Biomechanical Device for Measurement of Adductors Strength and Aid in Self-Catheterisation of Spastic Patients
Abstract
1. Introduction
2. Materials and Methods
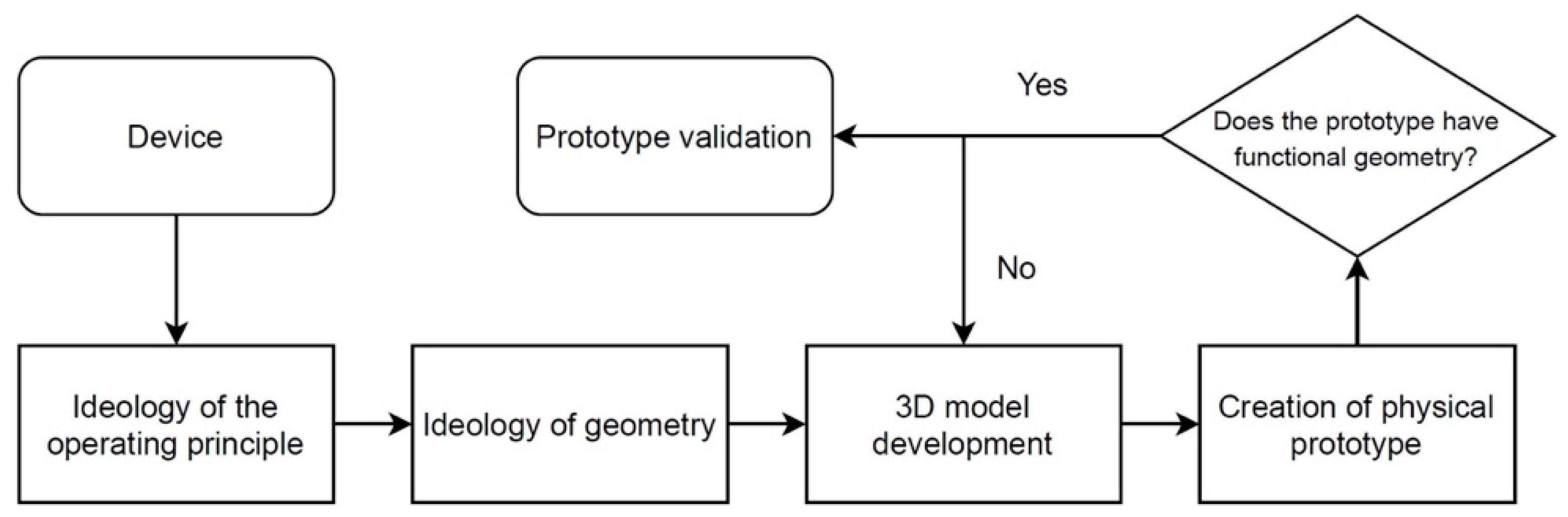
2.1. Device Development—3D Models
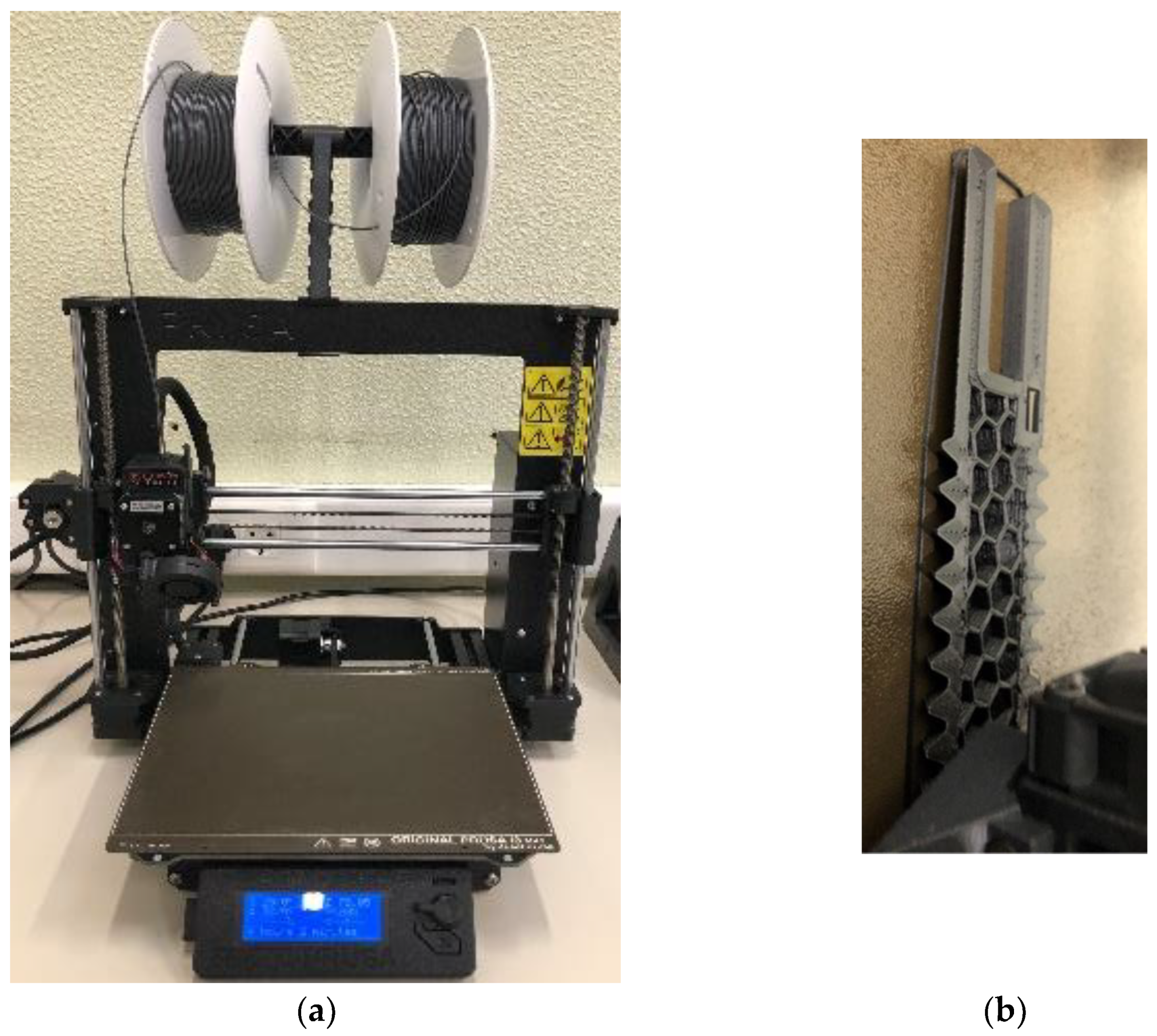
2.2. Production of Prototypes
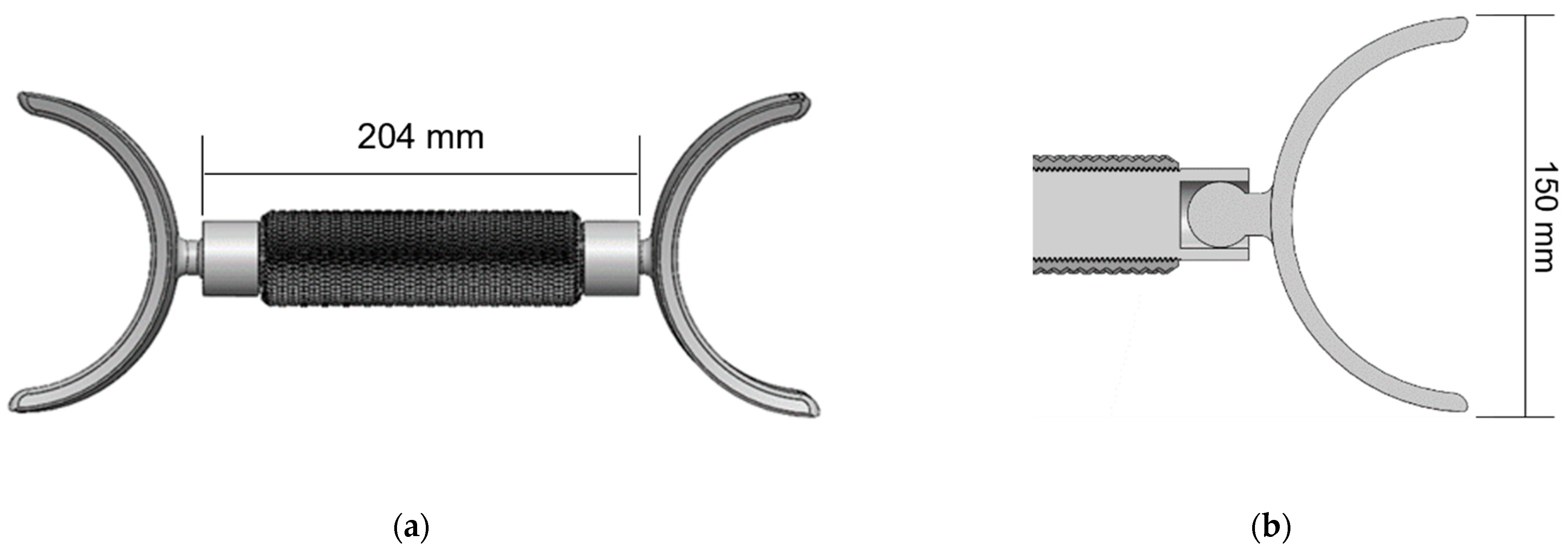
2.3. Real Devices
2.4. Finite Element Models
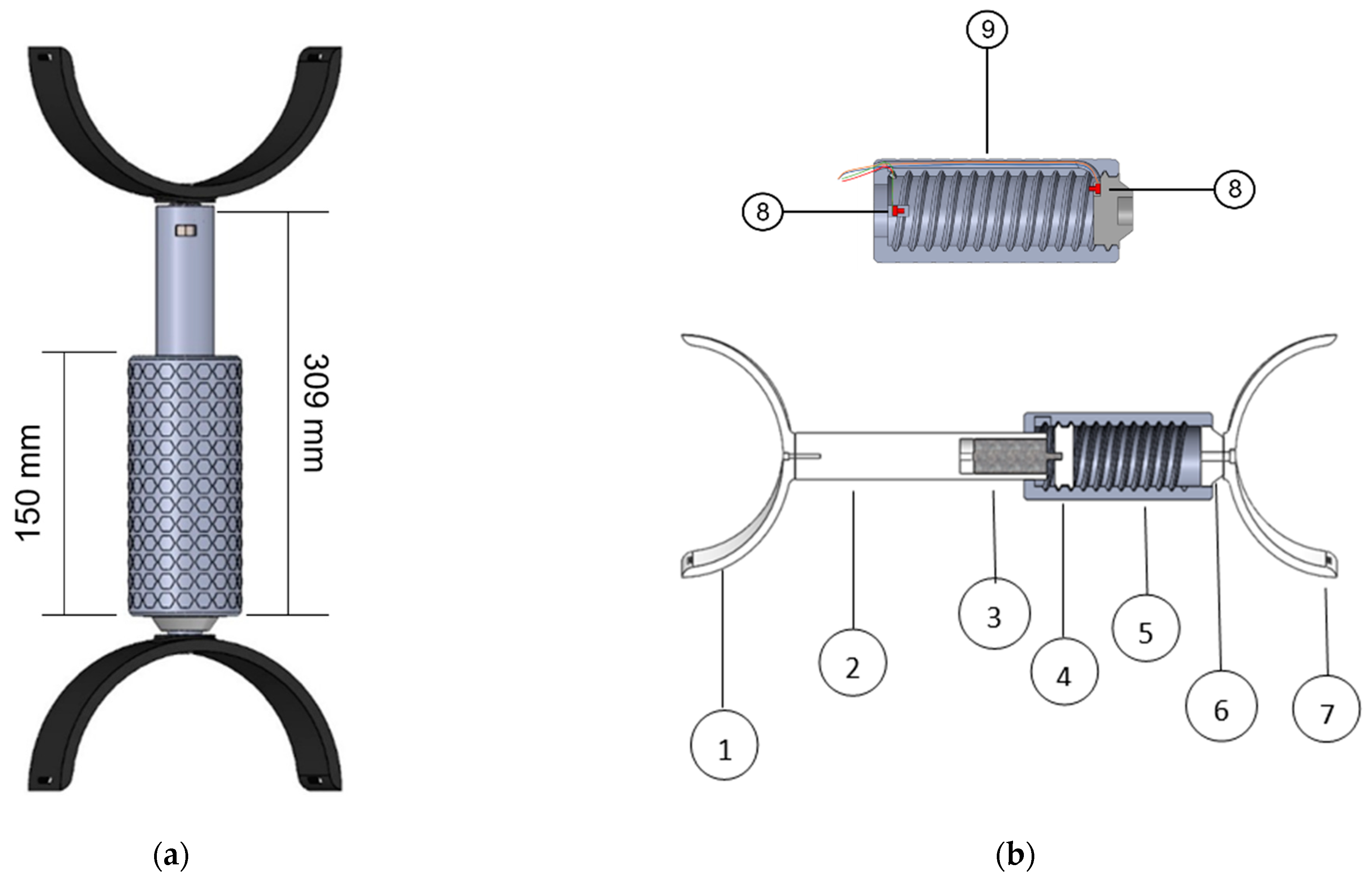
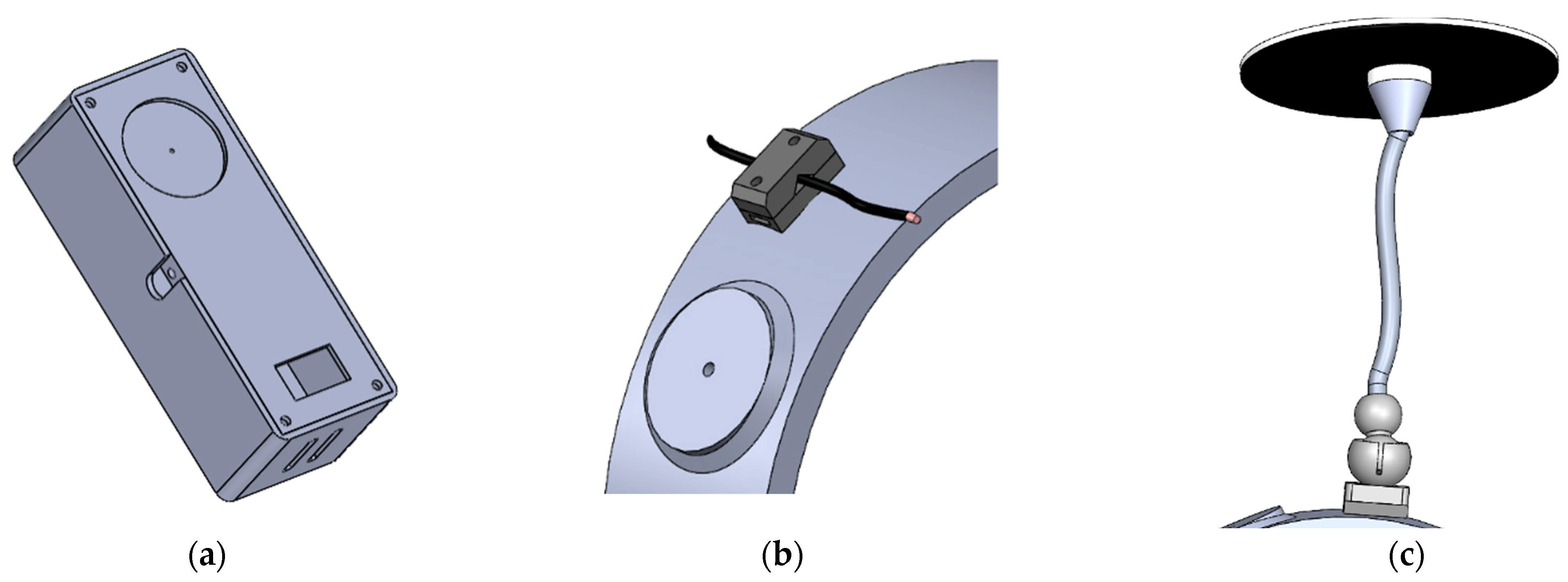
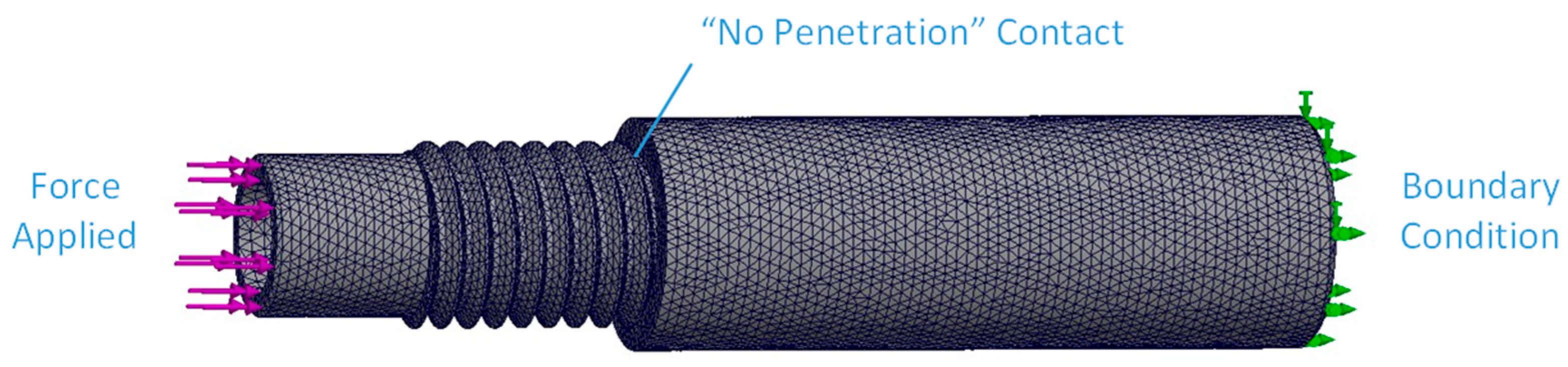
2.4.1. Instrumented Device
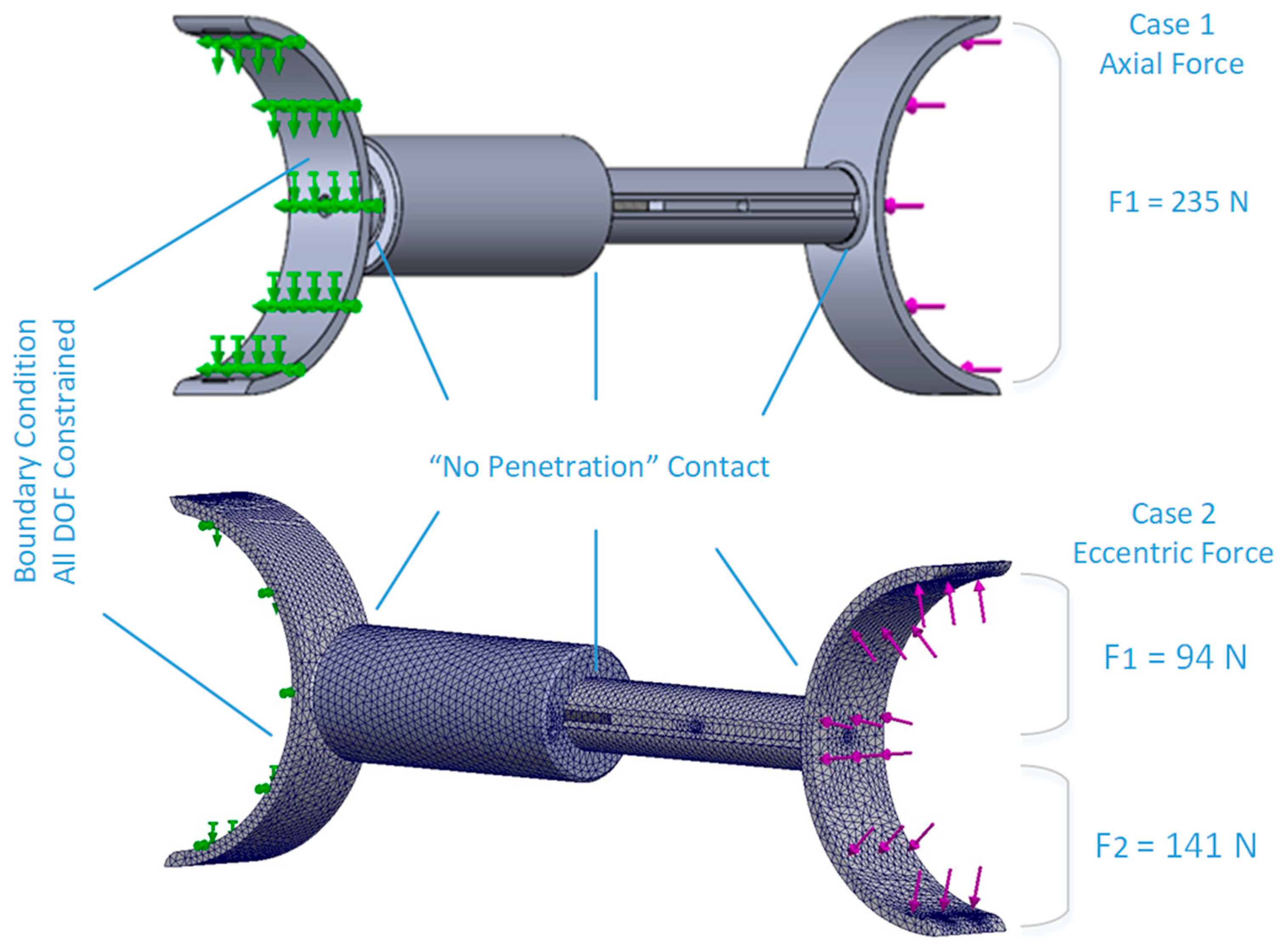
2.4.2. Motorised Device
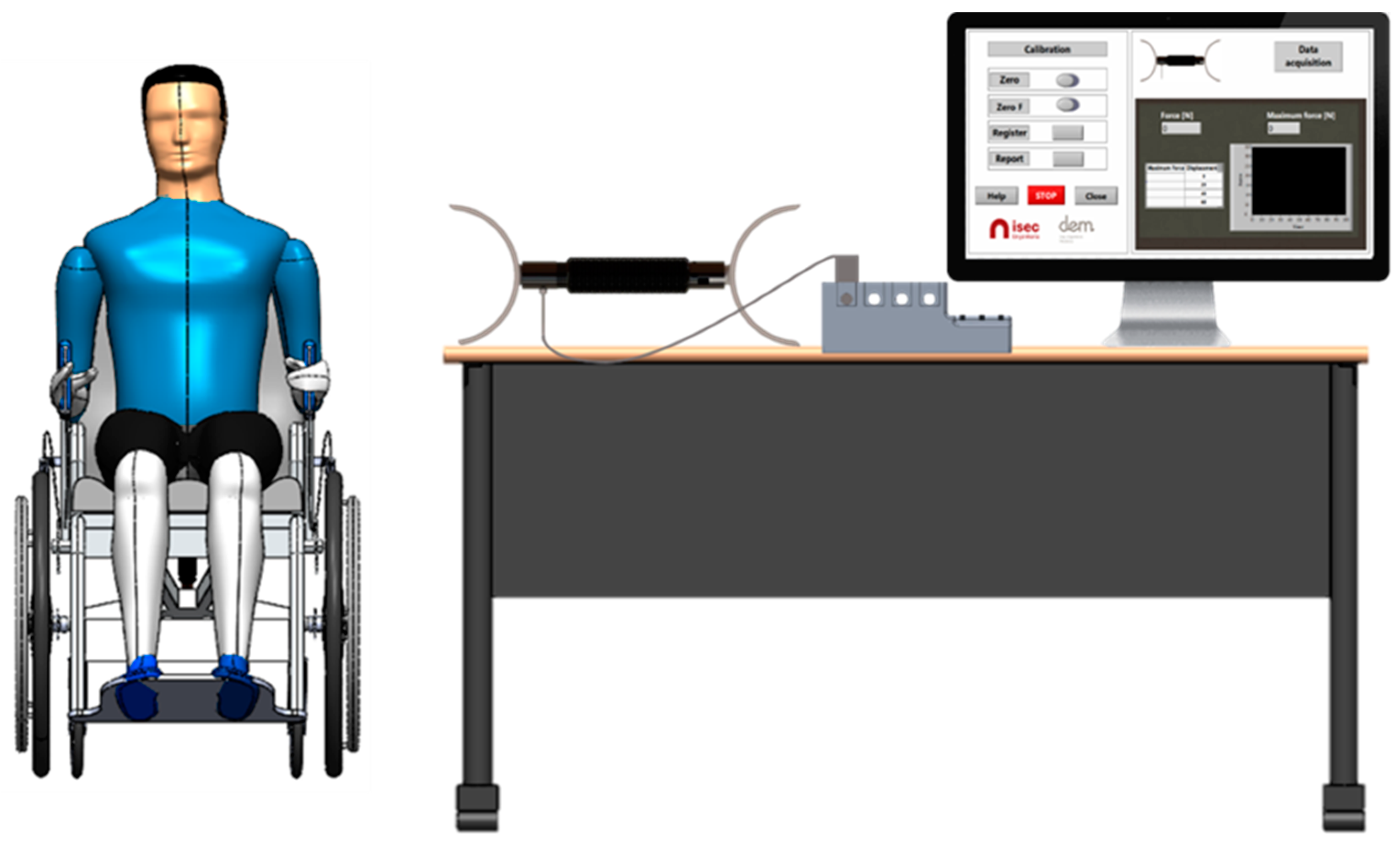
2.5. Experimental Tests
2.5.1. Tests with Healthy Young Volunteers
2.5.2. Tests with Volunteers with Spinal Cord Injury
3. Results
3.1. Numerical Results
3.1.1. Instrumented Device
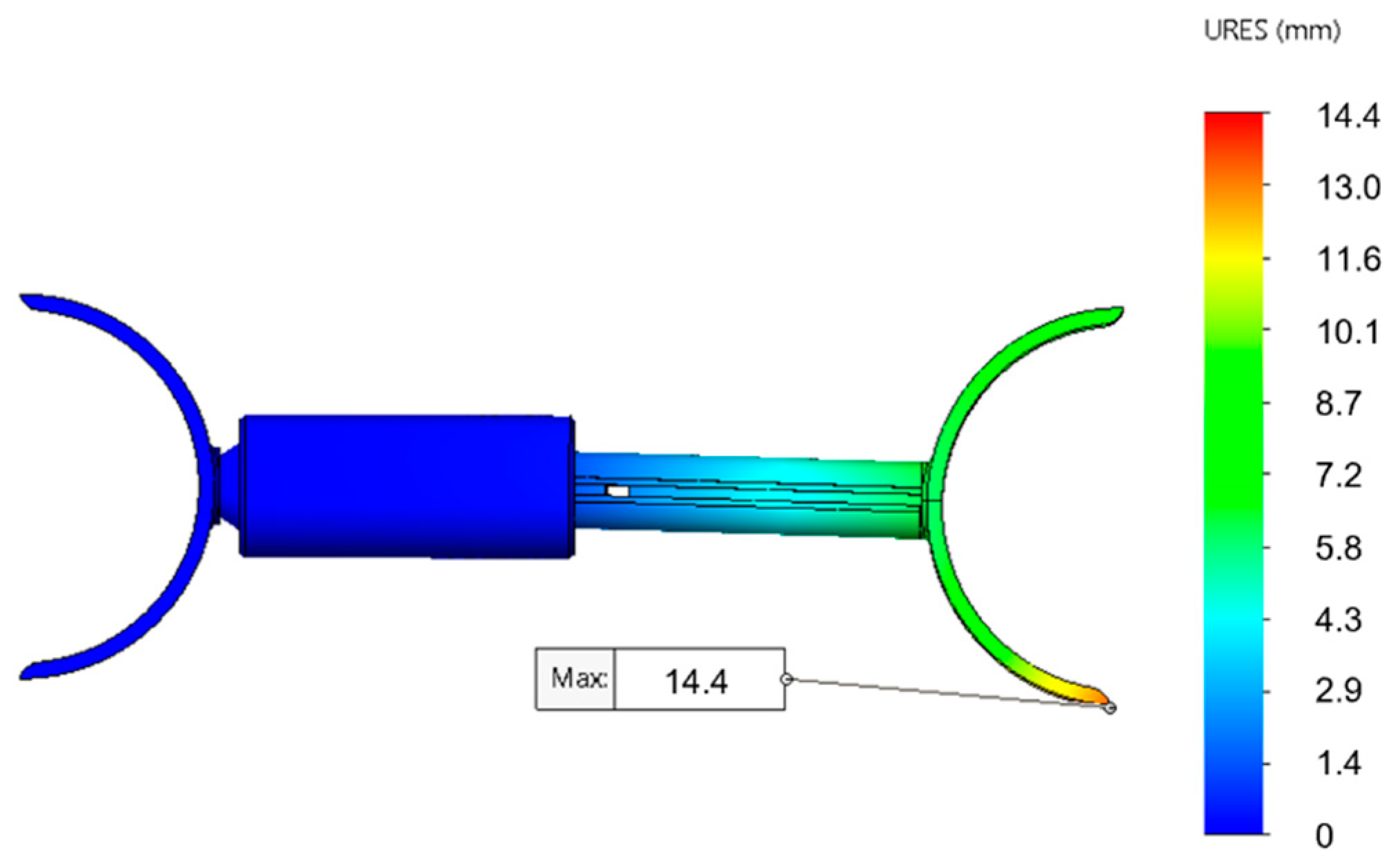
3.1.2. Motorised Device
3.2. Experimental Results
3.2.1. Tests with Healthy Volunteers
3.2.2. Tests with Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assis, G.M.; Faro, A.C.M. Clean intermittent self catheterization in spinal cord injury. Rev. da Esc. de Enferm. da USP 2011, 45, 282–286. [Google Scholar] [CrossRef]
- Shenot, P.J. Bexiga neurogénica. Available online: https://www.msdmanuals.com/pt/profissional/dist%C3%BArbios-geniturin%C3%A1rios/dist%C3%BArbios-miccionais/bexiga-neurog%C3%AAnica (accessed on 14 October 2020).
- Mazzo, A.; Souza-Junior, V.D.; Jorge, B.M.; Nassif, A.; Biaziolo, C.F.; Cassini, M.F.; Santos, R.C.R.; Mendes, I.A.C. Intermittent urethral catheterization—Descriptive study at a Brazilian service. Appl. Nurs. Res. 2014, 27, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Panicker, J.; Seth, J.; Haslam, C. Ensuring patient adherence to clean intermittent self-catheterization. Patient Prefer. Adher. 2014, 8, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Baczkoski, J.S. Mirror assembly for patients with personal hygiene problems. U.S. Patent 4,257,680, 24 March 1981. [Google Scholar]
- Levy, E.A. Mirror assembly to be held between the knees of a user. U.S. Patent 3,411,842, 19 November 1968. [Google Scholar]
- Billau, B.W.; Howland, D.R. Self-catheterization for the woman with quadriplegia. Am. J. Occup. Ther. 1991, 45, 366–369. Available online: https://ajot.aota.org/article.aspx?articleid=1875875 (accessed on 7 October 2020). [CrossRef][Green Version]
- Gerace, J.A. Mirror adapted for use in self-catheterizing procedures. U.S. Patent 5,311,366, 10 May 1994. [Google Scholar]
- Alvi, H.I.; Cozzie, N.L.; Wilkinson, M.R.; Ochoa, A.; Eaton, B. Self-inspection apparatus. U.S. Patent 8602574, 10 December 2013. [Google Scholar]
- Winther, S.B.; Foss, O.A.; Husby, O.S.; Wik, T.S.; Klaksvik, J.; Husby, V.S. A randomized controlled trial on maximal strength training in 60 patients undergoing total hip arthroplasty. Acta Orthop. 2018, 89, 295–301. [Google Scholar] [CrossRef]
- Trudelle-Jackson, E.; Smith, S.S. Effects of a late-phase exercise program after total hip arthroplasty: A randomized controlled trial. Arch. Phys. Med. Rehabilit. 2004, 85, 1056–1062. [Google Scholar] [CrossRef]
- Pinto, J.L.D. Dados Normativos e Normalizados de Força de Adução e Abdução da Anca em Jogadores de Futebol de Elite em Portugal. Ph.D. Thesis, Universidade Privada da Maia, Maia, Portugal, December 2020. [Google Scholar]
- Unlu, E.; Eksioglu, E.; Aydog, E.; Aydoð, S.T.; Atay, G. The effect of exercise on hip muscle strength, gait speed and cadence in patients with total hip arthroplasty: A randomized controlled study. Clin. Rehabilit. 2007, 21, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, B.; Cunningham, P.; Harrington, P.; Hing, W.; Blake, C.; Dohertya, D.O.; Cusack, T. Randomised controlled trial to evaluate a physiotherapy-led functional exercise programme after total hip replacement. Physiotherapy 2016, 103, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Muscle Controller by Kinvent. Hand-Held Dynamometer for Physios and Sport Pros. Available online: https://k-invent.com/k-forcemuscle-controller/ (accessed on 4 January 2022).
- ForceFrame: Strength Testing System. VALD Performance. Available online: https://valdperformance.com/forceframe/ (accessed on 4 January 2022).
- Cabrera, M.N.B.; Norris, J.A. Aparatus and method for evaluating hypertonic condition. U.S. Patent 2007/0027631A1, 1 February 2007. [Google Scholar]
- Wang, T.Y.; Wu, C.Y.; Sutton, O.M. Spasticity quantification device. U.S. Patent 2016/0317066A1, 3 November 2016. [Google Scholar]
- Husby, V.S.; Helgerud, J.; Bjørgen, S.; Husby, O.S.; Benum, P.; Hoff, J. Early maximal strength training is an efficient treatment for patients operated with total hip arthroplasty. Arch. Phys. Med. Rehabilit. 2009, 90, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Ström, H.; Huss, K.; Larsson, S. Unrestricted weight bearing and intensive physiotherapy after uncemented total hip arthroplasty. Scand. J. Surg. 2006, 95, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Bastiaenen, C.H.G.; Terrier, P.; Punt, I.; Ferrari, S.; Gold, G.; De Bie, R.; Allet, L. Evaluation of hip abductor and adductor strength in the elderly: A reliability study. Eur. Rev. Aging Phys. Act. 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Kempton, T.; Pacecca, E.; Coutts, A.J. Measurement properties of an adductor strength-assessment system in professional australian footballers. Int. J. Sports Physiol. Perform. 2019, 14, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Vega, E.C.; Jerez-Mayorga, D.; Payer, R.M.; Jara, C.C.; Guzman-Guzman, I.; Ponce, A.R.; Chirosa, L.J. Validity and reliability of evaluating hip abductor strength using different normalization methods in a functional electromechanical device. PLoS ONE 2018, 13, e0202248. [Google Scholar] [CrossRef]
- Neto, M.A.; Amaro, A.; Roseiro, L.; Cirne, J.; Leal, R. Engineering Computation of Structures: The Finite Element Method; Springer: Berlin, Germany, 2015; ISBN 978-3-319-17709-0. [Google Scholar]







| Material | Specific Mass ρ (kg/m3) | Young’s Modulus E (MPa) | Tensile Strength σc (MPa) | Poisson’s Ratio ν |
|---|---|---|---|---|
| PETG | 1420 | 2960 | 57.3 | 0.37 |
| PLA | 1240 | 3500 | 70.0 | 0.36 |
| TPU | 1120 | 26 | 45.0 | 0.39 |
| Elastosil M4512 * | 1190 | - | 3.5 | - |
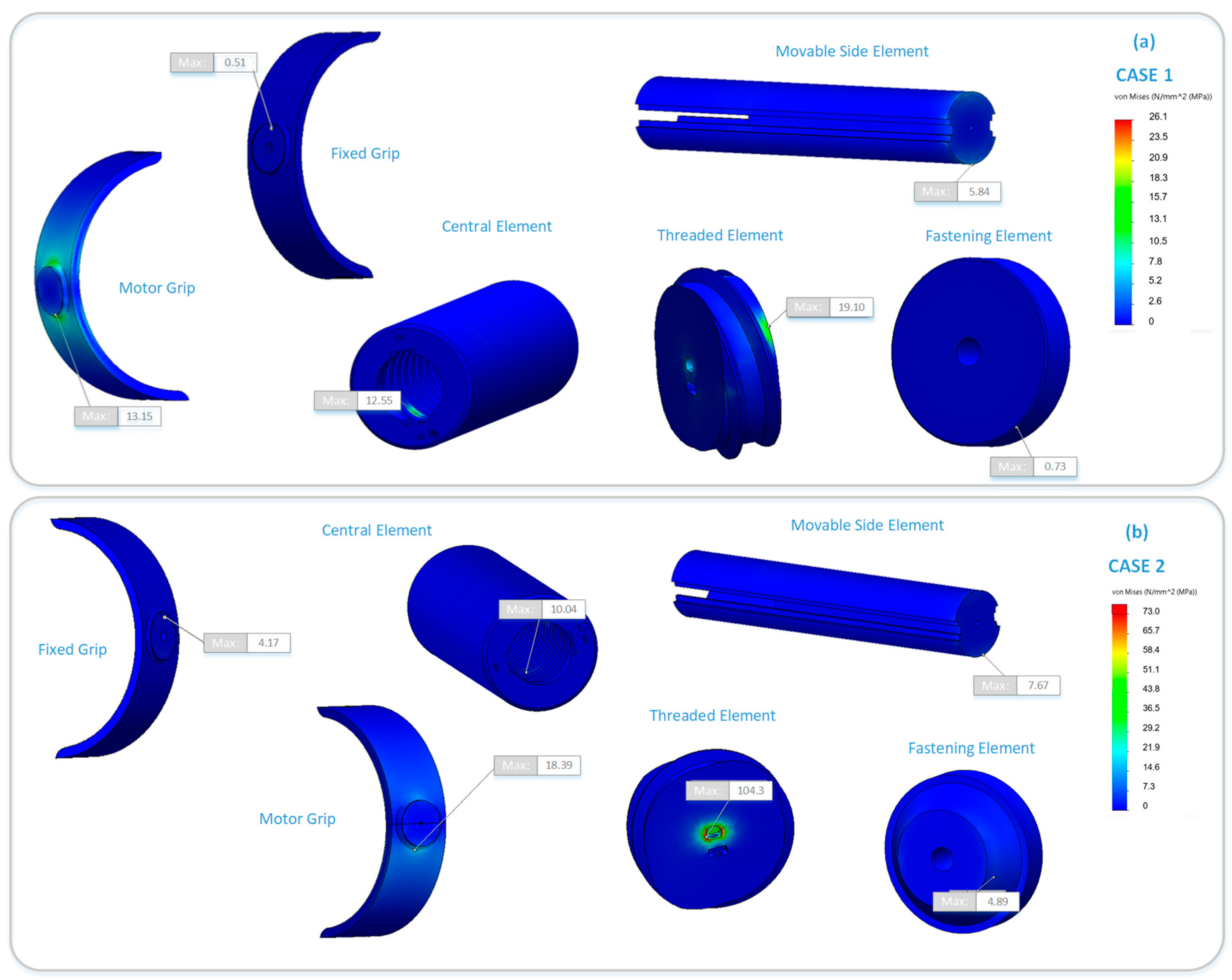
| Component | Maximum von Mises Stress (MPa) | |
|---|---|---|
| LOAD CASE 1 | LOAD CASE 2 | |
| Motor grip | 13.15 | 18.39 |
| Movable side element | 5.84 | 7.67 |
| Central element | 12.55 | 10.04 |
| Threaded element | 19.10 | 104.30 |
| Fastening element | 0.73 | 4.89 |
| Fixed Grip | 0.51 | 4.17 |
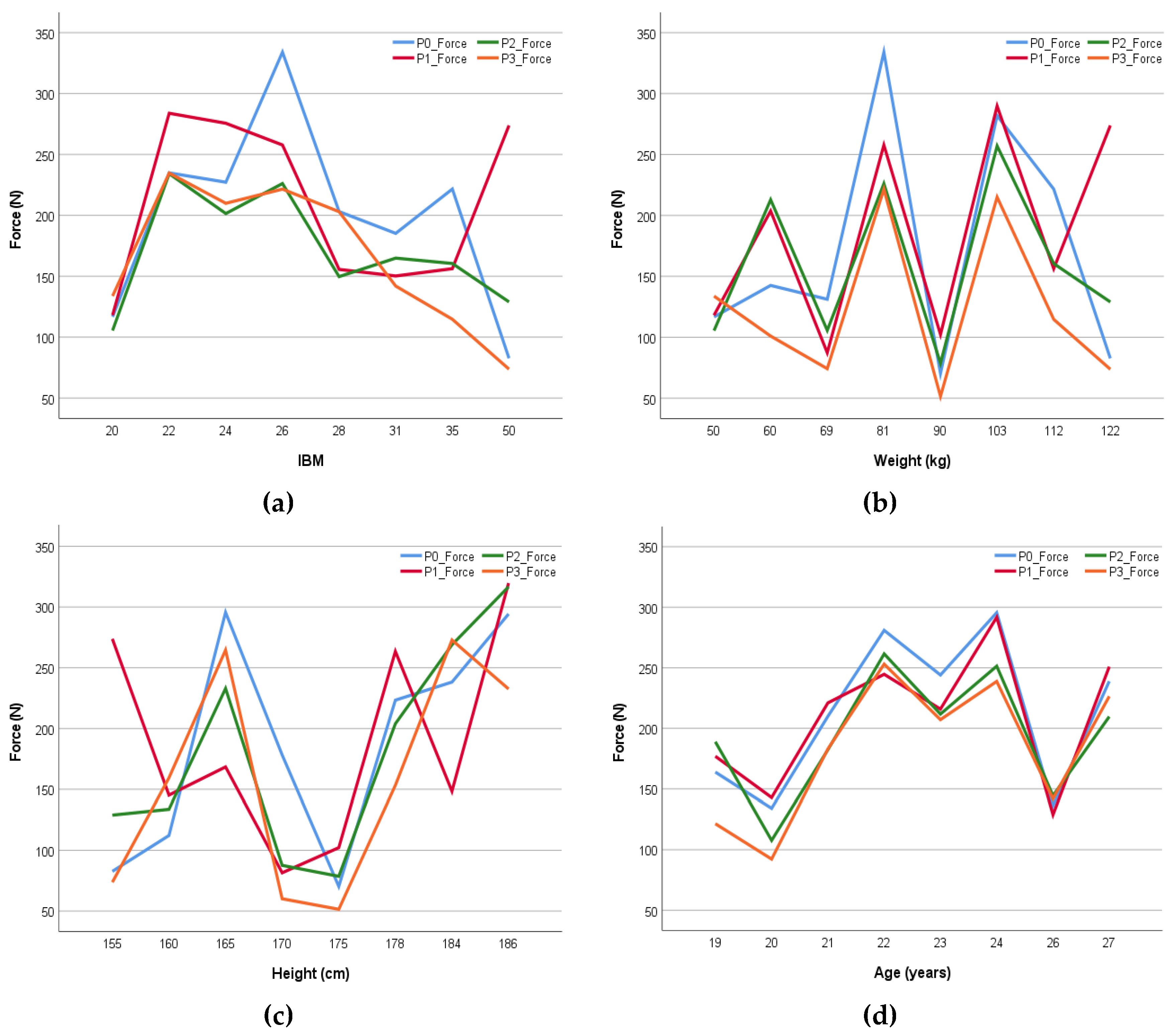
| Mean | Maximum | Minimum | |
|---|---|---|---|
| Age | 22 | 27 | 19 |
| Height (cm) | 169.95 | 186.00 | 155.00 |
| Weight (kg) | 75.51 | 121.80 | 47.50 |
| BMI | 26.16 | 50.70 | 18.80 |
| % Body Fat | 32.82 | 59.90 | 16.20 |
| % Skeletal Muscles | 30.44 | 43.00 | 15.90 |
| Total Sample (n = 32) | |||||
|---|---|---|---|---|---|
| M | SD | Median | Minimum | Maximum | |
| Force P0 | 212.76 | 88.60 | 224 | 70.33 | 416.92 |
| Force P1 | 206.28 | 78.12 | 197 | 75.77 | 345.22 |
| Force P2 | 188.52 | 71.80 | 207 | 65.40 | 371.81 |
| Force P3 | 179.04 | 80.02 | 207 | 46.10 | 327.27 |
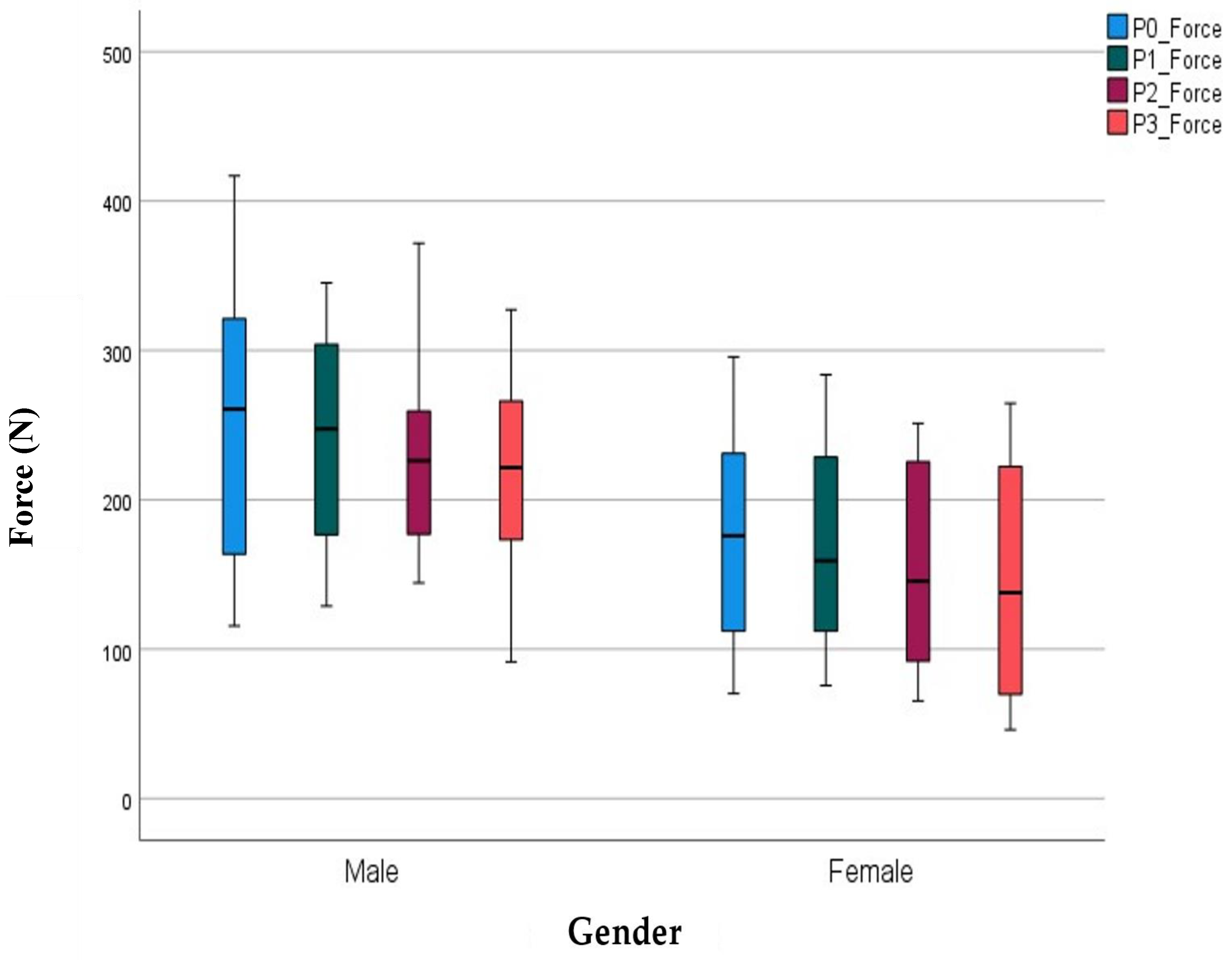
| Male (n = 16) | Female (n = 16) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Minimum | Maximum | M | SD | Minimum | Maximum | |
| Force P0 | 257.32 | 88.09 | 70.33 | 416.92 | 198.81 | 72.64 | 96.35 | 295.70 |
| Force P1 | 244.14 | 71.34 | 10.22 | 345.22 | 191.70 | 71.10 | 87.32 | 283.84 |
| Force P2 | 225.22 | 57.04 | 68.00 | 371.81 | 159.92 | 68.04 | 65.40 | 251.24 |
| Force P3 | 212.76 | 68.39 | 51.48 | 327.27 | 150.50 | 77.78 | 60.13 | 264.66 |
| Force | Equal Variances | Levene’s Test Equality of Variances | t-Test for Equality of Means | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t | df | p-Value | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||||
| F | Sig. | Lower | Upper | |||||||
| P0 | Assumed | 0.691 | 0.413 | 2.71 | 30.00 | 0.011 | 77.30 | 28.55 | 18.99 | 135.59 |
| Not Assumed | 2.71 | 28.95 | 0.011 | 77.30 | 28.55 | 18.99 | 135.59 | |||
| P1 | Assumed | 0.054 | 0.818 | 2.70 | 30.00 | 0.011 | 68.02 | 25.18 | 16.60 | 119.44 |
| Not Assumed | 2.70 | 30.00 | 0.011 | 68.02 | 25.18 | 16.60 | 119.44 | |||
| P2 | Assumed | 2.333 | 0.137 | 3.25 | 30.00 | 0.003 | 72.05 | 22.20 | 26.72 | 117.39 |
| Not Assumed | 3.25 | 29.11 | 0.003 | 72.05 | 22.20 | 26.72 | 117.39 | |||
| P3 | Assumed | 1.121 | 0.298 | 2.65 | 30.00 | 0.013 | 68.56 | 25.89 | 15.68 | 121.44 |
| Not Assumed | 2.65 | 29.52 | 0.013 | 68.56 | 25.89 | 15.68 | 121.44 | |||
| Force P0 | Force P1 | Force P2 | Force P3 | |
|---|---|---|---|---|
| Force P0 | 1 | 0.671 | 0.716 | 0.732 |
| Force P1 | 0.671 | 1 | 0.784 | 0.626 |
| Force P2 | 0.716 | 0.784 | 1 | 0.814 |
| Force P3 | 0.732 | 0.626 | 0.814 | 1 |
| Total Sample (n = 10) | |||||
|---|---|---|---|---|---|
| M | SD | Median | Minimum | Maximum | |
| Force P0 | 106.29 | 78.40 | 87 | 19.76 | 235.47 |
| Force P1 | 118.95 | 69.46 | 89 | 33.12 | 233.07 |
| Force P2 | 123.10 | 65.28 | 121 | 44.17 | 214.44 |
| Force P3 | 130.32 | 76.33 | 109 | 33.29 | 235.47 |
| Male (n = 5) | Female (n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Minimum | Maximum | M | SD | Minimum | Maximum | |
| Force P0 | 163.01 | 66.00 | 60.60 | 235.47 | 49.56 | 37.79 | 19.76 | 112.51 |
| Force P1 | 173.34 | 52.03 | 89.58 | 233.07 | 64.55 | 27.40 | 33.12 | 87.41 |
| Force P2 | 175.58 | 36.79 | 118.10 | 214.44 | 70.62 | 36.76 | 44.17 | 123.52 |
| Force P3 | 194.78 | 44.63 | 122.16 | 235.47 | 65.86 | 27.01 | 33.29 | 96.20 |
| Force | Equal Variances | Levene’s Test Equality of Variances | t-Test for Equality of Means | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | df | p-Value | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||||
| F | Sig. | Lower | Upper | |||||||
| P0 | Assumed | 0.718 | 0.422 | 3.34 | 8.00 | 0.010 | 113.45 | 34.01 | 35.02 | 191.88 |
| Not Assumed | 3.34 | 6.37 | 0.014 | 113.45 | 34.01 | 31.38 | 195.52 | |||
| P1 | Assumed | 0.342 | 0.575 | 4.14 | 8.00 | 0.003 | 108.80 | 26.30 | 48.15 | 169.44 |
| Not Assumed | 4.14 | 6.06 | 0.006 | 108.80 | 26.30 | 44.60 | 172.99 | |||
| P2 | Assumed | 0.164 | 0.696 | 4.51 | 8.00 | 0.002 | 104.96 | 23.26 | 51.33 | 158.59 |
| Not Assumed | 4.51 | 8.00 | 0.002 | 104.96 | 23.26 | 51.33 | 158.59 | |||
| P3 | Assumed | 0.666 | 0.438 | 5.53 | 8.00 | 0.001 | 128.91 | 23.33 | 75.12 | 182.71 |
| Not Assumed | 5.53 | 6.58 | 0.001 | 128.91 | 23.33 | 73.03 | 184.79 | |||
| Force P0 | Force P1 | Force P2 | Force P3 | |
|---|---|---|---|---|
| Force P0 | 1 | 0.900 | 0.757 | 0.789 |
| Force P1 | 0.900 | 1 | 0.942 | 0.948 |
| Force P2 | 0.757 | 0.942 | 1 | 0.975 |
| Force P3 | 0.789 | 0.948 | 0.975 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, M.; Maranha, V.; Moita, F.; Cruz, N.; Rasteiro, D.; Carvalho, F.; Lains, J.; Roseiro, L. Biomechanical Device for Measurement of Adductors Strength and Aid in Self-Catheterisation of Spastic Patients. Designs 2022, 6, 7. https://doi.org/10.3390/designs6010007
Cruz M, Maranha V, Moita F, Cruz N, Rasteiro D, Carvalho F, Lains J, Roseiro L. Biomechanical Device for Measurement of Adductors Strength and Aid in Self-Catheterisation of Spastic Patients. Designs. 2022; 6(1):7. https://doi.org/10.3390/designs6010007
Chicago/Turabian StyleCruz, Maria, Vítor Maranha, Fernando Moita, Nuno Cruz, Deolinda Rasteiro, Filipe Carvalho, Jorge Lains, and Luis Roseiro. 2022. "Biomechanical Device for Measurement of Adductors Strength and Aid in Self-Catheterisation of Spastic Patients" Designs 6, no. 1: 7. https://doi.org/10.3390/designs6010007
APA StyleCruz, M., Maranha, V., Moita, F., Cruz, N., Rasteiro, D., Carvalho, F., Lains, J., & Roseiro, L. (2022). Biomechanical Device for Measurement of Adductors Strength and Aid in Self-Catheterisation of Spastic Patients. Designs, 6(1), 7. https://doi.org/10.3390/designs6010007








