazolothienyl imide-based wide bandgap copolymers for efficient polymer solar cells. J. Mater. Chem. C 2019, 7, 11142–11151. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the Photoelectronic and Photocatalytic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous Solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Ezike, S.C. Effect of Concentration Variation on Optical and Structural Properties of TiO2 Thin Films. J. Mod. Mater. 2020, 7, 1–6. [Google Scholar] [CrossRef]





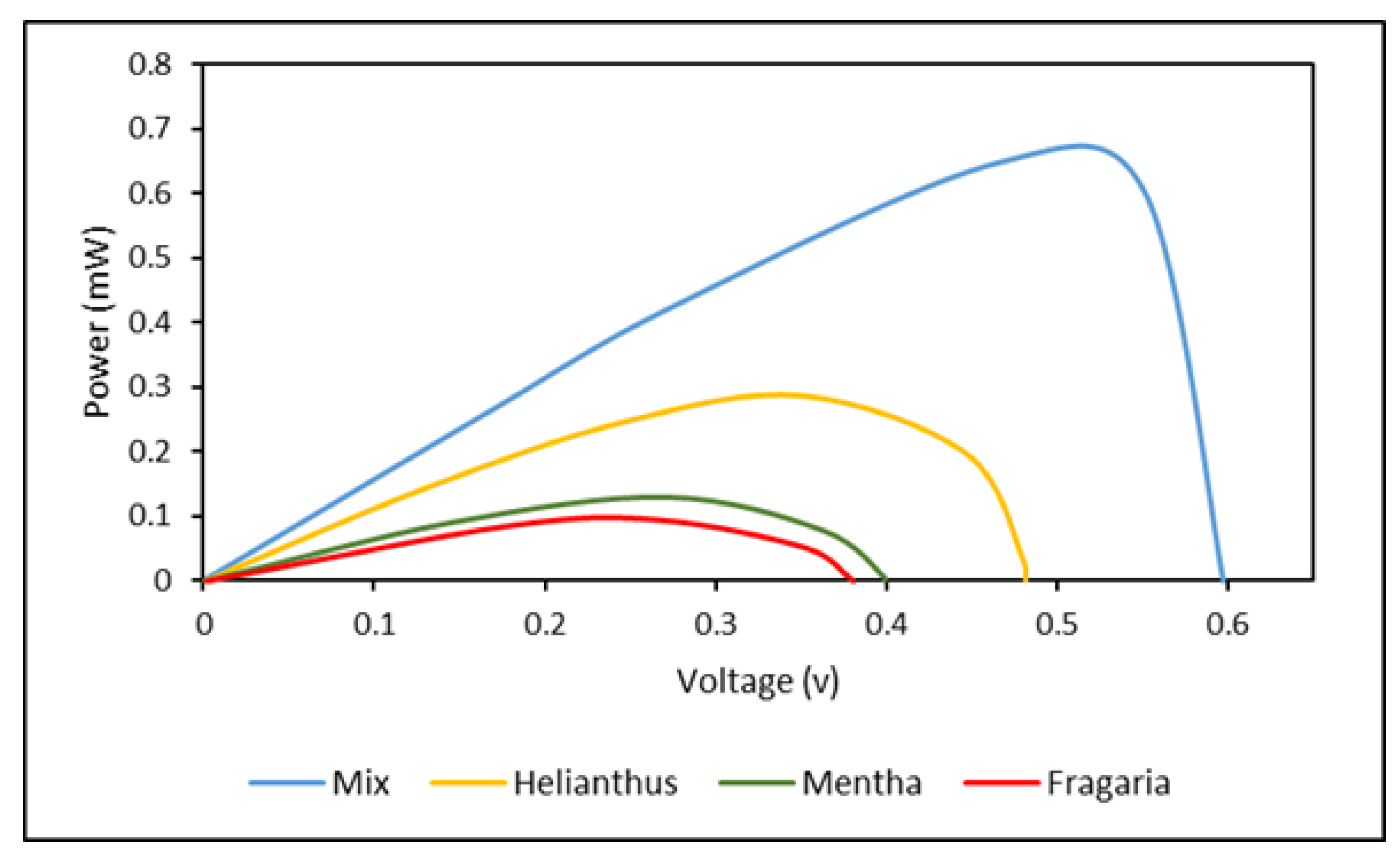
| Dye | ɳ (%) | F (%) | ||
|---|---|---|---|---|
| Fragaria | 0.38 | 0.51 | 0.09 | 46.44 |
| Mentha leaves | 0.41 | 0.64 | 0.15 | 57.16 |
| Helianthus annuus leaves | 0.48 | 1.19 | 0.29 | 50.77 |
| Mix | 0.59 | 1.59 | 0.69 | 73.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, Z.H.; Hachim, D.M.; Al-murshedi, A.S.N.; Kamil, F.; Al-Manea, A.; Yusaf, T. Comparative Performances of Natural Dyes Extracted from Mentha Leaves, Helianthus Annuus Leaves, and Fragaria Fruit for Dye-Sensitized Solar Cells. Designs 2022, 6, 100. https://doi.org/10.3390/designs6060100
Abdulrahman ZH, Hachim DM, Al-murshedi ASN, Kamil F, Al-Manea A, Yusaf T. Comparative Performances of Natural Dyes Extracted from Mentha Leaves, Helianthus Annuus Leaves, and Fragaria Fruit for Dye-Sensitized Solar Cells. Designs. 2022; 6(6):100. https://doi.org/10.3390/designs6060100
Chicago/Turabian StyleAbdulrahman, Zainab Haider, Dhafer Manea Hachim, Ahmed Salim Naser Al-murshedi, Furkan Kamil, Ahmed Al-Manea, and Talal Yusaf. 2022. "Comparative Performances of Natural Dyes Extracted from Mentha Leaves, Helianthus Annuus Leaves, and Fragaria Fruit for Dye-Sensitized Solar Cells" Designs 6, no. 6: 100. https://doi.org/10.3390/designs6060100
APA StyleAbdulrahman, Z. H., Hachim, D. M., Al-murshedi, A. S. N., Kamil, F., Al-Manea, A., & Yusaf, T. (2022). Comparative Performances of Natural Dyes Extracted from Mentha Leaves, Helianthus Annuus Leaves, and Fragaria Fruit for Dye-Sensitized Solar Cells. Designs, 6(6), 100. https://doi.org/10.3390/designs6060100









