One Factor at a Time Analysis to Modify Potting Technique for Manufacturing of Bubble-Free High-Voltage Polyester Insulated Automotive Coils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Curing Procedure
2.2. Manufacturing of Ignition Coil
2.3. One Factor at a Time Experiments
2.4. F and T Statistics
2.5. Testing Sample Coils
3. Results
3.1. Pre and Process Heating of Coils
3.2. Effect of Initiator and Accelerator
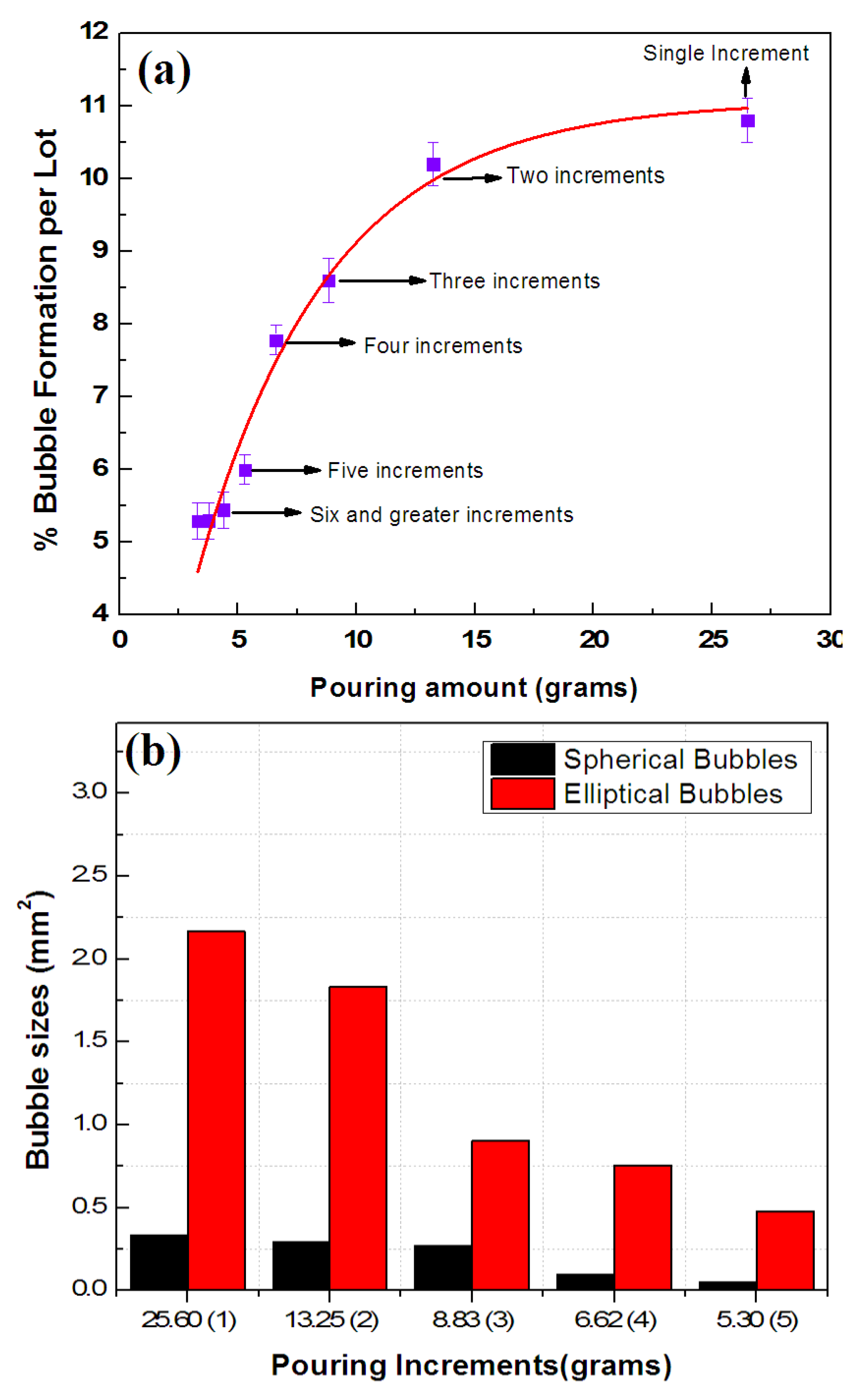
3.3. Incremental Pouring
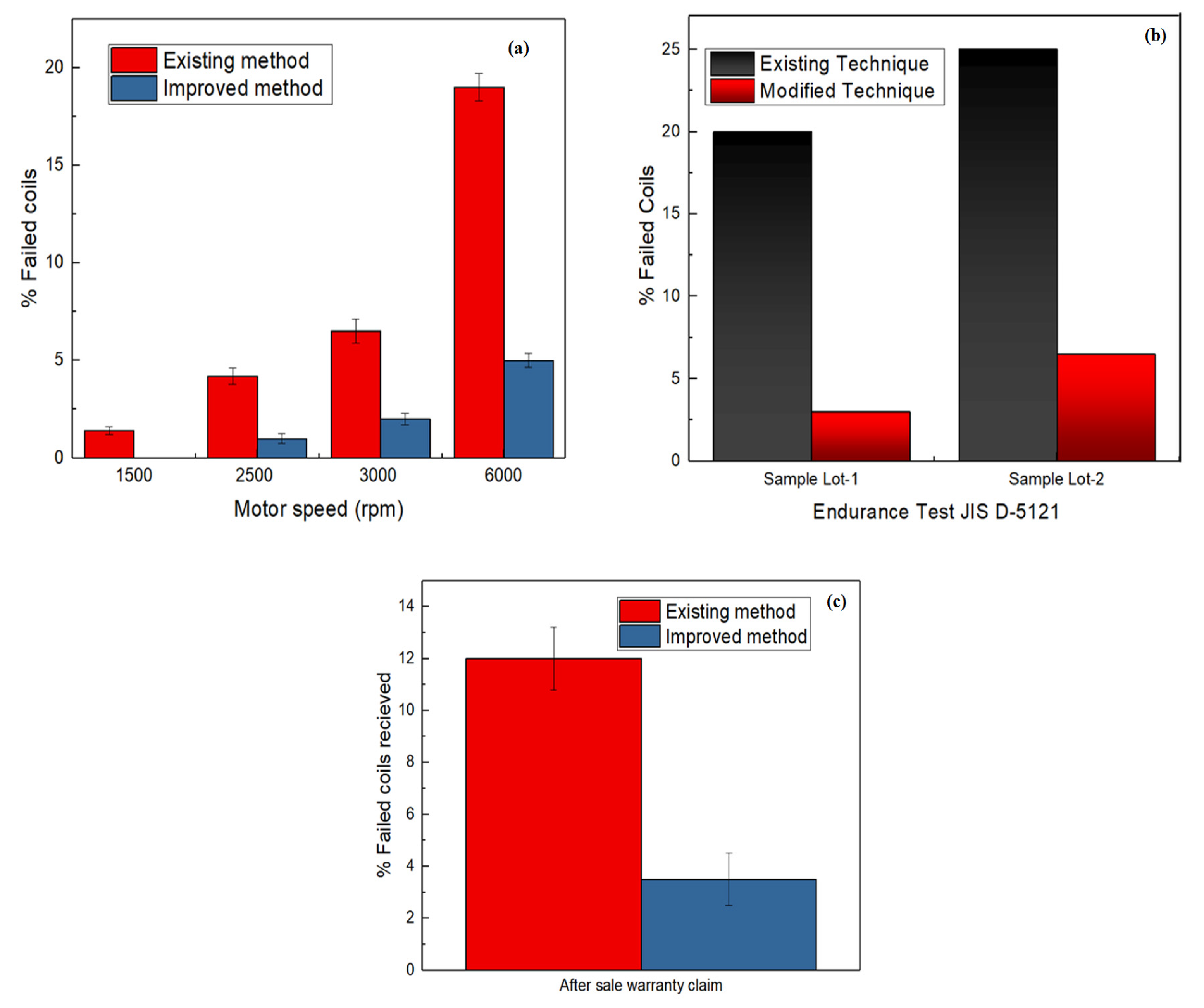
3.4. Dipping Electric Stirrer at Various Speeds
3.5. Testing of Coils and Analysis of Results
4. Discussion
5. Conclusions
- In the existing potting technique, 210–250 bubbled coils per lot are produced. These manufactured coils are wasted or usually received in market claims bearing considerable financial losses. Therefore, the aforesaid technique is improved by pre- and process heating of the coil for ~55–60 min for 50 ± 5 °C before and after pouring UPR, which reduces bubbled coils 1.31 times. Moreover, the UPR heated at 50 ± 5 °C is mixed with the accelerator/initiator (at 0.3 and 1.2%); poured in five increments; and further reduced to 1.89 and 3.36 folds, respectively.
- Dipping the electric stirrer inside the capsule forces elliptical bubbles towards the capsule’s inner-surface. Hence, most of the air escapes from the environment. Furthermore, lots manufactured using this modified technique were almost minimised 25.6 folds.
- IS-13480 and JIS-D5121 quality tests validate that non-qualified or failed specimens decreased by applying the OFAT approach stepwise. Additionally, after-sales claim tests also validate the effectiveness of the improved technique in producing high-quality coils.
- Based on the abovementioned results, suitable combinations of pre-treatment temperature, initiator/catalyst amount, pouring amount, and stirring speeds are extracted to alter the process and achieve minimum bubbled coils. This modification may reduce the waste material to approximately ~85%, which is received as a market claim within two years.
- The surface treatment of polymeric materials, i.e., laser and plasma, can be performed in future studies to tailor the dielectric strength and constant for high-voltage and other applications. Similarly, functionalised and non-functionalised micro fillers can also be investigated in making ignition coils and in the measurement of their properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohwein, G.; Camilli, L.S. Automotive Ignition Transfer Efficiency; Technical Paper for SAE: Warrendale, PA, USA, 2002. [Google Scholar]
- Yu, X.; Yu, S.; Yang, Z.; Tan, Q.; Ives, M.; Li, L.; Liu, M.; Zheng, M. Improvement on energy efficiency of the spark ignition system. In Proceedings of the ECX 17: SAE World Congress Experience, Detroit, MI, USA, 4 April 2017. [Google Scholar]
- Schwaner, J. The Magneto Ignition System, 1st ed.; Sacramento Sky Ranch Publisher: Sacramento, CA, USA, 1992. [Google Scholar]
- Ogata, K.; Kataoka, T. A high energy ignition system for EGR combustion engine. Trans. Automot. Soc. Jpn. 2016, 47, 357–362. [Google Scholar]
- Ruan, G.; Kienzle, D.; Roth, D.; Becker, M. Dual coil ignition for gasoline EGR engines. MTZ Worldwide 2014, 74, 18–21. [Google Scholar] [CrossRef]
- GmbH, R.B. Bosch Automotive Electrics and Automotive Electronics Systems and Components, Networking and Hybrid Drive, 1st ed.; Springer: Wiesbaden, Germany, 2014. [Google Scholar]
- Reif, K. System and components. In Gasoline Engine Management, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Chen, C.M.; Sun, C.H.; Chang, H.L. Environmental impact analysis of an automotive ignition coil in a supply chain. Carbon Manag. 2020, 11, 69–80. [Google Scholar] [CrossRef]
- Sanchez, R.O. A method for encapsulating high voltage power transformers. In Proceedings of the 11th Capacitor and Resistor Technology Symposium (CARTS), Las Vegas, NV, USA, 4–7 March 1991; pp. 42–47. [Google Scholar]
- Berk, C.L.; Phillip, J.D.; Hageman, D.E. System and Method for Minimizing Cure Induced Residual Stresses in an Epoxy Impregnated Coil. U.S. Patent 0197297A1, 23 October 2003. [Google Scholar]
- Nawaz, A. Study of the Problem of Void Formation in Electrical Lamination Parts and Its Removal. Master’s Thesis, University of Engineering & Technology, Peshawar, Pakistan, 2013. [Google Scholar]
- Kim, H.K.; Shi, F.G. Thickness dependent dielectric strength of a low-permittivity dielectric film. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 248–252. [Google Scholar] [CrossRef]
- Li, W.; Ren, X.; Ruan, L.; Zhu, S. Simulation of the effect of bubble defect on the distribution of electric field in epoxy resin pouring type insulated tubular bus-bar. In Proceedings of the 20th International Conference on Electrical Machines and Systems (ICEMS), Sydney, Australia, 11–14 August 2017; pp. 1–5. [Google Scholar]
- Cao, Y.; Irwin, P.C.; Younsi, K. The future of nanodielectrics in the electrical power industry. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 797–807. [Google Scholar]
- Cao, Y.; Irwin, P.C. The electrical conduction in polyimide nanocomposites. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Albuquerque, NW, USA, 19–22 October 2003; pp. 116–119. [Google Scholar]
- Tan, D.Q. The search for enhanced dielectric strength of polymer-based dielectrics: A focused review on polymer nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49379. [Google Scholar] [CrossRef]
- Sharma, C.P.; Vadera, K.L.; Agarwal, R.P. Impregnation of electrical machines with solvent less resins. Pigment Resin Technol. 1993, 22, 10–11. [Google Scholar] [CrossRef]
- Nawaz, A.; Islam, B.; Khattak, M.S.; Ali, L.; Saleem, U.; Ullah, A.; Ijaz, M.Z.; Mao, W.G. Polyester Usage in Manufacturing of Electrical and Mechanical Products and Assemblies. In Polyester-Production, Characterization and Innovative Applications; Camlibel, N.O., Ed.; Intech Publishers: London, UK, 2018; Volume 1, pp. 41–54. [Google Scholar]
- Dodiuk, H.; Goodman, S. Handbook of Thermoset Plastics, 3rd ed.; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Kosar, V.; Gomzi, Z. In-depth analysis of the mathematical model of polyester thermosets curing. Eur. Polym. J. 2004, 40, 2793–2802. [Google Scholar] [CrossRef]
- Fontalba, F. Method for Producing Individual Coil Thus Obtained. Patent No. US2007/0006858A1, 11 January 2007. [Google Scholar]
- IS 14380; Automotive Vehicles—Ignition Coils for Magneto Ignition Systems. Bureau of Indian Standards: New Delhi, India, 1996. Available online: https://archive.org/details/gov.in.is.14380.1996 (accessed on 1 December 2021).
- Judge, A.W. Modern Electrical Equipment for Automobile, 2nd ed.; Hall and Chapman: London, UK, 1970. [Google Scholar]
- Nawaz, A.; Islam, B.; Akhtar, R.; Alamgir, K.; Noor, S. Failure of polyester laminated automotive ignition coils influenced by environmental factors. Arab. J. Sci. Eng. 2015, 40, 3337–3344. [Google Scholar] [CrossRef]
- Islam, B. Study of Parameters Reducing Curing Time for Hot Plate Glass Capsule Impregnation Technique. Master’s Thesis, University of Engineering & Technology, Peshawar, Pakistan, 2016. [Google Scholar]
- Kurematsu, K.; Koshi, M. Kinetic Studies on void formation during liquid epoxy resin impregnation through polyester non-woven fabric. Colloid Polym. Sci. 1995, 263, 454–461. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Mashelkar, R.A. Advances in Transport Processes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Tong, H.M.; Ouano, A.C. Bubble formation mechanism during heat treatment of polymer films cast from solutions. Polym. Eng. Sci. 1985, 25, 75–82. [Google Scholar] [CrossRef]
- Adhhikari, D.; Hepburn, D.M.; Stewart, B.G. Characterization of PD occurring in injected air bubbles in block polymeric insulation. In Proceedings of the Electrical Insulation and Dielectric Phenomena (CEIDP) Conference, Montreal, QC, Canada, 14–17 October 2012; pp. 48–51. [Google Scholar]
- Yahaya, E.A.; Jacob, T.; Nwohu, M.; Abubakar, A. Power loss due to Corona on high voltage transmission lines. IOSR J. Electr. Electron. Eng. 2013, 8, 14–19. [Google Scholar] [CrossRef]
- Waigaonkar, S.; Babu, B.C.; Rajput, A. Curing studies of un-saturated polyester resin used in FRP products. Indian J. Eng. Mater. Sci. 2011, 18, 31–39. [Google Scholar]
- Jankovic, B. The Kinetic analysis of isothermal curing reaction of an unsaturated polyester resin: Estimation of density distribution function of the apparent activation energy. Chem. Eng. J. 2010, 162, 331–340. [Google Scholar] [CrossRef]
- Groβ, H.; Kollek, H.; Brockmann, H. The nucleophilicity as the determining parameter in the curing of epoxy resins. Int. J. Adhes. Adhes. 1988, 8, 225–235. [Google Scholar]
- Satapathi, A.; Patnaik, A. Analysis of dry sliding wear behavior of red mud filled polyester composites using the Taguchi method. J. Reinf. Plast. Compos. 2010, 29, 2883–2897. [Google Scholar] [CrossRef]
- Prabu, V.A.; Manikandan, V.; Uthayakumar, M.; Kalirasu, S. Investigations on the mechanical properties of red mud filled sisal and banana fiber reinforced polyester composites. Mater. Phys. Mech. 2012, 15, 173–179. [Google Scholar]
- Matyjaszewski, K.; Moller, M. Polymer Science: A Comprehensive Reference, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 779–831. [Google Scholar]
- Islam, S.; Arbab, M.N.; Nawaz, A.; Islam, B.; Bibi, S.; Hussain, I. Appropriate polymer selection for insulation of high voltage ignition coils by combination of material index determination and novel heuristical approach. Trans. Indian Inst. Met. 2021, 74, 1387–1396. [Google Scholar] [CrossRef]

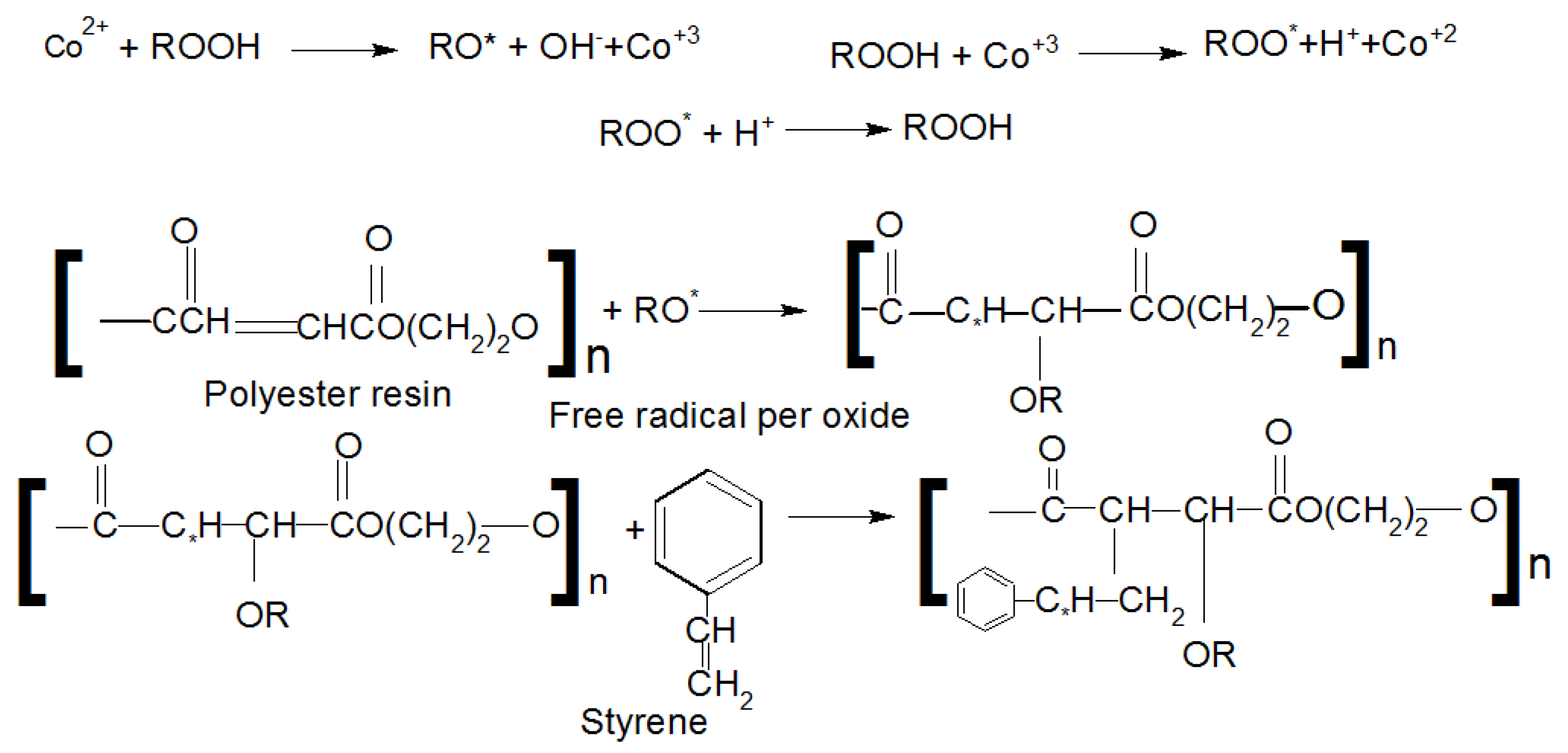
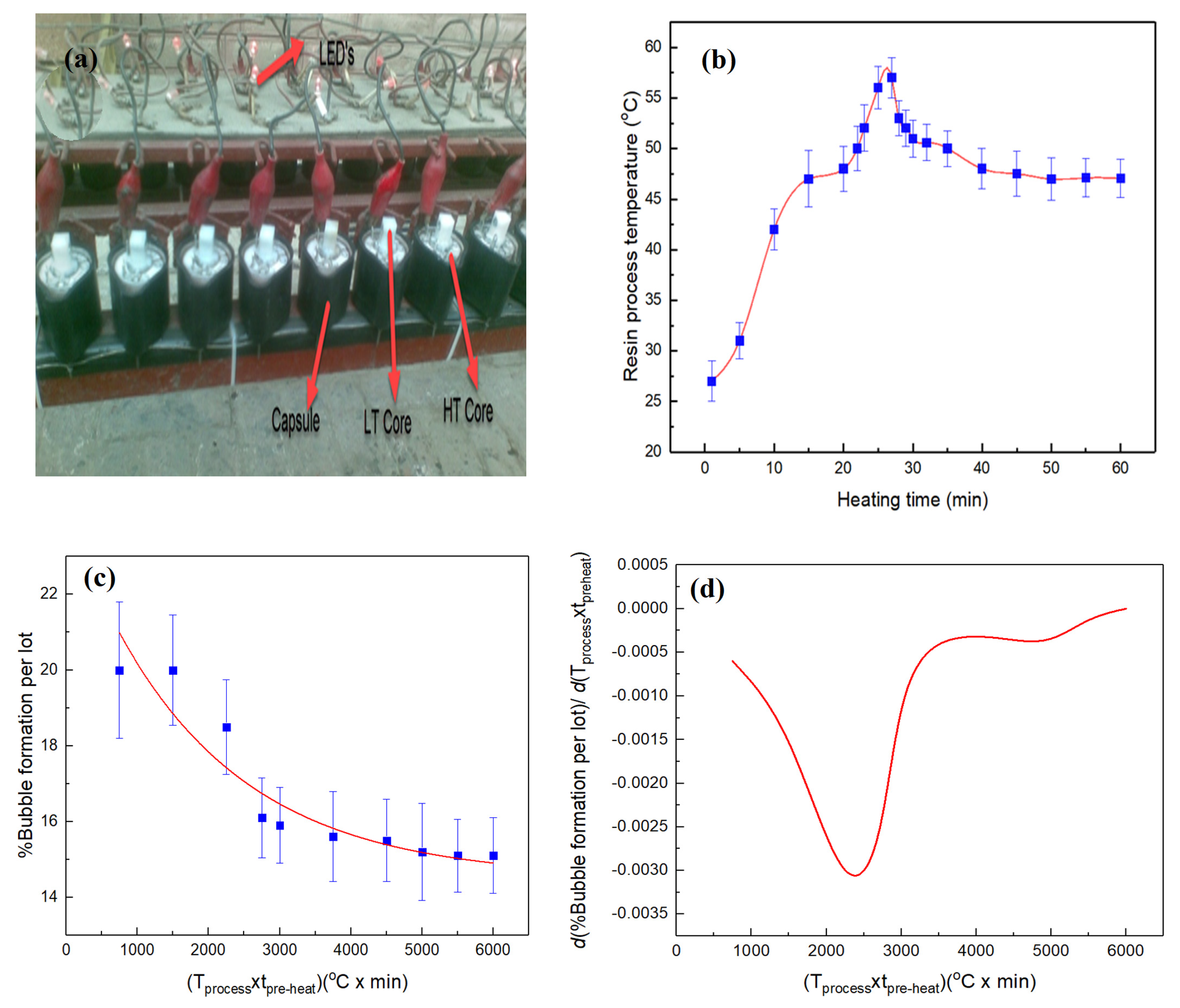
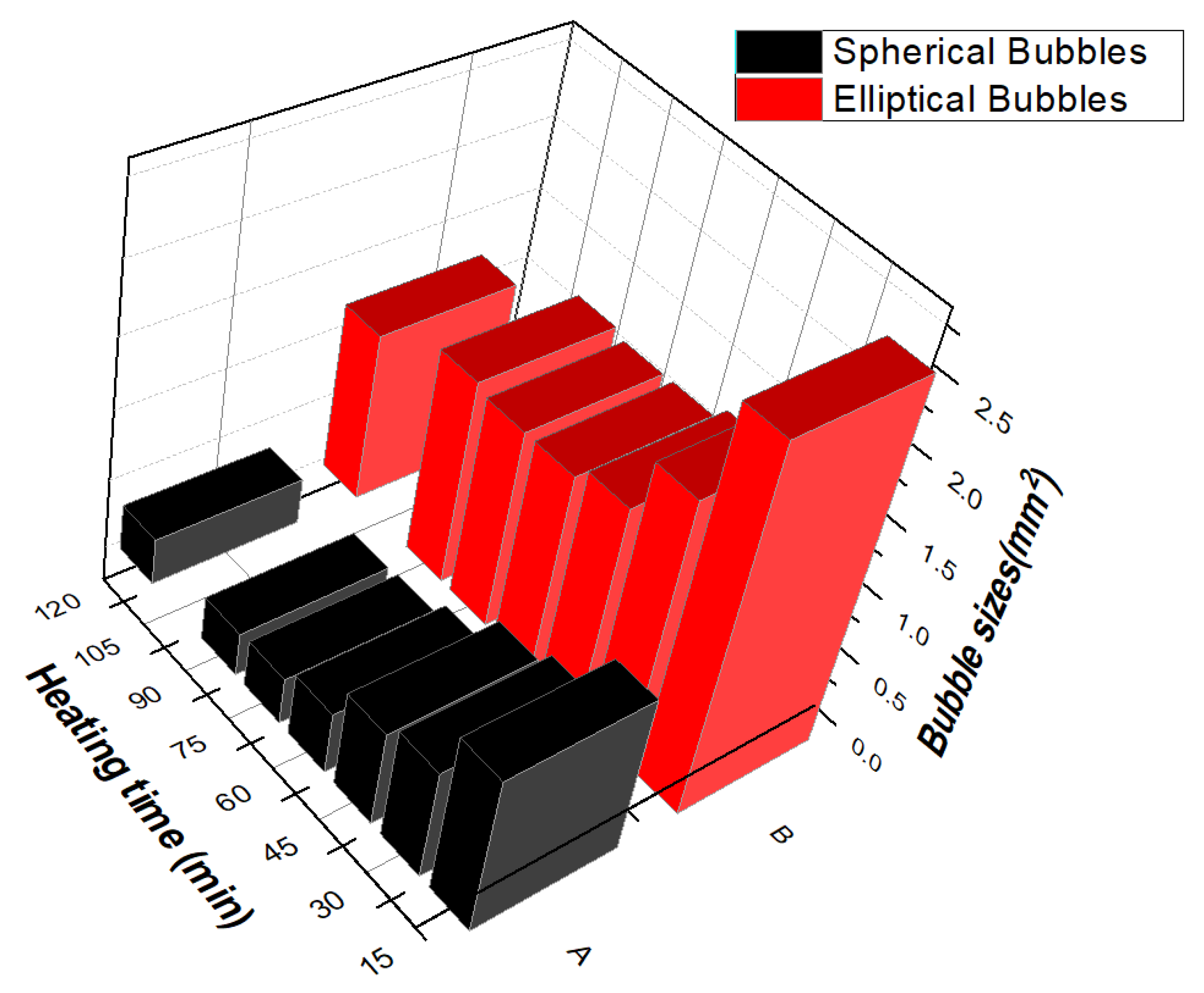
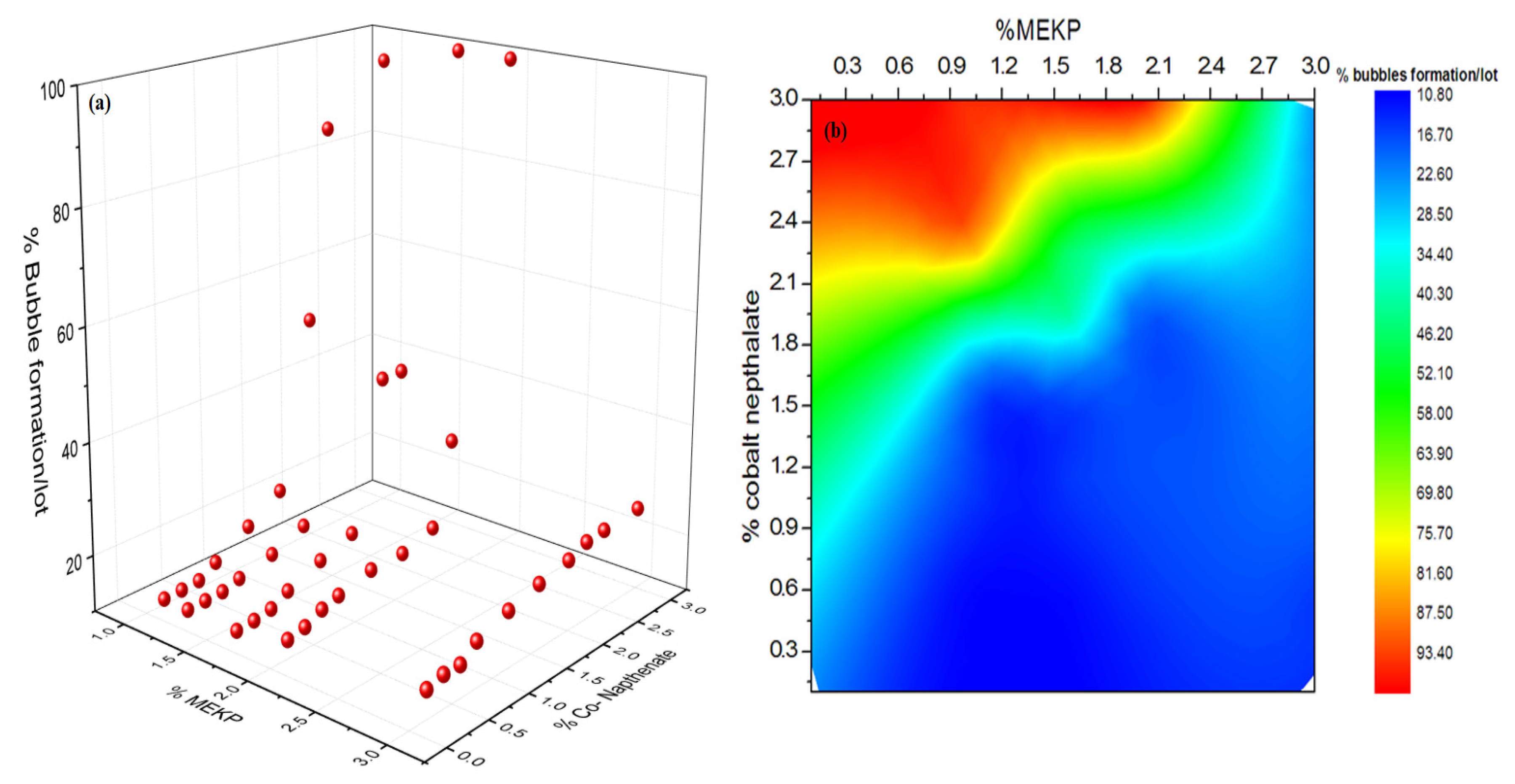

| Variables | DOF | Sum of Squares | Mean Square | FValue ˃ F0.1,v1,v2 | p-Value |
|---|---|---|---|---|---|
| Regression | 3 | 2008.71 | 669.57 | 1914.61 ˃ 3.07 | ˂0.1 |
| Residual | 7 | 2.448 | 0.34972 | ||
| Uncorrected Total | 10 | 2011.16 | |||
| Corrected Total | 9 | 18.6066 |
| Predictor | Coefficient (β) | Standard Error (SE) | t = β/SE | p-Value |
|---|---|---|---|---|
| Correction factor (βo) | 11.576 | 7.00 | 1.65 | 0.098 |
| MEKP wt% | −6.96 | 3.299 | −2.11 | 0.041 |
| Cobalt naphthenate wt% | 20.25 | 2.741 | 7.46 | 0.001 |
| Variables | DOF | Sum of Squares | Mean Square | FValue ˃ F0.1,v1,v2 | p-Value |
|---|---|---|---|---|---|
| Regression | 4 | 7041.148 | 1760.287 | 385.484 ˃ 4.11 | ˂0.1 |
| Residual | 4 | 18.26573 | 4.56643 | ||
| Uncorrected Total | 8 | 7059.413 | |||
| Corrected Total | 7 | 473.4712 |
| Variables | DOF | Sum of Squares | Mean Square | FValue ˃ F0.1,v1,v2 | p-Value |
|---|---|---|---|---|---|
| Regression | 3 | 1170.99016 | 390.33005 | 186.24 ˃ 4.19 | ˂0.1 |
| Residual | 4 | 8.38318 | 2.09579 | ||
| Uncorrected Total | 7 | 1179.37333 | |||
| Corrected Total | 6 | 635.32739 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, A.; Ahmad, I.; Akram, W.; Islam, B.; Pimenov, D.Y.; Giasin, K.; Aamir, M. One Factor at a Time Analysis to Modify Potting Technique for Manufacturing of Bubble-Free High-Voltage Polyester Insulated Automotive Coils. Designs 2022, 6, 44. https://doi.org/10.3390/designs6030044
Nawaz A, Ahmad I, Akram W, Islam B, Pimenov DY, Giasin K, Aamir M. One Factor at a Time Analysis to Modify Potting Technique for Manufacturing of Bubble-Free High-Voltage Polyester Insulated Automotive Coils. Designs. 2022; 6(3):44. https://doi.org/10.3390/designs6030044
Chicago/Turabian StyleNawaz, Ahmad, Ishaq Ahmad, Waseem Akram, Bilal Islam, Danil Yurievich Pimenov, Khaled Giasin, and Muhammad Aamir. 2022. "One Factor at a Time Analysis to Modify Potting Technique for Manufacturing of Bubble-Free High-Voltage Polyester Insulated Automotive Coils" Designs 6, no. 3: 44. https://doi.org/10.3390/designs6030044
APA StyleNawaz, A., Ahmad, I., Akram, W., Islam, B., Pimenov, D. Y., Giasin, K., & Aamir, M. (2022). One Factor at a Time Analysis to Modify Potting Technique for Manufacturing of Bubble-Free High-Voltage Polyester Insulated Automotive Coils. Designs, 6(3), 44. https://doi.org/10.3390/designs6030044









